Abstract
Increased coexistence of Alzheimer disease (AD) and type 2 diabetes mellitus (T2DM) suggests that insulin resistance abets neurodegenerative processes, but linkage mechanisms are obscure. Here, we examined insulin signaling factors in brains of insulin-resistant high-fat–fed mice, ob/ob mice, mice with genetically impaired muscle glucose transport, and monkeys with diet-dependent long-standing obesity/T2DM. In each model, the resting/basal activities of insulin-regulated brain protein kinases, Akt and atypical protein kinase C (aPKC), were maximally increased. Moreover, Akt hyperactivation was accompanied by hyperphosphorylation of substrates glycogen synthase kinase-3β and mammalian target of rapamycin and FOXO proteins FOXO1, FOXO3A, and FOXO4 and decreased peroxisome proliferator–activated receptor γ coactivator-1α (PGC-1α) expression. Akt hyperactivation was confirmed in individual neurons of anterocortical and hippocampal regions that house cognition/memory centers. Remarkably, β-amyloid (Aβ1–40/42) peptide levels were as follows: increased in the short term by insulin in normal mice, increased basally in insulin-resistant mice and monkeys, and accompanied by diminished amyloid precursor protein in monkeys. Phosphorylated tau levels were increased in ob/ob mice and T2DM monkeys. Importantly, with correction of hyperinsulinemia by inhibition of hepatic aPKC and improvement in systemic insulin resistance, brain insulin signaling normalized. As FOXOs and PGC-1α are essential for memory and long-term neuronal function and regeneration and as Aβ1–40/42 and phospho-tau may increase interneuronal plaques and intraneuronal tangles, presently observed aberrations in hyperinsulinemic states may participate in linking insulin resistance to AD.
Introduction
Insulin-resistant obesity and the metabolic syndrome are present in one of three adults (1) and progress to type 2 diabetes mellitus (T2DM) in one of four people over 65 years of age. Alzheimer disease (AD) afflicts one of eight people over 65 years of age and one of two people over 85 years of age. Moreover, AD risk in people with T2DM and vice versa is increased twofold (2,3), and fasting glucose intolerance or T2DM was present in four of five Mayo Clinic AD patients (4). It is therefore suspected that insulin-resistant states may predispose the brain to development of late-onset AD.
Mechanisms that might link insulin-resistant states with AD are obscure. Obesity/T2DM-associated vascular disease may contribute, but alterations in insulin signaling factors in AD brains raise the suspicion of a relationship. In particular, human AD brains show diminished insulin receptor levels and impairments in insulin receptor substrate (IRS)-1 (5,6), which, in some tissues, activates both protein kinase B (mammalian homolog to viral-Akt [Akt]) and atypical protein kinase C (aPKC), the two intracellular mediators of most insulin effects. However, in liver, although IRS-1 activates Akt, IRS-2 activates aPKC (7), and information on IRS-1 and IRS-2 in brain is lacking. Nevertheless, as systemic insulin resistance is often considered to be generalized, it is postulated that the brain is similarly insulin resistant and abets AD development (8–11). To reinforce this idea, it is theorized that insulin-stimulated Akt phosphorylates/inhibits glycogen synthase kinase-3β (GSK3β), which, by phosphorylating threonine (Thr)-231-tau (12,13), increases intraneuronal phospho (p)-tau tangles, which, along with interneuronal β-amyloid (Aβ1–40/42) peptide–containing plaques, promotes AD pathology (14). With this rationale, intranasal insulin therapy is now in clinical trials for the treatment of putative brain insulin resistance in AD (15).
However, insulin resistance is not necessarily generalized. Indeed, insulin-sensitive pathways extrinsic to that responsible for impaired glucose metabolism may be intact and hyperactivated. For example, in the initial phases of insulin resistance in high-fat–fed (HFF) (16) and ob/ob (17) mice, the level of hepatic aPKC is inordinately increased by diet-derived ceramide; aPKC selectively impairs insulin/Akt regulation of hepatic FOXO1; and subsequent increases in peroxisome proliferator–activated receptor γ coactivator-1α (PGC-1α) provoke increases in gluconeogenesis, glucose intolerance, systemic insulin resistance, and hyperinsulinemia, which increases still-intact hepatic Akt activity and lipogenesis (16,17). Additionally, in livers of obese humans and humans with T2DM, insulin activation of IRS-2 is conserved and further activates aPKC and lipogenesis, even when IRS-1 and Akt are downregulated (18,19).
To explore the relationships between systemic insulin resistance and neurodegenerative disorders, we examined insulin signaling in brains of mice with diet-induced obesity (DIO), HFF mice, and hyperphagic ob/ob mice consuming high-carbohydrate chow (16,17); mice in which insulin resistance is provoked by impaired muscle glucose transport owing to heterozygous muscle-specific PKC-λ knockout (Het-MλKO) (20,21); and monkeys with long-standing diet-dependent obesity and T2DM. In these models, brain insulin-signaling factors were maximally activated, thus leading to 1) hyperphosphorylation/inactivation of FOXO proteins and subsequent decreases in PGC-1α that together maintain neuronal function/integrity and 2) insulin-dependent increases in Aβ1–40/42 peptides and p-tau that may promote AD plaque and tangle formation.
Research Design and Methods
Mouse Studies
Brains were harvested from mice used in previous studies of insulin signaling and resistance in liver and muscle: 1) 4- to 6-month-old male C57BL/6 ob/ob and lean ob+ mice (The Jackson Laboratory, Bar Harbor, ME) (17), 2) 4- to 6-month-old male and female C57BL/6 mice used in studies of high-fat feeding (colony in University of South Florida [USF] vivarium) (16), and 3) 10- to 12-month-old C57BL/6 male and female Het-MλKO mice and littermate wild-type mice (colony in USF vivarium) (20,21). Males and females were comparably present in experimental groups 2 and 3, as sex did not alter the combined findings; hepatic alterations and clinical characteristics were reported previously (16,17,20,21). Mice were housed in environmentally controlled rooms and randomly fed standard mouse chow supplying 10% of calories from fat or diets supplying either 40% or 60% of calories from fat (Harlan Laboratories, Madison, WI) (16). Note that insulin-signaling results in brains (and liver and muscle) of mice fed 40% kcal fat and 60% kcal fat diets were comparable and therefore combined.
Where indicated, Het-MλKO mice were randomly injected subcutaneously daily for 8 days with aurothiomalate (ATM) (60 mg/kg body wt [bw]) or [1H-imidazole-4-carboxamide,5-amino]-[2,3-dihydroxy-4-[(phosphono-oxy)methyl]cyclopentane-[1R-(1a,2b,3b,4a)] (ICAPP) (0.4 mg/kg/bw) in saline to selectively inhibit hepatic aPKC and reduce serum insulin levels (21). Where indicated, mice were randomly treated with insulin (1 unit/kg/bw i.p.) 15 min before being euthanized by administration of xylazine/ketamine, followed by whole-body perfusion with PBS and rapid removal of brain and other tissues.
To summarize, the following schedules were used: HFF mice, age 4–6 months, normal or HFF diet × 10 weeks, intraperitoneal insulin administration 15 min before killing; ob/ob mice, age 4–6 months, normal diet, intraperitoneal insulin administration 15 min before killing; and Het-MλKO mice, age 10–12 months, normal diet, once-daily subcutaneous ATM administration for 8 days before killing, intraperitoneal insulin administration 15 min before killing.
All experimental procedures involving mice were approved by the institutional animal care and use committees of the USF Morsani College of Medicine or the Roskamp Institute, and the James A. Haley Veterans Hospital Research and Development Committee.
Monkey Studies
Brains were obtained immediately postmortem from 16- to 30-year-old male and female lean nondiabetic and obese/T2DM Macaca mulatta rhesus monkeys. Diabetic monkeys had been maintained on ad libitum standard monkey chow and treated with standard daily insulin regimens similar to those of patients with advanced T2DM, as described previously (22,23). Euthanitization was initiated with ketamine-HCl (10–15 mg/kg/bw) followed by intravenous Euthasol (0.22 mL/kg/bw). Brain samples were frozen in liquid N2 and stored at −160°. Experimental procedures involving monkeys, including euthanitization, were approved by the USF Morsani College of Medicine Institutional Animal Care and Use Committee.
Tissue Preparations
Mouse brains were hemisected sagittally, and one half was frozen in liquid N2, stored at −80°C, and subsequently homogenized in buffer containing 0.25 mol/L sucrose, 20 mmol/L Tris/HCl (pH 7.5), 2 mmol/L EGTA, 2 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, 20 μg/mL leupeptin, 10 μg/mL aprotinin, 2 mmol/L Na4P2O7, 2 mmol/L Na3VO4, 2 mmol/L NaF, and 1 μmol/L microcystin and then supplemented with 1% Triton X-100, 0.6% Nonidet, and 150 mmol/L NaCl. The remaining brain halves were fixed in 4% paraformaldehyde and embedded in paraffin, and 4-μm sagittal sections were prepared on a microtome for immunohistochemical staining.
Western Analyses
Western analyses were conducted with rabbit antisera or mouse monoclonal antibodies (16–21) using the following: anti-aPKC (Santa Cruz Biotechnology, Santa Cruz, CA); anti–p-Thr-560/555-PKC-ζ/λ/ι (Invitrogen, Carlsbad, CA); anti–p-serine (Ser)-256-FOXO1 and anti-FOXO1 (Abnova, Walnut, CA); anti-Akt (mouse monoclonal antibodies), anti-p-Ser-473-Akt, anti–p-Ser-9-GSK3β, anti-GSK3β, anti–p-Ser-253-FOXO3a, anti-FOXO3a, anti–p-Ser-256-FOXO1, anti–p-Ser-193-FOXO4, anti-FOXO1, anti–p-Ser-2448-mammalian target of rapamycin (mTOR), anti-mTOR, anti–Aβ1–40/42 (5–10 kDa), and anti–amyloid precursor protein (APP; 120 kDa) (Cell Signaling Technology, Danvers, MA); and anti–p-Ser-202-tau and anti–p-Thr-231-tau (GeneTex, Irvine, CA). Samples from experimental groups were compared on the same blots and routinely checked with loading controls. Note that 75-kDa aPKC is largely PKC-λ in mouse brain and 98% homologous PKC-ι in brains of monkeys, humans, and other primates; PKC-ζ in brain exists largely as a 50-kDa moiety that, lacking a regulatory domain, is constitutively active and unresponsive to insulin; 50-kDa PKC-ζ (also called PKM-ζ) is produced by the operation of an intronal promoter and downstream transcription start site and is postulated to function in long-term memory potentiation (24); however, this idea has been challenged (25,26).
Immunohistochemistry
The 4-μm brain sections were deparaffinized in an automated system (Discovery XT; Ventana Medical Systems) with EZ Prep solution. A heat-induced antigen retrieval method was used in Cell Conditioning 1 solution. Rabbit primary antibody reacting with pAkt (ab81283; Abcam, Cambridge, MA) was used at a 1:200 dilution in PSS Diluent for antibodies (Ventana Medical Systems) and was incubated for 32 min, followed by OmniMap anti-rabbit secondary antibody (Ventana Medical Systems) for 20 min. For detection, the Discovery ChromoMap DAB Kit (Ventana Medical Systems) was used; slides were then counterstained with hematoxylin, dehydrated, and coverslipped. A BX53 Microscope (Olympus) and cellSens Dimension software were used to obtain pictures with ×10 and ×60 objectives.
Statistical Analyses
Data are expressed as the mean ± SEM and were compared using one-way ANOVA and Tukey post hoc test for analysis of significance.
Results
Studies in HFF and ob/ob Mice
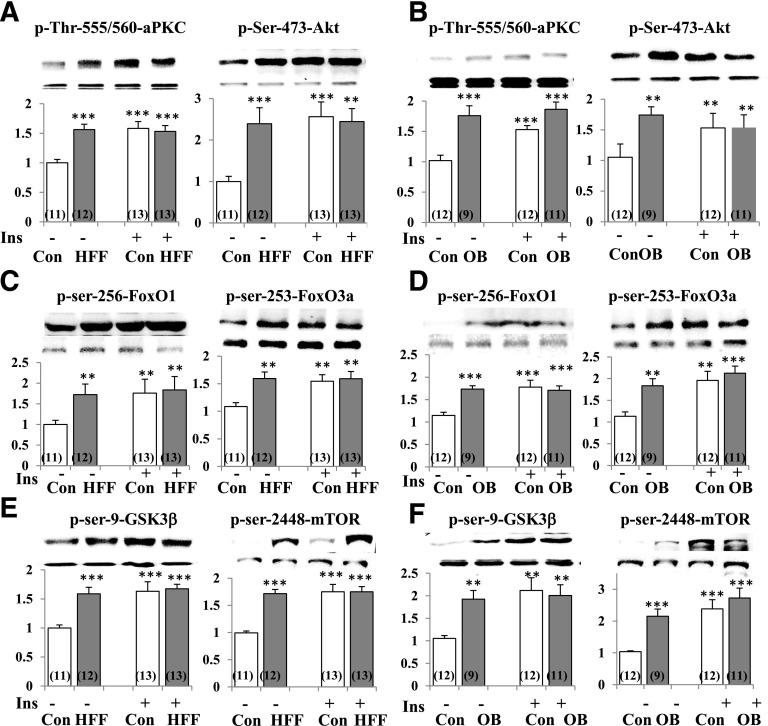
In control mice, short-term maximal insulin treatment provoked increases in brain activities (as per phosphorylation) of Akt and aPKC (Fig. 1). Surprisingly, however, resting/“basal” activities of Akt and aPKC were maximally or near maximally elevated, presumably by preexisting hyperinsulinemia (16,17), in brains of HFF and ob/ob mice because there was little or no response of either Akt or aPKC to exogenous insulin (Fig. 1). With Akt activation, phosphorylation of multiple Akt substrates, namely, GSK3β, mTOR, FOXO1, FOXO3a, and FOXO4, was elevated in the resting/basal state in brains of HFF and ob/ob mice and, here again, maximally or near maximally because short-term insulin treatment had little or no additive effect (Fig. 1) (the level of p-FOXO4 was similarly increased but not shown).
Figure 1.
Resting/basal and insulin-stimulated phosphorylation/activity of aPKC and Akt (A and B) and phosphorylation of Akt substrates FOXO1, FOXO3a (C and D), GSK3β, and mTOR (E and F) in brains of control (Con), HFF, and ob/ob (OB) mice. Where indicated, mice were treated with insulin (Ins) (1 unit/kg bw i.p.) 15 min before being killed. Representative blots of phosphoproteins (top bands) and unaltered total protein levels (bottom bands) are shown. Portrayed values of phosphoprotein levels are mean ± SEM of (N) mice. **P < 0.01 and ***P < 0.001 for comparison of HFF or OB values to control values (ANOVA).
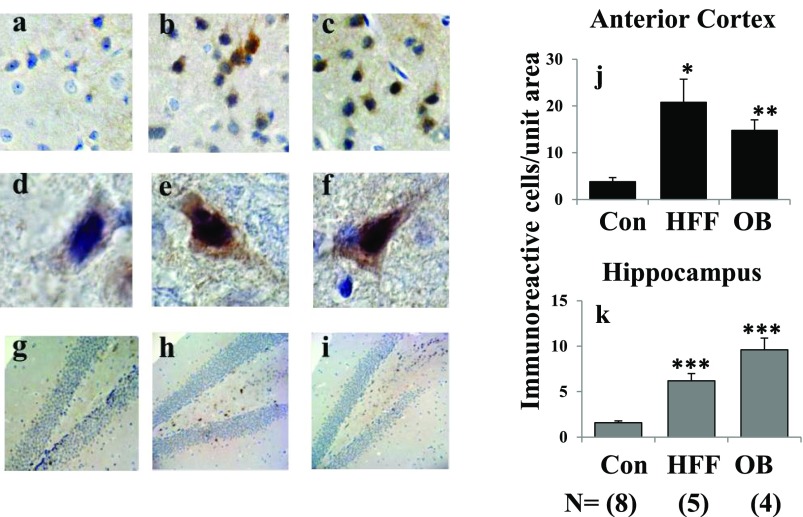
We also used immunohistochemical methods to examine Akt activation in individual neurons in the anterior cortex and hippocampus. In this regard, it has previously been shown (27) that insulin-signaling responses are seen largely in neurons, rather than glial or endothelial cells, most notably in neurons of the hippocampus and hypothalamus. Similarly, Akt activity was largely confined to neurons, most prominently and significantly increased in hippocampal and anterocortical regions of HFF and ob/ob mice (Fig. 2).
Figure 2.
Phosphorylation/activation of Akt in anterior cortical and hippocampal regions of brains of control (Con) (panels a, d, and g), HFF (panels b, e, and h), and ob/ob (OB) (panels c, f, and i) mice. Pictures show representative examples of (brown) immunostaining of p-Ser-473-Akt: top row, ×10 magnification, anterocortical sagittal sections; middle row, ×60 magnification, anterocortical neurons; and bottom row, ×10 magnification, hippocampus. Portrayed values are mean ± SEM of (N) mice and reflect relative staining of p-Ser-473-Akt per standard area of anterior cortex (j) and hippocampus (k). *P < 0.05, **P < 0.01, and ***P < 0.001 for HFF or ob/ob vs. Con mice (ANOVA).
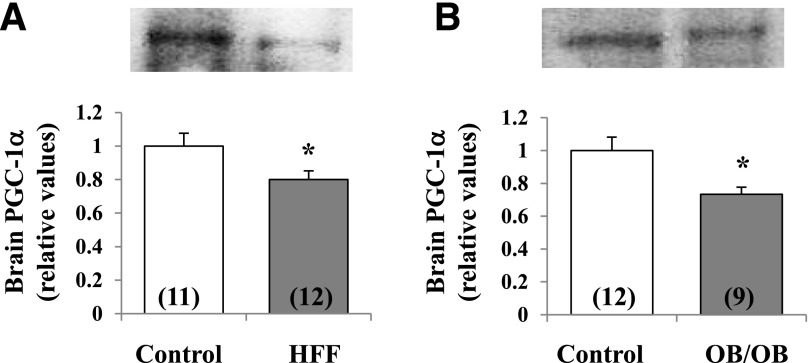
In keeping with the fact that FOXO1 controls PGC-1α expression and FOXO1 phosphorylation would diminished its activity, PGC-1α levels were diminished in HFF and ob/ob mice (Fig. 3).
Figure 3.
Diminished levels of PGC-1α in brains of HFF (A) and ob/ob (B) mice. Portrayed values are mean ± SEM of (N) mice. *P < 0.05 for comparison of HFF or OB control mice (ANOVA).
Studies in Het-MλKO Mice and Reversal of Brain-Signaling Aberrations by Correction of Insulin Resistance
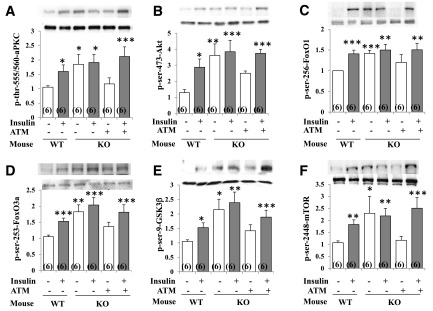
In addition to DIO mice, in which insulin resistance starts in liver (15,16), we studied Het-MλKO mice, in which insulin resistance is initiated by impaired glucose transport in muscle; and resultant hyperinsulinemia hyperactivates hepatic aPKC, causing excessive activation of gluconeogenic and lipogenic pathways that contribute importantly to glucose intolerance, insulin resistance, abdominal obesity, hyperlipidemia, hepatosteatosis, and mild/moderate T2DM (20,21). As in HFF and ob/ob mice, resting/basal activities of Akt and aPKC and phosphorylation levels of FOXO1, FOXO3a, GSK3β, and mTOR were maximally or near maximally increased in brains of Het-MλKO mice because short-term insulin treatment increased phosphorylation of these factors in control wild-type mice but not in Het-MλKO mice (Fig. 3).
As reported previously (21), tissue-selective inhibition of hepatic aPKC by ATM not only effectively diminished hepatic biochemical and clinical abnormalities but, moreover, reduced serum insulin levels to normal in Het-MλKO mice. It was therefore important to find that this marked improvement in hyperinsulinemia in ATM-treated Het-MλKO mice was attended by reductions in resting/basal activities of brain Akt and aPKC to levels comparable to those seen in wild-type mice; further, with diminished Akt activity, the phosphorylation of FOXO1, FOXO3a, GSK3β, and mTOR similarly diminished to near-normal levels (Fig. 4). Moreover, with decreases in resting/basal Akt and aPKC activities and Akt substrate phosphorylations after treatment of Het-MλKO mice with ATM, short-term insulin treatment provoked essentially normal increases in these parameters (Fig. 4), indicating a restoration of normal brain insulin-signaling mechanisms.
Figure 4.
Effects of liver-selective aPKC inhibitor ATM on resting/basal and insulin-stimulated phosphorylation/activity of aPKC and Akt and phosphorylation of Akt substrates FOXO1, FOXO3a, GSK3β, and mTOR in brains of wild-type (WT) control and Het-MλKO (KO) mice. Where indicated, ad libitum–fed mice were treated with insulin (1 unit/kg bw i.p.) 15 min before being killed. Where indicated, Het-MλKO mice were injected once daily for 8 days with aPKC inhibitor ATM (60 mg/kg bw s.c.), which reversed hyperinsulinemia (21). Portrayed values of phosphoproteins are reported as the mean ± SEM of 6 mice. *P < 0.05, **P < 0.01, and ***P < 0.001 (ANOVA) for comparison of values of indicated groups vs. values of the WT control group.
In addition to ATM, treatment of Het-MλKO mice with another liver-selective aPKC inhibitor, ICAPP, similarly reduced serum insulin levels in Het-MλKO mice to normal (21), and this normalization was attended by reductions of resting/basal activities of brain Akt and aPKC, comparable to those seen with ATM treatment (data not shown).
Studies in T2DM Monkeys
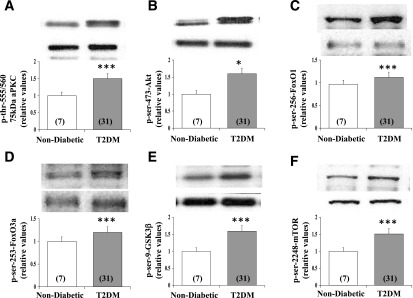
As mouse insulin resistance syndromes were relatively short-lived and mild, we studied monkeys with long-standing obesity and insulin-requiring T2DM (Supplementary Table 1, clinical characteristics). As in mouse models, phosphorylation/activities of Akt and aPKC and phosphorylation of Akt substrates FOXO1, FOXO3a, GSK3β, and mTOR were increased in brains of T2DM monkeys (Fig. 5).
Figure 5.
Resting/basal and insulin-stimulated phosphorylation/activity of the 75-kDa aPKC (largely PKC-ι) (A) and Akt (B) and phosphorylation of Akt substrates FOXO1 (C), FOXO3a (D), GSK3β (E), and mTOR (F) in brains of nondiabetic and T2DM monkeys. Portrayed values of phosphoprotein levels are the mean ± SEM of (N) monkeys. *P < 0.05 and ***P < 0.001 for comparison of T2DM and nondiabetic monkeys (ANOVA).
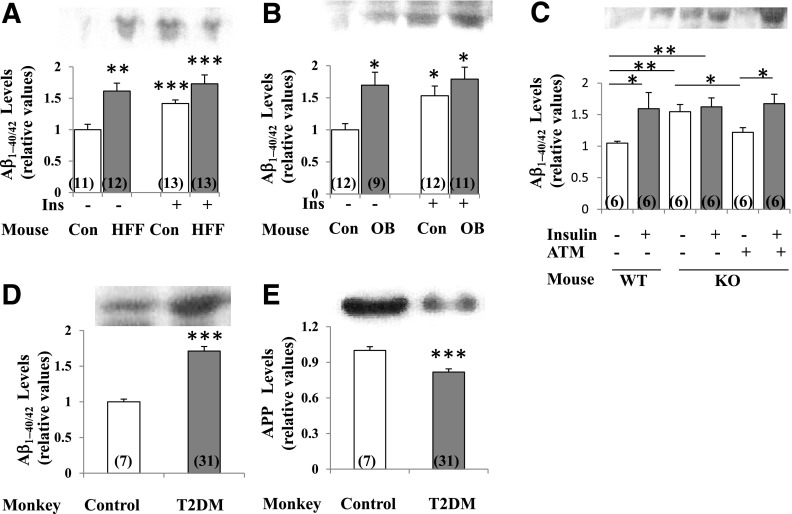
Aβ1–40/42 Peptide and APP Levels in Mouse and Monkey Brain
Particularly important, the levels of Aβ1–40/42 peptide, which may accumulate to form amyloid plaques, were increased in the short term by insulin in brains of control mice and in the resting/basal state in HFF, ob/ob, and Het-MλKO mice and obese/T2DM monkeys (Fig. 6A–D). Moreover, levels of 120-kDa APP, the Aβ1–40/42 precursor, were diminished in obese/T2DM monkeys (Fig. 6E), but not in HFF or ob/ob mice (data not shown). Also note that the correction of hyperinsulinemia in Het-MλKO mice diminished resting/basal levels of Aβ1–40/42 peptides and restored the ability of insulin to increase levels of Aβ1–40/42 peptides (Fig. 6C).
Figure 6.
Levels of Aβ1–40/42 peptides in brains of HFF mice (A), ob/ob mice (B), Het-MλKO (KO) mice (C), and T2DM monkeys (D); levels of APP levels in brains of monkeys (E); reversal of basal increases in Aβ1–40/42 peptides by treatment of hepatic aPKC with liver-selective aPKC inhibitor ATM (60 mg/kg bw/day) and reversal of hyperinsulinemia (21) (C); and effects of 15-min insulin treatment (1 unit/kg bw) on Aβ1–40/42 peptide levels in mouse models (A–C). Representative blots for 5- to 10-kDa Aβ1–40/42 and 120-kDa APP are shown. Portrayed values of Aβ1–40/42 and APP are the mean ± SEM of (N) mice or monkeys. *P < 0.05, **P < 0.01, and ***P < 0.001 (ANOVA) for comparison of HFF mice, ob/ob mice, or T2DM monkeys to respective controls. Con, control mice; Ins, insulin; OB, ob/ob mice; WT, wild type.
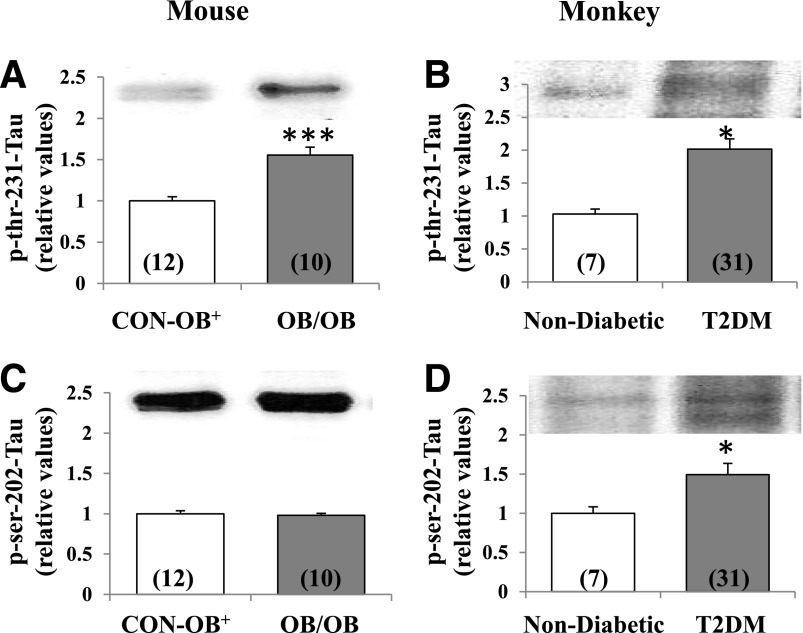
Tau Phosphorylation in Mouse and Monkey Brain
Despite hyperactivation of Akt and subsequent hyperphosphorylation of GSK3β in brains of HFF and Het-MλKO mice, phosphorylation of Thr-231-tau was not diminished, as might be expected with Akt-dependent decreases in GSK3β activity (13,14); also, phosphorylation of Thr-231-tau and Ser-202-tau was similarly not significantly altered in these mice (data not shown). Moreover, despite increased phosphorylation/inactivation of GSK3β, phosphorylation of Thr-231-tau was increased in both ob/ob mice and T2DM monkeys (Fig. 7). Further, phosphorylation of Ser-202-tau was increased in brains of T2DM monkeys (Fig. 7).
Figure 7.
Resting/basal phosphorylation of Thr-231-tau and Ser-202-tau in brains of lean control ob+ (CON-OB+) vs. ob/ob (OB/OB) mice (A and C), and nondiabetic vs. T2DM monkeys (B and D). Portrayed values of phosphoprotein levels are the mean ± SEM of (N) mice or monkeys. *P < 0.05 and ***P < 0.001 (ANOVA) for comparison of ob/ob mice or T2DM monkeys with lean ob+ control mice or nondiabetic monkeys.
Insulin Receptor Levels in Mouse and Monkey Brains
Although brain insulin receptor levels may be diminished in the AD brain (5,6), levels of the α-subunit and β-subunit of the insulin receptor were not altered in HFF mice, ob/ob mice, and T2DM monkeys (Supplementary Fig. 1).
Discussion
Our findings show that insulin signaling to the two major protein kinases that elicit many/most insulin effects, Akt and aPKC, is increased in brain, both at early stages of insulin resistance in HFF, ob/ob, and Het-MλKO mice and at later stages in obese/T2DM monkeys. The findings of increased insulin signaling in brains of insulin-resistant mice and monkeys are important because this hyperinsulinization leads to or is accompanied by not only the downregulation of factors needed for maintaining neuronal function and longevity, FOXOs and PGC-1α, but also by the upregulation of factors that may contribute directly to AD pathology, Aβ1–40/42 peptides and p-tau. Accordingly, excessive activation of insulin signaling in brain may explain, in part, the linkage between obesity/T2DM and AD.
The present findings further show that, contrary to previous speculations (9–12), insulin resistance in brain and the resultant increases in the activity of GSK3β are unlikely to serve as long-standing predisposing factors for AD development, and impairments in insulin-signaling factors that are seen in brains of human AD patients (5,6,9–12) are either later-appearing predisposing abnormalities or consequences, rather than predisposing causes, of AD pathology.
It may seem surprising that unlike muscle, where insulin signaling to Akt and aPKC is downregulated in insulin-resistant DIO mice (16,17) and humans with T2DM (28), and unlike liver, where insulin signaling to Akt (but not aPKC, which is hyperactivated by lipids plus hyperinsulinemia) is downregulated in obese and humans with T2DM (18,19), insulin signaling to both Akt and aPKC is hyperactive in brain. This paradoxical retention of central nervous system (CNS) insulin signaling in insulin-resistant states may reflect that insulin controls Akt and aPKC by different IRSs in different tissues. Thus, in muscle IRS-1 controls both Akt and aPKC, and IRS-1 is commonly downregulated in insulin-resistant conditions; however, in liver, whereas IRS-1 mainly controls Akt and is commonly downregulated, IRS-2 controls aPKC and is well conserved in T2DM (18,19). That total brain and anterior cortical and hippocampal areas were resistant to downregulation in presently studied insulin resistance models may reflect that IRS-2 preferentially mediates certain CNS functions (29); however, the role of IRS-1 in relation to IRS-2 throughout the CNS remains uncertain.
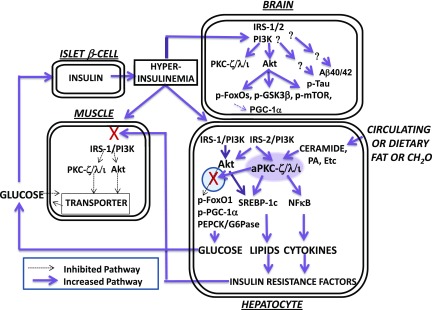
It may be noted that presently observed increases in brain FOXO phosphorylation are the opposite of decreases in hepatic FOXO1 that are seen in insulin-resistant states (Fig. 8). In this regard, during insulin action in normal liver, Akt-dependent phosphorylation of FOXO1 occurs in a subcellular compartment defined by the 40-kDa scaffolding protein, WD(tryp-asp-x-x) repeat, propeller-like, FYVE-containing protein (WD40/ProF), and, similar to Akt, aPKC is recruited to the WD40/ProF platform and may restrain Akt but still allows sufficient FOXO1 phosphorylation to inhibit PGC-1α and gluconeogenic enzyme expression (16,17). However, in livers of HFF (16), ob/ob (17), and Het-MλKO (M.S., R.I., R.V.F., unpublished observations) mice and obese human and humans with T2DM (18,19), aPKC accumulation on the WD40/ProF platform is excessive owing to increases in ceramide (16,17,19), a potent noninsulin activator of hepatic aPKC (16) (Fig. 8). Moreover, these excessive increases in hepatic aPKC activity in insulin-resistant states markedly reduce Akt2 levels on the WD40/ProF platform (16–19), thus specifically and substantially impairing FOXO1 phosphorylation, thereby increasing PGC-1α level and the expression of gluconeogenic enzymes (Fig. 7). The ensuing hyperinsulinemia, at least initially, hyperactivates hepatic Akt and further activates ceramide-activated hepatic aPKC, thus increasing hepatic lipogenesis (16–19). Most importantly, in vivo treatment of HFF (16), ob/ob (17), and Het-MλKO (21) mice and in vitro treatment of hepatocytes of humans with T2DM (18,19) with liver-selective aPKC inhibitors diminishes hepatic aPKC association with WD40/ProF, restores hepatic Akt association with WD40/ProF, increases hepatic FOXO1 phosphorylation, decreases the level of PGC-1α, and decreases the expression of hepatic gluconeogenic and lipogenic enzymes. These hepatic improvements are followed by reductions in serum insulin levels (21), which, as presently seen in Het-MλKO mice, were sufficient to largely normalize brain insulin signaling.
Figure 8.
Hyperactivation of insulin-dependent signaling factors in brain of mice with DIO and systemic insulin resistance. Note that lipid- and carbohydrate-rich diets provoke increases in hepatic ceramide, which potently activates hepatic aPKC, which thereupon selectively inhibits hepatic Akt-dependent FOXO1 phosphorylation within a subcellular compartment (defined by the presence of scaffolding protein WD40/ProF) shared by Akt and aPKC. As a result, there are increases in hepatic FOXO1 and PGC-1α activity and subsequent expression and abundance of PGC-1α and gluconeogenic enzymes, PEPCK and G6Pase. With increased release of glucose from liver, hyperinsulinemia ensues and stimulates “open” (unblocked) insulin-regulated pathways. Thus, in early stages (as depicted here and further reviewed in the study by Sajan et al. [16]), hyperinsulinemia provokes increases in hepatic Akt activity and further increases in hepatic aPKC activity. These increases in the activities of hepatic Akt and aPKC, in turn, provoke increases in the expression and abundance of hepatic lipogenic enzymes and proinflammatory factors, and these, and/or other liver-derived factors, impair insulin signaling to IRS-1, IRS-1–dependent phosphatidylinositol 3-kinase (PI3K), Akt, and aPKC in muscle, thereby impairing glucose transport and glycogen synthesis therein. In later stages (not depicted here), the activation of hepatic IRS-1, IRS-1–dependent PI3K, and Akt diminishes (also secondary to, or abetted by, excessive aPKC activity), but, in contrast, the activation of hepatic IRS-2 and IRS-2–dependent PI3K remains intact and contributes to continued hyperactivation of hepatic aPKC. In human hepatocytes, these abnormalities are further heightened by the fact that the expression and abundance of the primate-specific aPKC, PKC-ι, is strongly autostimulated by aPKC itself through a positive-feedback loop that is intensified by insulin and ceramide, thus creating a vicious cycle within the liver. As a result of hyperinsulinemia, the activities of Akt and aPKC are increased in brain, and phosphorylation of FOXO family members and other Akt substrates are similarly increased. As a result of increases in brain FOXO phosphorylation, FOXO activity is diminished, and this leads to decreases in brain PGC-1α activity and levels. Tau phosphorylation is also increased in brains of insulin-resistant mice and monkeys, but the responsible protein kinase remains uncertain. Although not depicted here (but described in the text), tissue-selective inhibition of hepatic aPKC can reverse abnormalities in liver, muscle, and brain. NFκB, nuclear factor-κB; PA, phosphatidic acid.
Increases in Akt-dependent phosphorylation of FOXO proteins in brains of presently studied insulin-resistant models are noteworthy because considerable evidence suggests that phosphorylation-induced inhibition of FOXO1, FOXO3a, FOXO4, and FOXO6 may diminish transcriptional functions of FOXOs and FOXO1-dependent PGC-1α, which, over long periods, may be detrimental. Thus, note that the brain contains FOXO1, FOXO3a, FOXO4, FOXO6, and insulin via Akt phosphorylates and inhibits all brain FOXOs (30–32) and FOXO1-regulated PGC-1α. Moreover, FOXOs have critically important functions in brain, as revealed by the fact that neuronal-specific knockout of either FOXO3a (33) or FOXO1/FOXO3a/FOXO4 (34) provokes initial increases in neuronal proliferation (perhaps reflecting initially decreased apoptosis), followed later, but perhaps more importantly, by impairments in neuronal differentiation, proliferation, survival, self-renewal, and atrophy as these knockout mice aged. Accordingly, it was proposed that, with diminished FOXO action, there were reduced responses to oxidative stress and hypoxia, upregulation of Wnt signaling, increased expression of the abnormal spindle-like microcephaly-associated gene (Aspm), and a failure to promote reparative quiescence that, in time, led to cellular exhaustion (33,34). And, more recently, it was found (35) that knockout of FOXO6, found almost exclusively in brain, or specific inhibition of FOXO6 in hippocampal centers by viral-mediated expression of inactive FOXO6 leads to defects in recent memory consolidation and neuronal dendrite structure.
As to presently observed insulin/Akt-dependent decreases in FOXO and PGC-1 activities in brains of insulin-resistant animals, also note the following: 1) FOXOs promote DNA repair, resistance to oxidative stress, and cellular and whole-organism longevity (33,34); 2) selective neuronal knockout of the insulin receptor or IGF-I receptor and expected activation of FOXOs and PGC-1α diminish Aβ1–40/42 peptides in transgenic Tg2576/AD mice (36); 3) in Caenorhabditis elegans, knockdown of the insulin/IGF-I–like receptor DAF-2, and subsequent activation of nematode FOXO DAF-16, diminishes Aβ1–40/42 peptide expression and toxicity (37); 4) increases in Akt activity and phosphorylation of Akt substrates are seen in temporal cortical neurons of AD patients, and there are eventual losses of Akt-phosphorylated neurons in the hippocampus in end stages of AD (38,39); 5) increases in Akt activity are seen in enterorhinal, hippocampal, and temporal lobe neurons of AD patients and may contribute to neurofibrillary tangles (40); 6) FOXO3a and FOXO6 expression is decreased in all brain cortical regions in HFF mice (41); 7) resveratrol-induced activation of sirtuin-1 (which, by deacetylation, activates both FOXOs and PGC-1α) protects against neuronal degeneration (42,43); and 8) sirtuin-1, by deacetylation, activates FOXO and its salutary effects on longevity (30,31,43,44).
In concert with our findings, increased Akt and GSK3β phosphorylation has recently been observed in brains of mice consuming a 60% kcal diet (45). Some findings, however, seemed to suggest that brain insulin signaling is diminished with caloric excess and high-fat feeding and furthermore seem to be at odds with the idea that FOXO has long-term salutary effects on neurons. Thus, caloric restriction over 6 months in transgenic Tg2576/AD mice expressing a mutated human APP gene led to seemingly increased phosphorylation of cortical Akt and FOXO3a and attenuated cortical AD pathology (46). Also, 5–6 months of high-fat feeding of Tg2576/AD mice (8) and normal mice (47) led to seemingly diminished phosphorylation of brain Akt and GSK3α/β and increased Aβ1–40/42 peptide generation and AD pathology. In both Tg2576/AD studies (8,46), signaling in normal mice and signaling responses to administered insulin were not determined, and the circulating levels of insulin when brains were taken are unclear. In any case, it was surmised that brain insulin resistance was increased by high-fat feeding and diminished by caloric restriction, and findings were interpreted as suggesting that insulin levels, via Akt, improved AD development and FOXO abetted AD development. The reason for differences in alterations in brain insulin signaling after dietary alterations is uncertain but may reflect the following: differences in mouse age and strain; duration of dietary alterations; and absence of preexisting AD pathology in presently studied mice, as opposed to brains with more advanced AD pathology, especially in Tg2576/AD mice, in which brain insulin resistance may have occurred secondarily. Further, Tg2576 mice develop spontaneous hyperphagia, obesity, and insulin resistance (48,49) and may therefore have increased sensitivity to a high-fat diet with inhibitory effects of fats on brain Akt signaling that are similar to those seen in liver (16,17,19). As to alterations in Aβ1–40/42 peptides seen in previous dietary studies (8,46,47), HFF-induced increases and caloric restriction–induced decreases in plasma insulin levels, rather than reported alterations in Akt signaling (which may not be relevant to insulin effects on Aβ1–40/42 peptide generation), may have contributed to respective increases and decreases in Aβ1–40/42 peptide production. Finally, although long-term loss of brain FOXO activity may be detrimental by impairing FOXO/PGC-1α–dependent neuronal stem cell function (as suggested from neuronal-specific FOXO knockout studies [33–35]), short-term loss of brain FOXO activity may be beneficial by diminishing neuronal apoptosis in established AD.
It was particularly interesting to find that, along with increased phosphorylation/inhibition of FOXO1, the levels of PGC-1α, and presumably its FOXO-dependent activity, were diminished in HFF and ob/ob mice because PGC-1α is important for maintaining mitochondrial biogenesis, oxidative processes, and antioxidant protection; moreover, PGC-1α levels are diminished in human and experimental AD and correlate inversely with Aβ1–40/42 peptide accumulation and mitochondrial dysfunction (50–52). Whether diminished FOXO1 activity was responsible for decreases in PGC-1α remains to be determined because PGC-1α is diversely regulated.
As to increases in tau phosphorylation in brains of ob/ob mice and T2DM monkeys, Ser-202 is phosphorylated by extracellular signal–related kinase and increased by hyperinsulinemia (27). However, the kinase responsible for increased Thr-231-tau hyperphosphorylation in ob/ob mice and T2DM monkeys is uncertain, as GSK3β was hyperphosphorylated (i.e., reduced in Akt-dependent activity). On the other hand, this increase in p-Thr-231-tau may reflect a more advanced stage of glucose intolerance, because increased Thr-231-tau phosphorylation is seen in diabetic db/db and streptozotocin-induced diabetic mice (53) and may reflect activation of extracellular signal–related kinase 2, c-Jun N-terminal kinase, p38 kinase, PKC, or aPKC. Irrespective of the mechanism, increases in p-tau may contribute to intraneuronal fibrillary tangles.
Because β-amyloid plaques in AD are composed of insoluble highly polymerized Aβ1–40/42 peptides, it was particularly important to find that Aβ1–40/42 peptide levels were increased not only basally as seen here and by others (45) in hyperinsulinemic HFF mice but also in ob/ob mice and obese/T2DM monkeys and, perhaps most intriguingly, after short-term insulin treatment in normal mice. Moreover, in monkeys with long-standing insulin resistance, but not in HFF and ob/ob mice with short-lived insulin resistance, the levels of APP were diminished. Taken together, these findings suggest that, in the short term, insulin increases the proteolytic release of Aβ1–40/42 from APP and that this conversion is incremental at first but, over time, leads to measureable decreases in APP levels; further, the released Aβ1–40/42 may be cleared, ironically, by insulin-degrading enzyme but, over time, may also accumulate to produce characteristic AD β-amyloid plaques that impair cognition/memory and promote neuronal atrophy. In this scenario, the underlying mechanism for plaque formation may involve altered activities of α/β/γ-secretases that regulate Aβ1–40/42 peptide release from APP or altered insulin-degrading enzyme activity. Further studies are needed to evaluate these possibilities.
Although the present findings suggest that the brain is initially hyperinsulinized in insulin-resistant states of obesity, the metabolic syndrome, and T2DM, it should be emphasized that with the development and progression of AD pathology, the brain may secondarily become insulin resistant. This transition, however, may be spotty. In any case, until we have more information on the efficacy of intranasal insulin and until we are able to determine whether important brain centers have reached the stage of being insulin resistant, it seems prudent to avoid, or exercise due caution in, using intranasal insulin for the treatment or prevention of AD or other cognitive disorders.
To summarize, the present findings suggest that hyperinsulinemia provokes excessive increases in activities of Akt and aPKC in brains of insulin-resistant mice and monkeys, and this hyperinsulinization leads to decreases in FOXOs and PGC-1α levels and increases in levels of Aβ1–40/42 peptides and p-tau (i.e., changes that may reasonably link insulin-resistant states to AD development). Fortunately, the correction of systemic insulin resistance and hyperinsulinemia can restore normal CNS insulin signaling and abolish this linkage.
Article Information
Funding. This research was supported by funds from the Department of Veterans Affairs Merit Review Program and National Institutes of Health grant RO1-DK-065969-09 to R.V.F.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.S. designed and performed the experiments and assisted in the interpretation and analysis of the data. B.H. provided samples and data for obese type 2 diabetic monkeys. R.I., J.S., C.A., S.S., and M.F.-H. performed the experiments and assays, and assembled the data. U.B. and M.L. genotyped Het-MλKO mice. R.V.F. conceived, designed, and directed the studies; analyzed the data; and wrote the paper. R.V.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1428/-/DC1.
The opinions expressed in this article are those of the authors and do not represent the views of the Department of Veterans Affairs or the U.S. Government.
References
- 1.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–359 [DOI] [PubMed] [Google Scholar]
- 2.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes—systematic overview of prospective observational studies. Diabetologia 2005;48:2460–2469 [DOI] [PubMed] [Google Scholar]
- 3.Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MMB. Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia 1996;39:1392–1397 [DOI] [PubMed] [Google Scholar]
- 4.Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 2004;53:474–481 [DOI] [PubMed] [Google Scholar]
- 5.Frölich L, Blum-Degen D, Bernstein HG, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm (Vienna) 1998;105:423–438 [DOI] [PubMed] [Google Scholar]
- 6.Hoyer S. The aging brain. Changes in the neuronal insulin/insulin receptor signal transduction cascade trigger late-onset sporadic Alzheimer disease (SAD). A mini-review. J Neural Transm (Vienna) 2002;109:991–1002 [DOI] [PubMed] [Google Scholar]
- 7.Sajan MP, Standaert ML, Miura A, Kahn CR, Farese RV. Tissue-specific differences in activation of atypical protein kinase C and protein kinase B in muscle, liver, and adipocytes of insulin receptor substrate-1 knockout mice. Mol Endocrinol 2004;18:2513–2521 [DOI] [PubMed] [Google Scholar]
- 8.Ho L, Qin W, Pompl PN, et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J 2004;18:902–904 [DOI] [PubMed] [Google Scholar]
- 9.Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res 2007;4:147–152 [DOI] [PubMed] [Google Scholar]
- 10.de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res 2012;9:35–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Felice FG. Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest 2013;123:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn RC, Suzuki R. Insulin action in the brain and the pathogenesis of Alzheimer’s disease. In Diabetes, Insulin and Alzheimer’s Disease. Craft S, Ed. New York, Springer-Verlag, 2010, p. 1–20 [Google Scholar]
- 13.Lucas JJ, Hernández F, Gómez-Ramos P, Morán MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J 2001;20:27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J-Z, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci 2007;25:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 2012;69:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sajan MP, Acevedo-Duncan ME, Standaert ML, Ivey RA III, Lee MC, Farese RV. Akt-dependent phosphorylation of hepatic FoxO1 is compartmentalized on a WD40/Propeller/FYVE scaffold and is selectively inhibited atypical PKC in early phases of diet-induced obesity. Diabetes 2014;63:2690–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sajan MP, Ivey RA III, Lee MC, Farese RV. Hepatic insulin resistance in ob/ob mice involves increases in ceramide, aPKC activity, and selective impairment of Akt-dependent FoxO1 phosphorylation. J Lipid Res 2015;56:70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sajan MP, Farese RV. Insulin signalling in hepatocytes of type 2 diabetic humans. Excessive expression and activity of PKC-ι and dependent processes and reversal by PKC-ι inhibitors. Diabetologia 2012;55:1446–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sajan MP, Ivey RA III, Farese RV. BMI-related progression of atypical PKC-dependent aberrations in insulin signaling through IRS-1, Akt, FoxO1 and PGC-1α in livers of obese and type 2 diabetic humans. Metabolism 2015;64:1454–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farese RV, Sajan MP, Yang H, et al. Muscle-specific knockout of PKC-λ impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 2007;117:2289–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sajan MP, Nimal S, Mastorides S, et al. Correction of metabolic abnormalities in a rodent model of obesity, metabolic syndrome, and type 2 diabetes mellitus by inhibitors of hepatic protein kinase C-ι. Metabolism 2012;61:459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen BC. Investigation and treatment of type 2 diabetes in nonhuman primates. Methods Mol Biol 2012;933:177–185 [DOI] [PubMed] [Google Scholar]
- 23.Lee HW, Muniyappa R, Yan X, et al. Comparison between surrogate indexes of insulin sensitivity/resistance and hyperinsulinemic euglycemic glucose clamps in rhesus monkeys. Endocrinology 2011;152:414–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacktor TC. PKMzeta, LTP maintenance, and the dynamic molecular biology of memory storage. Prog Brain Res 2008;169:27–40 [DOI] [PubMed] [Google Scholar]
- 25.Lee AM, Kanter BR, Wang D, et al. Prkcz null mice show normal learning and memory. Nature 2013;493:416–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volk LJ, Bachman JL, Johnson R, Yu Y, Huganir RL. PKM-ζ is not required for hippocampal synaptic plasticity, learning and memory. Nature 2013;493:420–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freude S, Plum L, Schnitker J, et al. Perpheral hyperinsulinemia promotes tau phosphorylation in vivo. Diabetes 2005;54:3343–3348 [DOI] [PubMed] [Google Scholar]
- 28.Beeson M, Sajan MP, Dizon M, et al. Activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes 2003;52:1926–1934 [DOI] [PubMed] [Google Scholar]
- 29.White MF. IRS2 integrates insulin/IGF1 signalling with metabolism, neurodegeneration and longevity. Diabetes Obes Metab 2014;16(Suppl. 1):4–15 [DOI] [PubMed] [Google Scholar]
- 30.Polter A, Yang S, Zmijewska AA, et al. Forkhead box, class O transcription factors in brain: regulation and behavioral manifestation. Biol Psychiatry 2009;65:150–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ausserlechner MJ, Haugenbuchner J, Fuchs S, Geiger K, Obexer P. FOXO transcription factors as potential therapeutic targets in neuroblastoma. In Neuroblastoma: Present and Future. Shimada H, Ed. Rijecha, Croatia, InTech, 2012 [Google Scholar]
- 32.Salih DAM, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol 2008;20:126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renault VM, Rafalski VA, Morgan AA, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell 2009;5:527–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paik J-H, Ding Z, Narurkar R, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 2009;5:540–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salih DAM, Rashid AJ, Colas D, et al. FoxO6 regulates memory consolidation and synaptic function. Genes Dev 2012;26:2780–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stöhr O, Schilbach K, Moll L, et al. Insulin receptor signaling mediates APP processing and β-amyloid accumulation without altering survival in a transgenic mouse model of Alzheimer’s disease. Age (Dordr) 2013;35:83–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science 2006;313:1604–1610 [DOI] [PubMed] [Google Scholar]
- 38.Griffin RJ, Moloney A, Kelliher M, et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem 2005;93:105–117 [DOI] [PubMed] [Google Scholar]
- 39.Rickle A, Bogdanovic N, Volkman I, Winblad B, Ravid R, Cowburn RF. Akt activity in Alzheimer’s disease and other neurodegenerative disorders. Neuroreport 2004;15:955–959 [DOI] [PubMed] [Google Scholar]
- 40.Pei J-J, Khatoon S, An W-L, et al. Role of protein kinase B in Alzheimer’s neurofibrillary pathology. Acta Neuropathol 2003;105:381–392 [DOI] [PubMed] [Google Scholar]
- 41.Zemva J, Schilbach K, Stöhr O, et al. Central FoxO3a and FoxO6 expression is down-regulated in obesity induced diabetes but not in aging. Exp Clin Endocrinol Diabetes 2012;120:340–350 [DOI] [PubMed] [Google Scholar]
- 42.Anekonda TS. Resveratrol—a boon for treating Alzheimer’s disease? Brain Res Brain Res Rev 2006;52:316–326 [DOI] [PubMed] [Google Scholar]
- 43.Kim D, Nguyen MD, Dobbin MM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J 2007;26:3169–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diatoku H, Sakamaki J, Fukamizu A. Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochim Biophys Acta 2011;1813:1954–1960 [DOI] [PubMed] [Google Scholar]
- 45.MacPherson REK, Baumeister P, Peppler WT, Wright DC, Little JP. Reduced cortical BACE1 content with one bout of exercise is accompanied by declines in AMPK, Akt, and MAPK signaling in obese, glucose-intolerant mice. J Appl Physiol (1985) 2015;119:1097–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin W, Zhao W, Ho L, et al. Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer’s disease-type amyloid neuropathology and spatial memory deterioration. Ann N Y Acad Sci 2008;1147:335–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nuzzo D, Picone P, Baldassano S, et al. Insulin resistance as a common molecular denominator linking obesity to Alzheimer’s disease. Curr Alzheimer Res 2015;12:723–735 [DOI] [PubMed] [Google Scholar]
- 48.Pedersen WA, Flynn ER. Insulin resistance contributes to aberrant stress responses in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol Dis 2004;17:500–506 [DOI] [PubMed] [Google Scholar]
- 49.Kohjima M, Sun Y, Chan L. Increased food intake leads to obesity and insulin resistance in the Tg2576 Alzheimer’s disease mouse model. Endocrinology 2010;151:1532–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin W, Haroutunian V, Katsel P, et al. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol 2009;66:352–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheng B, Wang X, Su B, et al. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem 2012;120:419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gong B, Pan Y, Vempati P, et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging 2013;34:1581–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim B, Backus C, Oh S, Hayes JM, Feldman EL. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology 2009;150:5294–5301 [DOI] [PMC free article] [PubMed] [Google Scholar]