Abstract
Although the autoimmune destruction of pancreatic β-cells underlying type 1 diabetes (T1D) development is ultimately mediated by T cells in NOD mice and also likely in humans, B cells play an additional key pathogenic role. It appears that the expression of plasma membrane–bound Ig molecules that efficiently capture β-cell antigens allows autoreactive B cells that bypass normal tolerance induction processes to be the subset of antigen-presenting cells most efficiently activating diabetogenic T cells. NOD mice transgenically expressing Ig molecules recognizing antigens that are (insulin) or are not (hen egg lysozyme [HEL]) expressed by β-cells have proven useful in dissecting the developmental basis of diabetogenic B cells. However, these transgenic Ig specificities were originally selected for their ability to recognize insulin or HEL as foreign, rather than autoantigens. Thus, we generated and characterized NOD mice transgenically expressing an Ig molecule representative of a large proportion of naturally occurring islet-infiltrating B cells in NOD mice recognizing the neuronal antigen peripherin. Transgenic peripherin-autoreactive B cells infiltrate NOD pancreatic islets, acquire an activated proliferative phenotype, and potently support accelerated T1D development. These results support the concept of neuronal autoimmunity as a pathogenic feature of T1D, and targeting such responses could ultimately provide an effective disease intervention approach.
Introduction
Although autoreactive CD4 and CD8 T cells ultimately mediate the pancreatic β-cell destruction underlying type 1 diabetes (T1D) development, in the NOD mouse model and likely also in humans, B cells play an additional key pathogenic role (1,2). The diabetogenic role of B cells in NOD mice was originally identified by findings that their ablation through either a genetic approach (introducing the Igµnull mutation) or antibody treatments had strong disease-protective effects (3–10). Other NOD mouse studies indicated that their unique ability to specifically take up pancreatic β-cell antigens through an Ig-mediated capture mechanism allows B cells to be the antigen-presenting cell (APC) subtype most efficiently supporting the expansion of diabetogenic T cells (11,12). These collective findings indicated that defects in both the immunological tolerance induction processes that normally cull or inactivate autoreactive B cells as well as T cells underlie T1D development. Several NOD-based model systems have been developed to dissect the genetic and mechanistic basis for diabetogenic B-cell development. These models entail NOD mice transgenically expressing Ig molecules specific for antigens that are (insulin) or are not (hen egg lysozyme [HEL]) expressed by β-cells resulting respectively in acceleration or inhibition of T1D development (11,13). However, both of these transgenic Ig specificities were originally selected for their ability to recognize insulin or HEL as foreign, rather than as autoantigens (14). Because of potential differences in Ig binding affinities and perhaps other factors, the selection and/or activation characteristics of B cells recognizing normally foreign antigens versus naturally occurring autoreactive diabetogenic clonotypes may not be identical. Thus, the goal of the current study was to develop and characterize NOD mice with B cells transgenically expressing an Ig specificity that naturally contributes to T1D.
The majority of hybridomas generated from pancreatic islet–associated B cells in NOD mice were unexpectedly found to recognize the autoantigen peripherin (15,16). Peripherin is expressed widely in neuronal cell bodies and axons of the peripheral and central nervous systems (17,18). The expression of peripherin also occurs in the peri-insular areas of postnatal mice (19). Peripherin-reactive autoantibodies have been paradoxically found in the sera of healthy humans and non–autoimmune-prone mice, albeit at lower titers than in the NOD strain (20). However, the potential contribution of peripherin-reactive B cells to T1D remains unclear. Thus, we generated and characterized a new NOD stock transgenically expressing the Ig molecule derived from a naturally occurring islet-infiltrating, peripherin-autoreactive B cell (designated NOD-PerIg mice). This model revealed that peripherin-autoreactive B cells are indeed potent contributors to T1D pathogenesis.
Research Design and Methods
Mice
NOD/LtDvs mice are maintained at The Jackson Laboratory and The University of Lleida (Spain) under specific pathogen-free conditions. B cell–deficient NOD.Igµnull and total lymphocyte-deficient NOD-scid mice have been described previously (3,21). A NOD stock transgenically expressing an HEL reactive, but with no other Ig specificities (NOD-IgHEL.Igµnull), has also been described (11,12). NOD mice deficient in T-cell receptor-α (TCRα) chain expression were obtained from the Type 1 Diabetes Repository (http://type1diabetes.jax.org/). NOD-PerIg mice were generated as follows: the Ig heavy (H) chain (PerH) and light (L) chain (PerL) DNA coding sequences from the islet-derived, peripherin-reactive B-cell hybridoma H280 (15,16) were respectively subcloned into the pESAC38 and AC38K vectors (22). The pESAC38 vector also encodes a constant region gene element enabling the transgenic H chain to be expressed as an IgM/D isotype of the Iga rather than the Igb allotype naturally characterizing NOD mice. These transgene constructs were separately directly microinjected into NOD zygotes. The resulting progeny carrying the PerH transgene are detected by PCR using the primers 5′-TCCTGTGTTGCCTCTGGATTCACT-3′ and 5′-GACATCGAAGTACCACCCGCCTGT-3′. PerL transgene carriers are detected using the primers 5′-AACTGTCACCATCACATGTCGAGC-3′ and 5′-CCTCCACCGAACGTCGGAGGAGTA-3′. A total of three PerH and two PerL founder lines were originally produced. A single line from each was selected for analysis based on transgenic IgH or IgL expression levels most closely matching the corresponding endogenous molecules in standard NOD mice. An intercross strategy generated NOD mice coexpressing the PerH and PerL transgenes (NOD-PerIg). The scid mutation was subsequently fixed to homozygosity in NOD mice carrying the PerH or PerL transgenes. An intercross strategy then produced NOD-scid mice carrying both the PerH and PerL transgenes. All mice were maintained under required U.S. or European legal standards.
Flow Cytometry
Indicated leukocyte suspensions were examined for various lymphocyte subsets by flow cytometry using FACSCalibur and FACS LSR instrumentation (BD Biosciences, San Jose, CA) and FlowJo software (Tree Star, Inc., Ashland, OR). The following fluorochrome-conjugated monoclonal antibodies were used: CD21, CD95, CD19, IgMa, IgMb, CD43, CD80, CD86, H2-Kd, and H2-Ag7 (7G6, JO2, ID3, DS-1, AF6–78, S7, 16-10A1, GL1, S1-1.1, and 10-3.6; BD Biosciences); CD4 (RM4–5; Invitrogen); B220, CD8, ICOS, CD23, GL7, CD138, CXCR5, and IgD (RA3–6B2, 53–6.7, C398.4A, B3B4, GL7, 281–2, L138D7, 11–26c.2a; BioLegend); and CD44 and CD62L (IM7, MEL-14; eBioscience). Ki-67 expression was assessed using a kit from BD Biosciences. Viability was determined using propidium iodide.
Diabetes Incidence
Mice were monitored weekly for glycosuria with Diastix Ames Reagent Test Strips (Bayer Healthcare Pharmaceuticals, Elkhart, IN) with diabetes onset determined by two consecutive values of ≥3.
Histology
Quantitative mean insulitis scores ranging from 0 (no visible lesions) to 4.0 (complete destruction of islets) were determined by a blinded observer using the previously described calculation approach (23).
Islet-Associated Lymphocyte Isolation
Pancreatic islet-infiltrating leukocytes were isolated as previously described (16,24) for flow cytometric analyses.
Adoptive Transfer Studies
Indicated NOD-scid recipients were injected intravenously with either 5 × 106 splenic leukocytes from NOD or NOD-scid.PerIg mice or 1 × 106 purified T cells from NOD-IgHEL.Igµnull donors. In other experiments lethally irradiated (1,200 rad) NOD.Igμnull recipients were injected intravenously with 5 × 106 syngeneic bone marrow (BM) cells admixed with an equal number of the indicated B cells, as previously described (3). Another study used lethally irradiated NOD.Igμnull recipients injected intravenously with the indicated populations of single (5 × 106) or mixed BM cells (2.5 × 106 for each donor type).
Sequencing of Possible Ig Somatic Mutation Variants
DNA was extracted from the indicated B cells using a DNeasy Blood & Tissue Kit (Qiagen, Waltham, MA). Primers flanking the variable regions of the PerH and PerL chain transgenes (5′-TCGACGTTAGGACTCACCTG-3′ and 5′-GAGTCTGGAGGAGGCTTGGT-3′, and 5′-CGTTTGATTTCCAGCTTGGT-3′ and 5′-CTCAGTCTCCAGCCTCCCTA-3′, respectively) were used to create PCR amplicons (354 and 310 bp, respectively). PCR-free workflow from the Kapa Hyper Prep Kit (Kapa Biosystems, Inc., Wilmington, MA) was used to create sequencing libraries from the PCR amplicons by The Jackson Laboratory Scientific Services Genome Technologies Group. Libraries were quantified by quantitative PCR, pooled, and pair end sequenced on an MiSeq Sequencer (Illumina, San Diego, CA). Samples were subjected to quality control, and only reads ≥230 bp containing no more than 2 bp with quality scores of <20 were used in the analysis. The reads were converted to FASTA files and subsequently split into files containing <500,000 sequences using the FASTA Splitter (http://kirill-kryukov.com/study/tools/fasta-splitter/), then were submitted to IMGT/HighV-QUEST (25) for mouse Ig analysis. Resulting output files were combined by strain and analyzed using the “bcRep” package for R (http://CRAN.R-project.org/package=bcRep, http://www.R-project.org).
Results
Restricting B-Cell Recognition to Peripherin Accelerates T1D Development in NOD Mice
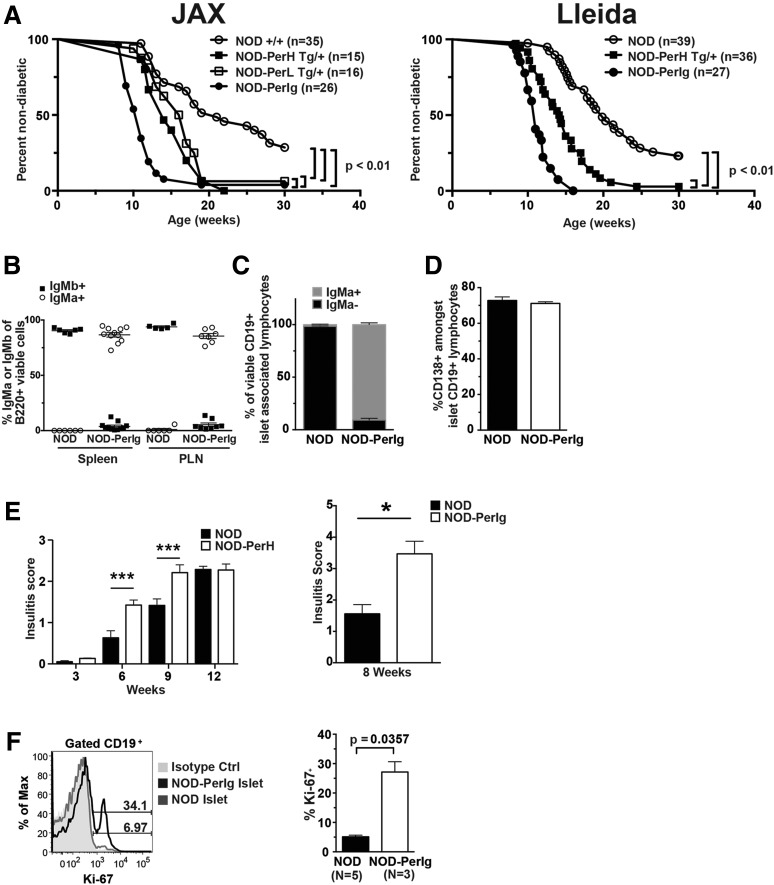
T1D is respectively inhibited or accelerated in NOD mice transgenically expressing Ig molecules originally selected to recognize HEL or insulin as foreign antigens (11,13). Thus, we tested whether transgenic expression of an Ig specificity representative of a sizeable proportion of islet-infiltrating B cells in NOD mice recognizing the naturally occurring β-cell autoantigen peripherin (16,26) also accelerated T1D onset. All tested peripherin-autoreactive B cells from NOD mice recognize a C-terminal epitope shared by two of the four isoforms of this protein, PRPH61 and PRPH58 (27). We created NOD mice separately expressing transgenes encoding IgM/D-locked Ig H or L chains characterizing the representative islet-derived, peripherin-autoreactive B-cell hybridoma H280 (15,16) (designated “NOD-PerH” and “NOD-PerL” mice). The H280 clone was chosen because it was isolated from the islets of a NOD mouse with recent T1D onset, exhibited strong peripherin binding, and, similar to most other islet-derived peripherin-autoreactive B cells from this strain, neither its H nor its L chain demonstrated any evidence of affinity maturation (16,26). An intercross strategy produced progeny expressing both the PerH and PerL chains (bitransgenic designated NOD-PerIg mice). Each transgenic stock was evaluated in a hemizygous state. The separate expression of both the PerH and PerL transgenes significantly accelerated T1D onset (Fig. 1A). Coexpression of both the PerH and PerL constructs further accelerated T1D development (Fig. 1A). PerIg-induced acceleration of T1D was observed in NOD mice housed at both The Jackson Laboratory and The University of Lleida. Endogenous B cells and those expressing the PerH transgene in NOD mice are, respectively, of the IgMb and IgMa allotype. B cells in both spleens and pancreatic lymph nodes (PLNs) of standard NOD and NOD-PerIg mice were, respectively, almost entirely of the IgMb and IgMa allotype (Fig. 1B). Before succumbing to T1D, there were no obvious neurological abnormalities in any of the transgenic lines.
Figure 1.
Peripherin-autoreactive B cells infiltrate the pancreatic islets of NOD mice, acquire an activated phenotype, and subsequently potently support T1D development. A: Diabetes incidence is significantly accelerated over standard nontransgenic NOD littermate controls (Ctrl) in the presence of the single PerH or PerL transgenes, with the disease-onset rate increased further in the NOD-PerIg stock expressing both constructs. Statistical comparisons were made by log-rank (Mantel-Cox) test. Left and right panels, respectively, depict T1D incidence data for groups of experimental mice housed at The Jackson Laboratory (JAX) and the University of Lleida (Lleida). B: Proportion of B cells expressing IgMa or IgMb H chains in spleens and PLNs of 10- to 14-week-old NOD and NOD-PerIg female mice. C: Proportions of B cells within islet-infiltrating leukocytes from 8- to 10-week-old NOD (n = 4) or NOD-PerIg (n = 3) female mice expressing IgMa or IgMb H chains. D: Percentage of viable CD19+, CD138+ islet-associated B cells in 8- to 10-week-old NOD (n = 4) and NOD-PerIg (n = 3) female mice. E, Left panel: Mean insulitis scores ± SEM at the indicated ages for female NOD and NOD-PerH mice. ***P < 0.001, Mann-Whitney U analyses. E, Right panel: Mean insulitis scores ± SEM for 8-week-old female NOD (n = 7) and bitransgenic NOD-PerIg (n = 4) mice. *P = 0.0121, Mann-Whitney U analyses. F: Representative flow cytometric profiles (left panel) and summary comparison (right panel) of proportions of proliferating B cells marked by Ki-67 staining among islet-associated leukocytes from 9- to 11-week-old NOD and NOD-PerIg mice. Statistical comparison was performed by Mann-Whitney U test.
Progressive development of insulitis, including B cells, is a hallmark of T1D pathogenesis in NOD mice (28–30). Insulitic B cells from NOD-PerIg mice were almost entirely of the transgenic IgMa allotype (Fig. 1C). As previously observed (31), a significant proportion of islet-associated B cells in standard NOD mice had converted to a CD138+ plasma cell–like phenotype consistent with autoantigen-induced activation (Fig. 1D). A similarly large proportion of B cells in the islets of NOD-PerIg mice also had a plasma cell–like phenotype (Fig. 1D). However, although theoretically possible based on the nature of the transgene expression vectors, to date using the previously described protocol (15,16) we have not detected secreted peripherin-reactive antibodies in NOD-PerIg mice. Histologic scoring revealed that transgenic expression of the PerH chain alone was sufficient to significantly accelerate insulitis development in NOD mice (Fig. 1E, left). This was also the case in NOD-PerIg bitransgenic mice (Fig. 1E, right). Furthermore, based on Ki-67 staining, a significant proportion of islet-associated B cells in NOD-PerIg mice were undergoing proliferation at levels exceeding those of NOD controls (Fig. 1F). These collective results indicated that peripherin-autoreactive B cells infiltrate the pancreatic islets of NOD mice, acquire an activated proliferative phenotype, and subsequently potently support T1D development. The H280-derived Ig molecule that we transgenically expressed in NOD mice strongly binds peripherin (16). However, it cannot be absolutely excluded that H280 Ig–expressing B cells can cross-react with other islet autoantigens, contributing to their diabetogenic activity and/or alternatively possibly taking up other proteins potentially complexed to peripherin.
B-Cell Subset Distributions and Cell Surface Phenotypes Differ in NOD and NOD-PerIg Mice
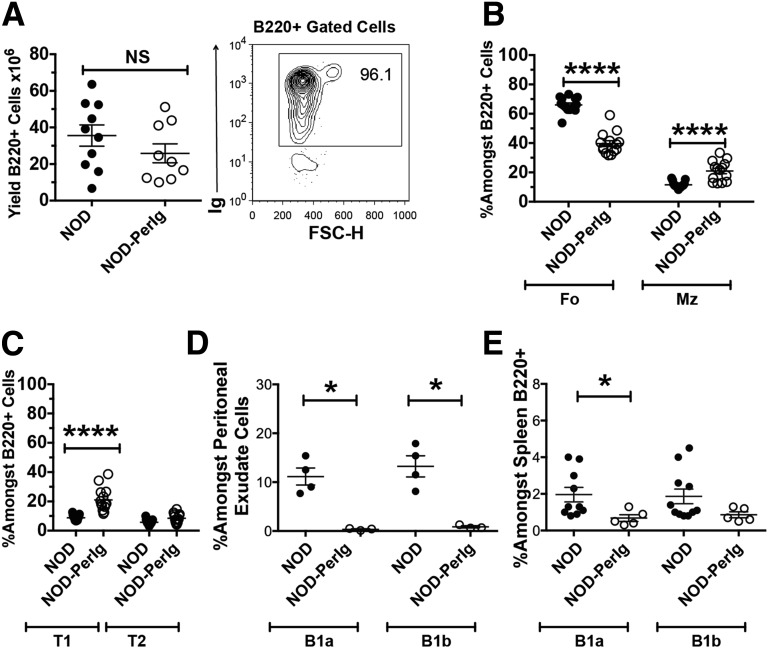
Flow cytometric gating strategies for comparing proportions of various B-cell subsets in NOD and NOD-PerIg mice are depicted in Supplementary Figure 1. Despite different maturation rates in BM (Supplementary Fig. 2), the numbers of total splenic B cells were similar in NOD-PerIg and standard NOD mice (Fig. 2A, left). The expression of both functional H and L chain transgenes is confirmed by the presence of B cells in NOD-scid.PerIg mice (Fig. 2A, right). NOD-scid.PerIg mice were insulitis free (data not shown), indicating that this process also requires T cells. Compared with NOD controls, the proportion of marginal zone (MZ) subset B cells was significantly increased in NOD-PerIg mice, with a corresponding decrease in the follicular (FO) population (Fig. 2B). These shifts are consistent with reports in other Ig transgenic mice (32). Proportions of splenic immature transitional stage 1 (T1) but not T2 B cells were also significantly greater in NOD-PerIg than standard NOD mice (Fig. 2C). Although present in NOD controls, intraperitoneal B1a and B1b subset B cells were virtually absent in NOD-PerIg mice (Fig. 2D, left). Proportions of B1a, but not B1b, B cells were also lower in the spleens of NOD-PerIg mice than in NOD controls (Fig. 2D, right).
Figure 2.
B-cell subset distribution differs in NOD-PerIg and standard NOD mice. A, Left panel: Absolute numbers of B220+ B cells among total leukocytes from spleens of 12-week-old NOD and NOD-PerIg female mice. A, Right panel: Representative presence of B cells in spleens of NOD-scid.PerIg mice document that both the H and L chain transgenes are functional. FSC-H, forward scatter height. B: Percentages of FO (B220+, CD23+, CD21+) and MZ (B220+, CD23dull, CD21hi) B-cell subsets of viable B220+ cells in spleens from 6-week-old female NOD and NOD-PerIg mice. C: Proportions of immature T1 (B220+ CD21neg, CD23neg) and T2 (B220+ CD21hi, CD23hi) stage B cells among B220+ cells in spleens from 6-week-old female NOD and NOD-PerIg mice. D: Proportions of B1a (B220int CD5+) and B1b (B220dull CD5−) B-cell subsets among peritoneal exudate cells (left panel) and B220+ spleen cells (right panel) from 6-week-old female NOD and NOD-PerIg mice. In panels B–D: *P < 0.05, ****P < 0.0001, Mann-Whitney U test.
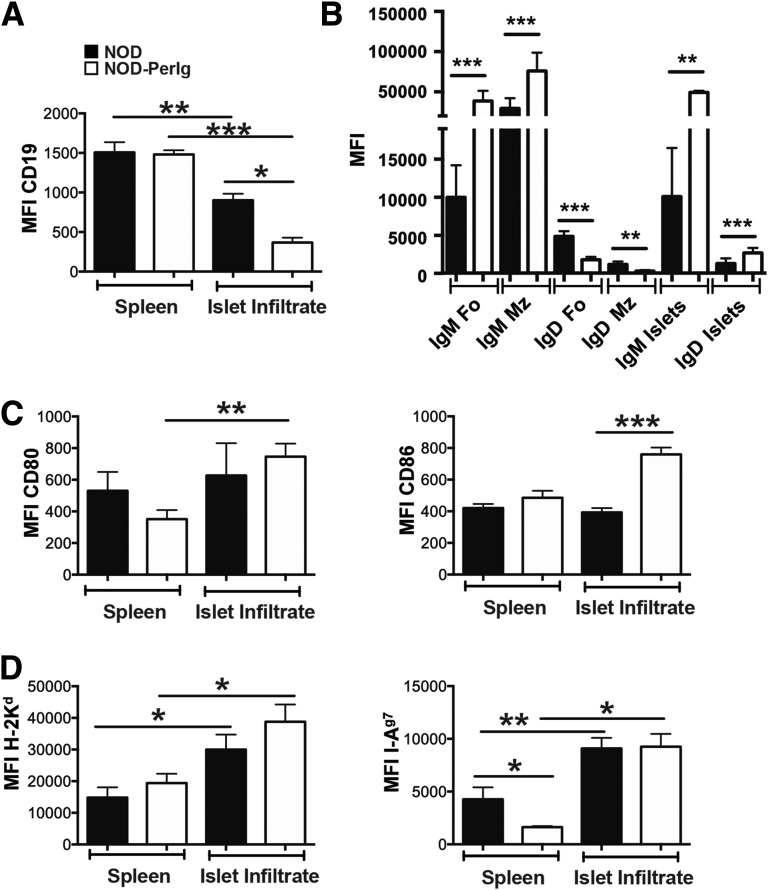
Cell surface phenotypes of B cells in the spleens and islets of standard NOD and NOD-PerIg mice also differed. CD19 expression was similar in splenic B cells in NOD and NOD-PerIg mice (Fig. 3A). Islet-infiltrating B cells from both strains expressed CD19 at lower levels than those in spleens, but with a more pronounced decrease in NOD-PerIg mice (Fig. 3A). This latter finding is consistent with islet-infiltrating B cells in both strains becoming activated and converting to plasma-like cells, but to a greater extent in NOD-PerIg mice. IgM expression by both FO and MZ subset B cells in spleens was significantly greater in NOD-PerIg mice than in NOD mice (Fig. 3B). Conversely, IgD expression by splenic FO and MZ subset B cells was significantly lower in NOD-PerIg mice than in NOD mice (Fig. 3B). The greater preponderance of splenic B cells with an IgMhi IgDlow phenotype in NOD-PerIg mice than in NOD mice is consistent with the MZ subpopulation enlargement in the former strain. Islet-infiltrating B cells in NOD-PerIg mice also expressed IgM at significantly higher levels than those in NOD controls (Fig. 3B). In contrast to that in the spleen, IgD expression was significantly higher in islet-infiltrating B cells from NOD-PerIg mice than from NOD mice (Fig. 3B). The levels of IgM and IgD expression by islet-infiltrating B cells in both NOD and NOD-PerIg mice were more similar to the splenic FO subset than the MZ subset. This suggests that it is the FO rather than the MZ subset of B cells that exerts diabetogenic effects in both standard NOD and NOD-PerIg mice. Immature splenic T1 and T2 subset B cells from both strains expressed IgM, but IgD was expressed at low levels (data not shown). CD80 was expressed at higher levels by islet-infiltrating than splenic B cells in NOD-PerIg, but not in standard NOD mice (Fig. 3C, left). Although not differing in the spleen, CD86 was expressed at significantly higher levels by islet-infiltrating B cells from NOD-PerIg mice than in NOD mice (Fig. 3C, right). MHC class I expression was significantly higher in islet-infiltrating than in splenic B cells in both strains (Fig. 3D, left). Although MHC class II expression was significantly lower in splenic B cells from NOD-PerIg mice than from standard NOD mice, it was significantly increased to a similarly higher level in the islet-infiltrating populations of both strains (Fig. 3D, right). Collectively, these data provide further evidence that PerIg-expressing B cells infiltrate the pancreatic islets of NOD mice, where they acquire an activation-like phenotype.
Figure 3.
Differing cell surface phenotypes of total B cells in the spleens and islets of standard NOD and NOD-PerIg mice. A: CD19 expression by splenic and islet-infiltrating B cells from 10-week-old female NOD (n = 7–11) and NOD-PerIg (n = 3) mice. B: IgM and IgD expression by gated splenic FO and MZ subsets and total islet-infiltrating B cells in 10-week-old female NOD (n = 8–11) and NOD-PerIg (n = 3–7) mice. C: CD80 (left panel) and CD86 (right panel) expression by splenic and islet-infiltrating B cells in 10-week-old female NOD (n = 8–13) and NOD-PerIg (n = 5–7) mice. D: H2-Kd MHC class I (left panel) and H2-Ag7 MHC class II (right panel) expression by splenic and islet-infiltrating B cells in 10-week-old female NOD (n = 8–11) and NOD-PerIg (n = 3–5) mice. In panels A–D: *P < 0.05, **P < 0.01, ***P < 0.001, Mann-Whitney U test. MFI, mean fluoresence intensity of antibody staining.
Cognate Interactions With CD4+ T Cells Are Increased Within the PLNs of NOD-PerIg Mice
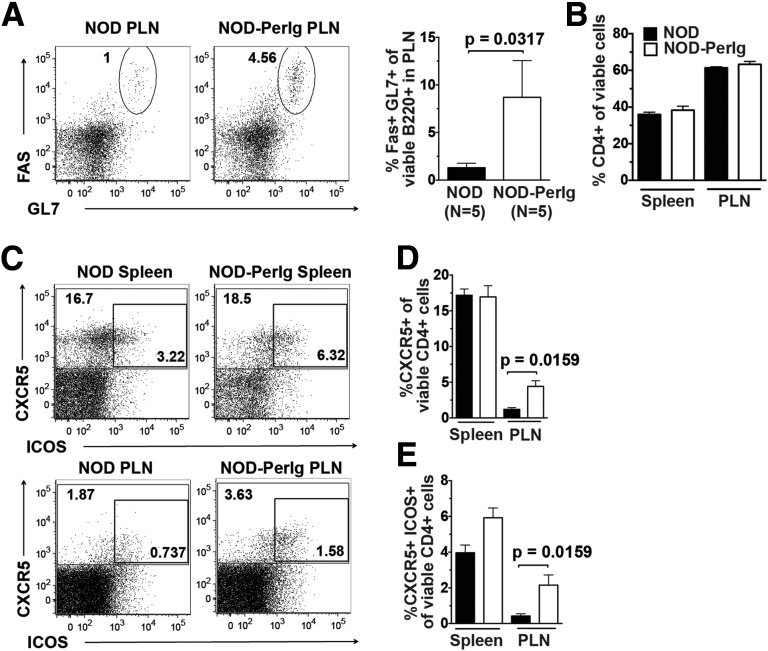
An ability to specifically take up β-cell molecules through Ig-mediated capture appears to allow B cells to critically contribute to T1D development in NOD mice, and likely also in humans, by serving as an APC subset preferentially activating pathogenic T cells (3,6,12,33–36). As a result of this process, antigen receptors expressed by diabetogenic T cells recognize epitopes derived from the same β-cell molecule that pathogenic interactive antigen-presenting B cells had taken up by Ig-mediated capture. Only if a sufficient number of cognate CD4+ T cells are present could the diabetogenic autoimmune response be amplified by peripherin-autoreactive B cells. Such cognate interactions take place in germinal centers, where B cells undergo proliferation and maturation into CD138+ plasmablasts/plasma cells. Germinal center B-cell levels were greater in the PLNs of NOD-PerIg mice than in those of standard NOD mice (Fig. 4A). No such differences were observed in spleens. After interacting with cognate B cells, CD4+ T cells can differentiate into T FO helper (Tfh) cells expressing both CXCR5 and ICOS (37). Proportions of the total number of CD4+ T cells were similar in the spleens and PLNs of NOD-PerIg and standard NOD mice (Fig. 4B). Levels of total splenic CD8+ T cells were paradoxically lower in NOD-PerIg mice than in standard NOD mice, while not differing in PLNs (Supplementary Fig. 3A). There were no strain differences in the activation status of CD8 T cells at either site (Supplementary Fig. 3B). However, the proportions of pre-Tfh (CXCR5+) and full Tfh cells (CXCR5+ ICOS+) within PLNs were significantly greater in NOD-PerIg mice than in NOD controls (Fig. 4C–E). These results indicate that there are significantly higher levels of cognate T- and B-cell interactions within the PLNs of NOD-PerIg mice than within those of standard NOD mice. By extension, these collective findings support the presence of peripherin-autoreactive CD4+ T cells contributing to T1D development in NOD mice.
Figure 4.
B cells transgenically expressing PerIg molecules undergo cognate interactions with CD4+ T cells within the PLNs of NOD mice. A, Left panel: Representative flow cytometric profiles of germinal center B cells among gated B220+ cells in PLNs from NOD and NOD-PerIg mice. A, Right panel: Percentage of germinal center B cells (B220+, Fas/CD95hi, GL7hi) in PLNs. B: Percentage of CD4+ T cells among viable leukocytes from spleen and PLN. C: Representative flow cytometric profiles of pre-Tfh cells (CXCR5+) and full Tfh cells (CXCR5+ ICOS+) among gated CD4+ cells from the spleens and PLNs of NOD and NOD-PerIg mice. D: Percentage of CXCR5+ pre-Tfh cells among viable CD4+ T cells within spleens and PLNs. E: Percentage of full CXCR5+ ICOS+ Tfh cells among viable CD4+ T cells within spleens and PLNs. For the above data sets, 12-week-old female NOD and NOD-PerIg mice (n = 5 per strain) were used. In panels A–E, statistical comparisons were performed using the Mann-Whitney U test.
NOD-PerIg B Cells Do Not Undergo Further Affinity Maturation
The recombined VDJ and VJ components encoding, respectively, the H and L Ig chains of the original peripherin-autoreactive H280 hybridoma were germline in nature (26). However, it was possible that somatic hypermutation events occur in transgenic NOD-PerIg B cells, enhancing their diabetogenic activity. To test this possibility, we isolated PLN-derived NOD-PerIg B cells and, as controls, those from NOD-scid.PerIg mice lacking T cells that induce affinity maturation, and sequenced their transgenic H and L chain coding regions using MiSeq (Supplementary Fig. 4). Analyses of over 106 reads showed no deviation from the original germline sequences in the H280 hybridoma. These results indicated that somatic hypermutation modifications within PLNs did not contribute to the ability of transgenic PerIg B cells to accelerate T1D development. It should also be noted that the original H280 clonotype was isolated from the islets of a NOD mouse with recent T1D onset, where it also demonstrated no evidence for undergoing Ig H or L chain affinity maturation (26). Furthermore, because they express the identical Ig molecule, transgenic PerIg B cells will retain the peripherin binding characteristics of the original H280 hybridoma.
Mature NOD-PerIg B Cells Fail to Engraft After Transfer
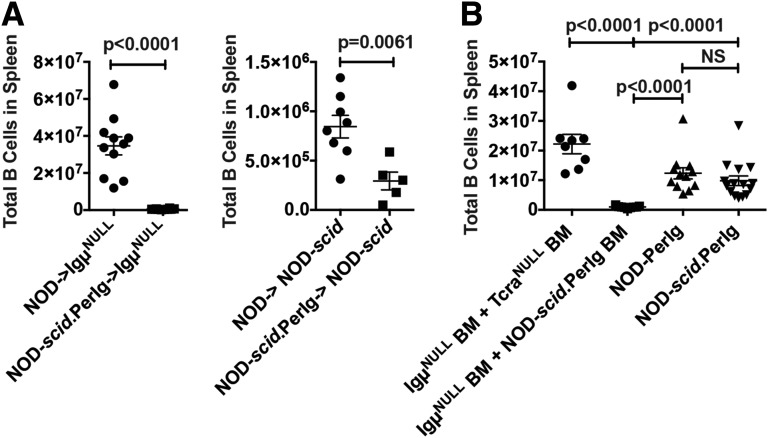
An important criterion for identifying a particular leukocyte population as contributing to an autoimmune pathology is documenting its ability to individually or in conjunction with other cell types adoptively transfer disease to immunodeficient recipients. Thus, we tested whether engraftment with NOD-scid.PerIg B cells abrogated the normal T1D resistance of B cell–deficient NOD.Igμnull recipients. B cells directly infused into NOD.Igμnull recipients are rejected by a host CD8+ T-cell response (3). This difficulty is bypassed by engrafting lethally irradiated NOD.Igμnull recipients with syngeneic BM admixed with the B cells of choice (3). This strategy allowed engraftment of NOD.Igμnull recipients by mature splenic NOD control B cells (Fig. 5A, left). In contrast, mature NOD-scid.PerIg B cells unexpectedly failed to engraft NOD.Igμnull recipients (Fig. 5A, left). To assess whether this was due to some feature of the NOD.Igμnull host environment, we also tested whether mature splenic NOD-scid.PerIg B cells could engraft lymphocyte-deficient NOD-scid recipients. Again, unlike those of standard NOD origin, mature NOD-scid.PerIg B cells poorly engrafted NOD-scid recipients (Fig. 5A, right).
Figure 5.
Mature NOD-PerIg B cells cannot be adoptively transferred, but such effectors differentiate from engrafted BM precursor cells. A: Unlike those of standard NOD origin, adoptively transferred NOD-scid.PerIg splenic CD19+ B cells from 10- to 14-week-old female donors engraft poorly in either NOD.Igµnull (n = 10, left panel) or NOD-scid (n = 5, right panel) recipients. B: NOD-PerIg and NOD-scid.PerIg BM transplants into NOD.Igµnull recipients resulted in successful CD19+ B-cell reconstitution. NOD.Igµnull recipients of 1:1 mixed BM transplants from 8- to 15-week-old NOD-scid.PerIg and NOD.Igµnull female donors failed to develop B cells compared with NOD.Igµnull control recipients receiving mixed BM from NOD.TCRαnull and NOD.Igµnull donors. In panels A and B, recipient mice were evaluated at 12–13 weeks after engraftment.
The results cited above led us to hypothesize that mature NOD-PerIg B cells might be short lived, and their presence might require an ability to be continually renewed from stem cell precursors in BM. Thus, we assessed whether B cells developed in lethally irradiated NOD.Igμnull recipients reconstituted with NOD-PerIg or NOD-scid.PerIg BM. This was indeed the case (Fig. 5B). We next determined whether, when present in conjunction with appropriate pathogenic T cells, NOD-scid.PerIg B cells differentiating from BM precursors could support T1D development. This was accomplished by engrafting lethally irradiated NOD.Igμnull recipients with a 1:1 mixture of syngeneic and NOD-scid.PerIg BM serving as stem cell sources for, respectively, T and B cells. Unexpectedly, B cells failed to develop in these mixed BM chimeras (Fig. 5B). This was due to an intrinsic inability of NOD-scid.PerIg B cells to differentiate from stem cell precursors when competing with other precursors under mixed BM chimeric conditions. This conclusion is based on the finding that mixed chimeric controls, consisting of NOD.Igμnull mice engrafted with a 1:1 mixture of syngeneic and NOD.TCRαnull BM, developed B cells (Fig. 5B), with T1D resulting in three of three mice from a separate cohort of such recipients.
As described earlier, islet-infiltrating NOD-PerIg B cells clearly exhibited signs of an activated phenotype, including proliferation. Conversely, but similar to the case for the insulin-reactive 125Tg clonotype (36,38), splenic NOD-PerIg B cells were more refractive to in vitro proliferation induced by costimulation of Ig/CD40 than those from NOD controls (Supplementary Fig. 5). This feature may contribute to the inability of mature NOD-PerIg B cells to undergo the homeostatic expansion process necessary to engraft NOD.Igμnull or NOD-scid recipients. However, NOD-PerIg B cells proliferated robustly in response to both Toll-like receptor (TLR) 9 and TLR4 stimulation (Supplementary Fig. 6). Insulin-reactive 125Tg B cells from NOD mice were reported to be insensitive to TLR4 stimulation (38), but possible TLR9 responses were not indicated. The ability of NOD-PerIg B cells to respond to TLR stimulation could also contribute to their ability to proliferate within islets and exert diabetogenic activity in vivo.
Initiation of Diabetogenic T-Cell Activity Is Enhanced by PerIg B Cells
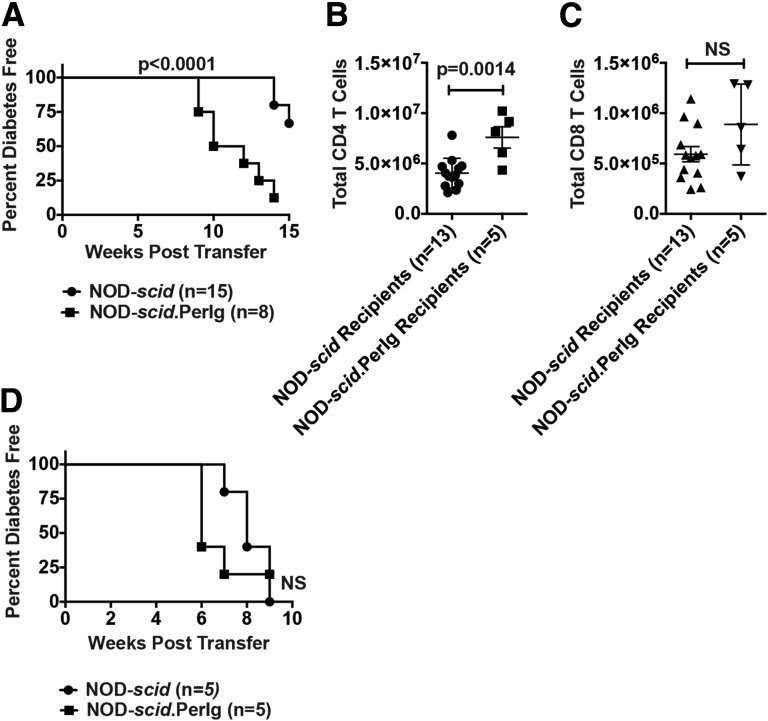
Because of their inability to be adoptively transferred, we used an alternative approach to assess whether PerIg-expressing B cells support diabetogenic T-cell activation. This approach was to compare the ability of NOD-scid.PerIg and standard NOD-scid recipient mice to develop T1D after engraftment with T cells having a NOD background origin that never had a previous opportunity to interact with pathogenic B cells. NOD-IgHEL.Igµnull mice having normal numbers of B cells, but none capable of recognizing β-cell autoantigens, provided such a T-cell donor. After engraftment, purified NOD-IgHEL.Igµnull T cells induced a significantly faster rate of T1D development in NOD-scid.PerIg recipients than in standard NOD-scid recipients (Fig. 6A). This differential T1D transfer efficiency was associated with a greater engraftment of NOD-IgHEL.Igµnull CD4+, but not CD8+, T cells in NOD-scid.PerIg than in NOD-scid recipients (Fig. 6B and C). In a control study, purified T cells from standard NOD donors that could have interacted with pathogenic B cells transferred T1D with equal efficiency to NOD-scid.PerIg and standard NOD-scid recipients (Fig. 6D). These collective results confirmed that PerIg-expressing B cells efficiently support the initiation of diabetogenic T-cell responses.
Figure 6.
Diabetogenic T-cell activity is enhanced in the presence of PerIg-expressing B cells. NOD-scid and NOD-scid.PerIg recipients were injected intravenously with 1 × 106 purified total splenic T cells from 5- to 6-week-old NOD-IgHEL.Igµnull or 8-week-old NOD donors, and subsequently were monitored for T1D development. A: T1D developed at a significantly more rapid rate in NOD-scid.PerIg recipients than in NOD-scid recipients of NOD-IgHEL.Igµnull T cells. Statistical comparison by log-rank (Mantel-Cox) test. B and C: Comparison of engraftment by donor NOD-IgHEL.Igµnull CD4+ and CD8+ T cells in NOD-scid.PerIg and NOD-scid recipients. D: T1D developed at a similar rate between NOD-scid.PerIg than NOD-scid recipients receiving NOD T cells.
Discussion
In NOD mice, and likely also in humans, B cells make important contributions to T cell–mediated autoimmune T1D. Indeed, a B-cell–targeting strategy has shown some clinical success as a T1D intervention approach (39). The current study demonstrates through an Ig transgenic approach that the high proportion of peripherin-reactive B cells previously reported to characterize the insulitic infiltrates in NOD mice (15,16) potently contributes to T1D pathogenesis in NOD mice and, by extension, potentially also in humans. However, it should be stressed that our current results do not preclude the possibility that B cells with other autoantigenic specificities additionally contribute to T1D development, as evidenced by the fact that transgenic expression of an insulin-specific Ig also accelerates disease onset in NOD mice (11,13). Similar to the case with the transgenic insulin-specific Ig (38), an increased percentage of NOD-PerIg B cells display an MZ phenotype. Despite this shift, islet-infiltrating B cells in NOD-PerIg mice seem to more phenotypically resemble the FO subset. Assessment of transgenic gene sequences also indicated that PerIg-expressing B cells do not have to undergo affinity maturation within PLNs to contribute to T1D development.
Because of an ability to efficiently take up β-cell autoantigens through Ig-mediated capture, B cells most likely contribute to T1D by serving as an APC subset that most efficiently activates pathogenic T cells (3,6,12,33–36). This elicits cognate interactions with diabetogenic T cells recognizing epitopes derived from the same β-cell molecule that pathogenic antigen-presenting B cells had internalized by Ig-mediated capture. The insoluble nature of the peripherin protein precluded an ability to use it in T- and B-cell interaction assays. However, the proportions of germinal center B cells and Tfh cells were significantly greater in the PLNs of NOD-PerIg mice than in NOD control mice. This observation supports the conclusion that, at least in part, T1D development in NOD mice entails cognate interactions between peripherin-autoreactive T and B cells. There are reports that T1D development in NOD mice requires an initial autoreactive T-cell response that destroys Schwann cells of neuronal origin enveloping pancreatic islets (40,41). Our current results support the possibility that B cell–dependent peripherin-autoreactive T-cell responses could be a component of any neuronal Schwann cell destruction contributing to T1D development.
Islet-infiltrating NOD-PerIg B cells clearly exhibited signs of activation, including proliferation. However, adoptively transferred mature splenic NOD-PerIg B cells failed to engraft in immunodeficient recipients. BM chimera studies indicated that this is likely due to mature NOD-PerIg B cells being short lived in nature and thus constantly having to be replenished from a stem cell source. Furthermore, in vitro studies found that costimulation with Ig/CD40 failed to induce significant proliferation of mature B cells from NOD-PerIg mice. This feature may contribute to the inability of mature NOD-PerIg B cells to engraft immunodeficient recipients. Conversely, NOD-PerIg B cells do proliferate in response to TLR stimulation that may contribute to their ability to function in vivo as diabetogenic APCs.
In conclusion, the current study indicates that peripherin-autoreactive B cells are potent contributors to T1D development in NOD mice and also potentially in humans, likely by undergoing cognate APC interactions with T cells recognizing autoantigenic epitopes derived from the same protein. Our studies also support the possibility that autoreactive responses against neuronal components of pancreatic islets are an important pathogenic feature of T1D. These findings indicate that strategies targeting peripherin-autoreactive B cells could, at least in part, provide a future clinically applicable T1D intervention approach.
Article Information
Acknowledgments. The authors thank Lucy Rowe and Mary Barter of The Jackson Laboratory for their technical assistance with the MiSeq project and Anuj Srivastava, also of The Jackson Laboratory, for sequence analysis.
Funding. The research reported in this publication was partially supported by the National Cancer Institute (grant P30CA034196); Plan Nacional de I+D+i of the Spanish Ministry of Science and Innovation (grant SAF2013-45140-R); CIBERDEM, which is an initiative from Instituto de Salud Carlos III (Spain); and JDRF innovative research grant 5-2005-1133. C.M.L. was partially supported by National Institutes of Health (NIH) grant 1K08-DK-101735. J.Carra. was supported by an FPI predoctoral fellowship (BES-2007-15221) from the Spanish Ministry of Science and Innovation. J.V. is an associate professor in the Serra-Hunter Program of the Catalan Government. D.V.S. is supported by NIH grants DK-46266, DK-95735, and OD-020351, as well as by grants from the JDRF, the American Diabetes Association, and Helmsley Charitable Trust (2014PG-T1D048).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.M.L. researched the data and wrote the manuscript. J.Rac., H.D.C., Q.W., J.Rat., L.E.-M., and E.R.-M. researched the data. B.A., J.Carri., and J.Carra. researched the data and reviewed the manuscript. T.S. researched the data, contributed to discussion, and reviewed the manuscript. J.V. coconceived the study, contributed to discussion, and reviewed the manuscript. D.V.S. coconceived the study, evaluated the data, and reviewed and edited the manuscript. D.V.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1606/-/DC1.
See accompanying article, p. 1794.
References
- 1.Driver JP, Serreze DV, Chen YG. Mouse models for the study of autoimmune type 1 diabetes: a NOD to similarities and differences to human disease. Semin Immunopathol 2011;33:67–87 [DOI] [PubMed] [Google Scholar]
- 2.Todd JA. Etiology of type 1 diabetes. Immunity 2010;32:457–467 [DOI] [PubMed] [Google Scholar]
- 3.Serreze DV, Chapman HD, Varnum DS, et al. . B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J Exp Med 1996;184:2049–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akashi T, Nagafuchi S, Anzai K, et al. . Direct evidence for the contribution of B cells to the progression of insulitis and the development of diabetes in non-obese diabetic mice. Int Immunol 1997;9:1159–1164 [DOI] [PubMed] [Google Scholar]
- 5.Wong FS, Visintin I, Wen L, Granata J, Flavell R, Janeway CA. The role of lymphocyte subsets in accelerated diabetes in nonobese diabetic-rat insulin promoter-B7-1 (NOD-RIP-B7-1) mice. J Exp Med 1998;187:1985–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes 1997;46:941–946 [DOI] [PubMed] [Google Scholar]
- 7.Bouaziz JD, Yanaba K, Venturi GM, et al. . Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci USA 2007;104:20878–20883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorina P, Vergani A, Dada S, et al. . Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes 2008;57:3013–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu CY, Rodriguez-Pinto D, Du W, et al. . Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest 2007;117:3857–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiu Y, Wong CP, Bouaziz JD, et al. . B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J Immunol 2008;180:2863–2875 [DOI] [PubMed] [Google Scholar]
- 11.Hulbert C, Riseili B, Rojas M, Thomas JW. B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. J Immunol 2001;167:5535–5538 [DOI] [PubMed] [Google Scholar]
- 12.Silveira PA, Johnson E, Chapman HD, Bui T, Tisch RM, Serreze DV. The preferential ability of B lymphocytes to act as diabetogenic APC in NOD mice depends on expression of self-antigen-specific immunoglobulin receptors. Eur J Immunol 2002;32:3657–3666 [DOI] [PubMed] [Google Scholar]
- 13.Silveira PA, Dombrowsky J, Johnson E, Chapman HD, Nemazee D, Serreze DV. B cell selection defects underlie the development of diabetogenic APCs in nonobese diabetic mice. J Immunol 2004;172:5086–5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojas M, Hulbert C, Thomas JW. Anergy and not clonal ignorance determines the fate of B cells that recognize a physiological autoantigen. J Immunol 2001;166:3194–3200 [DOI] [PubMed] [Google Scholar]
- 15.Carrillo J, Puertas MC, Alba A, et al. . Islet-infiltrating B-cells in nonobese diabetic mice predominantly target nervous system elements. Diabetes 2005;54:69–77 [DOI] [PubMed] [Google Scholar]
- 16.Puertas MC, Carrillo J, Pastor X, et al. . Peripherin is a relevant neuroendocrine autoantigen recognized by islet-infiltrating B lymphocytes. J Immunol 2007;178:6533–6539 [DOI] [PubMed] [Google Scholar]
- 17.Leonard DG, Gorham JD, Cole P, Greene LA, Ziff EB. A nerve growth factor-regulated messenger RNA encodes a new intermediate filament protein. J Cell Biol 1988;106:181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barclay M, Noakes PG, Ryan AF, Julien JP, Housley GD. Neuronal expression of peripherin, a type III intermediate filament protein, in the mouse hindbrain. Histochem Cell Biol 2007;128:541–550 [DOI] [PubMed] [Google Scholar]
- 19.Durant S, Geutskens S, Van Blokland SC, et al. . Proapoptosis and antiapoptosis-related molecules during postnatal pancreas development in control and nonobese diabetic mice: relationship with innervation. Lab Invest 2003;83:227–239 [DOI] [PubMed] [Google Scholar]
- 20.Strom A, Sonier B, Chapman HD, et al. . Peripherin-reactive antibodies in mouse, rabbit, and human blood. J Proteome Res 2010;9:1203–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serreze DV, Leiter EH, Hanson MS, et al. . Emv30null NOD-scid mice: an improved host for adoptive transfer of autoimmune diabetes and growth of human lymphohematopoietic cells. Diabetes 1995;44:1392–1398 [DOI] [PubMed] [Google Scholar]
- 22.Koenig-Marrony S, Soulas P, Julien S, et al. . Natural autoreactive B cells in transgenic mice reproduce an apparent paradox to the clonal tolerance theory. J Immunol 2001;166:1463–1470 [DOI] [PubMed] [Google Scholar]
- 23.Johnson EA, Silveira P, Chapman HD, Leiter EH, Serreze DV. Inhibition of autoimmune diabetes in nonobese diabetic mice by transgenic restoration of H2-E MHC class II expression: additive, but unequal, involvement of multiple APC subtypes. J Immunol 2001;167:2404–2410 [DOI] [PubMed] [Google Scholar]
- 24.Niens M, Grier AE, Marron M, Kay TW, Greiner DL, Serreze DV. Prevention of “Humanized” diabetogenic CD8 T-cell responses in HLA-transgenic NOD mice by a multipeptide coupled-cell approach. Diabetes 2011;60:1229–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alamyar E, Duroux P, Lefranc MP, Giudicelli V. IMGT(®) tools for the nucleotide analysis of immunoglobulin (IG) and T cell receptor (TR) V-(D)-J repertoires, polymorphisms, and IG mutations: IMGT/V-QUEST and IMGT/HighV-QUEST for NGS. Methods Mol Biol 2012;882:569–604 [DOI] [PubMed] [Google Scholar]
- 26.Carrillo J, Puertas MC, Planas R, et al. . Anti-peripherin B lymphocytes are positively selected during diabetogenesis. Mol Immunol 2008;45:3152–3162 [DOI] [PubMed] [Google Scholar]
- 27.Garabatos N, Alvarez R, Carrillo J, et al. . In vivo detection of peripherin-specific autoreactive B cells during type 1 diabetes pathogenesis. J Immunol 2014;192:3080–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrero JA, Calderon B, Towfic F, Artyomov MN, Unanue ER. Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse. PLoS One 2013;8:e59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA. Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and beta-cell destruction in NOD mice. Diabetes 1994;43:667–675 [DOI] [PubMed] [Google Scholar]
- 30.Alanentalo T, Hörnblad A, Mayans S, et al. . Quantification and three-dimensional imaging of the insulitis-induced destruction of beta-cells in murine type 1 diabetes. Diabetes 2010;59:1756–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serreze DV, Chapman HD, Niens M, et al. . Loss of intra-islet CD20 expression may complicate efficacy of B-cell-directed type 1 diabetes therapies. Diabetes 2011;60:2914–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol 2009;9:767–777 [DOI] [PubMed] [Google Scholar]
- 33.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol 1998;161:1163–1168 [PubMed] [Google Scholar]
- 34.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol 1998;161:3912–3918 [PubMed] [Google Scholar]
- 35.Noorchashm H, Lieu YK, Noorchashm N, et al. . I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol 1999;163:743–750 [PubMed] [Google Scholar]
- 36.Kendall PL, Case JB, Sullivan AM, et al. . Tolerant anti-insulin B cells are effective APCs. J Immunol 2013;190:2519–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramiscal RR, Vinuesa CG. T-cell subsets in the germinal center. Immunol Rev 2013;252:146–155 [DOI] [PubMed] [Google Scholar]
- 38.Acevedo-Suárez CA, Hulbert C, Woodward EJ, Thomas JW. Uncoupling of anergy from developmental arrest in anti-insulin B cells supports the development of autoimmune diabetes. J Immunol 2005;174:827–833 [DOI] [PubMed] [Google Scholar]
- 39.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al.; Type 1 Diabetes TrialNet Anti-CD20 Study Group . Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009;361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winer S, Tsui H, Lau A, et al. . Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nat Med 2003;9:198–205 [DOI] [PubMed] [Google Scholar]
- 41.Tsui H, Chan Y, Tang L, et al. . Targeting of pancreatic glia in type 1 diabetes. Diabetes 2008;57:918–928 [DOI] [PubMed] [Google Scholar]