Abstract
Maternal metabolites and metabolic networks underlying associations between maternal glucose during pregnancy and newborn birth weight and adiposity demand fuller characterization. We performed targeted and nontargeted gas chromatography/mass spectrometry metabolomics on maternal serum collected at fasting and 1 h following glucose beverage consumption during an oral glucose tolerance test (OGTT) for 400 northern European mothers at ∼28 weeks' gestation in the Hyperglycemia and Adverse Pregnancy Outcome Study. Amino acids, fatty acids, acylcarnitines, and products of lipid metabolism decreased and triglycerides increased during the OGTT. Analyses of individual metabolites indicated limited maternal glucose associations at fasting, but broader associations, including amino acids, fatty acids, carbohydrates, and lipids, were found at 1 h. Network analyses modeling metabolite correlations provided context for individual metabolite associations and elucidated collective associations of multiple classes of metabolic fuels with newborn size and adiposity, including acylcarnitines, fatty acids, carbohydrates, and organic acids. Random forest analyses indicated an improved ability to predict newborn size outcomes by using maternal metabolomics data beyond traditional risk factors, including maternal glucose. Broad-scale association of fuel metabolites with maternal glucose is evident during pregnancy, with unique maternal metabolites potentially contributing specifically to newborn birth weight and adiposity.
Introduction
Offspring of mothers with preexisting or gestational diabetes mellitus (GDM) are at risk for higher birth weight (BW) and adiposity as well as childhood metabolic disorders, including obesity, impaired glucose tolerance, and dyslipidemia (1–3). Mechanisms underlying these risks are not well defined but likely relate to fetal overnutrition in the setting of available maternal fuels (2,3).
One fuel present in increased supply in GDM is glucose. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study, a population-based study of >23,000 women conducted from 2000 to 2006, and others demonstrated a linear relationship between maternal glucose and offspring BW and fatness (2,4). This is likely mediated through glucose-stimulated insulin secretion in the fetus. Additional fuels also likely contribute (5). For example, pregnant women with GDM have increased circulating triglycerides in the third trimester. Maternal free fatty acids, which are derived from triglycerides, cross the placenta, serve as substrates for triglyceride synthesis, and contribute to fetal growth (2,3,6). Amino acids in maternal fasting plasma have been correlated with BW among women with diet-controlled GDM (7). The role of these and other fuels in risks associated with maternal hyperglycemia is unknown. To address possible metabolic linkages between maternal hyperglycemia and offspring phenotypes, we performed targeted and nontargeted metabolomics together with pathway, network, and random forest analyses in 400 European ancestry HAPO mothers.
Research Design and Methods
Participants
We studied 400 HAPO Caucasian mother-offspring dyads of northern European ancestry from Belfast, U.K., and Brisbane and Newcastle, Australia, field centers (Table 1). Dyads were sampled so that maternal glucose and BMI, newborn BW, and sum of skinfolds (SS) spanned the range observed in HAPO (4).
Table 1.
Demographics of mothers and their offspring
| Field center, N (%) | |
| Belfast, U.K. | 188 (47.0) |
| Brisbane, Australia | 136 (34.0) |
| Newcastle, Australia | 76 (19.0) |
| Maternal parity, N (%) | |
| First child | 203 (50.7) |
| Second or third child | 197 (49.2) |
| Newborn sex, N (%) | |
| Male | 209 (52.2) |
| Female | 191 (47.8) |
| Maternal characteristics | |
| Age at OGTT (years) | 29.4 (5.12) |
| BMI at OGTT (kg/m2) | 29.0 (4.89) |
| Mean arterial pressure (mmHg) | 83.1 (6.89) |
| FPG (mg/dL) | 82.1 (6.14) |
| One-hour plasma glucose (mg/dL) | 131.7 (27.24) |
| Two-hour plasma glucose (mg/dL) | 111.0 (20.34) |
| Sample storage time (years) | 9.9 (1.25) |
| Newborn characteristics | |
| Gestational age at OGTT (weeks) | 28.6 (1.37) |
| Gestational age at delivery (weeks) | 40.2 (1.15) |
| Cord C-peptide (μg/L) | 1.1 (0.51) |
| BW (g) | 3,667.6 (485.28) |
| SS (mm) | 12.8 (2.74) |
Data are means (SD) unless otherwise indicated.
Data and Sample Collection
HAPO (conducted 2000–2006) methods have been described previously (4). Eligible women underwent a 75-g oral glucose tolerance test (OGTT) at 24–32 weeks' gestation. Fasting plasma glucose (FPG) and 1-h plasma glucose were measured. Serum samples collected during the HAPO OGTT were stored at −80°C until the present metabolomics assays. Maternal height and weight and newborn BW and SS were measured by standard procedures with calibrated equipment. Gestational age was determined as previously described (4). Demographic and lifestyle characteristics were collected through questionnaire. Participants, caregivers, and HAPO staff remained blinded to glucose values unless glucose exceeded predefined levels. Unblinded participants were excluded.
Conventional Metabolite and Targeted Amino Acid and Acylcarnitine Analyses
Conventional metabolites (lactate, triglycerides, β-hydroxybutyrate, glycerol) were measured as previously described (8) with the addition of nonesterified fatty acids (NEFAs), using reagents from Wako (Mountain View, CA). Targeted assays of amino acids and acylcarnitines (ACs) were performed by using stable isotope–labeled internal standards on an Acquity TQD system (Waters Corporation, Milford, MA) (8). In total, 63 conventional and targeted metabolites were analyzed to complement nontargeted gas chromatography/mass spectrometry (GC/MS) analyses to the fullest extent possible.
Nontargeted GC/MS Analyses
Nontargeted assays designed to analyze the full range of metabolites present in serum were performed by GC/MS. Methanol, the extraction solvent, was spiked with a retention time locking internal standard of perdeuterated myristic acid. Extracts were dried, prepared by methoximation and trimethylsilylation (8,9), and run on a 6890N GC/5975 Inert MS (Agilent Technologies, Santa Clara, CA). Programmed temperature vaporization in the inlet and postrun, midcolumn, hot back-flushing of the GC column minimized analyte decomposition, carryover, and fouling of GC and MS. Peaks were deconvoluted with AMDIS freeware (10) and parsed against the Fiehn GC/MS Metabolimics RTL library (9) with additions from our laboratory. Detected peak areas were log2 transformed for analysis. Manual curation included identification of coeluting groups of isomeric metabolites and selection of reliable peaks (8). Some fatty acids are methylated during sample preparation, and the methylated fatty acid was used in analyses. α- and β-Monopalmitin likely represented both endogenous metabolite and contaminant from sample preparation. In total, 84 GC/MS metabolites not assayed through targeted approaches were used for data analysis.
Batches of fasting and 1-h maternal serum sample pairs were constructed to balance field center, maternal glucose, and BMI and newborn outcomes across batches. Quality control pools were prepared with equal volumes from all maternal samples and prepared for analysis as described above. These pools were injected as first, middle, and last samples of each GC/MS run. Batches of equal size were run over 50 consecutive days.
Data Analysis
GC/MS data were normalized to control technical variability attributable to batch and run order by using a mixture model approach in the R package metabomxtr (11). The mixture model can be viewed as a combination of a linear and logistic regression model, with the linear portion modeling quantifiable metabolite abundance and the logistic portion modeling detectability or lack thereof for a metabolite in a given sample. Given batch-specific detection thresholds, this approach is uniquely suited to GC/MS data. We specified separate mixture models for each metabolite by using quality control data, with metabolite level as the outcome, categorical batch variables in the logistic and linear model components, and log-transformed run order in the linear component. Variations in metabolite levels due to batch and run order were identified through the quality control data and then subtracted from analytical sample data to control technical noise for each metabolite.
Differences between maternal fasting and 1-h metabolite levels were evaluated with paired t tests for metabolites with ≥90% observed data. Associations between maternal fasting and 1-h glucose with metabolites at corresponding time points were evaluated using separate linear models, with metabolites as outcomes and glucose as predictors. For GC/MS metabolites undetectable in >10% of samples, mixture models were used (11) similar to normalization. Linear models were also used to investigate associations between maternal metabolite predictors and newborn SS and BW outcomes. GC/MS metabolites undetectable in >10% of samples were treated as four categories: one for undetected values and three for tertiles of normalized log2 peak areas. To model longitudinal associations for glucose and metabolites with detected values at both time points in ≥90% of mothers, we computed percent changes by dividing the difference between 1-h and fasting levels by the fasting level and repeated linear model analyses. Models were also examined for absolute differences between 1 h and fasting. All models included adjustment for field center, gestational age, maternal age, BMI, and mean arterial pressure at OGTT, newborn sex, and sample storage time. Adaptive Benjamini-Hochberg false discovery rate (FDR) correction was applied separately for targeted and nontargeted data (12).
Pathway analyses were conducted with MetaboAnalyst 3.0 (13) and its included set of human Kyoto Encyclopedia of Genes and Genomes pathways (14). Quantitative enrichment globaltest analysis (15) was used to identify maternal metabolites that are members of the same pathways and demonstrate collective associations with maternal fasting or 1-h glucose.
Because metabolites are not independent of one another but, rather, act in coordinated fashion, we conducted network analyses to complement individual metabolite analyses by simultaneously modeling metabolite correlations and phenotype associations. First, we constructed separate correlation networks for metabolites at fasting and 1 h. Nodes represented metabolites, and edges represented partial correlation of metabolite pairs at each time point with magnitude >0.25 after adjustment for all covariates used in the aforementioned regression analyses. After correlation networks were constructed from all metabolites, we identified subnetworks comprising metabolites that demonstrated joint association with our phenotypes. To do this, we applied a subnetwork identification algorithm that incorporated node scores and edge weights based on phenotype associations to find the optimal scoring subnetwork for that phenotype (16).
We first identified subnetworks of maternal metabolites associated with maternal glucose at fasting and 1 h. Given the P value (p) for association between the metabolite and glucose, the node score was defined as S = −log(p) + log(0.10). This assigns high positive scores for P < 0.10, modest positive scores for P close to but <0.10, and increasingly negative scores for P > 0.10. Edge weights were assigned by using algorithm defaults, giving higher weight to edges whose adjacent nodes had negative scores and low degree. Subnetworks associated with phenotype were determined by identifying connected sets of positively scoring nodes and evaluating whether uniting positive sets through negatively scoring nodes resulted in a positive sum of node scores. If so, the lowest weight edge path was used to connect nodes. To characterize local connectivity in networks, we applied spinglass community detection using the R package igraph (17,18). Maternal glucose networks can be interpreted as sets of correlated metabolites that are all associated with fasting or 1-h glucose, with the highest correlation evident within spinglass communities.
Networks for maternal metabolites associated with both maternal glucose and newborn outcomes were identified by the same approach with a modified node score. Given P values pm and pn for a metabolite’s association with maternal glucose and newborn outcome, respectively, we calculated an aggregate P value pa for the maximum of pm and pn based on two random draws from a uniform distribution (16). We then set S = −log(pa) + log(0.10).
Random forest analyses were conducted using the R package party to identify maternal metabolites that improved prediction of newborn BW and SS beyond known risk factors maternal glucose and BMI. Random forests are data-driven learning methods designed for prediction (19), in this case applied to continuous newborn BW and SS outcomes. Overall model accuracy is measured as percent variation explained, and contributions of individual predictors are measured by variable importance. Given known high correlations among metabolites, we used conditional permutations to evaluate variable importance (20). This approach evaluates predictor contributions independent of other correlated predictors. Variable importance scores >0 indicate higher percent variation explained when a predictor is included in the model. Variables with importance scores <0 may decrease prediction accuracy. We examined random forest models as follows: M0 = maternal BMI at OGTT, gestational age at delivery, field center, and sample storage time; M1 = M0 + maternal glucose; M2 = M1 + highest scoring metabolite within N spinglass communities for the network; M3 = M1 + N metabolites with lowest pa values; and M4 = M1 + metabolites with variable importance scores >0 after running a model that included all metabolites with pa < 0.10.
Results
Study Population
Study population characteristics are shown in Table 1. Mothers spanned the range of maternal BMI and glucose observed in the HAPO Study. Roughly equal numbers of males and females were represented among offspring.
Maternal Metabolites During the OGTT
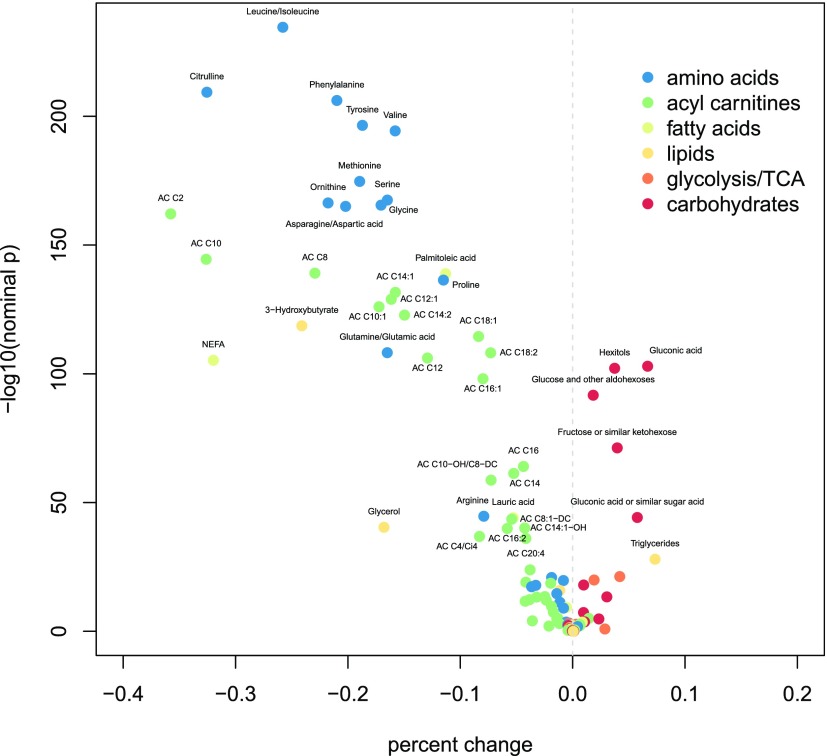
Changes in multiple metabolites between fasting and 1 h were observed (Fig. 1 and Supplementary Table 1). All targeted amino acids, several long- and medium-chain fatty acids, and multiple products of lipid metabolism, including ACs, glycerol, and β-hydroxybutyrate, decreased after glucose ingestion. In contrast, triglycerides, carbohydrates, and metabolic intermediates, including pyruvate and citrate/isocitrate, increased.
Figure 1.
Volcano plot of maternal metabolites demonstrating differences from fasting to 1 h with FDR-adjusted P < 0.05. The y-axis represents the negative log10 transformation of the nominal P value from paired t tests. The x-axis represents mean within-individual percent changes from fasting to 1 h. Points are colored according to metabolite class.
Maternal Metabolites Associated With Maternal Glucose
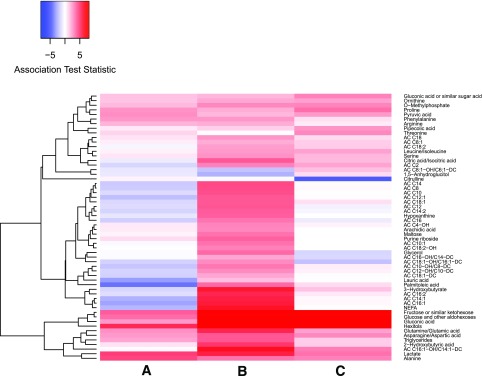
A limited number of fasting metabolites were positively associated with FPG after FDR adjustment, including gluconeogenic substrates alanine and lactate as well as hexitols and fructose. Lauric acid, a medium-chain fatty acid, and palmitoleic acid and its AC were negatively associated with FPG (Fig. 2 and Supplementary Table 2).
Figure 2.
Heat map showing positive (red) and negative (blue) associations of maternal glucose and metabolites at fasting (lane A) and 1 h (lane B) during the OGTT as well as percent change associations from fasting to 1 h for maternal glucose and metabolites (lane C). All metabolites with FDR-adjusted P < 0.05 for at least one time point or for percent change analyses are shown.
At 1 h, more associations between metabolites and glucose were significant (Fig. 2 and Supplementary Table 2). Similar to fasting, positive associations were observed for 1-h alanine, lactate, and fructose. Positive associations were also observed for NEFA, β-hydroxybutyrate, triglycerides, glycerol, asparagine/aspartate, glutamine/glutamate, leucine/isoleucine, ornithine, phenylalanine, proline and serine, and multiple ACs and fatty acids. Also distinct were positive associations of gluconic acid, a marker of oxidative stress, and 2-hydroxybutyrate, a previously reported biomarker for insulin resistance (21), and a negative association of 1-h 1,5-anhydroglucitol.
Longitudinal analyses identified similar associations observed at individual time points with a few additions (Fig. 2 and Supplementary Table 2). Percent changes in citrulline and threonine exhibited significant associations with percent change in glucose. Analyses of absolute differences from fasting to 1 h were consistent with percent change associations (data not shown).
Pathway Analyses
Pathway analyses identified multiple pathways related to amino acid, triglyceride, and sugar and carbohydrate metabolism whose metabolite members were jointly associated with FPG or 1-h glucose at corresponding time points (Table 2). There was substantial overlap in the pathways associated with glucose at the two time points, with a greater number of pathways demonstrating association at 1 h, including the tricarboxylic acid cycle (TCA) and metabolism of ketone bodies.
Table 2.
MetaboAnalyst pathway analysis results with FDR-adjusted P < 0.05 for fasting and/or 1-h maternal metabolites
| Pathway name | No. of metabolites annotated to pathway | No. of measured metabolites | FDR-adjusted P value at fasting | FDR-adjusted P value at 1 h |
|---|---|---|---|---|
| Amino acid metabolism | ||||
| Selenoamino acid metabolism | 22 | 1 | 3.6e-05 | 0.00084 |
| Taurine and hypotaurine metabolism | 20 | 3 | 4.7e-05 | 0.0031 |
| Alanine, aspartate, glutamate metabolism | 24 | 5 | 4.7e-05 | 6.2e-08 |
| Cysteine and methionine metabolism | 56 | 6 | 0.0013 | 0.00012 |
| Arginine and proline metabolism | 77 | 9 | 0.0027 | 1.6e-06 |
| Aminoacyl-tRNA biosynthesis | 75 | 15 | 0.0044 | 0.00013 |
| D-Glutamine and D-glutamate metabolism | 11 | 1 | 0.015 | 1.2e-07 |
| Nitrogen metabolism | 39 | 6 | 0.016 | 3.7e-05 |
| Histidine metabolism | 44 | 3 | 0.016 | 7.3e-07 |
| Phenylalanine metabolism | 45 | 4 | 0.016 | 0.011 |
| Lysine biosynthesis | 32 | 3 | 0.036 | 0.00026 |
| Phenylalanine, tyrosine, tryptophan biosynthesis | 27 | 2 | 0.036 | 0.026 |
| Glycine, serine, and threonine metabolism | 48 | 7 | 0.058 | 0.0041 |
| D-Arginine and D-ornithine metabolism | 8 | 2 | 0.079 | 0.015 |
| β-Alanine metabolism | 28 | 4 | 0.12 | 0.00053 |
| Cyanoamino acid metabolism | 16 | 3 | 0.12 | 0.0018 |
| Sugar and carbohydrate metabolism | ||||
| Galactose metabolism | 41 | 4 | 4.5e-07 | 1.6e-57 |
| Glycolysis or gluconeogenesis | 31 | 3 | 4.5e-07 | 9.8e-51 |
| Fructose and mannose metabolism | 48 | 1 | 4.5e-07 | 1.6e-20 |
| Pyruvate metabolism | 32 | 2 | 3.1e-05 | 7.3e-07 |
| Pentose phosphate pathway | 32 | 4 | 9.2e-05 | 2.2e-62 |
| Starch and sucrose metabolism | 50 | 3 | 0.0031 | 5.4e-45 |
| Amino sugar, nucleotide sugar metabolism | 88 | 3 | 0.0098 | 6.6e-42 |
| Citrate cycle (TCA) | 20 | 3 | 0.12 | 0.00027 |
| Lipid metabolism | ||||
| Fatty acid biosynthesis | 49 | 2 | 0.00015 | 0.0018 |
| Propanoate metabolism | 35 | 5 | 0.0017 | 5.9e-08 |
| Butanoate metabolism | 40 | 5 | 0.015 | 1.2e-12 |
| Glycerolipid metabolism | 32 | 3 | 0.112 | 9.0e-05 |
| Fatty acid metabolism | 50 | 2 | 0.22 | 0.00064 |
| Synthesis and degradation of ketone bodies | 6 | 2 | 0.33 | 7.2e-09 |
| Sphingolipid metabolism | 25 | 1 | 0.50 | 0.012 |
| Vitamin metabolism | ||||
| Nicotinate and nicotinamide metabolism | 44 | 2 | 0.0050 | 9.0e-05 |
| Pantothenate and CoA biosynthesis | 27 | 3 | 0.0098 | 0.00081 |
| Vitamin B6 metabolism | 32 | 1 | 0.025 | 0.086 |
| Biotin metabolism | 11 | 1 | 0.073 | 0.033 |
| Ascorbate and aldarate metabolism | 45 | 5 | 0.12 | 0.024 |
| Other | ||||
| Terpenoid backbone biosynthesis | 33 | 1 | 0.025 | 0.086 |
| Glutathione metabolism | 38 | 4 | 0.050 | 2.8e-06 |
| Porphyrin and chlorophyll metabolism | 104 | 3 | 0.05 | 2.7e-05 |
| Glyoxylate and dicarboxylate metabolism | 50 | 4 | 0.11 | 0.00076 |
| Sulfur metabolism | 18 | 2 | 0.74 | 0.027 |
Maternal Metabolite Associations With Newborn Outcomes
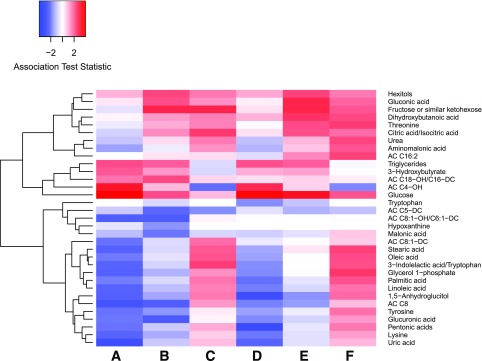
Although P values were not significant after FDR adjustment, several association trends between maternal metabolites and both newborn SS and BW were evident (Fig. 3 and Supplementary Table 3). Maternal fasting and 1-h triglycerides, fasting AC C4-OH, 1-h fructose, gluconic acid, and hexitols were positively associated. Maternal fasting 1,5-anhydroglucitol, lysine, and pentonic acids were negatively associated.
Figure 3.
Heat map showing positive (red) and negative (blue) associations of maternal metabolites at fasting and 1 h and percent change with newborn SS (lanes A–C, respectively) and BW (lanes D–F, respectively). Glucose in this figure refers to the original HAPO OGTT maternal glucose measurements. All metabolites with a nominal P < 0.05 for at least one time point or in percent change analyses are shown.
Other metabolites demonstrated unique association trends with either outcome. Fasting maternal tyrosine, glycerol 1-phosphate, and linoleic and stearic acids; 1-h hypoxanthine and malonic acid; and both fasting and 1-h AC C8, AC C8:1-OH/C6:1-DC, and glucuronic acid were negatively associated with newborn SS. Fasting β-hydroxybutyrate was positively associated with SS. Other ACs demonstrated several associations in both directions with SS at either time point or longitudinally. For newborn BW, positive associations included 1-h dihydroxybutanoic acid, threonine, and citric acid. Fasting palmitic acid was negatively associated with BW. Percent changes in AC C16:2, glycerol 1-phosphate, threonine, urea, aminomalonic acid, fructose, and several fatty acids were positively associated with BW.
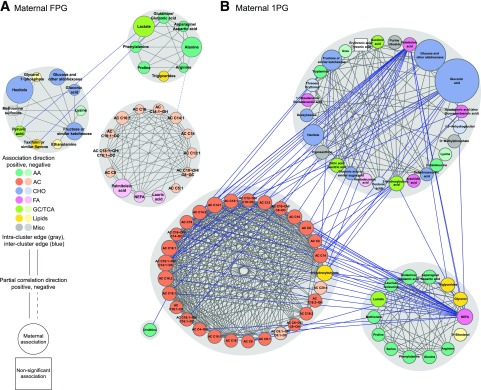
Maternal Glucose Networks
Network analyses were conducted to contextualize individual metabolite associations and describe joint associations on behalf of correlated metabolites. Several metabolites demonstrating associations in separate models were part of identified networks, whereas others were excluded because they do not correlate with other metabolites. Some metabolites were included that did not reach individual statistical significance. The network associated with glucose at fasting includes three spinglass communities of carbohydrates and organic acids, amino acids, and ACs and fatty acids. The 1-h network is substantially larger and, again, sorted primarily into communities of carbohydrates and organic acids, amino acids, and ACs (Fig. 4).
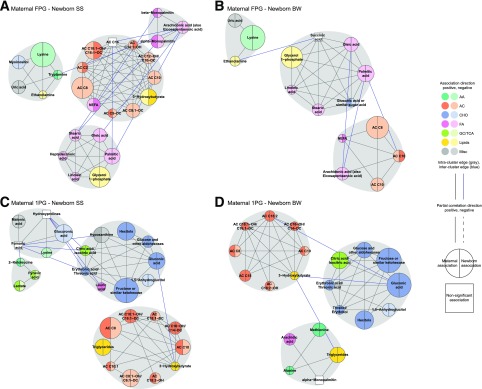
Figure 4.
Subnetworks of maternal metabolites associated with maternal fasting and 1-h glucose. Nodes represent metabolites, and edges represent partial correlation >0.25 for metabolite pairs. Nodes are sized according to node score–based P values, with larger nodes corresponding to higher scores. Nodes are colored according to metabolite class. Gray shading identifies spinglass communities within the subnetworks. Direction of association is noted by color shading in the nodes. Positive associations are darker, and negative associations are lighter. A: The subnetwork of maternal fasting metabolites associated with maternal FPG. B: The subnetwork of maternal 1-h metabolites associated with maternal 1-h plasma glucose (1PG). AA, amino acid; CHO, carbohydrate; FA, fatty acid; GC, glycolysis; Misc, miscellaneous.
Maternal Glucose and Newborn Outcome Networks
Networks for metabolites associated with maternal glucose and newborn outcomes were smaller with more granular spinglass communities at both fasting and 1 h (Fig. 5). Unlike the networks associated with maternal glucose alone, amino acids were largely absent from networks incorporating associations with newborn outcomes. Also striking was the transition of ACs from primarily negative associations with fasting glucose to largely positive associations with 1-h glucose and the larger size and stronger associations of this community with newborn SS compared with BW (Fig. 5).
Figure 5.
Subnetworks of maternal metabolites associated with maternal glucose and newborn outcomes. Nodes represent metabolites, and edges represent partial correlation >0.25 for metabolite pairs. Nodes are sized according to node score based on aggregate P values, with larger nodes corresponding to higher scores. Nodes are colored according to metabolite class. Gray shading identifies spinglass communities within the subnetworks. Direction of association for maternal glucose and newborn outcome is noted by color shading in the left and right sides of the nodes, respectively. Positive associations are darker, and negative associations are lighter. A: The subnetwork of maternal fasting metabolites associated with maternal FPG and newborn SS. B: The subnetwork of maternal fasting metabolites associated with maternal FPG and newborn BW. C: The subnetwork of maternal 1-h metabolites associated with maternal 1PG and newborn SS. D: The subnetwork of maternal 1-h metabolites associated with maternal 1PG and newborn BW. 1PG, 1-h plasma glucose; AA, amino acid; CHO, carbohydrate; FA, fatty acid; GC, glycolysis; Misc, miscellaneous.
Random Forest Analyses
To determine whether metabolites associated with maternal glucose contribute to the prediction of newborn BW and SS beyond traditional risk factors, including glucose, random forest analyses were performed (Table 3 and Supplementary Fig. 1). Model M1, which includes maternal glucose at either fasting or 1 h, explained higher overall percent variation for both newborn outcomes compared with model M0, which includes maternal BMI and other baseline covariates. This reflects the well-substantiated role of maternal glucose in newborn size outcomes. As seen in Supplementary Fig. 1, the relative contribution of glucose as a predictor of newborn outcomes (measured by conditional variable importance) decreased in models M2, M3, and M4 compared with M1 as additional metabolites were included. This decrease suggests that maternal metabolites that correlate with glucose account in part for the effect of glucose on these newborn outcomes. Importantly, in models M2, M3, and M4 where metabolites associated with both glucose and newborn outcomes are included as predictors, the percent variation explained is consistently higher than in M1 for both BW and SS. Taken together, the decrease in variable importance for glucose in M2, M3, and M4 and the increase in percent overall variation explained by these models indicate that metabolites that are part of the broad-scale changes associated with maternal glycemia are independent contributors to newborn size outcomes.
Table 3.
Overall percent variation explained for random forest models predicting newborn size outcomes
| Percent variation explained |
|||||
|---|---|---|---|---|---|
| M0 | M1 | M2 | M3 | M4 | |
| Maternal FPG and fasting metabolites—newborn SS | 3.99 | 6.03 | 7.59 | 8.13 | 10.08 |
| Maternal FPG and fasting metabolites—newborn BW | 3.02 | 4.35 | 8.01 | 7.47 | 7.16 |
| Maternal 1-h PG and 1-h metabolites—newborn SS | 3.99 | 5.35 | 7.08 | 6.98 | 8.52 |
| Maternal 1-h PG and 1-h metabolites—newborn BW | 3.02 | 7.07 | 8.49 | 7.89 | 9.22 |
M0, baseline model including maternal BMI, gestational age at delivery, field center, and sample storage time; M1, M0 + maternal glucose at fasting or 1 h; M2, M3, and M4, M1 + metabolites selected according to strategies described in research design and methods. Individual metabolites are listed in Supplementary Fig. 1.
Discussion
Maternal metabolism during pregnancy differs from the pregravid state due to metabolic adaptations to meet the mother’s and growing fetus’s energy needs (22–24). Pregnancy has been described as accelerated starvation during fasting to meet fetal demands for glucose, amino acids, and other nutrients and facilitated anabolism following nutrient ingestion to allow repletion of maternal reserves (22). For example, women in the third trimester of pregnancy exhibit a larger decrease in total free fatty acids and an increase in triglycerides compared with the nongravid state following glucose ingestion (5,22). The current study characterized the maternal metabolome in women with glucose levels across the range observed in a population-based study of women who underwent an OGTT at ∼28 weeks' gestation (4). To date, studies characterizing the metabolome of pregnant women have largely been limited in size and have focused on fasting women with GDM compared with healthy pregnant women (25). To our knowledge, the current study is the largest and first in pregnant women that examined two OGTT time points to identify maternal metabolites associated with glucose as a quantitative trait.
Prior studies examining the effect of glucose ingestion on the metabolome have been limited to nonpregnant populations (26–32). Similar to those studies, we demonstrated a glucose-induced decrease in multiple metabolites, including glycerol, β-hydroxybutyrate, NEFA, medium- and long-chain fatty acids, ACs, and amino acids. Pyruvate increased as did lactate after glucose ingestion; the latter has been previously reported (26). Previous findings with Krebs cycle intermediates have varied (26,32). We observed an increase in circulating citrate/isocitrate but no change in lactate, fumarate, and malate. Thus, despite pregnancy-induced insulin resistance and attendant changes in maternal metabolism, glucose-stimulated insulin secretion in pregnancy inhibits lipolysis, proteolysis, and ketogenesis and stimulates glycolysis similar to nonpregnant populations.
To date, metabolomic studies during pregnancy have focused largely on women with GDM, a state of relative insulin insufficiency due to inadequate maternal β-cell compensation for pregnancy-induced insulin resistance (33). Differences among the metabolomes between women without and with GDM have been reported, but few metabolites have demonstrated consistent changes across studies, likely due to small sample sizes and various technologies and study designs (25). Total and individual free fatty acids have been reported as either higher or both higher and lower during the second or third trimester in women with GDM and/or an impaired glucose challenge test (25,34–36). Previous studies of amino acid levels in women with GDM have been inconsistent (25). We identified many metabolites and metabolic pathways associated with maternal glucose at 28 weeks' gestation independent of maternal BMI. In the fasting state, a limited number of metabolites were positively associated with maternal glucose, most notably lactate and alanine. A potential explanation for the higher levels of these gluconeogenic substrates in the setting of maternal hyperglycemia is inefficient glucose utilization and relative mitochondrial inefficiency with diversion of glucose to alanine and lactate as opposed to entering the TCA. This finding is consistent with the observation that alanine, lactate, and organic acids are higher early in pregnancy (∼16 weeks' gestation) in women subsequently given a diagnosis of GDM (37). After glucose ingestion, many more metabolites were associated with maternal glucose, including a positive association of many amino acids and ACs. This is consistent with blunted insulin-induced inhibition of proteolysis and lipolysis in women with higher glucose and suggests that metabolic changes during pregnancy are not limited to GDM.
A unique aspect of this study was the availability of fetal phenotypes in addition to maternal phenotype and metabolomic data. Association of maternal glucose and triglyceride levels with BW and fetal adiposity has been demonstrated (3,4,38–42). Maternal fatty acids increase during pregnancy, are transported across the placenta, and contribute to fetal growth (6), whereas levels of amino acids, which are important for fetal protein accretion and growth, fall (23,43). Maternal fatty acids and glycerol have been shown to be associated with BW and fat mass in mothers with GDM but not in control subjects (39,44). Findings with amino acids have been inconsistent; some found no association with BW of maternal amino acids late in gestation, whereas others showed positive associations of maternal serine, lysine, proline, ornithine, and arginine and a negative association of methionine (45,46). Others found positive associations of aspartate, alanine, ornithine, and arginine levels at 25 weeks' gestation with BW (47). In the current study, individual maternal metabolites failed to demonstrate FDR-adjusted associations with newborn BW or SS. Fasting and 1-h triglycerides were nominally positively associated with BW and SS, and fasting levels of tyrosine and several fatty acids were nominally negatively associated with SS or BW. The reasons for the difference between the current results and earlier studies are not known, but the earlier studies were small with different study designs.
Recognizing that metabolites likely act in concert rather than individually, we performed network analyses to identify correlated metabolites associated with both maternal and fetal phenotypes. These network studies identified interrelated groups of maternal metabolites that collectively demonstrate consistent, often subtle, associations with both maternal glucose and newborn outcomes. At fasting, clusters of fatty acids and the carnitine esters of long- and medium-chain fatty acids were associated with maternal fasting glucose and newborn SS and/or BW. At 1 h, a cluster of ACs of long- and medium-chain fatty acids together with the ketone body β-hydroxybutyrate demonstrated an association with maternal 1-h glucose and both SS and BW. Different from the fasting state, clusters of sugars and metabolic intermediates at 1 h were also associated with maternal and newborn phenotypes. These include potential products of the polyol pathway, fructose and hexitols. The polyol pathway increases susceptibility to oxidative stress and contributes to diabetic complications (48). Of note, amino acid clusters were associated with maternal fasting or 1-h glucose alone but not with newborn phenotypes.
The Pedersen hypothesis states that higher transplacental transport of glucose and resulting fetal insulin secretion in the setting of maternal hyperglycemia contribute to macrosomia (5). The HAPO Study confirmed this hypothesis (4). Freinkel (5) modified the hypothesis to suggest that nutrients in addition to glucose also contribute to fetal growth and fat accretion in the setting of maternal hyperglycemia. We used random forest analysis to confirm Freinkel’s modification of the Pedersen hypothesis by identifying multiple metabolites associated with maternal glucose that contribute to newborn BW and SS independent of maternal glucose and BMI. As seen in Supplementary Fig. 1, a broad array of maternal metabolites makes some contribution to BW and/or SS. In the fasting state, this includes a number of lipid-related metabolites (fatty acids, triglycerides, glycerol 1-phosphate, ACs of medium-chain fatty acids), uric acid, and sugars (hexitols, myoinositol). Of note, lysine is the only amino acid in the fasting state that contributes to newborn size in these analyses. Previously, lysine levels helped to explain variability in the development of GDM (49), and higher levels of lysine have been demonstrated in some studies of women with GDM (45,46,50). At 1 h, a similar pattern was evident with lipid-related metabolites (β-hydroxybutyrate among others), sugars (fructose, hexitols, disaccharides, maltose), products of carbohydrate metabolism (citrate/isocitrate, lactate), and amino acids (methionine, phenylalanine), which all contributed to newborn outcomes. Together, these analyses demonstrate that beyond glucose, additional metabolites associated with glucose are independent contributors to newborn size at birth and, in aggregate, may have the potential to predict fetal size at birth.
The current study had several strengths. It is the largest to date to examine the association of maternal metabolic traits with maternal metabolites and the first in pregnant women to examine associations across the full range of glucose and associations of metabolites with glucose at both fasting and 1 h following a glucose load. Because pregnancy has been described as a state of accelerated starvation and facilitated anabolism, the examination of metabolites in both the fasting and the postprandial state is important. This is also the first metabolomic study to include mother-newborn dyads to allow for the examination of associations between the maternal metabolome and newborn outcomes and the identification of metabolites associated with glucose important for newborn size at birth. One limitation is the use of nontargeted assays that are not strictly quantitative. These assays allow unbiased examination of metabolite-phenotype associations, but findings of interest will ultimately require the development of targeted assays for confirmation. Moreover, the network and random forest analyses will require replication in independent studies.
In conclusion, we demonstrate broad-scale association of metabolites with either maternal fasting or 1-h glucose by using population-based data and provide new insight into metabolic changes characteristic of maternal hyperglycemia. We also found evidence for a role of maternal triglycerides, fatty acids, and their metabolites together with sugars and metabolic intermediates in newborn outcomes, whereas random forest analyses suggest that these and other metabolites are independent contributors to newborn BW and SS. Further studies relating these findings to the fetal metabolome will provide additional insight into mechanisms underlying fetal size at birth.
Article Information
Acknowledgments. The authors thank Stephan Baumann and Steven Fischer at Agilent Technologies, Inc., for assistance in developing the nontargeted GC/MS platform and Raji Balasubramanian at University of Massachusetts Amherst for helpful discussions on network analyses.
Funding. This study was funded by National Institute of Diabetes and Digestive and Kidney Diseases (grant R01-DK-095963) by the National Institute of Child Health and Human Development (grants R01-HD-34242 and R01-HD-34243).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.M.S. contributed to the study design, data analysis, interpretation of findings, and primary drafting of the manuscript. J.R.B. and M.J.M contributed to the conventional metabolite assays and nontargeted metabolomics, interpretation of findings, and manuscript writing. A.C.R. and M.N. contributed to the data analysis. R.D.S. and O.I. contributed to the development and performance of targeted metabolomics assays. L.P.L. contributed to the study design. B.E.M. and C.B.N. contributed to the study design and interpretation of findings. W.L.L. conceived the hypothesis and contributed to the study design, interpretation of findings, and primary drafting of the manuscript. D.M.S. and W.L.L. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1748/-/DC1.
References
- 1.Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol 2007;50:972–979 [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes 2011;60:1849–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol 2011;204:479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metzger BE, Lowe LP, Dyer AR, et al.; HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 5.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes 1980;29:1023–1035 [DOI] [PubMed] [Google Scholar]
- 6.Haggarty P. Fatty acid supply to the human fetus. Annu Rev Nutr 2010;30:237–255 [DOI] [PubMed] [Google Scholar]
- 7.Metzger BE. Biphasic effects of maternal metabolism on fetal growth. Quintessential expression of fuel-mediated teratogenesis. Diabetes 1991;40(Suppl. 2):99–105 [DOI] [PubMed] [Google Scholar]
- 8.Scholtens DM, Muehlbauer MJ, Daya NR, et al.; HAPO Study Cooperative Research Group . Metabolomics reveals broad-scale metabolic perturbations in hyperglycemic mothers during pregnancy. Diabetes Care 2014;37:158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kind T, Wohlgemuth G, Lee Y, et al. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal Chem 2009;81:10038–10048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halket JM, Przyborowska A, Stein SE, Mallard WG, Down S, Chalmers RA. Deconvolution gas chromatography/mass spectrometry of urinary organic acids—potential for pattern recognition and automated identification of metabolic disorders. Rapid Commun Mass Spectrom 1999;13:279–284 [DOI] [PubMed] [Google Scholar]
- 11.Nodzenski M, Muehlbauer MJ, Bain JR, Reisetter AC, Lowe WL Jr, Scholtens DM. Metabomxtr: an R package for mixture-model analysis of non-targeted metabolomics data. Bioinformatics 2014;30:3287–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat 2000;25:60–83 [Google Scholar]
- 13.Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0—making metabolimics more meaningful. Nucleic Acids Res 2015;43:W251–W257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res 2016;44(D1):D457–D462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goeman JJ, van de Geer SA, de Kort F, van Houwelingen HC. A global test for groups of genes: testing association with a clinical outcome. Bioinformatics 2004;20:93–99 [DOI] [PubMed] [Google Scholar]
- 16.Dittrich MT, Klau GW, Rosenwald A, Dandekar T, Müller T. Identifying functional modules in protein-protein interaction networks: an integrated exact approach. Bioinformatics 2008;24:i223–i231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reichardt J, Bornholdt S. Statistical mechanics of community detection. Phys Rev E Stat Nonlin Soft Matter Phys 2006;74:016110. [DOI] [PubMed] [Google Scholar]
- 18.Csardi G, Nepusz T. The igraph software package for complex network research [Internet]. InterJournal, Complex Systems 1695 2006. Available at http://igraph.org. Accessed 27 October 2015
- 19.Breiman L. Random forests. Mach Learn 2001;45:5–32 [Google Scholar]
- 20.Strobl C, Boulesteix AL, Kneib T, Augustin T, Zeileis A. Conditional variable importance for random forests. BMC Bioinformatics 2008;9:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrannini E, Natali A, Camastra S, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 2013;62:1730–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freinkel N, Metzger BE, Nitzan M, Daniel R, Surmaczynska B, Nagel T. Facilitated anabolism in late pregnancy: some novel maternal compensations for accelerated starvation. In Diabetes: Proceedings of the Eighth Congress of the International Diabetes Federation, Brussels, Belgium, 1973. Amsterdam, The Netherlands, Excerpta Medica International Congress Series, p. 474–488 [Google Scholar]
- 23.Hadden DR, McLaughlin C. Normal and abnormal maternal metabolism during pregnancy. Semin Fetal Neonatal Med 2009;14:66–71 [DOI] [PubMed] [Google Scholar]
- 24.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr 2000;71(Suppl.):1256S–1261S [DOI] [PubMed] [Google Scholar]
- 25.Huynh J, Xiong G, Bentley-Lewis R. A systematic review of metabolite profiling in gestational diabetes mellitus. Diabetologia 2014;57:2453–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho JE, Larson MG, Vasan RS, et al. Metabolite profiles during oral glucose challenge. Diabetes 2013;62:2689–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geidenstam N, Spégel P, Mulder H, Filipsson K, Ridderstråle M, Danielsson AP. Metabolite profile deviations in an oral glucose tolerance test-a comparison between lean and obese individuals. Obesity (Silver Spring) 2014;22:2388–2395 [DOI] [PubMed] [Google Scholar]
- 28.Spegel P, Danielsson AP, Bacos K, et al. Metabolomic analysis of a human oral glucose tolerance test reveals fatty acids as reliable indicators of regulated metabolism. Metabolomics 2010;6:56–66 [Google Scholar]
- 29.Bentley-Lewis R, Xiong G, Lee H, Yang A, Huynh J, Kim C. Metabolomic analysis reveals amino acid responses to an oral glucose tolerance test in women with prior history of gestational diabetes mellitus. J Clin Transl Endocrinol 2014;1:38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krug S, Kastenmüller G, Stückler F, et al. The dynamic range of the human metabolome revealed by challenges. FASEB J 2012;26:2607–2619 [DOI] [PubMed] [Google Scholar]
- 31.Zhao X, Peter A, Fritsche J, et al. Changes of the plasma metabolome during an oral glucose tolerance test: is there more than glucose to look at? Am J Physiol Endocrinol Metab 2009;296:E384–E393 [DOI] [PubMed] [Google Scholar]
- 32.Shaham O, Wei R, Wang TJ, et al. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol 2008;4:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest 2005;115:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Scholl TO, Leskiw M, Savaille J, Stein TP. Differences in maternal circulating fatty acid composition and dietary fat intake in women with gestational diabetes mellitus or mild gestational hyperglycemia. Diabetes Care 2010;33:2049–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prentice KJ, Luu L, Allister EM, et al. The furan fatty acid metabolite CMPF is elevated in diabetes and induces β cell dysfunction. Cell Metab 2014;19:653–666 [DOI] [PubMed] [Google Scholar]
- 36.Dudzik D, Zorawski M, Skotnicki M, et al. Metabolic fingerprint of gestational diabetes mellitus. J Proteomics 2014;103:57–71 [DOI] [PubMed] [Google Scholar]
- 37.Enquobahrie DA, Denis M, Tadesse MG, Gelaye B, Ressom HW, Williams MA. Maternal early pregnancy serum metabolites and risk of gestational diabetes mellitus. J Clin Endocrinol Metab 2015;100:4348–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulkarni SR, Kumaran K, Rao SR, et al. Maternal lipids are as important as glucose for fetal growth: findings from the Pune Maternal Nutrition Study. Diabetes Care 2013;36:2706–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008;31:1858–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Cianni G, Miccoli R, Volpe L, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med 2005;22:21–25 [DOI] [PubMed] [Google Scholar]
- 41.Kushtagi P, Arvapally S. Maternal mid-pregnancy serum triglyceride levels and neonatal birth weight. Int J Gynaecol Obstet 2009;106:258–259 [DOI] [PubMed] [Google Scholar]
- 42.Knopp RH, Magee MS, Walden CE, Bonet B, Benedetti TJ. Prediction of infant birth weight by GDM screening tests. Importance of plasma triglyceride. Diabetes Care 1992;15:1605–1613 [DOI] [PubMed] [Google Scholar]
- 43.Kalhan SC. Protein metabolism in pregnancy. Am J Clin Nutr 2000;71(Suppl.):1249S–1255S [DOI] [PubMed] [Google Scholar]
- 44.Schaefer-Graf UM, Meitzner K, Ortega-Senovilla H, et al. Differences in the implications of maternal lipids on fetal metabolism and growth between gestational diabetes mellitus and control pregnancies. Diabet Med 2011;28:1053–1059 [DOI] [PubMed] [Google Scholar]
- 45.Cetin I, de Santis MS, Taricco E, et al. Maternal and fetal amino acid concentrations in normal pregnancies and in pregnancies with gestational diabetes mellitus. Am J Obstet Gynecol 2005;192:610–617 [DOI] [PubMed] [Google Scholar]
- 46.McClain PE, Metcoff J, Crosby WM, Costiloe JP. Relationship of maternal amino acid profiles at 25 weeks of gestation to fetal growth. Am J Clin Nutr 1978;31:401–407 [DOI] [PubMed] [Google Scholar]
- 47.Kalkhoff RK, Kandaraki E, Morrow PG, Mitchell TH, Kelber S, Borkowf HI. Relationship between neonatal birth weight and maternal plasma amino acid profiles in lean and obese nondiabetic women and in type I diabetic pregnant women. Metabolism 1988;37:234–239 [DOI] [PubMed] [Google Scholar]
- 48.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park S, Park JY, Lee JH, Kim SH. Plasma levels of lysine, tyrosine, and valine during pregnancy are independent risk factors of insulin resistance and gestational diabetes. Metab Syndr Relat Disord 2015;13:64–70 [DOI] [PubMed] [Google Scholar]
- 50.Butte NF, Hsu HW, Thotathuchery M, Wong WW, Khoury J, Reeds P. Protein metabolism in insulin-treated gestational diabetes. Diabetes Care 1999;22:806–811 [DOI] [PubMed] [Google Scholar]