Abstract
Objective
Pelvic and paraortic lymph nodal regions are frequent sites of relapse in women with endometrial cancer who have not undergone adjuvant external beam radiation. We investigated outcomes after definitive management of nodal relapses of endometrial cancer with intensity modulated radiation therapy (IMRT).
Methods
Between 2002-2012, 38 patients with endometrial cancer who had no prior external beam radiation were treated definitively using IMRT for regionally confined pelvic or paraortic nodal recurrences. Thirteen (34%) had chemotherapy prior to radiation, and 21 (55%) received concurrent chemotherapy. The nodal basins were typically treated to 45-50 Gy, with a boost to the gross tumor to a median total of 64.7 Gy (range 59 – 73 Gy).
Results
The median overall survival from date of recurrence was 46.1 months and the 2-year survival was 71%. Patients who received concurrent chemotherapy had a significantly longer median survival (61.9 months versus 28.7 months, p=0.034). In-field failures were more frequent in patients who received chemotherapy prior to radiation, had a shorter recurrence-free interval, received a lower radiation dose, and had higher tumor grade. Three patients (8%) experienced grade 3-4 late gastrointestinal (GI) toxicity.
Conclusions
Long-term survival can be achieved in women with nodal recurrences of endometrial cancer. The use of concurrent chemotherapy and dose escalation with IMRT as feasible may improve survival for women with isolated nodal recurrences of endometrial cancer.
Introduction
Endometrial cancer is the most common gynecologic malignancy in developed countries, with over 50,000 new diagnoses and 8,600 deaths due to the disease per year in the United States [1]. Most patients are diagnosed with localized disease and have an excellent prognosis, with a 95% 5-year relative survival [2]. However, 4-20% of patients with early stage and up to 50% of patients with advanced stage endometrial cancer who do not receive pelvic radiation will experience a pelvic or paraortic nodal recurrence [3]. There is no consensus on the management of recurrent nodal disease, with surgery, chemotherapy, endocrine therapy, and radiation therapy as options [4].
Decreasing utilization of adjuvant pelvic radiation in early stage endometrial cancer may increase the number of nodal recurrences requiring salvage therapy. PORTEC-2 reported that vaginal brachytherapy (VBT) provides equivalent vaginal control as whole pelvic radiation therapy (WPRT) in patients with early endometrial cancer. WPRT decreased the risk of pelvic relapse but increased GI toxicity without a detectable improvement in survival [5]. Therefore, VBT is increasingly being utilized in place of WPRT in high-intermediate risk early stage endometrial cancer [6].
The published literature on radiation for the management of regional nodal recurrences is limited. However, salvage radiation therapy has been shown to be very effective for treatment of isolated vaginal recurrences [3, 7]. Intensity-modulated radiation therapy (IMRT) is a method of highly conformal radiation that permits the delivery of high doses of radiation to tumor while relatively sparing surrounding normal tissues, and has been used effectively in the treatment of gynecologic malignancies [8]. This study reviews the clinical outcomes of salvage IMRT for patients with regional nodal recurrences who had not received prior WPRT.
Methods
Patient Selection
Following institutional review board approval, our institution's tumor registry and radiation oncology databases were used to identify patients with endometrial cancer who received definitive IMRT for the treatment of measurable recurrent disease in the pelvic and/or paraortic nodes between 2002-2012. Patients who received WPRT at initial diagnosis were excluded, but patients who received VBT alone were included. Definitive IMRT was defined as delivery of at least 59 Gy to measurable gross disease, which was the minimum dose used to treat gross disease based on our institutional practice. All patients underwent hysterectomy initially, with pelvic lymph node dissection performed in 8 patients (21%), and pelvic and paraortic lymph node dissection performed in 17 patients (45%). Patients who first underwent resection of recurrent disease were included as long as there was residual measurable disease evident on postoperative imaging. One patient who underwent resection of a sidewall mass at presentation was found to have measurable disease on PET-CT 1.7 months afterwards and was included in the study. Patients were included if they received definitive IMRT for progressive or persistent disease following chemotherapy given after hysterectomy. Patients with additional synchronous disease in a non-nodal site were included if the additional site was regionally confined: vagina (n = 6), rectus abdominus (n=1), peritoneal reflection (n=1), and pericolonic node (n=1).
Pelvic recurrences were defined as any recurrences located inferior to the L4 vertebral body, along the common iliac, external iliac, or internal iliac vasculature, pelvic sidewall, or psoas muscle. Paraortic recurrences were defined as any disease located along the abdominal aorta or vena cava.
Thirty-eight patients met eligibility criteria as described above and were included in this retrospective analysis. Institutional and radiation oncology records were used to obtain data regarding patient characteristics, pathology, imaging, radiation treatment plans, toxicity, and survival.
Treatment following initial diagnosis of endometrial cancer
All patients underwent hysterectomy after initial diagnosis of endometrial cancer. Surgical lymph node evaluation, adjuvant systemic therapy, and adjuvant radiation therapy on initial presentation varied (Table 1).
Table 1.
Patient, Tumor and Treatment Characteristics
| Characteristic | |
|---|---|
| Stage - No. (%) | |
| I | 16 (42%) |
| II | 5 (13%) |
| III | 12 (32%) |
| IV | 5 (13%) |
| Age at Hysterectomy | |
| Median (range) | 63.5 (34-85) |
| ≤ 65 | 21 (55%) |
| >65 | 17 (45%) |
| Initial Grade - No. (%) | |
| 1 | 7 (18%) |
| 2 | 14 (37%) |
| 3 | 11 (29%) |
| unknown | 6 (16%) |
| Initial Histology - No. (%) | |
| Endometrioid | 16 (42%) |
| Clear Cell | 5 (13%) |
| Carcinosarcoma | 7 (18%) |
| Serous | 10 (26%) |
| Initial LN Dissection - No. (%) | |
| Not performed | 13 (34%) |
| Pelvic only | 8 (21%) |
| Pelvic + Paraortic | 17 (45%) |
| Initial Adjuvant Treatment | |
| Brachytherapy | 4 (11%) |
| Chemotherapy | 16 (42%) |
| None | 18 (47%) |
| Sites of Recurrence - No. (%) | |
| Pelvic Only | 13 (34%) |
| PAN Only | 8 (21%) |
| Pelvic + PAN/Other | 17 (45%) |
| Size of Largest Recurrence Site - No. (%) | |
| ≥ 3 cm | 17 (45%) |
| <3 cm | 21 (55%) |
| Total GTV Dose (Gy) | |
| Median (range) | 64.7 (59-73) |
| Initial Chemotherapy - No. (%) | |
| Yes | 13 (34%) |
| No | 25 (66%) |
| Concurrent Chemotherapy - No. (%) | |
| Yes | 21 (55%) |
| No | 17 (45%) |
LN, lymph node; PAN, paraortic node; GTV, gross tumor volume
Treatment following diagnosis of recurrent endometrial cancer
Patients were diagnosed with recurrence due to routine imaging (n=19), symptoms (n=15), and elevated CA-125 (n=4). Thirteen patients (34%) received treatment with chemotherapy before radiation for recurrence, defined as any chemotherapy given within three months prior to the start of salvage radiation, which was most commonly carboplatin and paclitaxel. Twenty-one patients (55%) received concurrent chemotherapy with radiation. Of these, 19 patients received weekly cisplatin (40 mg/m2), and two patients received weekly paclitaxel (40 mg/m2). Five patients (13%) received chemotherapy before and during radiation. Six patients (16%) had planned chemotherapy following concurrent chemoradiation. Three patients (8%) had surgery before radiation with residual measurable disease.
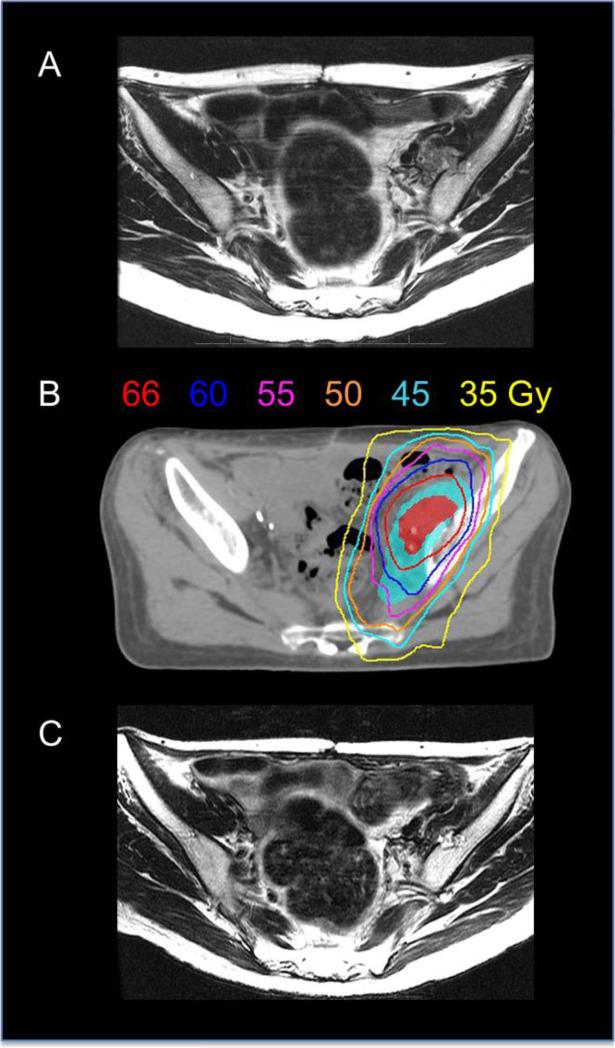
Radiation was delivered using IMRT technique (Figure 1 illustrates a typical plan). The clinical target volume was contoured around the adjacent nodal basins at risk, which generally included one nodal echelon above the grossly involved region (e.g. common iliac nodes included with a grossly involved external iliac node, or low paraortic nodes included with a grossly involved common iliac node). A 0.5-1 cm (most commonly 0.7 cm) margin was typically added to this to generate the planning treatment volume (PTV), and it was prescribed to a dose of 45-50 Gy in 1.8-2 Gy per fraction. The goal of this approach was to treat only the adjacent nodal region at highest risk for microscopic disease in order to minimize toxicity. Twelve patients (32%) were treated to the whole pelvis only, including bilateral external, internal, and common iliac nodes up to the aortic bifurcation. Nineteen patients (50%) were treated to the whole pelvis plus the paraortic basin, up to the T12 vertebral body. The other treatment fields included: paraortic basin only (n=2), ipsilateral pelvic nodes (n=3), ipsilateral pelvic nodes plus paraortic basin (n=1), and pelvic recurrence area only (n=1). The gross tumor volume was also contoured, and a 0.5-1 cm (most commonly 0.7 cm) margin added to generate the boost PTV, which was treated to a higher dose using an integrated boost (n=16), sequential boost (n=7), or both (n=15). If an integrated boost was utilized, the median dose per fraction was 2.1 Gy with a range of 2-2.25 Gy. The type of boost was determined by the treating physician. In general, if there was adjacent bowel close to the target volume, a sequential boost was chosen over giving a higher dose per fraction as part of an integrated boost, to avoid potential late toxicity associated with treatment as a higher dose per fraction. The median total additive dose delivered to gross disease was 64.7 Gy (range 59 – 73 Gy; 25th to 75th quartile 63.2 – 66 Gy). .
Figure 1.
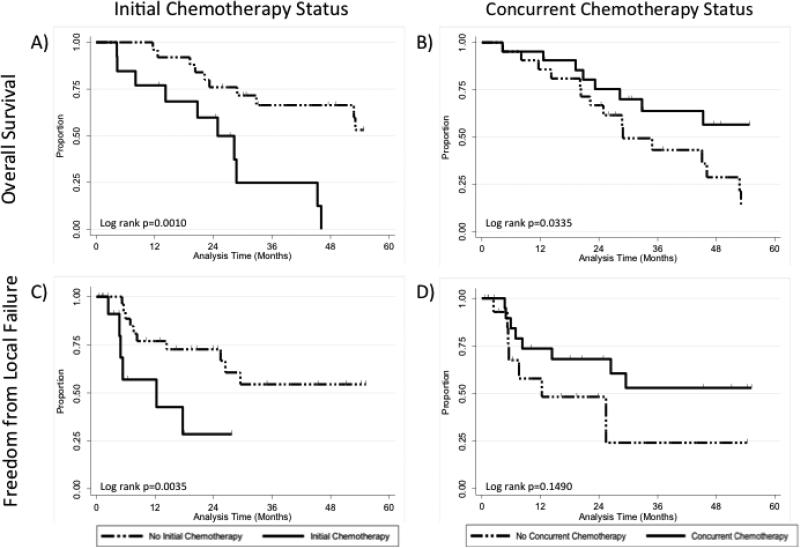
Kaplan-Meier analyses comparing: (A) overall survival of patients receiving (solid line) or not receiving (dashed line) initial chemotherapy, (B) overall survival of patients receiving (solid line) or not receiving (dashed line) concurrent chemotherapy, (C) freedom from local failure of patients receiving (solid line) or not receiving (dashed line) initial chemotherapy, and (D) freedom from local failure of patients receiving (solid line) or not receiving (dashed line) concurrent chemotherapy.
Adaptive treatment planning was utilized in some cases at the discretion of the treating physician to reduce the GTV volume in response to tumor regression to minimize toxicity (i.e. if there was an initially large tumor volume close to critical structures). There was not a uniform dose constraint used for small bowel. In general, the small bowel volume receiving greater than 60 Gy was limited to 2 cm3 or less. Image guided radiation therapy with daily kV images was typically utilized for daily treatment setup. Four patients also received VBT as part of their treatment of recurrence. Two received prophylactic high-dose rate brachytherapy (10 Gy in 2 fractions) prescribed to the surface of the vagina. Two received brachytherapy for gross disease: one to a dose of 24 Gy in 4 fractions, and one with pulsed-dose rate brachytherapy, receiving 28.8 Gy at the apical vaginal surface.
Follow-up after radiation therapy for recurrence
After completion of IMRT, patients were typically followed in clinic every 3 months for 2-3 years, then every 6 months for 2 additional years, then yearly. The median follow-up was 29.5 months (range 4.2 – 136.9 months) in all patients, and 31.9 months (range 12.7-136.9 months) for patients still alive. Local recurrence was defined as any recurrence within the radiation treatment field, including the interval development of new disease or any persistent, non-regressing disease after IMRT. Local failure in a high dose region refers to a recurrence arising in the region of the original recurrence, which received the IMRT boost. Local failure in a low dose region refers to the development of a new recurrence located in one of the nodal basins that received radiation to a dose of 45-50 Gy. Patients were imaged with PET or CT at variable intervals at the discretion of the physician. Toxicity was scored using the Common Terminology Criteria for Adverse Events version 4.0. Toxicity was recorded if radiation therapy was a possible cause, but complications thought to be due to progressive tumor were excluded. Patients no longer followed at our institution were contacted annually to obtain information about survival, disease and treatment status.
Statistical analysis
Survival rates were calculated from date of diagnosis of recurrence, and local control rates were calculated from the last date of radiation. Date of recurrence following salvage radiation was the date of biopsy, or, in cases without biopsy, date of radiologic study indicating recurrence. For disease free survival, local failure, distant failure, and death were scored as events. For freedom from local failure analysis, in-field failure was scored as an event and patients were censored at time of last follow up.
Data analysis was performed using Stata/MP 13.0 statistical software [9]. Fisher's exact test assessed measures of association in frequency tables. The equality of group medians was assessed with a nonparametric test for equality. The survival function was carried out using Kaplan-Meier estimates. The log rank test assessed the equality of the survivor function across groups. A p-value of 0.05 or less was considered to be statistically significant. Statistical tests were based on a two-sided significance level. The Cox's proportional hazard model assessed the effect of factors of significance on the survival end points. The estimated hazard ratio is reported.
Results
Patient, Tumor, and Recurrence Characteristics
Patient characteristics are listed in Table 1. The median age at hysterectomy was 63 years. Sixteen patients (42%) had FIGO stage I disease, 5 (13%) had stage II, 12 (32%) had stage III, two (5%) had stage IVA, and three (7%) had stage IVB (due to omental involvement). All histologic subtypes were included. Adenocarcinoma not otherwise specified was categorized under endometrioid histology. Sixteen patients (42%) had endometrioid histology; the remainder had high-risk histologic subtypes including papillary serous carcinoma, carcinosarcoma, and clear cell carcinoma.
The median time from hysterectomy to diagnosis of first recurrence and start of radiation was 20.0 months (range 1.7-185.7 months), and 26.1 months (range 1.9-187.9 months), respectively. Thirteen patients (34%) had pelvic nodal recurrences only; 8 (21%) had paraortic recurrences only; 9 (24%) had simultaneous pelvic and paraortic recurrences; 8 (21%) had simultaneous pelvic and vaginal and/or other regionally confined recurrences. Twenty-four patients (63%) were treated to more than one recurrent gross tumor volume (GTV). The median size of the largest nodal recurrence site was 2.9 cm (range 1.3-9.1 cm).
Survival following radiation therapy for recurrence
At the time of analysis, 25 patients (66%) had died. The median, one-year and two-year overall survival rates were 46.1 months, 89% (95% CI 74%-96%) and 71% (95% CI 53%-83%), respectively. The median, one and two-year disease free survival rates were 20.3 months, 60% (95% CI 43%-74%) and 47% (95% CI 30%-62%), respectively. The median freedom from local failure was 26.4 months.
Median overall survival in patients who received concurrent chemotherapy (61.9 months) was significantly higher than in patients who did not (28.7 months, p=0.0335; Figure 2 and Table 2). In contrast, patients who received chemotherapy prior to radiation for treatment of recurrence had a significantly lower median overall survival (24.9 versus 61.9 months, p=0.001; Figure 2 and Table 2). Patients who had received chemotherapy prior to radiation therapy had a shorter recurrence free interval. Short recurrence free interval (less than one year versus more than one year) was associated with a higher risk of death (HR 2.6, 95% CI 1.0-6.9, p=0.053), disease recurrence (HR 2.7, 95% CI 1.1-6.6, p=0.025) and local recurrence (HR 3.4, 95% CI 1.2-10.0, p=0.027; Table 2). Patients with initial FIGO stage III-IV disease (compared to stage I-II) had a higher risk of death (HR 2.5, 95% CI 1.1-5.8, p=0.024) and disease progression (HR 2.7, 95% CI 1.2-5.9, p=0.014; Table 2). Age, histology, size of the largest nodal recurrence site, total GTV dose, site of recurrence, or having more than one site of gross disease were not associated with overall or disease free survival.
Figure 2.
(A) Axial T2 MRI image of a patient with a left pelvic sidewall recurrence. (B) The patient's IMRT plan: the pelvic nodal basins at risk (cyan shaded region) received 50 Gy (cyan isodose line) in 25 fractions, followed by a boost to the gross tumor volume (red shaded region) with an additional 16 Gy in 8 fractions. In total, the gross tumor volume received 66 Gy (red isodose line). (C) T2 MRI axial image obtained 2 months after radiation, showing significant decrease in size of the left pelvic sidewall mass.
Table 2.
Univariate Analysis for Overall Survival, Disease Free Survival, and Local Control
| Overall Survival | Disease Free Survival | Local Control | |||||
|---|---|---|---|---|---|---|---|
| Variable | No. (%) | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Figo Stage | |||||||
| III-IV | 17 (45%) | 2.5 (1.1-5.8) | 0.024 | 2.7 (1.2-5.9) | 0.014 | 2.3 (0.8-6.6) | 0.123 |
| I-II | 21 (55%) | ||||||
| Age at Surgery | |||||||
| Continuous | 1.0 (1.0-1.1) | 0.091 | 1.0 (1.0-1.1) | 0.243 | 1.0 (1.0-1.1) | 0.384 | |
| >65 | 17 (45%) | 1.8 (0.8-4.1) | 0.158 | 1.4 (0.6-2.9) | 0.427 | 1.2 (0.4-3.2) | 0.776 |
| ≤ 65 | 21 (55%) | ||||||
| Histology | |||||||
| High Risk* | 22 (58%) | 1.0 (0.5-2.3) | 0.953 | 1.5 (0.7-3.3) | 0.291 | 2.1 (0.7-6.7) | 0.207 |
| Endometrial | 16 (42%) | ||||||
| No. Recurrent Sites | |||||||
| > 1 | 24 (63%) | 1.1 (0.5-2.5) | 0.82 | 1.1 (0.5-2.5) | 0.821 | 0.9 (0.3-2.4) | 0.766 |
| 1 | 14 (37%) | ||||||
| Size of Largest Recurrence | |||||||
| Continuous | 1.0 (0.8-1.2) | 0.988 | 0.9 (0.8-1.1) | 0.440 | 1.0 (0.8-1.3) | 0.928 | |
| ≥ 3 cm | 17 (45%) | 0.8 (0.4-1.9) | 0.669 | 0.7 (0.3-1.4) | 0.288 | 1.4 (0.5-3.8) | 0.564 |
| < 3 cm | 21 (55%) | ||||||
| Initial Chemotherapy | |||||||
| Yes | 13 (34%) | 4.4 (1.7-11.6) | 0.002 | 4.6 (2.0-10.8) | <0.001 | 4.9 (1.5-16.0) | 0.008 |
| No | 25 (66%) | ||||||
| Concurrent Chemotherapy | |||||||
| Yes | 21 (55%) | 0.4 (0.2-1.0) | 0.039 | 0.6 (0.3-1.2) | 0.148 | 0.5 (0.2-1.30 | 0.158 |
| No | 17 (45%) | ||||||
| Total GTV Dose (Gy) | |||||||
| < 64.7 | 19 (50%) | 1.2 (0.5-2.6) | 0.663 | 1.9 (0.9-4.2) | 0.099 | 3.2 (1.0-10.0) | 0.048 |
| ≥ 64.7 | 19 (50%) | ||||||
| Initial Grade | |||||||
| 3 | 11 (29%) | 2.4 (1.0-6.0) | 0.063 | 3.5 (1.4-8.9) | 0.008 | 4.8 (1.1-20.8) | 0.038 |
| 1, 2 | 21 (55%) | ||||||
| Type of Recurrence | |||||||
| Pelvic only | 13 (34%) | 1.0 (0.4-2.9) | 0.935 | 1.0 (0.3-2.8) | 0.961 | 1.2 (0.3-4.6) | 0.807 |
| Pelvic + other | 17 (45%) | 1.0 (0.4-2.9) | 0.943 | 1.1 (0.4-3.0) | 0.813 | 0.5 (0.1-2.3) | 0.391 |
| PAN only | 8 (21%) | ||||||
| Time from TAH to Recurrence | |||||||
| Continuous | 1.0 (0.99-1.01) | 0.72 | 0.99 (0.98-1.01) | 0.29 | 0.97 (0.93-1.0) | 0.119 | |
| ≤1 year | 12 (32%) | 2.6 (1.0-6.9) | 0.053 | 2.7 (1.1-6.6) | 0.025 | 3.4 (1.2-10.0) | 0.027 |
| >1 year | 26 (68%) | ||||||
High risk histology includes papillary serous, carcinosarcoma, and clear cell.
Abbreviations: HR, hazard ratio; CI, confidence interval; GTV, gross total volume; PAN, paraortic node; TAH, total abdominal hysterectomy.
Risk factors in patients treated for recurrent disease
In order to determine if better outcomes in patients receiving concurrent chemotherapy could be due to imbalances in risk factors, we compared features of patients treated with and without concurrent chemotherapy (Table 3). Patients receiving concurrent chemotherapy were younger (median age 58 versus 71, p=0.009). However, there were no other significant differences in risk factors including tumor size, higher initial stage, high-risk histology, tumor grade, site of recurrence, higher GTV radiation dose, more extensive recurrences, time from TAH to recurrence or treatment in earlier versus later time periods (Table 3).
Table 3.
Frequency of Characteristics by Type of Chemotherapy
| Initial Chemotherapy | Concurrent Chemotherapy | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Total | No | Yes | p-value | No | Yes | p-value |
| No. of Patients | 38 | 25 | 13 | 17 | 21 | ||
| Figo Stage | |||||||
| I-II | 21 | 14 | 7 | 1.000 | 7 | 14 | 0.190 |
| III-IV | 17 | 11 | 6 | 10 | 7 | ||
| Age at Surgery | |||||||
| Median | 63.5 | 63 | 65 | 1.000 | 71 | 58 | 0.009 |
| ≤ 65 | 21 | 14 | 7 | 1.000 | 5 | 16 | 0.008 |
| >65 | 17 | 11 | 6 | 12 | 5 | ||
| Pathology | |||||||
| Carcinosarcoma | 7 | 7 | 0 | 0.123 | 3 | 4 | 0.289 |
| Clear Cell | 5 | 2 | 3 | 2 | 3 | ||
| Endometrial | 18 | 10 | 6 | 5 | 11 | ||
| Serous | 10 | 6 | 4 | 7 | 3 | ||
| Endometrial | 16 | 10 | 6 | 0.742 | 5 | 11 | 0.197 |
| High Risk** | 22 | 15 | 7 | 12 | 10 | ||
| No. Recurrent Sites | |||||||
| 1 | 14 | 10 | 4 | 0.728 | 8 | 6 | 0.318 |
| >1 | 24 | 15 | 9 | 9 | 15 | ||
| Site of Recurrence | |||||||
| PAN only | 8 | 5 | 3 | 0.908 | 6 | 2 | 0.175 |
| Pelvic only | 13 | 8 | 5 | 4 | 9 | ||
| Pelvic /other | 17 | 12 | 5 | 7 | 10 | ||
| Size of Largest Recurrence Site | |||||||
| Median | 2.9 | 2.9 | 2.9 | 0.828 | 2.3 | 3.1 | 0.468 |
| <3 | 21 | 14 | 7 | 1.000 | 11 | 10 | 0.342 |
| ≥ 3 cm | 17 | 11 | 6 | 6 | 11 | ||
| Total GTV Dose (Gy) | |||||||
| Median | 64.7 | 64.8 | 64.5 | 1.000 | 64.5 | 65.8 | 1.000 |
| Grade | |||||||
| 1 | 7 | 4 | 3 | 0.453 | 3 | 4 | 0.669 |
| 2 | 14 | 11 | 3 | 5 | 9 | ||
| 3 | 11 | 6 | 5 | 6 | 5 | ||
| Unknown | 6 | 4 | 2 | 3 | 3 | ||
| Concurrent chemotherapy | |||||||
| No | 17 | 9 | 8 | 0.178 | |||
| Yes | 21 | 16 | 5 | ||||
| Treatment Period | |||||||
| 2002-2007 | 12 | 7 | 5 | 0.714 | 4 | 8 | 0.486 |
| 2007-2012 | 26 | 18 | 8 | 13 | 13 | ||
| Time from TAH to Recurrence | |||||||
| > 1 year | 26 | 20 | 6 | 0.064 | 12 | 14 | 1.000 |
| ≤ 1 year | 12 | 5 | 7 | 5 | 7 | ||
*High risk histology includes papillary serous, carcinosarcoma, and clear cell.
Abbreviations: HR, hazard ratio; CI, confidence interval; GTV, gross total volume; PAN, paraortic node.
To determine if patients treated with chemotherapy prior to radiation had higher-risk features, we also assessed possible differences in risk factors between patients receiving initial chemotherapy versus not. Patients receiving chemotherapy prior to radiation were more likely to have had time to recurrence less than one year (p=0.064, Table 3), but there were no other significant differences in risk factors (Table 3). Furthermore, there was no significant difference in the percentage of patients receiving concurrent chemotherapy among patients receiving or not receiving chemotherapy first, or in the percentage of patients receiving chemotherapy first among patients receiving or not receiving concurrent chemotherapy.
In-field recurrence following radiation therapy for recurrence
There were 15 patients (39%) who developed local in field failures after salvage IMRT (Supplementary Table). Thirteen of these failures were pelvic nodal failures; two were paraortic nodal failures. Nine failures occurred in the high dose radiation field, four in the low dose area, and two in the overlapping area between the high and low doses. The median time to local failure after radiation was 6.9 months (range 2.4-28.6).
Patients who received initial chemotherapy were more likely to have a local failure (HR 4.9, 95% CI 1.5 – 16.0, p=0.008, Table 2). Five out of 13 patients who received initial chemotherapy had a local failure, compared to 10 out of 25 patients not receiving initial chemotherapy. In addition, patients who had shorter time to recurrence (less than one year), who received less than the median total GTV dose of 64.7 Gy and with grade 3 tumors were more likely to have a local failure (HR 3.4, 95% CI 1.2-10.0, p=0.027, HR 3.2, 95% CI 1.0-10.0, p=0.048, and HR 4.8, 95% CI 1.1-20.8, p=0.038, respectively; Table 2). Histology, initial stage, age, number of recurrent sites, size of largest recurrent node, and the use of concurrent chemotherapy were not significant risk factors for local failure (Table 2).
Toxicity
No patients experienced grade 5 toxicity. Two patients experienced grade 3 early hematologic toxicity (requiring transfusion during radiation). Eight patients (21%) experienced any grade 3 to 4 late toxicity. Of these patients, three patients (7.9%) experienced late grade 3-4 GI toxicity (Supplementary Table 3). These three patients had gross disease immediately adjacent to bowel, resulting in small volumes of bowel receiving close to the prescribed dose (Supplementary Table 3). Two patients experienced grade 4 late GI toxicity (distal jejunal stricture requiring TPN; ileal perforation) and one patient experienced grade 3 late GI toxicity (small bowel obstruction not requiring surgery). Other grade 3 late toxicities included hematologic (n=3), secondary malignancy (n=1), vaginal stricture (n=1), and lower extremity edema (n=1). The majority of patients experienced grade 1-2 diarrhea during radiation.
Discussion
To our knowledge, this is the largest study on the use of IMRT for the management of nodal recurrences of endometrial cancer. We have shown that long-term survival is possible with IMRT after diagnosis of nodal recurrence, with a median and 2-year overall survival of 46.1 months and 71%, respectively.
Patients who received concurrent chemotherapy (given as weekly cisplatin in over 90% of cases) had significantly longer median survival as compared to patients treated with radiation therapy without concurrent chemotherapy (61.9 months versus 28.7 months, p=0.034). We did not identify any imbalance in known risk factors for patients treated with radiation alone as compared to those that received concurrent chemotherapy, except for age. Concurrent chemotherapy with radiation has been shown to improve survival in many cancers, including cervical, head and neck, and anal cancer, but this has not been demonstrated in recurrent endometrial cancer [10-12]. A currently ongoing GOG/NRG trial (NCT00492778) for women with pelvic and/or vaginal recurrences of endometrial cancer is evaluating the benefit of concurrent cisplatin chemotherapy with radiation therapy. A recently published study of radiation and concurrent bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor, for recurrent endometrial cancer showed a 3-year survival of 75% and no relapses in the radiation field [13].
Interestingly, we found that patients receiving chemotherapy within three months before salvage radiation had a nearly five-fold higher rate of local recurrence and lower rates of overall survival. However, patients receiving chemotherapy prior to radiation had a shorter recurrence free interval which we found to be associated with a lower survival. As a result, no definitive conclusions can be made about the impact of initial chemotherapy on survival in this series. We did not detect any other significant differences in risk factors among patients treated with chemotherapy before radiation as compared to those that did not receive initial chemotherapy (Table 3).
Two other studies have examined the outcomes following treatment of nodal disease with IMRT: one examining IMRT for any gross nodal disease (about half in the recurrent setting), and one examining IMRT for paraortic recurrences alone [14, 15]. Our survival outcomes (2-year survival of 71%) appear comparable to the results of these two studies, which showed a 2-year survival of 63% [14] and 77% [15]
We found that a lower radiation dose (lower than the median dose of 64.7 Gy to the GTV) was associated with a higher rate of local failure. There was no association of radiation dose with outcome in the two other studies of IMRT for endometrial nodal disease [14, 15]. This may be attributed to the large range of doses used in these patients, due to the variability in proximity to adjacent critical structures.
Limiting dose to the bowel and duodenum can reduce the risk of complications. Two studies showed that limiting the volume of duodenum receiving 55 Gy or more to below 15 cm3 or even below 1 cm3 can reduce the risk of duodenal complications [16, 17]. There were three patients (7.9%) in our series that experienced late grade 3 or 4 GI toxicity, two of which were nonduodenal small bowel toxicities (and the third was not localized). The maximum radiation doses prescribed for those three patients ranged from 60-66 Gy, similar to the median dose of 64.7 Gy in the entire cohort. Another study of extended field IMRT reported a similar late GI complication rate of 6.5%--those patients were treated to a median dose of 50.4 Gy, and also did not include duodenal toxicity [18]. In that study, there was no dose-volume relationship for GI toxicity reported.
Although histologic subtype, positive peritoneal cytology, and deep myometrial invasion have been shown to increase risk of relapse in advanced endometrial cancer [19], we did not find any differences in survival or control based on these features in the setting of recurrent disease. In addition, the size of recurrence, number of recurrent sites or location of recurrence did not impact outcome. These results suggest that nodal recurrences have a unique biology shared across histologic subtypes and that outcome is determined by response to therapy, rather than clinical or pathological features.
The steep dose gradient that can be achieved with IMRT allows delivery of high doses to disease within the nodal basin while reducing dose to the critical adjacent pelvic structures. Integrated boosts with a higher dose per fraction can increase the relative effective dose to the tumor compared to normal tissues. In this analysis the dose per fraction was increased only slighted (to 2.1-2.2 Gy per fraction) from the standard of 2 Gy per fraction. This small increase in dose per fraction has minimal effect on biologically equivalent doses, so biologically equivalent doses were not reported in this series. The safety of delivering higher doses per fraction with IMRT has not been established. However, two studies of integrated boosts (to 2.2 Gy per fraction) in the treatment of locally advanced gynecologic malignancies showed minimal late toxicity [20, 21]. In our experience, the use of integrated versus sequential boosts was variable, and we do not have enough data to recommend one type of boost technique over another. In current practice, if a sequential boost is chosen, the most common fractionation scheme is to deliver 2 Gy per fraction to the GTV and 1.8 Gy to the CTV for 25 fractions followed by a sequential boost to treat the GTV to 60-66 Gy. However, regardless of the boosting technique, careful attention must be paid to daily set up accuracy, internal organ motion and tumor response during treatment to minimize the risk of complications from higher doses delivered to normal tissues. Daily image guidance with CT may be helpful to ensure adequate tumor coverage and identify the need for adaptive treatment planning.
Conclusions from this study are applicable only to patients treated with IMRT for management of gross nodal disease and do not provide any insights into the role of surgical resection in this setting. This study included three patients who had undergone surgery with gross measurable disease on post-operative imaging but did not include patients treated surgically without residual disease post-operatively. Surgical debulking prior to radiation offers the benefit of having to treat less volume to a high dose and potentially higher rates of local control, but also carries increased potential risk of bowel and wound complications.
It is important to note that this study included many high-risk patients, including several with peritoneal disease at initial presentation and regional non-nodal disease at the time of IMRT. These patients were included since they received definitive IMRT to nodal recurrences after multidisciplinary consultation based on limited extent of disease at the time of radiation. We did find that patients with initial stage III-IV disease had lower survival following IMRT for recurrent nodal disease.
Other limitations of this study include retrospective design, limited size, and heterogeneous patient characteristics. However, we demonstrated that long-term survival is possible following salvage radiation for nodal recurrence of endometrial cancer, with acceptable rates of toxicity. Furthermore, our analysis showed that patients receiving concurrent chemotherapy had a decreased risk of local recurrence and improved overall survival, compared to those not receiving concurrent chemotherapy. Our results suggest that receipt of chemotherapy prior to radiation, short recurrence free interval, lower radiation dose, and higher tumor grade are risk factors for recurrence following definitive IMRT for treatment of nodal recurrence. The use of concurrent chemotherapy and dose escalation as feasible may improve survival for women with isolated nodal recurrences of endometrial cancer. Further studies are needed to validate this observation and to determine how the use of surgical resection of recurrent disease impacts outcome.
Supplementary Material
Highlights.
Long-term survival can be achieved following IMRT for endometrial nodal recurrences
Patients receiving concurrent chemotherapy had improved survival
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
To the best of my knowledge, I have no financial relationships or conflicts of interest to disclose, and there are no financial relationships or conflicts of interest to disclose among any of the authors on this manuscript.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.SEER Stat Fact Sheets: Endometrial Cancer. 2014 [Google Scholar]
- 3.Bradford LS, Rauh-Hain JA, Schorge J, Birrer MJ, Dizon DS. Advances in the Management of Recurrent Endometrial Cancer. Am J Clin Oncol. 2013 doi: 10.1097/COC.0b013e31829a2974. [DOI] [PubMed] [Google Scholar]
- 4.Fung-Kee-Fung M, Dodge J, Elit L, Lukka H, Chambers A, Oliver T, et al. Follow-up after primary therapy for endometrial cancer: a systematic review. Gynecol Oncol. 2006;101:520–529. doi: 10.1016/j.ygyno.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Nout RA, Smit VT, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 6.Chino JP, Jones E, Berchuck A, Secord AA, Havrilesky LJ. The influence of radiation modality and lymph node dissection on survival in early-stage endometrial cancer. Int J Radiat Oncol Biol Phys. 2012;82:1872–1879. doi: 10.1016/j.ijrobp.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 7.Jhingran A, Burke TW, Eifel PJ. Definitive radiotherapy for patients with isolated vaginal recurrence of endometrial carcinoma after hysterectomy. Int J Radiat Oncol Biol Phys. 2003;56:1366–1372. doi: 10.1016/s0360-3016(03)00414-0. [DOI] [PubMed] [Google Scholar]
- 8.Mundt AJ, Lujan AE, Rotmensch J, Waggoner SE, Yamada SD, Fleming G, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;52:1330–1337. doi: 10.1016/s0360-3016(01)02785-7. [DOI] [PubMed] [Google Scholar]
- 9.StataCorp . In: Stata: Release 13. Statistical Software. LP. S., editor. College Station, TX: 2013. [Google Scholar]
- 10.Adelstein DJ, Li Y, Adams GL, Wagner H, Jr., Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 12.Northover J, Glynne-Jones R, Sebag-Montefiore D, James R, Meadows H, Wan S, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer. 2010;102:1123–1128. doi: 10.1038/sj.bjc.6605605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viswanathan AN, Lee H, Berkowitz R, Berlin S, Campos S, Feltmate C, et al. A prospective feasibility study of radiation and concurrent bevacizumab for recurrent endometrial cancer. Gynecol Oncol. 2014;132:55–60. doi: 10.1016/j.ygyno.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Shirvani SM, Klopp AH, Likhacheva A, Jhingran A, Soliman PT, Lu KH, et al. Intensity modulated radiation therapy for definitive treatment of paraortic relapse in patients with endometrial cancer. Pract Radiat Oncol. 2013;3:e21–28. doi: 10.1016/j.prro.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Townamchai K, Poorvu PD, Damato AL, DeMaria R, Lee LJ, Berlin S, et al. Radiation dose escalation using intensity modulated radiation therapy for gross unresected node-positive endometrial cancer. Pract Radiat Oncol. 2014;4:90–98. doi: 10.1016/j.prro.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Verma J, Sulman EP, Jhingran A, Tucker SL, Rauch GM, Eifel PJ, et al. Dosimetric predictors of duodenal toxicity after intensity modulated radiation therapy for treatment of the para-aortic nodes in gynecologic cancer. Int J Radiat Oncol Biol Phys. 2014;88:357–362. doi: 10.1016/j.ijrobp.2013.09.053. [DOI] [PubMed] [Google Scholar]
- 17.Kelly P, Das P, Pinnix CC, Beddar S, Briere T, Pham M, et al. Duodenal toxicity after fractionated chemoradiation for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;85:e143–149. doi: 10.1016/j.ijrobp.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 18.Poorvu PD, Sadow CA, Townamchai K, Damato AL, Viswanathan AN. Duodenal and other gastrointestinal toxicity in cervical and endometrial cancer treated with extended-field intensity modulated radiation therapy to paraaortic lymph nodes. Int J Radiat Oncol Biol Phys. 2013;85:1262–1268. doi: 10.1016/j.ijrobp.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Milgrom SA, Kollmeier MA, Abu-Rustum NR, O'Cearbhaill RE, Barakat RR, Alektiar KM. Quantifying the risk of recurrence and death in stage III (FIGO 2009) endometrial cancer. Gynecol Oncol 2014. 134:297–301. doi: 10.1016/j.ygyno.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Boyle J, Craciunescu O, Steffey B, Cai J, Chino J. Methods, safety, and early clinical outcomes of dose escalation using simultaneous integrated and sequential boosts in patients with locally advanced gynecologic malignancies. Gynecol Oncol. 2014;135:239–243. doi: 10.1016/j.ygyno.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 21.Vargo JA, Kim H, Choi S, Sukumvanich P, Olawaiye AB, Kelley JL, et al. Extended field intensity modulated radiation therapy with concomitant boost for lymph node-positive cervical cancer: analysis of regional control and recurrence patterns in the positron emission tomography/computed tomography era. Int J Radiat Oncol Biol Phys. 2014;90:1091–1098. doi: 10.1016/j.ijrobp.2014.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.