Abstract
Six Veillonella species have been frequently isolated from human oral cavities including infectious sites. Recently, it was reported that diet, smoking, and possibly socioeconomic status can influence the bacterial profile in oral cavities. In addition, oral hygiene habits may also influence oral microbiota in terms of both numbers and diversity of microorganisms. In this study, the identification of Veillonella species in tongue biofilms of Thai children, divided into three groups dependent on their status of oral hygiene. For this, we used a novel one-step PCR method with species-specific primer sets based on sequences of the rpoB gene. As shown in the results, the number of isolates of Veillonella species was 101 strains from only 10 of 89 subjects. However, the total number of bacteria was high for all subjects. Since it was reported in previous studies that Veillonella species were easy to isolate in human tongue biofilms at high numbers, the results obtained in this study may suggest country- or age-specific differences. Moreover, Veillonella species were detected predominantly in subjects who had poor oral hygiene compared to those with good or moderate oral hygiene. From these results, there is a possibility that Veillonella species may be an index of oral hygiene status. Furthermore, V. rogosae was a predominant species in tongue biofilms of Thai children, whereas V. parvula and V. denticariosi were not isolated at all. These characteristics of the distribution and frequency of Veillonella species are similar to those reported in previous studies. Although further studies are needed in other countries, in this study, a successful novel one-step PCR method was established to detect Veillonella species in human oral cavities easily and effectively. Furthermore, this is the first report investigating the distribution and frequency of Veillonella species in tongue biofilms of Thai children.
Introduction
The genus Veillonella consists of small, strictly anaerobic, Gram-negative cocci that lack flagella, spores, and capsules. Members of this genus obtain energy from the utilization of short chain organic acids and have been isolated from the oral cavity and intestinal tract of humans and other animals [1, 2]. Currently, the genus Veillonella is subdivided into 13 species. Of these species, only V. atypica, V. denticariosi, V. dispar, V. parvula, V. rogosae, and V. tobetsuensis have been isolated from human oral cavities [3–7]. The main habitats of these oral Veillonella are the tongue, buccal mucosa, and saliva [3, 8–12]. Oral Veillonella, especially V. parvula, are associated with severe early childhood caries [13] and intraradicular infections [14, 15], including abscesses [16], apical root canals [17], and dental tubules [18]. Veillonella species are also predominantly found in subgingival biofilm samples of patients who have chronic periodontitis [19–21], and our previous study concluded that V. parvula is associated with the state of chronic periodontitis [21]. Furthermore, in periodontal patients undergoing therapy, Veillonella species, along with Streptococcus and Neisseria species, were found to be consistently resistant to tetracycline [22]. Moreover, tetracycline-resistant Veillonella species have the opportunity to come in close contact with, and consequently transfer resistance elements to other oral bacteria and bacteria that pass through the oral cavity [23]. Veillonella species were previously known to be sensitive to penicillin and ampicillin but are now frequently resistant to these antibiotics [24]. In addition, Delwiche et al. [25] reported that Veillonella species produce a large amount of lipopolysaccharides (LPS) and Metera et al. [26] reported that with V. parvula, LPS-stimulated cytokine induction, as well as p38 MAPK activation, are Toll-like receptor 4-dependent. It is thought that these properties of Veillonella species make it difficult to treat the associated periodontitis.
In the case of dental caries, Veillonella species are highly associated with lactic acid-producing species [27]. This is not surprising given their reliance on lactate as a nutrient source. This has potential clinical utility; Veillonella levels may serve as a sensitive biological indicator and early warning sign of acid production. In addition, among children without a history of caries, the presence of Veillonella or other acid-producing bacteria, including Streptococcus mutans, has predicted the development of future caries [27].
The bacterial communities found in the human oral cavities, with around 1000 species present [28], have been shown to be the second most complex in the body, after the colon [29]. It has been known for a long time that periodontitis and dental caries are caused by oral biofilms. Oral biofilms are composed of multiple species, whose development is initiated by adherence of pioneer species to the salivary proteins and glycoproteins adsorbed onto the tooth enamel. The biofilm is not formed by the random simultaneous colonization of these species, but rather by selective, reproducible, and sequential colonization [30, 31].
As mentioned above, it is evident that oral Veillonella are associated with oral biofilms, which cause many human oral infectious diseases, such as periodontitis and dental caries. Furthermore, Periasamy and Kolenbrander [32] reported that Veillonella species had a central role as an early colonizer in establishing multispecies oral biofilm communities having initial, middle, and late colonizers. Therefore, it would appear that understanding the distribution and frequency of Veillonella species in oral biofilms is important in considering treatments or preventions for these oral infectious diseases. Furthermore, several recent reports indicated that diet, smoking, and potentially socioeconomic status can influence the bacterial profile of oral cavities [33–35]. According to these reports, the possibility of having country-, community-, or family-specific distribution and frequency of bacteria in oral biofilms has been considered. In addition, it was reported that oral hygiene habits might also influence oral microbiota qualitatively and quantitatively [36, 37].
In this study, the distribution and frequency of Veillonella species in tongue biofilms of children in Thailand, in association with the level of oral hygiene, were examined. We then compared the results to previous reports of our group and others regarding the identification of Veillonella species in tongue biofilm. Moreover, the effective novel one-step PCR method using species-specific primer sets designed from the rpo B gene (encoding the β subunit of bacterial RNA polymerase) was established in this study.
Materials and Methods
Ethics Statement
This study received approval from the Ethics Committee, Mahidol University, Bangkok, Thailand under process number MU-DT/PY-IRB 2015/DT028, and samples were collected in March 2015. The participants and their parents were made aware of the objectives and procedures of the study and agreed to participate by providing written, informed consent.
Subjects
The 89 children selected consisted of 41 males and 48 females (age range: 7 to 15 years). Children with a history of immunosuppression or systemic diseases (e.g. diabetes and HIV), those using medications that reduce saliva flow, and those under treatment with antimicrobials in the previous three months, were excluded from the study. The participants were evaluated by the Simplified Oral Hygiene Index (OHI-S) according to the criteria of Greene &Vermillion [38] and divided into three groups. The first group (Good oral hygiene) was composed of 29 children (10 males and 19 females) with OHI-S scores of 0–1.2. The second group (Moderate oral hygiene) was composed of 30 children (15 males and 15 females) with OHI-S scores of 1.3–3.0. The third group (Poor oral hygiene) was composed of 30 children (16 males and 14 females) with OHI-S scores of 3.1–6.0.
Sample Collection
Tongue biofilm samples were collected at Dental Hospital, Mahidol University Faculty of Dentistry using sterile cotton wool swabs to swab the tongue 5 times each per subject. Samples in reduced transport fluids were transported in an anaerobic box (HIRASAWA WORKS Inc.) containing 80% N2, 10% CO2, and 10% H2. The samples were immediately placed in 1 mL of sterile saline (< 1 hour from the time of collection). Samples were homogenized for 3 min with a BioMasher®II(Nippi, Incorporated Protein Engineering Office, Tokyo, Japan) to disperse the biofilm and were serially diluted 10-fold with sterile saline from 10−3 to 10−8.
Culture Conditions
Aliquots of serial 10-fold dilutions (100 μL) were used to inoculate Bacto™ Brain Heart Infusion (Difco Laboratories, BD) supplemented with 5% (volume/volume) defibrinated sheep blood (BHI agar), hemin (10 μg/mL), and menadione (5 μg/mL), and also the selective medium, Veillonella agar [39]. After inoculation, all media were incubated in an anaerobic box containing 80% N2, 10% CO2, and 10% H2 at 37°C; Veillonella agar was incubated for 5 days, while BHI agar was incubated for 7 days.
The total number of bacteria in the samples was determined by counting the total number of colonies on BHI agar, while the number of Veillonella was determined by counting the total number of typical Veillonella colonies on Veillonella agar. Bacterial cells of typical Veillonella colonies were confirmed by light microscopy after Gram staining.
Bacterial Strains
V. atypica ATCC 17744T, V. denticariosi JCM 15641T, V. dispar ATCC 17748T, V. parvula ATCC 10790T, V. rogosae JCM 15642T and V. tobetsuensis ATCC BAA-2400 (= JCM 17976T) were used as control oral Veillonella to confirm the specificity of the primer sets. They were cultured on BHI agar at 37°C in the anaerobic box for 5 days.
DNA Extraction
Genomic DNA was extracted from individual bacterial cells using an InstaGene Matrix Kit (Bio-Rad) and the DNA concentration was determined based on fluorescence using a Qubit® 3.0 Fluorometer (Invitrogen life technologies) according to the manufacturer’s instructions.
Design of Primer Sets
According to the DNA sequence of a conserved region of the partial sequence of the rpo B gene, species-specific primer sets for six Veillonella species were designed by the standard manual method [40]. The accession numbers of the rpo B gene sequences of reference strains from the GenBank database (Database ID: BA123456) were EF185159 for V. atypica, EF185162 for V. denticariosi, EF185161 for V. dispar, EF185158 for V. parvula, EF211831 for V. rogosae, and AB698646 for V. tobetsuensis. Pairwise similarities among six Veillonella species were estimated by MEGALINE including CLUSTAL W in the LASERGENE program (DNASTAR).
Identification of Veillonella Species
Specific primer sets were used to obtain the PCR products described below. For identification of oral Veillonella at the genus level, one primer pair: Veill-rpoBF (5’-GTAACAAAGGTGTCGTTTCTCG-3’) and Veill-rpoBR (5’-GCACCRTCAAATACAGGTGTAGC-3’) was used before identification of oral Veillonella at the species level [41, 42].
PCR Protocol
PCR at the species level was performed using 1 μL of template DNA, 1 μL of each primer (10 pmol/mL), 17 μL of PCR grade water, and 25 μL of master mix from an AmpliTaq Gold® 360 Master Mix (Applied Biosystems). When using specific primer sets for six Veillonella species, PCR mixtures were subjected to preheating at 94°C for 15 min; followed by 30 cycles of 92°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s; with a final extension at 72°C for 5 min. The PCR products were applied to a 3.0% agarose gel. In the case of PCR at the genus level, the PCR protocol was performed in accordance with the protocols described by Arif et al. and Beighton et al. [6, 41]. After electrophoresis, the gel was stained with SYBR® Safe DNA gel stain (Invitrogen™).
Clonal Analysis of Unknown Strains
Unknown strains of genus Veillonella (PCR reaction was positive with genus-specific primers, but was negative with species-specific primer sets in this study) were examined clonal analysis. The representative 11 strains in the 40 unknown strains were chosen in this study.
DNA was extracted from the individual bacterial cells isolated on Veillonella agar by using the InstaGene Matrix kit (Bio-Rad), according to the manufacturer’s instructions. PCR-based amplification and partial sequence analyses of rpoB were performed using previously described specific primers for genus Veilloenlla [41, 42]. The sequence determined with an ABI PRISM 310 Genetic Analyzer were aligned with each other and connected by using SEQMAN IIof the LASERGENE program (DNASTAR). The programs MEGALIGN including CLUSTALW and NJPlot were used to compare sequences and the reconstruct the evolutionary tree by the neighbour-joining method. Also, confidence intervals were assessed by CLUSTAL W with bootstrap analysis. In particular, pairwise similarity values were determined with MEGALIGN in the LASERGENE program. The rpoB (559 nt) partial sequences of 11 strains were aligned against the sequences of the representative strains retrieved from GenBank.
Results
Species-specific Primer Sets for Oral Veillonella
A similarity search of the rpo B gene of six Veillonella species revealed a high degree of polymorphism in the region of position 2500 to 3100 in all oral Veillonella tested. The similarities among partial sequences of the rpo B gene regions of these six Veillonella species were found to be from 73.7 to 90.9% (Table 1).
Table 1. Level of rpoB partial sequence similarity among six species of oral Veillonella.
| Percentage of similarity with | ||||||
|---|---|---|---|---|---|---|
| Species | V. atypica | V. denticariosi | V. dispar | V. parvula | V. rogosae | V. tobetsuensis |
| V. atypica | ||||||
| V. denticariosi | 73.7 | |||||
| V. dispar | 81.4 | 76.6 | ||||
| V. parvula | 76.7 | 78.2 | 82.8 | |||
| V. rogosae | 75.8 | 81 | 83.2 | 90.9 | ||
| V. tobetsuensis | 80.7 | 74.1 | 81.9 | 76.9 | 78.5 | |
One forward primer, VF (5’-GTAACAAAGGTGTCGTTTCTCG-3’), was designed using the sequence of the conserved region of the rpo B gene for all Veillonella species (Fig 1). According to the sequences of the variable regions in the rpo B gene of oral Veillonella, six reverse primers were designed as species-specific primers. These specific reverse primers were designated as ATYR (5’-AGCAGCTTCTTCTACGTGACC-3’) for V. atypica, as DENR (5’-CAACCCGTTTCGCTTCGGCG-3’) for V. denticariosi, as DISR (5’-GCGAATAGCGTCAATTTGTC-3’) for V. dispar, as PARR (5’-CGTAACATCTTCCGAAACTTTC-3’) for V. parvula, as ROGR (5’-GATCCATTTCTGGAGCATCC-3’) for V. rogosae and as TOBR (5’-TTTCAATAGCTTTTAATTCCGC-3’) for V. tobetsuensis (Fig 1).
Fig 1. The location and sequence of species-specific primers for the rpoB gene of oral Veillonella.
Underlined red font indicates the nucleotide sequences of each reverse primer for different Veillonella species. Numbers indicate the nucleotide positions in the rpoB gene of Veillonella species. Black font indicates sequences of the rpoB gene of other species of Veillonella corresponding to the same nucleotide positions of the reverse primers indicated.
Specificity and Sensitivity of the Primer Sets
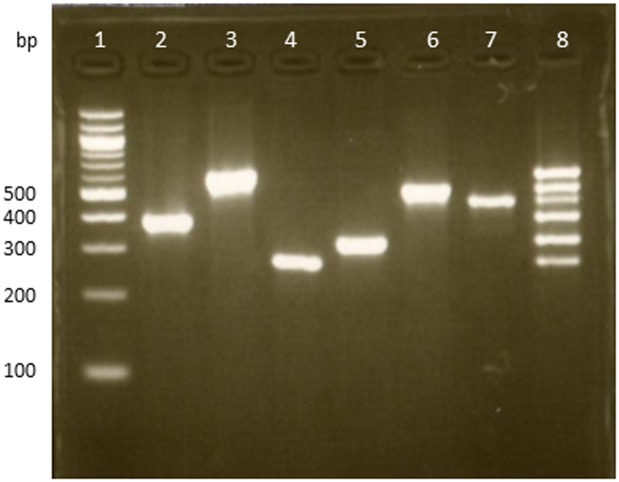
To achieve faster and more convenient identification of oral Veillonella, all six reverse primers (ATYR, DENR, DISR, PARR, ROGR, and TOBR) were examined for use with VF in single PCR mixture. PCR was performed using DNA templates from the type strain of six Veillonella species as described in Materials and Methods. The electrophoretically detected PCR amplicons were shown as species-specific products (Fig 2). The molecular weights of the PCR products were also identical to the theoretical values, which were 396 base pairs (bp) for V. atypica, 594 bp for V. denticariosi, 257 bp for V. dispar, 311 bp for V. parvula, 510 bp for V. rogosae and 447 bp for V. tobetsuensis (Fig 2).
Fig 2. The products of the polymerase chain reaction obtained by using a mixture of primers.
Molecular weight marker is in lane 1. Primers were a mixture of VF, ATYR, DENR, DISR, PARR, ROGR, and TOBR. Template DNA was from V. atypica, lane 2; V. denticariosi, lane 3; V. dispar, lane 4; V. parvula, lane 5; V. rogosae, lane 6; V. tobetsuensis, lane 7; the mixture of all PCR products of Veillonella species, lane 8.
Each of the six PCR products were mixed and applied to the agarose gel to use as a molecular marker (Fig 2) for reference when six Veillonella species were identified from tongue samples, through electrophoresis. These results demonstrated that six Veillonella species could be specifically and effectively identified by PCR using a mixture of primer sets, ATYR, DENR, DISR, PARR, ROGR, TOBR and VF.
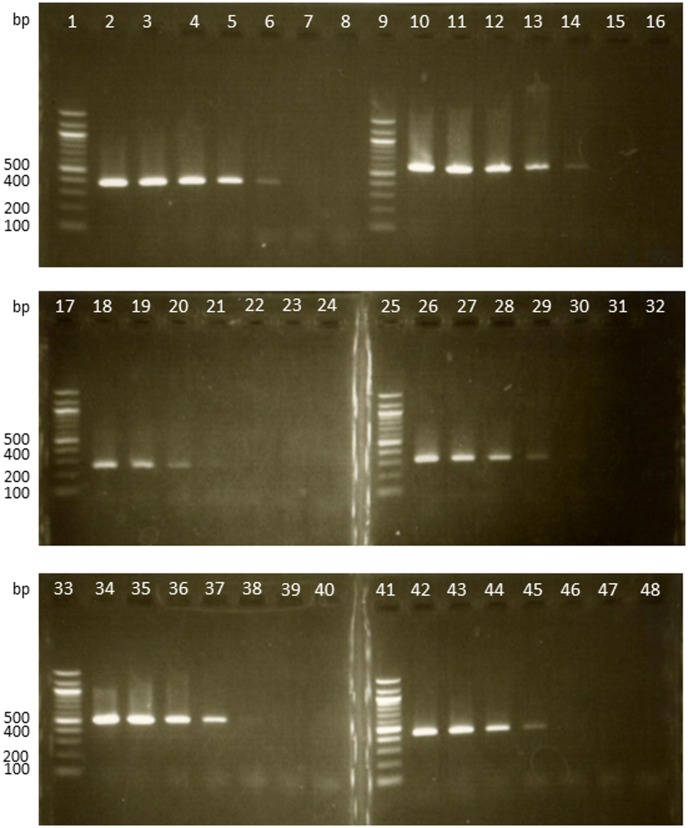
To examine the sensitivity of these primers, PCR was performed with 0.01pg– 10 ng of template DNA from the type strains of six Veillonella species (Fig 3). 1 pg of V. atypica and V. denticariosi DNA, 0.1–0.01 ng of V. dispar DNA, 0.01 ng of V. parvula and V. tobetsuensis DNA, and 0.01 ng- 1 pg of V. rogosae DNA could be detected using these primer sets (Fig 3).
Fig 3. Sensitivity of the PCR for decreasing concentrations of genomic DNA purified from Veillonella species using species-specific primer sets.
A molecular weight marker is shown in lanes 1, 9, 17, 25, 33, and 41. Lane 2 to lane 8: V. atypica, lane 10 to 16: V. denticariosi, lane 18 to 24: V. dispar, lane 26 to 32: V. parvula, lane 34 to 40: V. rogosae, lane 42 to 48: V. tobetsuensis. Amount of the target genomic DNA in the reaction mixture is as follows. Lane 2, 10, 18, 26, 34, and 42: 10 ng; lane 3, 11, 19, 27, 35, and 43: 1 ng; lane 4, 12, 20, 28, 36, and 44: 0.1 ng, lane 5, 13, 21, 29, 38, and 45: 0.01 ng; lane 6, 14, 22, 30, 38, and 46: 1 pg; lane 7, 15, 23, 31, 39, and 47: 0.1 pg; lane 8, 16, 24, 32, 40, and 48: 0.01 pg.
Detection of Oral Veillonella at the Species Level from Tongue Samples
Oral Veillonella were detected in only 10 subjects from 89 subjects from all oral hygiene groups as shown in Tables 2–4. However, the tongue biofilm swabs did indeed yield high numbers of bacterial colonies on BHI agar. Mean (SE) colony-forming units (CFU/mL) per swab were 2.2 (0.08) x 106 with a median of 9.0 x 105 in the first group (Good oral hygiene), Table 2, 5.6 (0.02) x 105 with a median of 9.0 x 104 in the second group (Moderate oral hygiene), Table 3, and 1.4 (0.04) x 106 with a median of 6.5 x 105 in the third group (Poor oral hygiene), Table 4. A total of 101 strains were isolated from Veillonella agar in all groups. Veillonella species were predominantly detected in subjects who had poor oral hygiene compared to those with good or moderate oral hygiene (Tables 2–4). Typical Veillonella colonies on the Veillonella agar were 2–4 mm in diameter, regular and slightly domed in shape and were entirely white; they were composed of small, gram-negative coccal cells, mainly existing as single cells but with some short chains visible. The detection limit was dependent on the number of bacteria in the sample and was < 0.1% of the total colony count.
Table 2. The first group (Good oral hygiene).
| The first group (Good oral hygiene [OHIs 0–1.2]) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age | Sex | Total number | Isolated Veillonella spp. | ||||||||
| All bacteria CFU/mL (× 107) | Veillonella spp. CFU/mL (× 103) | Total number (100%) | V. atypica number (%) | V. denticariosi number (%) | V. dispar number (%) | V. parvula number (%) | V. rogosae number (%) | V. tobetsuensis number (%) | Unknown species number (%) | |||
| G-1 | 10 | F | 2.4 | 0.006 | 6 | 0 (0.0) | 0 (0.0) | 2 (33.3) | 0 (0.0) | 2 (33.3) | 0 (0.0) | 2 (33.3) |
| G-2 | 11 | F | 0.02 | 0.009 | 9 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) | 0 (0.0) | 8 (88.9) |
| G-3 | 10 | M | 0.03 | 0 | - | - | - | - | - | - | - | - |
| G-4 | 9 | F | 0.002 | 0 | - | - | - | - | - | - | - | - |
| G-5 | 12 | M | 0.09 | 0 | - | - | - | - | - | - | - | - |
| G-6 | 15 | F | 0.002 | 0 | - | - | - | - | - | - | - | - |
| G-7 | 13 | M | 0.3 | 0 | - | - | - | - | - | - | - | - |
| G-8 | 11 | F | 0.006 | 0 | - | - | - | - | - | - | - | - |
| G-9 | 11 | M | 0.4 | 0 | - | - | - | - | - | - | - | - |
| G-10 | 10 | F | 0.02 | 0 | - | - | - | - | - | - | - | - |
| G-11 | 11 | M | 0.1 | 0 | - | - | - | - | - | - | - | - |
| G-12 | 10 | F | 0.2 | 0 | - | - | - | - | - | - | - | - |
| G-13 | 10 | M | 0.03 | 0 | - | - | - | - | - | - | - | - |
| G-14 | 10 | F | 0.002 | 0 | - | - | - | - | - | - | - | - |
| G-15 | 11 | F | 0.06 | 0 | - | - | - | - | - | - | - | - |
| G-16 | 12 | F | 0.4 | 0 | - | - | - | - | - | - | - | - |
| G-17 | 10 | F | 0.3 | 0 | - | - | - | - | - | - | - | - |
| G-18 | 9 | F | 0.1 | 0 | - | - | - | - | - | - | - | - |
| G-19 | 9 | F | 0.01 | 0 | - | - | - | - | - | - | - | - |
| G-20 | 12 | M | 0.005 | 0 | - | - | - | - | - | - | - | - |
| G-21 | 8 | F | 0.5 | 0 | - | - | - | - | - | - | - | - |
| G-22 | 13 | M | 0.01 | 0 | - | - | - | - | - | - | - | - |
| G-23 | 12 | M | 0.1 | 0 | - | - | - | - | - | - | - | - |
| G-24 | 11 | M | 0.2 | 0 | - | - | - | - | - | - | - | - |
| G-25 | 13 | F | 0.5 | 0 | - | - | - | - | - | - | - | - |
| G-26 | 9 | F | 0.06 | 0 | - | - | - | - | - | - | - | - |
| G-27 | 7 | F | 0.2 | 0 | - | - | - | - | - | - | - | - |
| G-28 | 10 | F | 0.2 | 0 | - | - | - | - | - | - | - | - |
| G-29 | 10 | F | 0.08 | 0 | - | - | - | - | - | - | - | - |
Total number of anaerobic bacterial colony counts, total number of Veillonella species counts and the number of isolates from each subject identified using species-specific primer sets. CFU: colony-forming unit; detection limit <0.1% of the total count. Individual species as a percentage of the number of isolates from each subject identified using species-specific primer sets.
Table 4. The third group (Poor oral hygiene).
| The third group (Poor oral hygiene [OHIs 3.1–6.0]) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age | Sex | Total number | Isolated Veillonella spp. | ||||||||
| All bacteria CFU/mL (× 107) | Veillonella spp. CFU/mL (× 103) | Total number (100%) | V. atypica number (%) | V. denticariosi number (%) | V. dispar number (%) | V. parvula number (%) | V. rogosae number (%) | V. tobetsuensis number (%) | Unknown species number (%) | |||
| P-1 | 11 | F | 0.07 | 0.002 | 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 1 (50.0) |
| P-2 | 9 | M | 0.02 | 2.0 | 20 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 10 (50.0) | 0 (0.0) | 10 (50.0) |
| P-3 | 13 | M | 0.1 | 0.002 | 2 | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) |
| P-4 | 12 | M | 0.7 | 0.001 | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) |
| P-5 | 11 | M | 0.1 | 2.0 | 20 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 19 (95.0) | 0 (0.0) | 1 (5.0) |
| P-6 | 9 | M | 0.1 | 0.001 | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| P-7 | 9 | M | 0.06 | 0 | - | - | - | - | - | - | - | - |
| P-8 | 12 | F | 0.4 | 0 | - | - | - | - | - | - | - | - |
| P-9 | 10 | M | 0.03 | 0 | - | - | - | - | - | - | - | - |
| P-10 | 11 | F | 0.1 | 0 | - | - | - | - | - | - | - | - |
| P-11 | 11 | F | 0.01 | 0 | - | - | - | - | - | - | - | - |
| P-12 | 11 | F | 0.005 | 0 | - | - | - | - | - | - | - | - |
| P-13 | 11 | M | 0.05 | 0 | - | - | - | - | - | - | - | - |
| P-14 | 10 | F | 0.01 | 0 | - | - | - | - | - | - | - | - |
| P-15 | 13 | F | 0.01 | 0 | - | - | - | - | - | - | - | - |
| P-16 | 11 | F | 0.2 | 0 | - | - | - | - | - | - | - | - |
| P-17 | 10 | F | 0.5 | 0 | - | - | - | - | - | - | - | - |
| P-18 | 11 | F | 0.06 | 0 | - | - | - | - | - | - | - | - |
| P-19 | 11 | M | 0.2 | 0 | - | - | - | - | - | - | - | - |
| P-20 | 9 | F | 0.2 | 0 | - | - | - | - | - | - | - | - |
| P-21 | 11 | M | 0.08 | 0 | - | - | - | - | - | - | - | - |
| P-22 | 9 | F | 1.0 | 0 | - | - | - | - | - | - | - | - |
| P-23 | 9 | M | 0.004 | 0 | - | - | - | - | - | - | - | - |
| P-24 | 12 | M | 0.005 | 0 | - | - | - | - | - | - | - | - |
| P-25 | 11 | F | 0.08 | 0 | - | - | - | - | - | - | - | - |
| P-26 | 7 | M | 0.03 | 0 | - | - | - | - | - | - | - | - |
| P-27 | 12 | M | 0.03 | 0 | - | - | - | - | - | - | - | - |
| P-28 | 9 | M | 0.02 | 0 | - | - | - | - | - | - | - | - |
| P-29 | 11 | M | 0.09 | 0 | - | - | - | - | - | - | - | - |
| P-30 | 11 | F | 0.01 | 0 | - | - | - | - | - | - | - | - |
Total number of anaerobic bacterial colony counts, total number of Veillonella species counts and the number of isolates from each subject identified using species-specific primer sets. CFU: colony-forming unit; detection limit <0.1% of the total count. Individual species as a percentage of the number of isolates from each subject identified using species-specific primer sets.
Table 3. The second group (Moderate oral hygiene).
| The second group (Moderate oral hygiene [OHIs 1.3–3.0]) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age | Sex | Total number | Isolated Veillonella spp. | ||||||||
| All bacteria CFU/mL (× 107) | Veillonella spp. CFU/mL (× 103) | Total number (100%) | V. atypica number (%) | V. denticariosi number (%) | V. dispar number (%) | V. parvula number (%) | V. rogosae number (%) | V. tobetsuensis number (%) | Unknown species number (%) | |||
| M-1 | 11 | F | 0.3 | 2.0 | 20 | 2 (10.0) | 0 (0.0) | 4 (20.0) | 0 (0.0) | 9 (45.0) | 0 (0.0) | 5 (25.0) |
| M-2 | 12 | F | 0.2 | 2.0 | 20 | 6 (30.0) | 0 (0.0) | 1 (5.0) | 0 (0.0) | 0 (0.0) | 1 (5.0) | 12 (60.0) |
| M-3 | 10 | M | 0.05 | 0 | - | - | - | - | - | - | - | - |
| M-4 | 13 | F | 0.004 | 0 | - | - | - | - | - | - | - | - |
| M-5 | 12 | M | 0.0004 | 0 | - | - | - | - | - | - | - | - |
| M-6 | 11 | M | 0.001 | 0 | - | - | - | - | - | - | - | - |
| M-7 | 10 | M | 0.08 | 0 | - | - | - | - | - | - | - | - |
| M-8 | 9 | F | 0.001 | 0 | - | - | - | - | - | - | - | - |
| M-9 | 10 | M | 0.04 | 0 | - | - | - | - | - | - | - | - |
| M-10 | 11 | F | 0.06 | 0 | - | - | - | - | - | - | - | - |
| M-11 | 10 | M | 0.4 | 0 | - | - | - | - | - | - | - | - |
| M-12 | 11 | F | 0.08 | 0 | - | - | - | - | - | - | - | - |
| M-13 | 10 | M | 0.06 | 0 | - | - | - | - | - | - | - | - |
| M-14 | 12 | M | 0.03 | 0 | - | - | - | - | - | - | - | - |
| M-15 | 12 | M | 0.004 | 0 | - | - | - | - | - | - | - | - |
| M-16 | 11 | F | 0.03 | 0 | - | - | - | - | - | - | - | - |
| M-17 | 13 | M | 0.001 | 0 | - | - | - | - | - | - | - | - |
| M-18 | 11 | F | 0.008 | 0 | - | - | - | - | - | - | - | - |
| M-19 | 11 | F | 0.0005 | 0 | - | - | - | - | - | - | - | - |
| M-20 | 9 | F | 0.002 | 0 | - | - | - | - | - | - | - | - |
| M-21 | 12 | F | 0.2 | 0 | - | - | - | - | - | - | - | - |
| M-22 | 11 | M | 0.0004 | 0 | - | - | - | - | - | - | - | - |
| M-23 | 11 | F | 0.01 | 0 | - | - | - | - | - | - | - | - |
| M-24 | 11 | F | 0.0001 | 0 | - | - | - | - | - | - | - | - |
| M-25 | 11 | M | 0.003 | 0 | - | - | - | - | - | - | - | - |
| M-26 | 11 | M | 0.008 | 0 | - | - | - | - | - | - | - | - |
| M-27 | 11 | M | 0.005 | 0 | - | - | - | - | - | - | - | - |
| M-28 | 11 | F | 0.03 | 0 | - | - | - | - | - | - | - | - |
| M-29 | 9 | F | 0.09 | 0 | - | - | - | - | - | - | - | - |
| M-30 | 15 | M | 0.001 | 0 | - | - | - | - | - | - | - | - |
Total number of anaerobic bacterial colony counts, total number of Veillonella species counts and the number of isolates from each subject identified using species-specific primer sets. CFU: colony-forming unit; detection limit <0.1% of the total count. Individual species as a percentage of the number of isolates from each subject identified using species-specific primer sets.
Using species-specific primer sets, 61 of the 101 isolates were identified as either V. atypica, V. dispar, V. rogosae, or V. tobetsuensis (Tables 2–4). Among them, V. rogosae was detected as the predominant species through all groups. However, V. parvula and V. denticariosi were not isolated from any subjects. V. dispar was isolated mainly from subjects who had good or moderate oral hygiene. In addition, of the 101 strains isolated in this study, zero or multiple PCR products were detected using species-specific primer sets with DNA from 40 strains isolated from eight subjects (Tables 2–4). These 40 strains made distinctive PCR products with Veillonella genus primers (Data not shown).
Clonal Analysis of Unknown Strains
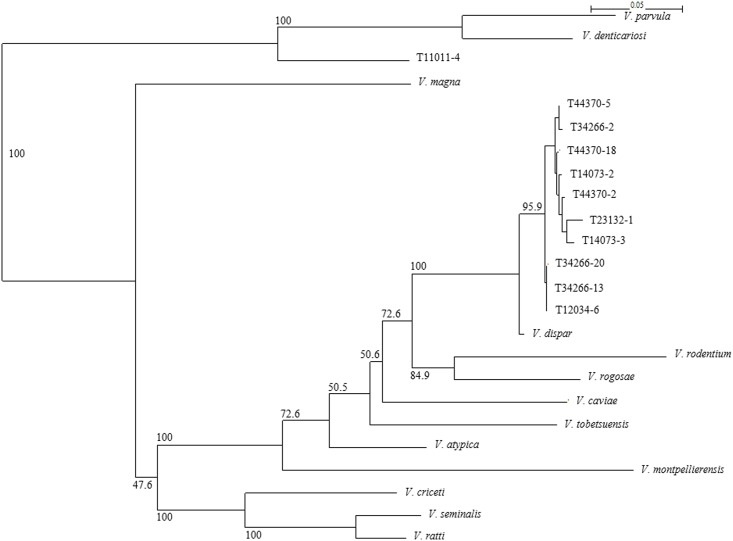
The 11 strains formed distinct taxa with robust bootstrap support (100 and 95.9%) in the rpoB tree (Fig 4) within the genus Veillonella. The rpoB partial sequence similarity among the 11 strains was 92.4–100%. The mean rpoB sequence similarity among the 11 strains belonged to V. dispar and the most closely related species was 98.3%.
Fig 4. Neighbour-joining tree based on rpoB gene sequences (559nt), showing the relationship between the representative 11 unknown strains T11011-4, T44370-5, T34266-2, T44370-18, T14073-2, T44370-2, T23132-1, T14073-3, T34266-20, T34266-13 and T12034-6 and the strains of the recognized members of the genus Veillonella.
The strain of each species and GenBank/EMBL/DDBJ accession numbers for rpoB gene sequences are shown as follows; V. atypica ATCC 17744T (EF185159), V. caviae ATCC 33540T (EF185163), V. criceti ATCC 17747T, V. denticariosi ATCC JCM 15641T (EF185162), V. dispar ATCC 17748T (EF185161), V. magna lac18T (GU479075), V. montpellierensis ADV 281.99T (EF411194), V. parvula ATCC 10790T (EF185158), V. ratti ATCC 17746T (EF185165), V. rodentium ATCC 17743T (EF185166), V. rogosae JCM 15642T (EF211831), V. seminalis strain 08/01/13-B-2228 (KJ580479), V. tobetsuensis ATCC BAA-2400T (AB698646), T; Type strain. Bootstrap values are indicated at corresponding nodes. Bar, 0.05 substitutions per site.
Discussion
Veillonella species are relatively easily identified at the genus level, but species-level identification of the members of this genus is difficult because of a lack of conventional phenotypic and biochemical tests [5, 42–44]. Molecular methods based on 16S ribosomal DNA (rDNA) gene sequencing, including PCR-random fragment length polymorphism analysis, have been used to identify Veillonella strains at the species level [44, 45]. However, recent studies have shown that identification of the members of this genus using 16S rDNA gene sequencing is not reliable [6, 7, 44, 46]. To overcome this problem, sequence analysis of housekeeping genes, including rpoB, dnaK, and gyrB, have been used to define Veillonella species [5–7, 46]. This approach has enabled clear discrimination between species that are well defined based on DNA-DNA homology but poorly defined based on 16S rDNA. V. denticariosi, V. rogosae, and V. tobetsuensis have been proposed as novel species of oral Veillonella based on the results of the molecular analysis using rpoB or dnaK gene sequencing in conjunction with sequence analysis of the 16S r DNA gene [5–7]. Beighton et al. [11] successfully used the rpoB gene sequence, rather than 16S rDNA gene sequence, to discriminate between 253 Veillonella isolates from human tongues of healthy adults at the species level. However, it is time consuming to obtain sequence data of many strains, and laborious to identify them at the species level.
Igarashi et al. [47] reported the successful design of species-specific primer sets for five species of oral Veillonella (V. atypica, V.denticariosi, V. dispar, V. parvula, and V. rogosae) based on a highly variable region (positions 2500–3100) of the rpoB gene. They distinguished between five species of oral Veillonella at the species level by using PCR products generated by a two-step PCR method with species-specific primer sets. This proved to be easier than using complete rpoB gene sequences. In addition, the distribution and frequency of five species of oral Veillonella in tongue biofilms of healthy adults were analyzed by PCR with these specific-primer sets as performed in our previous study [12]. Furthermore, species-specific primer pairs for V. tobetsuensis were designed using the sequence of the dnaK gene in the region displaying a high degree of polymorphism (positions 424–1048) [48]. In addition, the distribution and frequency of six species of oral Veillonella in the periodontal pockets were recently clarified by PCR using these primer sets and those of our previous study [21]. Thus, our previous study demonstrated that these species-specific primer sets were effective in identifying oral Veillonella at the species level, more so than sequence analysis of housekeeping genes. However, these previous procedures used three steps (a two-step PCR with species-specific primer sets for five Veillonella species and PCR with species-specific primer pair for V. tobetsuensis) to define all species of oral Veillonella. This study describes the establishment of the first successful one-step PCR method, using species-specific primer sets for the highly variable region of the rpoB gene of six species of oral Veillonella (positions 2,500–3,100). This one-step PCR method with species-specific primer sets is easier and more effective than previous PCR methods with species-specific primer sets and pairs for oral Veillonella described above.
For faster and more convenient identification of the six Veillonella species, our pilot study used a multiplex PCR method and was tested in many conditions, simultaneously using all species-specific primer sets and DNA templates of six Veillonella species in a single PCR mixture. Only the PCR product of VF and DISR, specific for V. dispar, could not be confirmed (Data not shown). Although the reason was not clarified, it was suspected to be due to the sensitivity of DISR. The specific primer set for V. dispar, VF and DISR, showed lower sensitivity than the other sets (Fig 3). Therefore, a one-step PCR method, with species-specific primer sets for six species of oral Veillonella, was developed in this study.
According to the results of Tables 2–4, Veillonella species had a tendency to be detected in subjects who had poor oral hygiene compared to those with good or moderate oral hygiene. However, the CFU count of all bacteria in tongue biofilms had no relationship with oral hygiene status. It was suggested that the appearance of Veillonella species in tongue biofilm of Thai children would be one of the indexes for deteriorating oral hygiene.
In our previous study, the distribution and frequency of oral Veillonella at a species level in the tongue biofilm of Japanese healthy young adults (Twenty-seven subjects: 12 males and 15 females, age: 20s) were investigated [12]. In that study, all subjects had Veillonella species (more than 0.6 x 106 CFU/mL), and the number of isolates was 416 from all subjects. Tongue biofilm samples from these subjects could be divided into two groups based on the distribution and frequency of five species of oral Veillonella. In one group, V. rogosae was predominant, while the other group consisted of mainly V. dispar and V. atypica. Beighton et al. [11] also reported the predominant cultivable Veillonella species in tongue biofilm of healthy adults (eleven subjects: the ratio of gender and range of age were not shown) in the UK. They also demonstrated that Veillonella species were isolated from all subjects, and the total number of Veillonella isolates was 253. In addition, they reported that the predominant species in tongue biofilms were V. atypica, V. dispar, and V. rogosae. In this study, only 10 of 89 subjects had Veillonella species, and the total number of isolates was 101 from 10 subjects. The ratio of Veillonella isolates was clearly low compared to these previous reports. As described in the introduction, it was reported that Veillonella species have a central role in biofilm formation as the early colonizer [32]. The results herein may be due to country- or age-specific differences, in which case, other oral bacterial species may have central roles as the early colonizer to form the biofilm and to cause various oral infectious diseases. As the source of these differences was not clarified here (for example, whether it is due to country- or age-specific differences), further studies are needed to investigate the distribution and frequency of Veillonella species from tongue biofilms of children in other countries including Japan.
V. rogosae was also isolated as the predominant species in tongue biofilms in this study. In addition, V. parvula and V. denticariosi were not isolated at all. Similarly, Beighton et al. [11] reported that V. parvula was isolated from only one subject while V. denticariosi was not isolated from any subjects in their study. In our previous study [12], V. parvula was isolated from four subjects and V. denticariosi was isolated from only one subject. Taken together, it appears that the distribution and frequency of Veillonella species in tongue biofilm have generational and universal commonality. In this study, V. tobetsuensis was isolated from only one subject. In addition, in our previous studies [12, 48], 12 strains of V. tobetsuensis were isolated from tongue biofilms of 5 of 27 subjects. Here, it was also considered that V. tobetsuensis is a minor species in the tongue biofilm. There are likely to be several factors that influence these differences in distribution and frequency of Veillonella species in tongue biofilms.
PCR products could not be identified in 40 strains, using species-specific primer sets for oral Veillonella species, but these 40 strains produced PCR products with the Veillonella genus-specific primer set used in the present study. This confirms that these 40 strains were members of the genus Veillonella. Although these 40 strains could not be classified as any of the established oral Veillonella, these results suggest the possibility that other Veillonella species also inhabit the human oral cavity.
In addition, according to the results of clonal analysis, although the representative 11 strains in the 40 unknown strains showed similar sequences with V. dispar, they formed distinct taxa with robust bootstrap support (100 and 95.9%) in the rpoB tree (Fig 4). These 40 isolates may be members of novel oral Veillonella species. Furthermore, there are at least two novel Veillonella species in the 40 unknown strains based on the evolutionary tree of rpoB gene (Fig 4). However, the sequence analysis of other housekeeping genes, such as 16S rDNA, dnaK and gyrB genes, are required to propose the novel species of genus Veillonella [5–7, 44–46]. At present time of writing this manuscript, the sequence analysis of other housekeeping genes of these representative 11 unknown strains are in the process.
In this study, a novel one-step PCR method with species-specific primer sets for oral Veillonella was established using a partial sequence of the rpoB gene. Moreover, this is the first report of the use of species-specific primer sets for determining the distribution and frequency of oral Veillonella in tongue biofilm of Thai children. However, as described above, further studies are needed to discuss the country- or age-specific differences in the distribution and frequency of oral Veillonella. The study of the distribution and frequency of oral Veillonella by using the tongue biofilms in other countries including Japan will be investigated in near future.
Acknowledgments
We are grateful to Dr. Masahiro Asakura in FUSO pharmaceutical industries, R&D center, Osaka, Japan, Dr. Natdhanai Chotprasert in Maxillofacial Prosthetic Unit, Faculty of Dentistry, Mahidol University, Thailand, and Dr. Doan Minh Tri in Faculty of Odonto-Stomatology, University of Medicine and Pharmacy Ho Chi Minh City, Vietnam.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported in part by grants-in-aid from the Japan Society for the Promotion of Sciences (JSPS) (https://www.jsps.go.jp/) Fellows (grant No. 15J30007), Scientific Research from KAKENHI (grant No. 26462793), 2014-2015 Research Project of the Research Institute of Personalized Health Sciences (No. 2014-200-3), Health Sciences University of Hokkaido (http://www.hoku-iryo-u.ac.jp/~kotaisa/index.html), and Faculty of Dentistry Grant (2015) (Grant No. 0517.031/00437), Mahidol University (http://www.dt.mahidol.ac.th). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mancl KA, Kirsner RS, Ajdic D. Wound biofilms: lessons learned from oral biofilms. Wound Rep Reg 2013. 21(3):352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keijser BJ, Zaura E, Huse SM, van der Vossen JM, Schuren FH, Montijn RC, et al. Pyrosequencing analysis of the oral microflora of healthy adults. J Dent Res 2008. 87(11):1016–1020. [DOI] [PubMed] [Google Scholar]
- 3.Mays TD, Holdeman LV, Moore WEC, Rogosa M, Johnson JL. Taxnomy of the genus Veillonella Prèvot. Int J Syst Bacteriol 1982. 32:28–36. [Google Scholar]
- 4.Rogosa M. The genus Veillonella. IV. Serological groupings, and genus and species emendations. J Bacteriol 1965. 90(3):704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byun R, Carlier JP, Jacques NA, Marchandin H, Hunter N. Veillonella denticariosi sp. nov., isolated from human carious dentine. Int J Syst Evol Microbiol 2007. 57:2844–2848. [DOI] [PubMed] [Google Scholar]
- 6.Arif N, Do T, Byun R, Sheehy E, Clark D, Gilbert SC, et al. Veillonella rogosae sp. nov., an anaerobic, Gram-negative coccus isolated from dental plaque. Int J Syst Evol Microbiol 2008. 58:581–584. 10.1099/ijs.0.65093-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mashima I, Kamaguchi A, Miyakawa H, Nakazawa F. Veillonella tobetsuensis sp. nov., an anaerobic, Gram-negative coccus isolated from human tongue biofilms. Int J Syst Evol Microbiol 2013. 63:1443–1449. 10.1099/ijs.0.042515-0 [DOI] [PubMed] [Google Scholar]
- 8.Sutter VL. Anaerobes as normal oral flora. Rev Infect Dis 1984. 6(Suppl 1):S62–S66. [DOI] [PubMed] [Google Scholar]
- 9.Rogosa M. Anaerobic Gram-negative cocci, p 680–685. In Krieg NR, Holt JG (ed), Bergey’s manual of systematic bacteriology, vol 1 1984. Williams & Wilkins, Baltimore, MD. [Google Scholar]
- 10.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacteria flora of the oral cavity. J Clin Microbiol 2005. 43(11):5721–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beighton D, Clark D, Hanakura B, Gilbert S, Do T. The predominant cultivable Veillonella spp. of the tongue of healthy adults identified using rpoB sequencing. Oral Microbiol Immunol 2008. 23(4):344–347. 10.1111/j.1399-302X.2007.00424.x [DOI] [PubMed] [Google Scholar]
- 12.Mashima I, Kamaguchi A, Nakazawa F. The distribution and frequency of oral Veillonella spp. in the tongue biofilm of healthy young adults. Curr Microbiol 2011. 63(5):403–407. 10.1007/s00284-011-9993-2 [DOI] [PubMed] [Google Scholar]
- 13.Kanasi E, Dewhirst FE, Chalmers NI, Kent R Jr, Moore A, Hughes CV, et al. Clonal analysis of the microbiota of severe early childhood caries. Caries Res 2010. 44(5):485–497. 10.1159/000320158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundqvist G. Associations between microbial species in dental root canal infections. Oral Microbiol Immunol 1992. 7(5):257–262. [DOI] [PubMed] [Google Scholar]
- 15.Wittgow WC Jr, Sabiston CB Jr. Microorganisms from pulpal chambers of intact teeth with necrotic pulps. J Endod 1975. 1(5):168–171. [DOI] [PubMed] [Google Scholar]
- 16.Khemaleelakul S, Baumgartner JC, Pruksakorn S. Identification of bacteria in acute endodontic infections and their antimicrobial susceptibility. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002. 94(6):746–755. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartener JC, Falkler WA Jr. Bacteria in the apical 5 mm of infected root canals. J Endod 1991. 17(8):380–383. [DOI] [PubMed] [Google Scholar]
- 18.Peters LB, Wesselink PR, Buijs JF, van Winkelhoff AJ. Viable bacteria in root dentinal tubules of teeth with apical periodontitis. J Endod 2001. 27(2):76–81. [DOI] [PubMed] [Google Scholar]
- 19.Heller D, Silva-Boghossian CM, do Souto RM, Colombo AP. Subgingival microbial profiles of generalized aggressive and chronic periodontal diseases. Arch Oral Biol 2012. 57(7):973–980. 10.1016/j.archoralbio.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 20.Silva-Boghossian CM, Neves AB, Resende FA, Colombo AP. Suppuration-associated bacteria in subjects with chronic and aggressive periodontitis. J Periodontol 2013. 84(9):e9–e16. 10.1902/jop.2013.120639 [DOI] [PubMed] [Google Scholar]
- 21.Mashima I, Fujita M, Nakatsuka Y, Kado T, Furuichi Y, Herastuti S, et al. The Distribution and Frequency of Oral Veillonella spp. Associated with Chronic Periodontitis. Int J Curr Microbiol App Sci 2015. 4(3): 150–160. [Google Scholar]
- 22.Williams BL, Osterberg SK, Jorgensen J. Subgingibal microflora of periodontal patients on tetracycline therapy. J Clin Periodontol 1979. 6(4):210–221. [DOI] [PubMed] [Google Scholar]
- 23.Ready D, Pratten J, Roberts AP, Bedi R, Mullany P, Wilson M. Potential role of Veillonella spp. as a reservoir of transferable tetracycline resistance in the oral cavity. Antimicrob Agents Chemother 2006. 50(8):2866–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ready D, Bedi R, Mullany P, Wilson M. Penicillin and amoxicillin resistance in oral Veillonella spp. Int J Antimicrob Agents 2012. 40(2):188–189. 10.1016/j.ijantimicag.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 25.Delwiche EA, Pestka JJ, Tortorello ML. The Veillonellae: gram negative cocci with unique physiology. Annu Rev Microbiol 1985. 39:175–193. [DOI] [PubMed] [Google Scholar]
- 26.Matera G, Muto V, Vinci M, Zicca E, Abdollahi-Roodsaz S, van de Veerdonk FL, et al. Receptor recognition of and immune intracellular pathways for Veillonella parvula lipopolysaccharide. Clin Vaccine Immunol 2009. 16(12):1804–1809. 10.1128/CVI.00310-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross EL, Beall CJ, Kutsch SR, Firestone ND, Leys EJ, Griffen AL. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One 2012. 7(10):e47722 10.1371/journal.pone.0047722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, et al. The human oral microbiome. J Bacteriol 2010. 192(19):5002–5017. 10.1128/JB.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012. 486:207–214. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diaz PI, Chalmers NI, Rickard AH, Kong C, Milburn CL, Palmer RJ Jr, et al. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl Environ Microbiol 2006. 72(4):2837–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyvad B, Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res 1987. 95(5):369–380. [DOI] [PubMed] [Google Scholar]
- 32.Periasamy S, Kolenbrander PE. Central role of the early colonizer Veillonella sp. in establishing multispecies biofilm communities with initial, middle, and late colonizers of enamel. J Bacteriol 2010. 192(12):2965–2972. 10.1128/JB.01631-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belstrøm D, Holmstrup P, Nielsen CH, Kirkby N, Twetman S, Heitmann BL, et al. Bacterial profiles of saliva in relation to diet, lifestyle factors, and socioeconomic status. J Oral Microbiol 2014. 6:23609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomczyk J. Comments on Soltysiak's paper: "Comment: low dental caries rate in Neandertals: the result of diet or the oral flora compositions?" Homo 2012. 63(4): 311–314. 10.1016/j.jchb.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 35.Brook I. The impact of smoking on oral and nasopharyngeal bacterial flora. J Dent Res 2011. 90(6):704–710. 10.1177/0022034510391794 [DOI] [PubMed] [Google Scholar]
- 36.Haffajee AD, Teles RP, Socransky SS. The effect of periodontal therapy on the composition of the subgingival microbiota. Periodontol 2000 2006. 42:219–258. [DOI] [PubMed] [Google Scholar]
- 37.Tanwir F, Altamash M, Gustafsson A. Effect of diabetes on periodontal status of a population with poor oral health. Acta Odontol Scand 2009. 67(3):129–133. 10.1080/00016350802208406 [DOI] [PubMed] [Google Scholar]
- 38.Greene JC, Vermillion JR. The simplified oral hygiene index. Journal of the American Dental Association 1964. 68:7–13. [DOI] [PubMed] [Google Scholar]
- 39.Rogosa M, Fitzgerald RJ, Mackintosh ME, Beaman AJ. Improved medium for selective isolation of Veillonella. J Bacteriol 1958. 76(4):455–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu DY, Ugozzoli L, Pal BK, Qian J, Wallace RB. The effect of temperature and oligonucleotide primer length on the specificity and efficiency of amplification by the polymerase chain reaction. DNA Cell Biol 1991. 10:233–238. [DOI] [PubMed] [Google Scholar]
- 41.Beighton D, Clark D, Hanakuka B, Gilbert S, Do T. The predominant cultivable Veillonella spp. of the tongue of healthy adults identified using rpoB sequencing. Oral Microbiol Immunol 2008. 23(4):344–347. 10.1111/j.1399-302X.2007.00424.x [DOI] [PubMed] [Google Scholar]
- 42.Kolenbrander PE, Moore LVH. The genus Veillonella In: Balows HG, Trüper M, Dworkin W, Harder W, Schleifer KH, editors. The prokaryotes. 2nd ed New York: Springer; 1992. p. 2034e47. [Google Scholar]
- 43.Haraszthy VI, Zambon JJ, Sreenivasan PK, Zambon MM, Gerber D, Rego R, et al. Identification of oral bacterial species associated with halitosis. J Am Dent Assoc 2007. 138(8):1113–1120. [DOI] [PubMed] [Google Scholar]
- 44.Marchandin H, Teyssier C, Siméon DB, Jean PH, Carriere C, Jumas BE. Intrachromosomal heterogeneity between the four 16S rRNA gene copies in the genus Veillonella: implications or phylogeny and taxonomy. Microbiology 2003. 149(Pt 6):1493–1501. [DOI] [PubMed] [Google Scholar]
- 45.Sato T, Matsuyama J, Sato M, Hoshino E. Differentiation of Veillonella atypica, Veillonella dispar, Veillonella parvula using restricted fragment-length polymorphism analysis of 16S rDNA amplified by polymerase chain reaction. Oral Mirobiol Immunol 1997. 12(6):350–353. [DOI] [PubMed] [Google Scholar]
- 46.Jumas BE, Carlier JP, Jean PH, Teyssier C, Gay B, Campos J, et al. Veillonella montpellierensis sp. nov., a novel anaerobic gram-negative coccus isolated human clinicalsamples. Int J Syst Evol Microbiol 2004. 54(Pt 4):1311–1316. [DOI] [PubMed] [Google Scholar]
- 47.Igarashi E, Kamaguchi A, Fujita M, Miyakawa H, Nakazawa F. Identification of oral species of the genus Veillonella by polymerase chain reaction. Oral Microbiol Immunol 2009. 24(4):1–4. [DOI] [PubMed] [Google Scholar]
- 48.Mashima I, Nakazawa F. Identification of Veillonella tobetsuensis in tongue biofilm by using a species-specific primer pair. Anaerobe 2013. 22:77–81. 10.1016/j.anaerobe.2013.04.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.