Abstract
Epithelial polarity genes are important for maintaining tissue architecture, and regulating growth. The Drosophila neoplastic tumor suppressor gene scribble (scrib) belongs to the basolateral polarity complex. Loss of scrib results in disruption of its growth regulatory functions, and downregulation or mislocalization of Scrib is correlated to tumor growth. Somatic scribble mutant cells (scrib-) surrounded by wild-type cells undergo apoptosis, which can be prevented by introduction of secondary mutations that provide a growth advantage. Using genetic tools in Drosophila, we analyzed the phenotypic effects of loss of scrib in different growth promoting backgrounds. We investigated if a central mechanism that regulates cell adhesion governs the growth and invasive potential of scrib mutant cells. Here we show that increased proliferation, and survival abilities of scrib- cells in different genetic backgrounds affect their differentiation, and intercellular adhesion. Further, loss of scrib is sufficient to cause reduced cell survival, activation of the JNK pathway and a mild reduction of cell adhesion. Our data show that for scrib cells to induce aggressive tumor growth characterized by loss of differentiation, cell adhesion, increased proliferation and invasion, cooperative interactions that derail signaling pathways play an essential role in the mechanisms leading to tumorigenesis. Thus, our study provides new insights on the effects of loss of scrib and the modification of these effects via cooperative interactions that enhance the overall tumorigenic potential of scrib deficient cells.
Introduction
Epithelial cells are the major cell-type for all organs in multicellular organisms that organize into elaborate stratified sheets via formation of intercellular junctions, and have a distinct apical-basal polarity that is maintained during cell division [1, 2]. In order to achieve correct organ size, epithelial tissues need mechanisms that limit their proliferation, and protect tissues from damage caused by defective epithelial cells [3–5]. In Drosophila, defective epithelial cells that arise due to disruption of apical-basal polarity trigger a cell non-autonomous response in which either neighboring cells [6] or circulating hemocytes induce apoptosis in the mutant cells [7]. Epithelial functions such as signaling across the epithelial layer, dynamic interactions of cells with the underlying basement membrane and extracellular matrix (ECM) depend on highly organized epithelial architecture that is orchestrated by apical and basolateral junctional complexes [1, 2, 8]. This highly organized epithelial architecture is damaged and eventually lost in cancer, where malignant cells lose polarity and connections to the basement membrane causing cancer cells to become proliferative, motile (by undergoing EMT [epithelial-mesenchymal transition]), and invasive (by degrading ECM) [2, 9, 10]. Thus, the proliferation of cancer cells depends on the influence of cell-cell contacts and the cell-microenvironment interactions [11–13].
The apical junctional complexes are landmarks for the evolutionarily conserved Crumbs/Par and the basolateral Scrib polarity modules [1, 5, 14, 15]. The Crumbs (Crb) complex is formed by the association of Crb with Stardust (Sdt) and PALS1 (protein associated with Lin seven 1)-associated TJ protein (PATJ), that together play a critical role in establishing the apical domain [2]. The Par complex consists of three components: Atypical Protein Kinase C (aPKC), Cell Division Cycle 42 (Cdc42) and Partitioning Defective 6 (Par-6) that act at the apical cortex to position Bazooka (Baz) at the Adherens Junction (AJs) [9]. The basolateral Scribble complex comprises of Lethal giant larvae (Lgl) [16], Discs large (Dlg), and Scribble (Scrib) [17, 18] that are required for the formation of septate junctions, and mutations in Scribble complex components cause massive neoplastic overgrowth of mutant tissues in addition to defects in cell polarity and are referred to as “neoplastic tumor suppressor genes”. Further, the basolateral proteins are required for assembly of other junctional complexes, and are a great model system to study mechanisms of cell polarity and growth control [19, 20].
In addition to the junctional complexes described above, epithelial cells are connected to each other via adhesion molecules at the AJs that mediate stable cohesion between cells [21, 22]. These junctional complexes comprise of E-Cadherin (E-Cad), which forms a trans-dimer on adjacent cells through its extracellular domain and intracellularly binds with β-catenin and α-catenin in a junctional complex [21–23]. During normal development, the intercellular junctions provide the structural foundation for maintaining tissue architecture, and AJs are actively reorganized to allow tissue remodeling [5, 21]. Further, junctional dynamics plays a key role in how a cell responds to stress, or other signals [13, 24, 25]. Thus, the organization and maintenance of junctional complexes in epithelial cells reflects a homeostatic state, which is disrupted when junctions are damaged in conditions like cancer. Therefore, it is possible that mutations in polarity genes change cell adhesion to promote aggressive tumor growth.
We tested this hypothesis in Drosophila scrib mutant cells that are known to have different growth potential depending on the genotype of the mutant or neighboring cells. The vast range of phenotypes includes the slow growing scrib mutant cells to tumors formed by oncogenic cooperation between scrib- and RasV12 [26–30]. These phenotypic variations lead us to investigate the effects of loss of scrib alone, and genetic combinations that provide a growth advantage to scrib mutant cells on proliferation, differentiation, survival, cell adhesion and invasiveness. We show that the increased proliferation and survival associated with scrib- cells in different genetic combinations co-relate with changes in cell adhesion. We further show that invasive potential of scrib- cell can be uncoupled from the invasive tumor phenotype, which is exhibited only in the presence of certain oncogenic insults like oncogenic RasV12.
Materials and Methods
Fly stocks and genetics
The Drosophila stocks used in this study are previously published and described in FlyBase. GFP positive MARCM clones [31] were generated in the eye-antennal imaginal discs by crossing eyFLP; AyGAL4 UAS GFP; FRT82B TubGAL80 flies with (i) FRT82B, (ii) FRT82B scribj7b3 or thlacZ, FRT82B scrib2, (iii) FRT82B wtsX1, (iv) UAS p35; FRT82B scrib2, (v) UAS RasV12; FRT82B, (vi) UAS RasV12 FRT82B scrib2, and (vii) thlacZ FRT82B scrib2 wtsX1 flies.
GFP negative scrib loss of function clones in Minute background [32] were generated by crossing eyFLP;; FRT82B M(95A) UbiGFP [33] flies with thlacZ FRT82B scrib2 or FRT82B scribj7b3 flies. All experiments, except for generation of FRT82BwtsX1 MARCM clones (which was performed at room temperature), were performed at 25°C. Discs from wandering third instar larvae were used for all phenotypic analyses.
Immunohistochemistry
Antibody staining was performed by using the following primary antibodies: mouse anti PH3 (1:200, Cell Signaling Technology), mouse anti DIAP1 (1:200, from Dr. Bruce Hay), rat anti ELAV (1:300, DSHB), mouse anti Armadillo (1:100, DSHB), mouse anti Fas2 (1:100, DSHB), rat anti E-Cad (1:100, DSHB), and mouse anti MMP1 (1:200, DSHB). The secondary antibodies used to detect primary antibodies were: Donkey Cy3 conjugated anti mouse IgG (1:200, Jackson ImmunoResearch) or Donkey Cy5 conjugated anti rat IgG (1:200, Jackson ImmunoResearch).
Immunohistochemistry was performed using standard protocol (Kango-Singh et al., 2002). Briefly, third instar larvae of appropriate genotypes were dissected in 1X PBS, fixed in 4% paraformaldehyde (PFA). The discs were incubated with appropriate primary (overnight incubation at 4°C), and secondary (2 hours at room temperature) antibodies. 1X PBST was used to permeabilize the tissue, and wash off unbound antibodies following each incubation. The processed tissue was mounted in Vectashield (Vector labs). A minimum of 15 discs were analyzed for each staining and genotype.
Confocal imaging
Images (at 40X magnification) were captured using Olympus Fluoview 1000 Laser Scanning Confocal Microscope. The images were edited using Phostoshop (Version CS5, and CC).
Results
scrib- cells proliferate ectopically in the presence of growth promoting mutations
It is well-documented that scrib- cells in wild-type background undergo apoptosis that masks their neoplastic potential [30]. We generated somatic scrib- clones in different genetic backgrounds where their elimination was compromised by (A) reducing fitness of neighboring cells by making them Minute heterozygous [32] [referred to as scrib-/M throughout the text], (B) blocking apoptosis due to overexpression of the pan-caspase inhibitor p35 [34] [referred to as p35+scrib- throughout the text], (C) by introducing loss of function mutation in warts (wts-) [35]–a key player that mediates growth functions of scrib through the Hippo pathway [29] [referred to as scrib-,wts- throughout the text], or (D) by activation of oncogenic Ras (UAS RasV12) [36] in scrib- clones [referred to as RasV12,scrib- throughout the text]. We specifically tested scrib-,wts- combination as recently we and others have shown that AJC components like Crb, aPKC, Scrib and Lgl interact with the Hippo pathway to regulate growth [37–44].
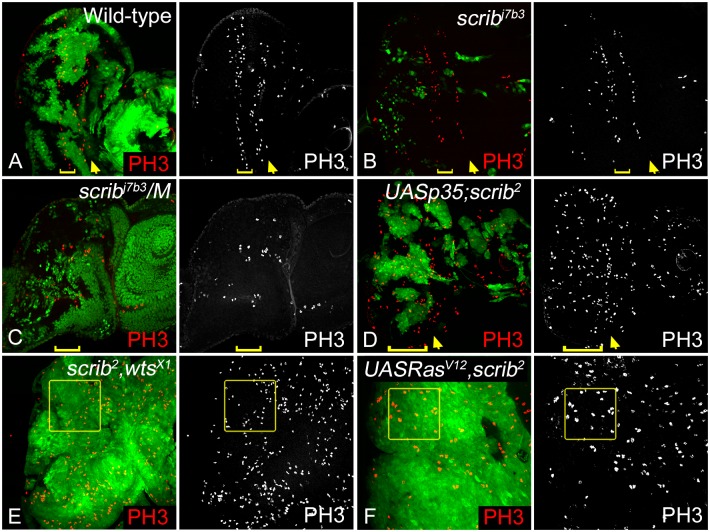
We first tested the effect of these genetic combinations on cell proliferation using an antibody against Phospho-histone H3 (PH3), which marks mitotic figures [45–47] (Fig 1). We compared PH3 profiles in eye imaginal discs containing MARCM clones that were either wild type (Fig 1A), or scrib- (Fig 1B) with the other genetic combinations (Fig 1C–1F). Consistent with the well-documented cell cycle regulation in wild-type third instar eye imaginal discs, PH3 positive cells are seen mainly anterior to the morphogenetic furrow (MF), and in the second mitotic wave (SMW) posterior to the MF (Fig 1A) [47, 48]. This pattern remains largely unaffected in scrib- cells in wild-type background (Fig 1B). In contrast, scrib-/M (Fig 1C), p35+scrib- (Fig 1D), scrib-,wts- (Fig 1E), and RasV12,scrib- (Fig 1F) show ectopic PH3 expression. Interestingly, of these genetic combinations only scrib-,wts- and RasV12,scrib- clones show massive overgrowth causing a disruption in the eye imaginal disc morphology which become enlarged in case of scrib-,wts-, and show multilayered neoplastic tumor phenotypes in RasV12,scrib- double mutants. Although ectopic proliferation is seen in wtsx1 clones (data not shown) [49], and in RasV12 overexpressing clones (data not shown) [33]. These data suggest that additional mutations in scrib mutant cells modify their growth profile. However, the effect of these additional mutations is variable as reflected by the difference in degree of proliferation and growth. The qualitative difference in clone growth and overall eye disc size led us to ask if differentiation, survival, adhesion and invasion potential are altered in scrib- cells in different genetic backgrounds.
Fig 1. Analysis of cell proliferation in scrib- cells with additional mutations that provide growth advantage.
M-phase cells were observed in third instar eye imaginal discs by using anti PH3 antibody (red, and greyscale in A-F) to mark the proliferating cells. The panels show disc of the genotypes (A) eyFLP; AyGAL4 UAS GFP; FRT82B TubGal80/FRT82B [Wild-type], (B) eyFLP; AyGAL4 UAS GFP; FRT82B TubGal80/FRT82B scribj7b3 [scrib-] (C) eyFLP;+; FRT82B (M95A) ubiGFP/FRT82B scribj7b3 [scrib-/M], (D) eyFLP; AyGAL4 UAS GFP/ UASP35; FRT82B TubGal80/FRT82B scrib2 [p35+scrib-] (E) eyFLP; AyGAL4 UAS GFP; FRT82B TubGal80/FRT82B wtsX1,scrib2 [scrib-,wts-], and (F) eyFLP; AyGAL4 UAS GFP; FRT82B TubGal80/UAS RasV12, FRT82B scrib2 [RasV12,scrib-]. Clones in A, B, D, E, and F are positively marked by GFP (generated by MARCM technique), and clones in C are marked by lack of GFP (generated by FLP/FRT technique). The yellow arrows (panels A-D) mark the morphogenetic furrow (MF), while the yellow brackets (panels A-D) mark the extent of the second mitotic wave (SMW). Yellow boxes in panels E and F mark ectopic PH3 within clones. All images are shown at identical magnification (40X), and orientated with posterior to the left, and anterior to the right. Genotypes, magnification, orientation and arrangement of panels mentioned in Fig 1 are consistent in Figs 2–4 and 6.
Increased survival of scrib- cells negatively regulates differentiation
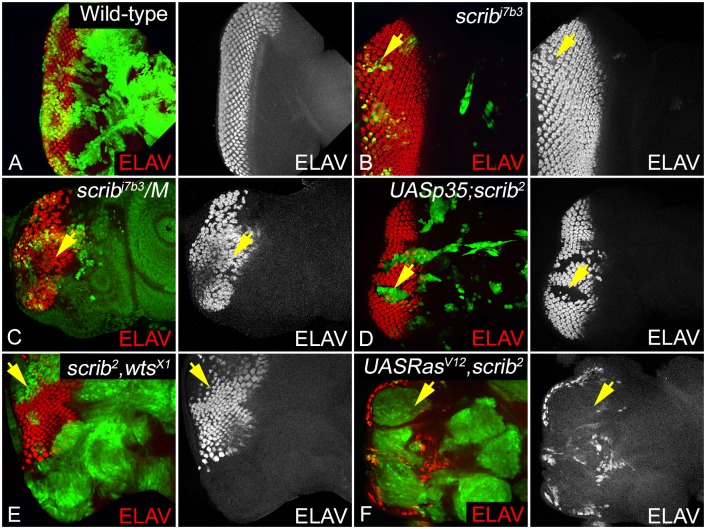
High grade tumors often have poorly differentiated cells, and altered morphology [50]. In contrast cells with poor growth potential, for example, mutations in ribosomal proteins (the Minute mutants) or signaling pathways, for example, loss of Drosophila S6 kinase (dS6k-), yorkie (yki-) or Target of Rapamycin (dTOR-) show poor growth but no effect on differentiation [32, 51–54]. Therefore, we checked if scrib- cells, or the different genetic backgrounds that modify the growth potential of scrib mutant cells show any effects on cell differentiation. We tested for expression of Embryonic Lethal Abnormal Vision (ELAV), which is expressed in the differentiated photoreceptor neurons as a marker in the third instar eye discs (Fig 2) [55]. Compared to wild-type (Fig 2A), we did not observe any noticeable differentiation defects in scrib- cells undergoing apoptosis (Fig 2B). However, we saw varying degree of effect on MF progression or differentiation of photoreceptor neurons when the growth potential of scrib mutant cells is modified in other genetic combinations (Fig 2C–2F). In scrib-/M discs photoreceptor neurons differentiate both in the scrib mutant and the neighboring M/+ cells, however, the spacing of the ommatidial clusters and the progression of the MF are affected (Fig 2C) suggesting misregulation of furrow progression. The scrib-,wts- double mutants show suppression of MF progression in the ventral eye margin and increased spacing between ommatidial clusters (Fig 2E), a phenotype that resembles wts mutant cells (S1A Fig). These phenotypes are typical of loss of Hippo pathway genes, and consistent with our earlier finding that scrib acts through wts to regulate its growth functions [29]. In comparison, p35+scrib- mutant cells (Fig 2D) and the RasV12,scrib- mutant cells (Fig 2F) show a complete suppression of differentiation. The RasV12 control clones (S1B Fig) show defects in photoreceptor organization, and regulation of furrow progression. One reason why suppression of cell death (p35) or overactivation of oncogenic Ras (RasV12) cause suppression of differentiation in scrib mutant cells is that the signals controlling MF progression are inhibited, or alternatively changes in cell survival or cell adhesion or combinations thereof cause tumor like growth by suppressing differentiation. Therefore, we tested if these factors contribute to increased growth and tumorigenesis in scrib mutant cells that have increased proliferation ability.
Fig 2. Effect on differentiation in scrib loss of function clones, and other genetic backgrounds.
Panels show eye imaginal discs containing clones of the following genotypes (A) Wild-type (GFP-positive), (B) scrib- (GFP-positive), (C) scrib-/M (GFP-negative), (D) p35+scrib- (GFP-positive), (E) scrib-,wts- (GFP-positive), and (F) RasV12,scrib- (GFP-positive) stained with antibody to the pan-neural marker ELAV (red, and greyscale in A-F) to assess changes in differentiation, morphogenetic furrow progression, and photoreceptor organization. Note that for all genotypes, only those clones posterior to the morphogenetic furrow are relevant for analysis. Yellow arrows in panels B-F highlight areas/mutant clones where changes in ELAV expression were assessed.
We assessed survival potential of clones by testing expression of Drosophila inhibitor of apoptosis-1 protein (DIAP1), which is known to protect cells from apoptosis in several contexts including developmentally regulated apoptosis, or stress induced apoptotic response [56–62]. Compared to the ubiquitous expression of DIAP1 seen in wild-type (S2A Fig), DIAP1 levels are down regulated in scrib- cells (S2B Fig), and p35+scrib- (S2D Fig) clones, but induced in scrib-/M clones (S2C Fig). Consistent with previous reports, DIAP1 levels are robustly induced in scrib-,wts- (S2E Fig), and RasV12,scrib- (S2F Fig) clones [28, 29]. Of note, forced suppression of apoptosis by expression of p35 in scrib- cells caused inhibition of differentiation despite suppression of DIAP1 suggesting that a net increase in survival of scrib mutant cells promotes growth and inhibits differentiation. Overall, these data suggest that upregulation of survival signaling in scrib mutant cells is correlates with increased growth and negative regulation of differentiation. Next, we tested if scrib mutant cells also induce changes in cell adhesion that enhance the overall tumorigenic potential of scrib mutants in the genetic backgrounds under study, or if increased survival of cells from loss of wts or Ras overexpression account for the growth phenotypes.
Is loss of differentiation linked to changes in cell adhesion?
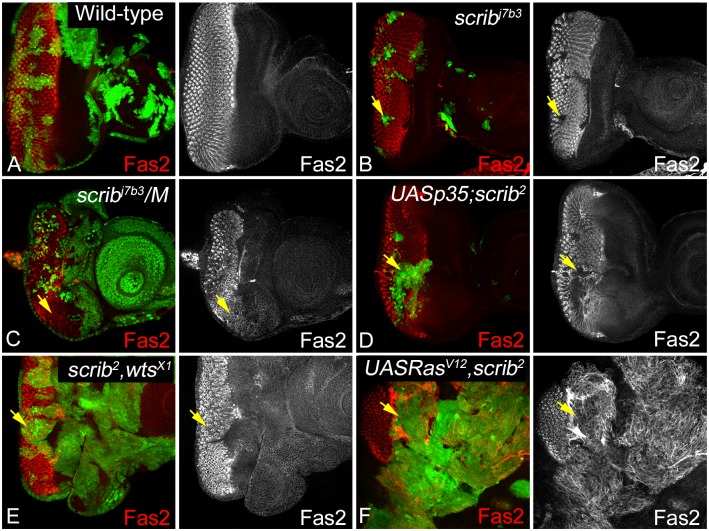
Epithelial cells establish specific adhesion complexes at the lateral and apical cell surface that appear to act as specialized sites of signal transmission [63]. Fasciclin 2 (Fas2), the Drosophila Neural Cell Adhesion Molecule (NCAM) ortholog, is a member of the immunoglobulin superfamily that functions in lateral adhesion, growth cone guidance, and in reintegrating misoriented cells into epithelial monolayers [64–66]. In wild-type eye discs, Fas2 is strongly upregulated just posterior to the morphogenetic furrow, and is expressed basolaterally in the differentiated ommatidial clusters (Fig 3A). Interestingly, Fas2 expression is lost in scrib- cells in wild type background (Fig 3B), and significantly reduced in scrib-/M (Fig 3C), p35+scrib- (Fig 3D), and RasV12,scrib- (Fig 3F) clones respectively. However, in scrib-,wts- (Fig 3E), wts- (S3A Fig) or UAS RasV12 (S3B Fig) clones, Fas2 expression is disrupted but not downregulated. Overall, these data suggest that loss of scrib is sufficient to downregulate Fas2 likely due to disruption of apical basal polarity and this effect is exaggerated in the different genetic backgrounds that modify the growth potential of scrib mutant cells. The loss of lateral adhesion generally makes cells susceptible to elimination from epithelial monolayers [64], however, the different growth potential of scrib mutant cells that co-express p35 or RasV12 suggests that other factors besides suppression of lateral adhesion or differentiation may contribute to the observed effects. Therefore, we tested if the apical adherence junctions are affected in scrib mutant cells in different genetic backgrounds.
Fig 3. Alterations in Fas2 expression due to loss of scrib.
Eye imaginal discs stained with antibody against Drosophila Fas2 (red, and greyscale in A-F) are shown in somatic clones of the genotypes (A) Wild-type (B) scrib- (C) scrib-/M, (D) p35+scrib-, (E) scrib-,wts-, and (F) RasV12,scrib-. The effect on Fas2 expression, and localization was assayed in clones that were present posterior to the MF where Fas2 is endogenously expressed. The yellow arrows in panels B-F point to the clones depicting changes in Fas2 expression.
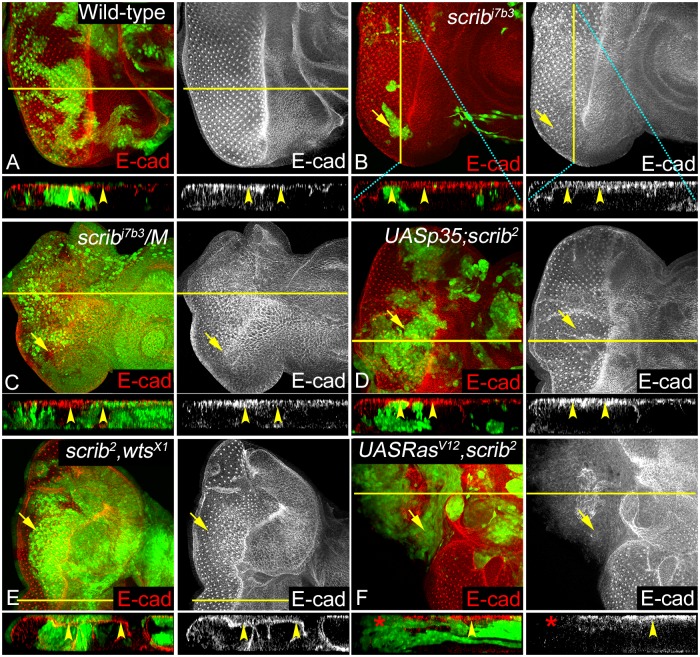
E-Cad localizes to the AJs in epithelial cells, and is often downregulated or mislocalized in cancers [67–71]. In wild-type eye discs, E-Cad was localized normally at the AJs (Fig 4A). Interestingly, in scrib- cells in wild-type background no obvious defect in E-Cad expression is seen (Fig 4B). Similarly, no appreciable change in E-Cad levels or localization (see Z-projections for all genotypes) is seen in scrib-/M (Fig 4C), p35+scrib- (Fig 4D), scrib-,wts- (Fig 4E), wts- (S4A Fig), or UAS RasV12 (S4B Fig) clones that show hyperplastic overgrowth. In contrast, in the RasV12,scrib- clones that show robust tumorigenic potential E-Cad is downregulated (Fig 4F) [30]. Overall, these data show that in all combinations except RasV12,scrib-, E-Cad can localize correctly suggesting that although adhesion is reduced in the different genetic backgrounds that modify the growth potential of scrib mutant cells, the apical AJs may not be severely affected.
Fig 4. Changes in AJ organization in response to scrib loss of function in epithelial cells.
Panels show eye imaginal discs stained with anti-E Cad antibody (red, and greyscale in A-F). Genotypes analyzed include (A) Wild-type (B) scrib- (C) scrib-/M, (D) p35+scrib-, (E) scrib-,wts-, and (F) RasV12,scrib- clones. (A-F) Panels show expression of E-Cad staining in XY plane, clones marked with yellow arrows. In addition, XZ (A, C-F) or YZ (B, marked by cyan line) projections of region highlighted by yellow lines are shown for each genotype to show E-Cad localization. Yellow arrowheads in XZ or YZ projections (A-F) show apical E-Cad localization at the AJs, and red asterisk (F) shows lack of E-Cad in RasV12, scrib- clones.
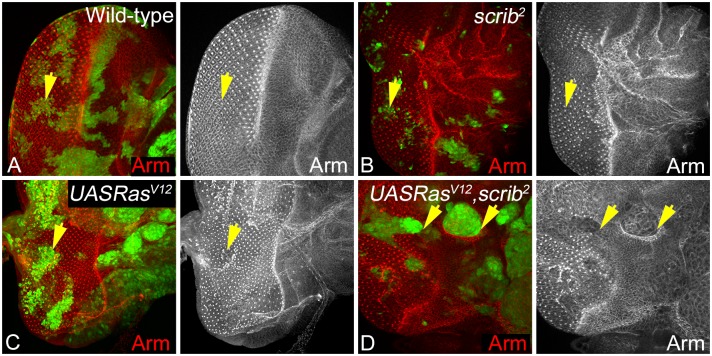
To further confirm our findings, we checked if levels and localization of another AJ protein- Armadillo (Arm) to test if AJs are affected in scrib- or RasV12,scrib- mutant cells (Fig 5). In epithelial cells, Arm is ubiquitously expressed in the cytoplasm and the apical AJs, and anchors them to the actin cytoskeleton [23, 72]. Compared to wild-type (Fig 5A), expression of Arm is mildly disrupted in scrib- clones especially posterior to the MF (Fig 5B). Similarly, in clones overexpressing RasV12 alone Arm expression appears normal (Fig 5C). In contrast, in RasV12,scrib- clones, Arm levels are downregulated and mislocalized from the membrane to the cytoplasm (Fig 5D). These changes in Arm expression and localization are consistent with our earlier observations with E-Cad, confirming that loss of scrib alone does not significantly impact apical junctional complexes. In summary, epithelial integrity of scrib mutant cells is weakened due to disruption of the lateral but not apical cell adhesion in genetic backgrounds like scrib-/M, p35+scrib-, or scrib-,wts- that enhance survival of scrib- cells. However, in RasV12,scrib- clones both apical and translateral adhesion complexes are disrupted suggesting that cell adhesion is severely compromised. Overall, these data suggest that loss of differentiation is tightly correlated to loss of adhesion in RasV12,scrib- but not in other genetic backgrounds like scrib-/M, p35+scrib-, or scrib-,wts- that enhance survival of scrib- cells.
Fig 5. Armadillo expression is disrupted at the AJs during neoplastic growth.
Arm expression and localization (red, and greyscale in A-D) in eye discs containing GFP positive eyFLP MARCM clones of the genotype (A) Wild-type, (B) scrib-, (C) RasV12, and (D) RasV12,scrib- clones (GFP, green) is shown. The yellow arrows in panels A-D highlight mutant cells in different genotypes.
In addition to the reversible association of Arm with the AJs, cytoplasmic pools of Arm are regulated by phosphorylation-based mechanisms by Wingless (Wg) [73], and Jun N-terminal kinase (JNK) signaling pathway [74–76]. Interestingly, polarity regulators (like PAR-1) positively regulate Wnt/β-catenin pathway and inhibit the JNK pathway [77]. Thus, it is possible that downregulation of Arm in RasV12,scrib- clones is linked with increased JNK signaling previously reported in RasV12,scrib- clones. Further, increased JNK signaling is required for growth and invasion in RasV12,scrib- clones [30, 44]. Therefore, we tested levels of JNK signaling in all genetic combinations under study.
JNK activation is not sufficient for Invasive growth of scrib mutant cells
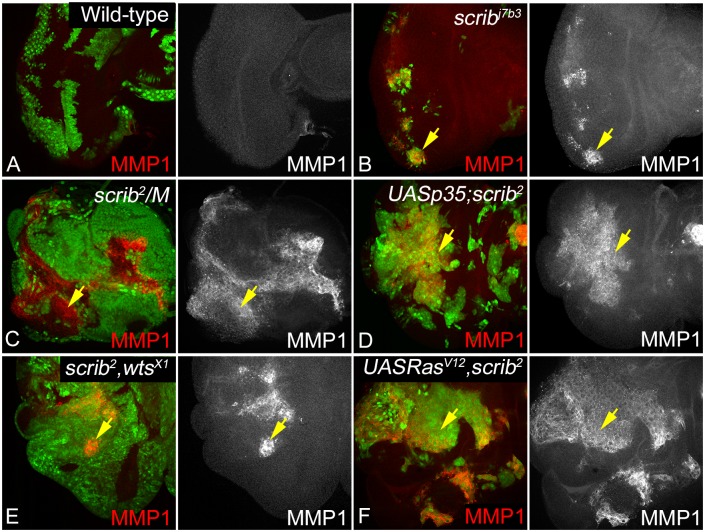
Matrix metalloproteinase-1 (MMP1) is a JNK regulated gene that is a well-described marker of invasion, and is known to be involved in ECM degradation following EMT [78–81]. MMP1 also plays an important role in tissue remodeling and cell migration during development [80, 82]. Loss of scrib is known to induce JNK activity [30], however, do all genetic combinations under study show a similar induction with JNK activity, and if JNK activation was sufficient to confer invasive phenotype remains unclear. We therefore tested expression of MMP1 in the genotypes of our interest (Fig 6). MMP1 is expressed ubiquitously at low levels in wild type cells (Fig 6A). We observed a strong upregulation of MMP1 in all combinations under study, however, the pattern of MMP1 induction is variable. MMP1 is strongly induced in large clones in scrib- (Fig 6B), scrib-/M (Fig 6C), p35+scrib- (Fig 6D), and RasV12,scrib- (Fig 6F); however, MMP1 is induced in patches in scrib-,wts- (Fig 6E) or wts- (S5A Fig) clones. Interestingly, MMP1 is not induced in UAS RasV12 control clones (S5B Fig). Taken together, these data suggest that loss of scrib is sufficient for JNK activation, however, JNK activation by itself does not correlate with tumorigenic growth. It is also interesting to note that MMP1 activation in all genetic combinations except RasV12,scrib- does not co-relate with invasion suggesting that invasive potential of scrib mutant cells is not just dependent on JNK activation but alteration of cell survival due to increased Epidermal Growth Factor/ Mitogen-Activated Protein Kinases (EGFR/MAPK) or Yki activity in case of RasV12 or loss of wts respectively.
Fig 6. Effect of loss of scrib on JNK/MMP1 signaling.
Panels show eye imaginal discs stained for MMP1 (red, greyscale in A-F) from the following genotypes: (A) Wild-type, (B) scrib-, (C) scrib-/M, (D) p35+scrib-, (E) scrib-,wts-, and (F) RasV12,scrib-. Clones in A-B and D-F are GFP positive, and clones in C are GFP-negative, and are marked by yellow arrows.
Discussion
Studies in Drosophila imaginal discs have provided important insights about effects of loss of apical basal polarity on cell proliferation, cell death and cooperative interactions that can lead to tumor growth and progression [1, 19, 20, 26, 27, 83]. In this study, we investigated if a central mechanism that regulates cell adhesion governs the growth and invasive potential of scrib mutant cells; and if this mechanism promotes tumorigenic growth via cooperative interactions. Over the last decade, a number of strategies have been used to study effects of loss of polarity by loss of function mutations in scrib, for example, loss of scrib in Minute background [28], or combining loss of scrib with UASp35 which suppresses cell death [26, 84] or inducing scrib mutant clones in eiger mutant background [28, 84]. All of these manipulations lead to formation of scrib mutant cells that are no longer eliminated, and the range of phenotypes observed by loss of scrib in combination with these mutations is comparable but not identical, suggesting that these genetic modifiers of scrib induce distinct effects on growth and tumorigenesis. To address the shared and distinct effects of loss of scrib in different genetic backgrounds, we compared effects on proliferation, differentiation, cell survival and cell adhesion. Our studies show that reduced cell survival, activation of the JNK pathway and reduced cell adhesion are central to loss of scrib, however, for scrib cells to induce aggressive growth cooperative interactions that derail signaling pathways play an essential role in the mechanisms leading to tumorigenesis (Fig 7).
Fig 7. Models depicting changes in scrib mutant cells in different genetic backgrounds.
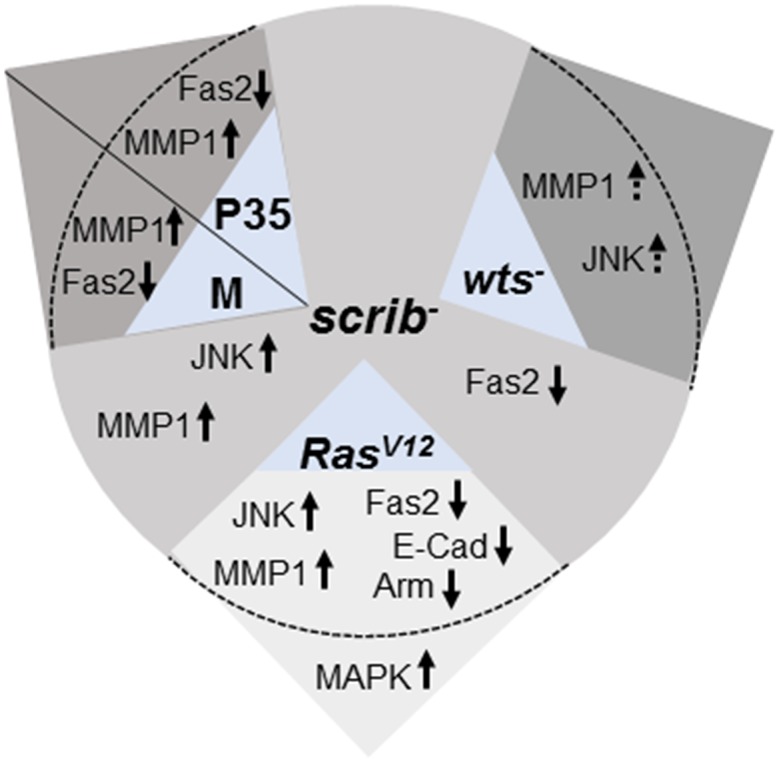
The image shows changes in signaling pathways, and cell adhesion in scrib mutant cells, and changes to these signals in scrib cells combined with different growth modifiers.
We initially compared differences in proliferation and differentiation, and their correlation to cell survival. In Drosophila eye imaginal discs, cell division is very tightly regulated in the differentiating cells posterior to the MF, where cells undergo a G1 arrest as they enter the MF, begin differentiating into the photoreceptor neurons to form the precluster (Fig 1). The cells then go through one round of cell division in the second mitotic wave and arrest in the G1 phase, and all the remaining cells that comprise the ommatidial clusters are differentiated [47]. We found that in scrib mutant clones generated in wild-type background, the cell division profile is largely unperturbed but in all other combinations mutant cells undergo ectopic proliferation and show defects in differentiation with respect to progression of the morphogenetic furrow or differentiation of photoreceptor neurons. Increased proliferation and loss of differentiation is linked to tumor progression, so we next tested if the genetic combinations that promote scrib growth affected differentiation (Fig 2). We found mild defects in morphogenetic furrow progression and spacing of ommatidial clusters in scrib/M cells, and scrib- wts- clones. We found that differentiation is suppressed in two genotypes (p35+scrib-, and RasV12,scrib-) (Fig 2) where increased survival either by upregulation of DIAP1 or suppression of apoptosis by expression of p35 promotes growth. However, p35+scrib- clones do not grow into invasive tumors, suggesting that although loss of differentiation is linked to tumor progression, cells require a potent growth-promoting signal to form aggressive tumors. Furthermore, the differences in phenotype with respect to regulation of furrow progression and differentiation of photoreceptor neurons also shows that these genetic combinations do not share a single molecular mechanism to enhance growth of scrib- cells.
Previous studies have shown that regulation of apical basal polarity and maintenance of junctional integrity plays a critical role in cellular functions and homeostasis [8, 25]. We tested if the genetic combinations that modify scrib growth and survival show any defects with respect to the lateral and apical adhesions formed by the homophilic binding of Fas2, E-Cad or Arm (Fig 7). Interestingly, Fas2 was misregulated in all genotypes, suggesting that loss of scrib resulted in disruption of photoreceptor differentiation and also defects in intercellular adhesion however, the severity of the phenotype is dependent on the modifying mutations. Furthermore, studies in other model systems have established an interesting reciprocal relationship between the regulation of NCAM (Fas2) and E-Cad in the initiation and maintenance of EMT [85]. Reduced levels of NCAM expression is shown to promote tumor dissemination in vivo [85]. NCAMs promote signaling changes in membrane microdomains, and promote interactions at the focal adhesion and AJs [9, 64]. Therefore, we extended our analysis to the stability of AJs (E-Cad, Arm) in the genetic combinations that promote growth of scrib mutant cells.
Consistent with previous data, where knockdown of scrib in wing discs caused no polarity defect [86], we found that loss of scrib in wild type or in M background did not result in loss of apical AJ proteins like E-Cad or Arm, suggesting that junctional organization is not immediately lost in these genetic combinations. Similarly, loss of scrib in combination with wts did not cause polarity defects. Comparison of AJ markers like E-Cad and Arm in p35+scrib-, and RasV12,scrib- sheds some light on the changes that may be critical for induction of invasive tumors. We found that both genotypes show loss of differentiation but adhesion is lost more severely in RasV12,scrib- mutant cells suggesting that apical basal polarity and cell adhesion are lost only in this genotype.
It is interesting to note that several key changes occur in p35+scrib- cells, for example, defects in differentiation, loss of Fas2 and activation of MMP1, but these clones fail to induce robustly growing tumors. One reason for this may be that changes in cell adhesion in a cell where apoptotic signals are induced by activity of caspases, differ fundamentally from the changes that occur due to oncogenic cooperation. Normally, apoptotic cells show several key changes like condensation of the cytoplasm, breakdown of nuclear integrity, cell rounding, membrane blebbing, and in epithelial cells the loss of cell polarity and cell junctions [87, 88]. These changes are caused by cleavage of key proteins by caspases [89]. A key early change in the apoptotic cell is the loss of contact and extrusion of these cells from the epithelium by neighboring cells. Since the integrity of the epithelial cells depends on the cadherin-catenin mediated establishment of the apical AJs, it is not surprising that several of these junctional proteins are targets of caspase activity during apoptosis. For example, the Drosophila Caspase 3 Drice targets Armadillo for cleavage, which is responsible for the loss of DE-Cad from cell junctions and thus might contribute to the degeneration of epithelial integrity during apoptosis [90]. Our data with p35+scrib- shows that expression of the caspase inhibitor prevents activation of caspases, thereby, causing no obvious change in the expression of E-Cad or Arm, which may be important for cells to change their signaling behavior and show robust tumorigenic growth. Taken together, our data show that loss of apical basal polarity and cell adhesion is critical for progression of scrib mutant cells to tumors. In addition, increased signaling from growth promoting pathways (Yki, TGF-β [Transforming growth factor beta], MAPK etc) synergistically contribute to tumor growth and progression.
We found that MMP1- a JNK regulated gene and marker for tumor invasion was induced in all genetic combinations under study (Figs 6 and 7). This was an interesting finding as scrib/M or p35+scrib- or scrib- wts- clones show induction of MMP1, and an increase in clone size but do not show signature changes of aggressive neoplastic tumors. Thus, JNK activation is clearly not sufficient but required for the changes that confer tumorigenic potential to scrib mutant cells. It is thought that in RasV12,scrib- clones, JNK undergoes a paradoxical switch from pro-apoptotic to pro-proliferation signal by modifying Yki activity via inactivation of Wts by F-actin mediated activation of Ajuba [91]. However, direct inactivation of wts in scrib mutant cells shows a phenotype that is qualitatively different where clones show hyperplastic growth, and there is no loss of cell adhesion. Thus, oncogenic Ras specifically contributes to tumorigenesis by activation of MAPK and Yki that synergistically cause tumor growth and progression. Alternatively, AJs are disrupted only when certain clonal mass is achieved in scrib- cells due to additional mutations such as RasV12. In summary, our studies show that some changes are caused by loss of scrib and are therefore shared in all genotypes (e.g., effect on Fas2, MMP1) but other defects are contributed by the modifying mutations, which synergistically interact and modify the phenotype. In all genetic combinations where one or more of these critical changes do not occur show improved survival of scrib mutant cells, but not highly proliferative and invasive tumors. Overall our data show that loss of apical basal polarity and cell adhesion are critical for progression of scrib mutant cells to tumors. In addition, increased signaling from growth promoting pathways (Yki, TGFβ, MAPK, etc.) may synergistically contribute to tumor growth and progression.
Supporting Information
Panels show ELAV (red, greyscale) expression in somatic clones (GFP, green) (A) wts- loss of function, or (B) overexpression of RasV12 in eye discs. ELAV staining within the clones is highlighted in yellow arrows.
(TIF)
Panels show DIAP1 expression (Red, greyscale) in eye discs containing clones (GFP, green) of the following genotypes (A) wild-type, (B) scrib-, (C) scrib-/M, (D) p35+scrib-, (E) scrib-,wts- and (F) RasV12,scrib- clones. Note that scrib-/M clones are marked by loss of GFP. DIAP1 expression in clones of the indicated genotypes is marked with yellow arrows.
(TIF)
Eye imaginal discs showing Fas 2 expression (red, greyscale) in clones (GFP, green) of the genotype (A) wts-, and (B) RasV12 are depicted. Note that clones located posterior to morphogenetic furrow (yellow arrows) are relevant for comparing changes in Fas2 expression.
(TIF)
E-Cad (red, greyscale) expression and localization in (A) wts-, and (B) RasV12 clones (GFP, green) is shown. Panels show cross sections (of regions corresponding to yellow lines) to highlight E-Cad localization, and expression (yellow arrowheads). Cyan lines in A show the orientation of the YZ projection. Yellow arrows highlight E-Cad levels in appropriate clones.
(TIF)
Panels show eye discs containing eyFLP MARCM clones (marked by yellow arrows) (GFP, green) of the genotype (A) wts-, and (B) RasV12 stained for MMP1 (red, greyscale).
(TIF)
Acknowledgments
The authors would like to thank the Bloomington Drosophila Stock Center (Indiana), the Drosophila Genetics Resource Center (Japan) for flies and, the Developmental Studies Hybridoma Bank for antibodies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by Start Up research support to MK-S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tepass U. The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu Rev Cell Dev Biol. 2012;28:655–85. 10.1146/annurev-cellbio-092910-154033 . [DOI] [PubMed] [Google Scholar]
- 2.Ellenbroek SI, Iden S, Collard JG. Cell polarity proteins and cancer. Seminars in cancer biology. 2012;22(3):208–15. 10.1016/j.semcancer.2012.02.012 . [DOI] [PubMed] [Google Scholar]
- 3.Adachi-Yamada T, O'Connor MB. Morphogenetic apoptosis: a mechanism for correcting discontinuities in morphogen gradients. Dev Biol. 2002;251(1):74–90. . [DOI] [PubMed] [Google Scholar]
- 4.Tamori Y, Deng WM. Cell competition and its implications for development and cancer. J Genet Genomics. 2011;38(10):483–95. Epub 2011/11/01. S1673-8527(11)00167-6 [pii] 10.1016/j.jgg.2011.09.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wodarz A. Establishing cell polarity in development. Nat Cell Biol. 2002;4(2):E39–44. 10.1038/ncb0202-e39 . [DOI] [PubMed] [Google Scholar]
- 6.Igaki T. Correcting developmental errors by apoptosis: lessons from Drosophila JNK signaling. Apoptosis. 2009;14(8):1021–8. Epub 2009/05/26. 10.1007/s10495-009-0361-7 . [DOI] [PubMed] [Google Scholar]
- 7.Cordero JB, Macagno JP, Stefanatos RK, Strathdee KE, Cagan RL, Vidal M. Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev Cell. 2010;18(6):999–1011. Epub 2010/07/16. S1534-5807(10)00250-9 [pii] 10.1016/j.devcel.2010.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson MC, Perrimon N. Apicobasal polarization: epithelial form and function. Curr Opin Cell Biol. 2003;15(6):747–52. . [DOI] [PubMed] [Google Scholar]
- 9.Bergstralh DT, St Johnston D. Epithelial cell polarity: what flies can teach us about cancer. Essays Biochem. 2012;53:129–40. 10.1042/bse0530129 . [DOI] [PubMed] [Google Scholar]
- 10.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Goswami S, Sahai E, Wyckoff JB, Segall JE, Condeelis JS. Tumor cells caught in the act of invading: their strategy for enhanced cell motility. Trends Cell Biol. 2005;15(3):138–45. . [DOI] [PubMed] [Google Scholar]
- 12.Pottier C, Wheatherspoon A, Roncarati P, Longuespee R, Herfs M, Duray A, et al. The importance of the tumor microenvironment in the therapeutic management of cancer. Expert Rev Anticancer Ther. 2015;15(8):943–54. 10.1586/14737140.2015.1059279 . [DOI] [PubMed] [Google Scholar]
- 13.Parisi F, Vidal M. Epithelial delamination and migration: lessons from Drosophila. Cell Adh Migr. 2011;5(4):366–72. Epub 2011/08/13. 17524 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778(3):614–30. 10.1016/j.bbamem.2007.08.029 . [DOI] [PubMed] [Google Scholar]
- 15.Flores-Benitez D, Knust E. Dynamics of epithelial cell polarity in Drosophila: how to regulate the regulators? Curr Opin Cell Biol. 2016;42:13–21. 10.1016/j.ceb.2016.03.018 . [DOI] [PubMed] [Google Scholar]
- 16.Mechler BM, McGinnis W, Gehring WJ. Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophila melanogaster. Embo J. 1985;4(6):1551–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289(5476):113–6. . [DOI] [PubMed] [Google Scholar]
- 18.Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403(6770):676–80. . [DOI] [PubMed] [Google Scholar]
- 19.Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18(16):1909–25. . [DOI] [PubMed] [Google Scholar]
- 20.Kango-Singh M, Halder G. Drosophila as an emerging model to study metastasis. Genome Biol. 2004;5(4):216 Epub 2004/04/03. 10.1186/gb-2004-5-4-216 gb-2004-5-4-216 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wirtz-Peitz F, Zallen JA. Junctional trafficking and epithelial morphogenesis. Curr Opin Genet Dev. 2009;19(4):350–6. 10.1016/j.gde.2009.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ragkousi K, Gibson MC. Cell division and the maintenance of epithelial order. J Cell Biol. 2014;207(2):181–8. 10.1083/jcb.201408044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31(12):2714–36. 10.1038/emboj.2012.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goehring NW, Grill SW. Cell polarity: mechanochemical patterning. Trends Cell Biol. 2013;23(2):72–80. 10.1016/j.tcb.2012.10.009 . [DOI] [PubMed] [Google Scholar]
- 25.West JJ, Harris TJ. Cadherin trafficking for tissue morphogenesis: control and consequences. Traffic. 2016. 10.1111/tra.12407 . [DOI] [PubMed] [Google Scholar]
- 26.Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. Embo J. 2003;22(21):5769–79. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302(5648):1227–31. . [DOI] [PubMed] [Google Scholar]
- 28.Chen CL, Schroeder MC, Kango-Singh M, Tao C, Halder G. Tumor suppression by cell competition through regulation of the Hippo pathway. Proc Natl Acad Sci U S A. 2012;109(2):484–9. Epub 2011/12/23. 1113882109 [pii] 10.1073/pnas.1113882109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verghese S, Waghmare I, Kwon H, Hanes K, Kango-Singh M. Scribble acts in the Drosophila fat-hippo pathway to regulate warts activity. PLoS One. 2012;7(11):e47173 Epub 2012/11/13. 10.1371/journal.pone.0047173 PONE-D-12-25889 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Igaki T, Pagliarini RA, Xu T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr Biol. 2006;16(11):1139–46. . [DOI] [PubMed] [Google Scholar]
- 31.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–4. [DOI] [PubMed] [Google Scholar]
- 32.Morata G, Ripoll P. Minutes: mutants of drosophila autonomously affecting cell division rate. Dev Biol. 1975;42(2):211–21. . [DOI] [PubMed] [Google Scholar]
- 33.Prober DA, Edgar BA. Ras1 promotes cellular growth in the Drosophila wing. Cell. 2000;100(4):435–46. . [DOI] [PubMed] [Google Scholar]
- 34.Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120(8):2121–9. . [DOI] [PubMed] [Google Scholar]
- 35.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumour suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–63. [DOI] [PubMed] [Google Scholar]
- 36.Karim FD, Rubin GM. Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development. 1998;125(1):1–9. . [DOI] [PubMed] [Google Scholar]
- 37.Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, Tao C, et al. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci U S A. 2010;107(36):15810–5. Epub 2010/08/28. 1004060107 [pii] 10.1073/pnas.1004060107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol. 2010;20(7):573–81. Epub 2010/04/07. S0960-9822(10)00151-X [pii] 10.1016/j.cub.2010.01.055 . [DOI] [PubMed] [Google Scholar]
- 39.Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr Biol. 2010;20(7):582–90. Epub 2010/04/07. S0960-9822(10)00338-6 [pii] 10.1016/j.cub.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, et al. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci U S A. 2010;107(23):10532–7. Epub 2010/05/26. 1004279107 [pii] 10.1073/pnas.1004279107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsons LM, Grzeschik NA, Allott ML, Richardson HE. Lgl/aPKC and Crb regulate the Salvador/Warts/Hippo pathway. Fly (Austin). 2010;4(4):288–93. Epub 2010/08/28. 13116 [pii] 10.1016/j.cub.2010.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doggett K, Grusche FA, Richardson HE, Brumby AM. Loss of the Drosophila cell polarity regulator Scribbled promotes epithelial tissue overgrowth and cooperation with oncogenic Ras-Raf through impaired Hippo pathway signaling. BMC Dev Biol. 2011;11:57 Epub 2011/10/01. 1471-213X-11-57 [pii] 10.1186/1471-213X-11-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skouloudaki K, Puetz M, Simons M, Courbard JR, Boehlke C, Hartleben B, et al. Scribble participates in Hippo signaling and is required for normal zebrafish pronephros development. Proc Natl Acad Sci U S A. 2009;106(21):8579–84. Epub 2009/05/15. 0811691106 [pii] 10.1073/pnas.0811691106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enomoto M, Igaki T. Deciphering tumor-suppressor signaling in flies: genetic link between Scribble/Dlg/Lgl and the Hippo pathways. J Genet Genomics. 2011;38(10):461–70. Epub 2011/11/01. S1673-8527(11)00166-4 [pii] 10.1016/j.jgg.2011.09.005 . [DOI] [PubMed] [Google Scholar]
- 45.Shibata K, Inagaki M, Ajiro K. Mitosis-specific histone H3 phosphorylation in vitro in nucleosome structures. Eur J Biochem. 1990;192(1):87–93. . [DOI] [PubMed] [Google Scholar]
- 46.Su TT, Sprenger F, DiGregorio PJ, Campbell SD, O'Farrell PH. Exit from mitosis in Drosophila syncytial embryos requires proteolysis and cyclin degradation, and is associated with localized dephosphorylation. Genes Dev. 1998;12(10):1495–503. Epub 1998/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker NE. Cell proliferation, survival, and death in the Drosophila eye. Semin Cell Dev Biol. 2001;12(6):499–507. . [DOI] [PubMed] [Google Scholar]
- 48.Wolff T, Ready DF. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development. 1991;113(3):841–50. . [DOI] [PubMed] [Google Scholar]
- 49.Tapon N, Harvey K, Bell D, Wahrer D, Schiripo T, Haber D, et al. salvador Promotes Both Cell Cycle Exit and Apoptosis in Drosophila and Is Mutated in Human Cancer Cell Lines. Cell. 2002;110(4):467 . [DOI] [PubMed] [Google Scholar]
- 50.Jogi A, Vaapil M, Johansson M, Pahlman S. Cancer cell differentiation heterogeneity and aggressive behavior in solid tumors. Ups J Med Sci. 2012;117(2):217–24. 10.3109/03009734.2012.659294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, Thomas G. Drosophila S6 kinase: a regulator of cell size. Science. 1999;285(5436):2126–9. . [DOI] [PubMed] [Google Scholar]
- 52.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122(3):421–34. . [DOI] [PubMed] [Google Scholar]
- 53.Oldham S, Montagne J, Radimerski T, Thomas G, Hafen E. Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev. 2000;14(21):2689–94. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 2000;14(21):2712–24. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinow S, White K. The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol. 1988;126(2):294–303. . [DOI] [PubMed] [Google Scholar]
- 56.Wang SL, Hawkins CJ, Yoo SJ, Muller HA, Hay BA. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell. 1999;98(4):453–63. . [DOI] [PubMed] [Google Scholar]
- 57.Igaki T, Yamamoto-Goto Y, Tokushige N, Kanda H, Miura M. Down-regulation of DIAP1 triggers a novel Drosophila cell death pathway mediated by Dark and DRONC. J Biol Chem. 2002;277(26):23103–6. Epub 2002/05/16. 10.1074/jbc.C200222200 C200222200 [pii] . [DOI] [PubMed] [Google Scholar]
- 58.Muro I, Hay BA, Clem RJ. The Drosophila DIAP1 protein is required to prevent accumulation of a continuously generated, processed form of the apical caspase DRONC. J Biol Chem. 2002;277(51):49644–50. Epub 2002/10/25. 10.1074/jbc.M203464200 M203464200 [pii] . [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez A, Chen P, Oliver H, Abrams JM. Unrestrained caspase-dependent cell death caused by loss of Diap1 function requires the Drosophila Apaf-1 homolog, Dark. EMBO J. 2002;21(9):2189–97. Epub 2002/05/01. 10.1093/emboj/21.9.2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryoo HD, Bergmann A, Gonen H, Ciechanover A, Steller H. Regulation of Drosophila IAP1 degradation and apoptosis by reaper and ubcD1. Nat Cell Biol. 2002;4(6):432–8. . [DOI] [PubMed] [Google Scholar]
- 61.Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7(4):491–501. Epub 2004/10/08. S1534580704003247 [pii] 10.1016/j.devcel.2004.08.019 . [DOI] [PubMed] [Google Scholar]
- 62.Betz A, Ryoo HD, Steller H, Darnell JE Jr. STAT92E is a positive regulator of Drosophila inhibitor of apoptosis 1 (DIAP/1) and protects against radiation-induced apoptosis. Proc Natl Acad Sci U S A. 2008;105(37):13805–10. Epub 2008/09/10. 0806291105 [pii] 10.1073/pnas.0806291105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLachlan RW, Yap AS. Not so simple: the complexity of phosphotyrosine signaling at cadherin adhesive contacts. J Mol Med (Berl). 2007;85(6):545–54. 10.1007/s00109-007-0198-x . [DOI] [PubMed] [Google Scholar]
- 64.Bergstralh DT, Lovegrove HE, St Johnston D. Lateral adhesion drives reintegration of misplaced cells into epithelial monolayers. Nat Cell Biol. 2015;17(11):1497–503. 10.1038/ncb3248 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szafranski P, Goode S. A Fasciclin 2 morphogenetic switch organizes epithelial cell cluster polarity and motility. Development. 2004;131(9):2023–36. 10.1242/dev.01097 . [DOI] [PubMed] [Google Scholar]
- 66.Harrelson AL, Goodman CS. Growth cone guidance in insects: fasciclin II is a member of the immunoglobulin superfamily. Science. 1988;242(4879):700–8. . [DOI] [PubMed] [Google Scholar]
- 67.Peifer M. Cancer, catenins, and cuticle pattern: a complex connection. Science. 1993;262(5140):1667–8. . [DOI] [PubMed] [Google Scholar]
- 68.Niessen CM, Leckband D, Yap AS. Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol Rev. 2011;91(2):691–731. 10.1152/physrev.00004.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11(7):502–14. 10.1038/nrm2927 . [DOI] [PubMed] [Google Scholar]
- 70.Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol. 2014;15(6):397–410. 10.1038/nrm3802 . [DOI] [PubMed] [Google Scholar]
- 71.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28(1–2):151–66. 10.1007/s10555-008-9179-y . [DOI] [PubMed] [Google Scholar]
- 72.Peifer M. The product of the Drosophila segment polarity gene armadillo is part of a multi-protein complex resembling the vertebrate adherens junction. J Cell Sci. 1993;105 (Pt 4):993–1000. . [DOI] [PubMed] [Google Scholar]
- 73.Bienz M. beta-Catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol. 2005;15(2):R64–7. 10.1016/j.cub.2004.12.058 . [DOI] [PubMed] [Google Scholar]
- 74.Jemc JC, Milutinovich AB, Weyers JJ, Takeda Y, Van Doren M. raw Functions through JNK signaling and cadherin-based adhesion to regulate Drosophila gonad morphogenesis. Dev Biol. 2012;367(2):114–25. 10.1016/j.ydbio.2012.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McEwen DG, Cox RT, Peifer M. The canonical Wg and JNK signaling cascades collaborate to promote both dorsal closure and ventral patterning. Development. 2000;127(16):3607–17. . [DOI] [PubMed] [Google Scholar]
- 76.McEwen DG, Peifer M. Wnt signaling: Moving in a new direction. Curr Biol. 2000;10(15):R562–4. . [DOI] [PubMed] [Google Scholar]
- 77.Sun TQ, Lu B, Feng JJ, Reinhard C, Jan YN, Fantl WJ, et al. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat Cell Biol. 2001;3(7):628–36. 10.1038/35083016 . [DOI] [PubMed] [Google Scholar]
- 78.Page-McCaw A, Serano J, Sante JM, Rubin GM. Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev Cell. 2003;4(1):95–106. . [DOI] [PubMed] [Google Scholar]
- 79.Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006;25(22):5294–304. 10.1038/sj.emboj.7601401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc Natl Acad Sci U S A. 2007;104(8):2721–6. Epub 2007/02/16. 0611666104 [pii] 10.1073/pnas.0611666104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beaucher M, Hersperger E, Page-McCaw A, Shearn A. Metastatic ability of Drosophila tumors depends on MMP activity. Dev Biol. 2007;303(2):625–34. 10.1016/j.ydbio.2006.12.001 . [DOI] [PubMed] [Google Scholar]
- 82.Glasheen BM, Robbins RM, Piette C, Beitel GJ, Page-McCaw A. A matrix metalloproteinase mediates airway remodeling in Drosophila. Dev Biol. 2010;344(2):772–83. 10.1016/j.ydbio.2010.05.504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brumby AM, Richardson HE. Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer. 2005;5(8):626–39. . [DOI] [PubMed] [Google Scholar]
- 84.Igaki T, Pastor-Pareja JC, Aonuma H, Miura M, Xu T. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev Cell. 2009;16(3):458–65. Epub 2009/03/18. S1534-5807(09)00030-6 [pii] 10.1016/j.devcel.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perl AK, Dahl U, Wilgenbus P, Cremer H, Semb H, Christofori G. Reduced expression of neural cell adhesion molecule induces metastatic dissemination of pancreatic beta tumor cells. Nat Med. 1999;5(3):286–91. 10.1038/6502 . [DOI] [PubMed] [Google Scholar]
- 86.Nakajima Y, Meyer EJ, Kroesen A, McKinney SA, Gibson MC. Epithelial junctions maintain tissue architecture by directing planar spindle orientation. Nature. 2013;500(7462):359–62. 10.1038/nature12335 . [DOI] [PubMed] [Google Scholar]
- 87.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. . [DOI] [PubMed] [Google Scholar]
- 88.Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3(4):339–45. 10.1038/35070009 . [DOI] [PubMed] [Google Scholar]
- 89.Thornberry NA. Caspases: key mediators of apoptosis. Chem Biol. 1998;5(5):R97–103. . [DOI] [PubMed] [Google Scholar]
- 90.Kessler T, Muller HA. Cleavage of Armadillo/beta-catenin by the caspase DrICE in Drosophila apoptotic epithelial cells. BMC Dev Biol. 2009;9:15 10.1186/1471-213X-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Enomoto M, Kizawa D, Ohsawa S, Igaki T. JNK signaling is converted from anti- to pro-tumor pathway by Ras-mediated switch of Warts activity. Dev Biol. 2015;403(2):162–71. 10.1016/j.ydbio.2015.05.001 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Panels show ELAV (red, greyscale) expression in somatic clones (GFP, green) (A) wts- loss of function, or (B) overexpression of RasV12 in eye discs. ELAV staining within the clones is highlighted in yellow arrows.
(TIF)
Panels show DIAP1 expression (Red, greyscale) in eye discs containing clones (GFP, green) of the following genotypes (A) wild-type, (B) scrib-, (C) scrib-/M, (D) p35+scrib-, (E) scrib-,wts- and (F) RasV12,scrib- clones. Note that scrib-/M clones are marked by loss of GFP. DIAP1 expression in clones of the indicated genotypes is marked with yellow arrows.
(TIF)
Eye imaginal discs showing Fas 2 expression (red, greyscale) in clones (GFP, green) of the genotype (A) wts-, and (B) RasV12 are depicted. Note that clones located posterior to morphogenetic furrow (yellow arrows) are relevant for comparing changes in Fas2 expression.
(TIF)
E-Cad (red, greyscale) expression and localization in (A) wts-, and (B) RasV12 clones (GFP, green) is shown. Panels show cross sections (of regions corresponding to yellow lines) to highlight E-Cad localization, and expression (yellow arrowheads). Cyan lines in A show the orientation of the YZ projection. Yellow arrows highlight E-Cad levels in appropriate clones.
(TIF)
Panels show eye discs containing eyFLP MARCM clones (marked by yellow arrows) (GFP, green) of the genotype (A) wts-, and (B) RasV12 stained for MMP1 (red, greyscale).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.