Abstract
Background
Psoriasis is a long-term immune-mediated inflammatory disorder mainly, but not only, affecting skin, and is associated with significant medical and psychological morbidity. Evidence suggests that sleep is disrupted in psoriasis, however high quality empirical evidence is lacking. Given the importance of sleep for health, characterisation of sleep disruption in psoriasis is an important goal. We therefore conducted a systematic review of the sleep-psoriasis literature.
Methods
Searches were conducted in Pubmed, SCOPUS and Web of Science from inception to May 2016. Studies were compared against inclusion/exclusion criteria and underwent a quality evaluation. Given the heterogeneity of studies, we conducted a narrative synthesis of the findings.
Results
Searches revealed 32 studies which met our predetermined inclusion/exclusion criteria. Whilst 93.7% of studies reported sleep disruption in this population, ranging from 0.05% to 85.4%, many had important methodological shortcomings. Over half of all quantitative studies (54.8%; 17/31) relied on non-validated measures, contributing to heterogeneity in study findings. In those that employed valid measures, assessing sleep was often not the primary objective. We frequently found the absence of adequate sample size calculations and poor statistical reporting.
Conclusion
This review showed that in psoriasis, reported sleep rates of sleep disturbance varied substantially. Most studies lacked a hypothesis driven research question and/or failed to use validated measures of sleep. We were unable to draw firm conclusions about the precise prevalence and nature of sleep disturbance within the psoriasis population. We offer suggestions to help advance understanding of sleep disturbance in psoriasis.
Introduction
Psoriasis is a long-term, immune mediated inflammatory skin disorder, characterised by scaly plaques on knees, elbows and scalp, but any skin surface may be affected [1]. Population-based cohort studies indicate that psoriasis is associated with other systemic inflammatory conditions, including: psoriatic arthritis (PsA) [2]; inflammatory bowel disease [3]; cardiovascular disease (CVD) [4]; and diabetes [5].
Contemporary evidence suggests that sleep is important for daytime functioning and health, [6–8] subserving optimal physiological [9] and psychological functioning [10–13]. Moreover, disturbed sleep may causally drive disease processes. For example, persistent sleep disturbance is a risk factor for the future development of diabetes [14], CVD [15, 16], hypertension [17,18], and depression [19–22]. Several studies [23–38], including four reviews, have examined sleep domains in psoriasis populations. One review evaluated factors linked to sleep disturbance in psoriasis, concluding that mood, obstructive sleep apnoea (OSA), itch and pain as possible sources of sleep disturbance [29]. Another review provided an overview of the sleep and dermatology literature with a focus on the role of itch, suggesting skin temperature, circadian rhythm and psychological factors, such as depression, influenced itch, which in turn disrupted sleep [30]. Similarly, a further review assessed the associations between sleep disorders and the broad category of skin disorders [31]. More recently, Gupta et al. conducted a systematic review of psoriasis and sleep disorders and found an increased prevalence of OSA and restless leg but inconclusive evidence of increased insomnia risk [32]. Whilst these reviews provide some insight into the nature and predictors of sleep in psoriasis the majority are narrative and none critically examine the measures used to assess sleep nor do they assess the quality of current research. Additionally the most recent review [32] only included papers where a sleep disorder diagnosis was made, thus excluding studies where sleep was assessed without a diagnosis, potentially missing research where sleep was assessed but no diagnosis was made. Accordingly, a more rigorous and nuanced appraisal of the sleep-psoriasis literature is required. We present a systematic review focusing on (1) the evidence for sleep disturbance in psoriasis and (2) the quality of measures and methodology employed to assess sleep in patients with psoriasis. We conclude by outlining a research agenda aimed at advancing understanding of sleep disturbance in the context of psoriasis.
Method
Search criteria
PubMed, SCOPUS and Web of Science databases were searched from inception to May 2016. We used the PICOS framework (Population, Interventions, Comparators/Control, Outcomes, Study design) [33] however the intervention category could not be applied due to poor reporting and/or too much variation in study design. In order to increase the sensitivity of the search, search terms were kept broad and were combined using ‘AND’ plus wildcard operators (*) and MeSH terms (Table 1). Studies were assessed against inclusion/exclusion criteria determined by an expert group of reviewers (Table 2). Reference lists of eligible studies were forward and backward searched for completeness. Reviews were included to identify additional studies that were not identified via database searches.
Table 1. Search terms used across databases.
| 1. Sleep /OR sleep disruption /OR sleep disturbance /OR sleep fragmentation |
| 2. Psoriasis/ OR chronic plaque psoriasis/ OR psori* |
| 3. Circadian*/ OR circadian rhythm |
| 4. 1 OR 3 |
| 5. 4 AND 2 |
Table 2. Inclusion criteria for studies.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Partial or whole psoriasis sample (including any subtype of psoriasis or psoriatic arthritis) | 1. Not peer reviewed |
| 2. Sleep was measured either directly using a valid measure of sleep, unvalid measure of sleep or by-proxy via another measure | 2. Animal studies |
| 3. Any study design | |
| 4. Any age group | |
| 5. Published in English | |
| 6. Accessible in full text |
Data extraction
Data extracted from studies included: authors, title, year, study aim(s), journal, study design, type of psoriasis, sample size, measure of sleep used and findings relating to sleep (Tables 3–5).
Table 3. Information (i.e. aim, study design, primary focus of the study, measure of sleep used, sample, pertinent results relating to sleep) and quality scores for each study using a validated measure of sleep included in the review.
| Type of measure of sleep | Study | Aim | Study Design | Primary focus on sleep | Measure of Sleep | Participants | Results pertaining to sleep | Quality Score | Quality Percentage | Quality Decision |
|---|---|---|---|---|---|---|---|---|---|---|
| Validated subjective | Gezer et al., 2014 | Determine the effects of PsA on sleep quality and associations between sleep, QoL and psychological state in PsA | Case-control study | Yes | PSQI | 41 patients with psoriatic arthritis and 38 healthy controls | Subjective SQ, SOL, SD, SE, sleep disturbance, daytime dysfunction and total PSQI scores were all significantly higher (worse) in PsA patients (9.70 ± 3.90) compared to controls (4.05 ± 1.85) (P < 0.05) | 26/34 | 76.5 | fair |
| Validated subjective | Stinco et al., 2013 | Investigate the influence of Psoriasis on sleep | Case-control study | Yes | PSQI | 202 patients with psoriasis and 202 healthy controls | No significant difference in PSQI score between psoriasis patients (5.56 ± 3.93) and controls (5.13 ± 4.16) (P > 0.05). | 25/34 | 73.5 | fair |
| Validated subjective | Balta et al., 2015 | To investigate sleep quality, general psychiatric symptoms and coping strategies in psoriasis | Case-control study | Yes | PSQI | 37 patients with psoriasis and 42 control subjects | Significant differences in subjective sleep quality between psoriasis patients (1.48 ± 0.17) and controls (1.02 ± 0.13) (P < 0.05) and in habitual sleep efficiency between psoriasis patients (0.79 ± 0.19) and controls (0.32 ± 0.14) (P < 0.05). | 24/34 | 73.5 | fair |
| Validated subjective | Shutty et al., 2013 | Measure prevalence of sleep disturbance in psoriasis | Case control study | Yes | PSQI, ISI, ESS | 35 patients with psoriasis and 44 controls | PSQI scores higher in psoriasis patients (8.8 ± 4.4) than controls (6.3 ± 4.4) (P < 0.05). ISI scores significantly higher for psoriasis patients (11.0 ± 7.0) than controls (6.3 ± 6.0). (P < 0.05). ESS scores were not significantly different. | 25/34 | 73.5 | fair |
| Validated subjective | Ljosaa et al., 2012 | Investigate the association between skin pain/discomfort and HRQoL and to explore whether sleep disturbance is a mediator of this relationship | Cross-sectional study | No | GSDS | 139 patients with psoriasis | Mean GSDS score = 52.8, with the highest levels being reported in the pain group (66.6). Sleep emerged as a partial mediator for the association between skin pain and HRQoL | 28/30 | 93.3 | good |
| Validated subjective | Mrowietz et al., 2014 | Characterize the extent of pruritus in moderate to severe psoriasis, and its association with QoL, pre and post exposure to varied doses of etanercept | RCT—post hoc analysis | No | MOS-SS | 270 patients with plaque psoriasis | Levels of pruritus were significantly associated with MOS-SS scores (p < 0.05) (no pruritus = 22.17; mild-moderate = 29.60; severe = 37.52) | 31/36 | 86.1 | good |
| Validated subjective | Strober et al., 2012 | Describe baseline sleep disturbance in psoriasis, factors associated with sleep disturbance, assess the impact of adalimumab on psoriasis and the correlation between sleep outcomes following treatment | Uncontrolled clinical trial | yes (but an embedded study) | MOS-SS | 152 patients with psoriasis | Poor sleep was associated with lower DLQI scores at baseline (P < 0.05). Depression and PsA were significantly associated with daytime somnolence and sleep adequacy respectively. Following treatment, sleep improved at a MCID (30% improvement for sleep disturbance, 32.2% for daytime somnolence and 37% for perceived sleep adequacy) | 19/30 | 63.3 | fair |
| Validated sleep questionnaire | Zachariae et al., 2008 | Validate a sensory and affective approach to understanding pruritus and explore associations between pruritus, psychological symptoms and perceived impairment of pruritus QoL. Evaluate the role of sleep disturbance as a mediator between pruritus, psychological symptoms and QoL. | Cross-sectional study | No | SQQ (3 items from PSQI) | 40 patients with psoriasis | Impaired sleep quality partially mediated the association between itch severity and psychological symptoms. | 24/30 | 80 | good |
| Validated subjective | Thaci et al., 2014 | Evaluate the safety and efficacy of etanercept alongside topical treatments on psoriasis | RCT | No | MOS-SS | 270 patients with psoriasis | Mean baseline scores indicated sleep impairment. At baseline, somnolence and symptoms of OSA were 37% and 39% worse than the pop. norm. At follow up these improved to within 10% of the pop. norm. Sleep adequacy was 10% worse, improving to within 1% at follow-up. Sleep disturbance improved from 43% worse to 9%. | 28/36 | 77.8 | fair |
| Validated objective | Buslau & Bentomane., 1999 | Assess the prevalence of OSA in psoriasis | Case control study | Yes | Polysomnography | 25 patients with psoriasis and 19 matched controls with chronic bronchitis | OSA occurred at greater rates in psoriasis patients compared to those with chronic bronchitis (AHI: 14.4 vs 8.8) | 20/34 | 58.8 | poor |
| Validated objective | Maari et al., 2014 | Assess the efficacy of adalimumab on sleeping parameters in patients with psoriasis and OSA | RCT | Yes | Polysomnography | 20 patients with psoriasis and OSA | No significant difference between adalimumab and placebo groups from baseline to follow-up on AHI, SOL, SE, TWT, FOSQ, ESS or daytime SOL (p > 0.05). | 30/40 | 75 | fair |
| Validated objective | Papadavid et al., 2013 | Determine the association between psoriasis and OSA taking into account demographic and metabolic parameters | Cross-sectional study | Yes | Polysomnography | 35 patients with psoriasis | No correlation between OSA and psoriasis. When adjusting for psoriasis, age and gender, there was a significant association between OSA, BMI and hypertension (P < 0.05). | 28/34 | 82.4 | good |
| Validated objective | Karaca et al., 2013 | Determine the frequency of OSA in psoriasis and its relationship with DLQI and psoriasis severity | Cross-sectional study | Yes | Polysomnography | 33 patients with psoriasis | Frequency of OSA in psoriasis patients was found to be higher than within the normal population (54.5% vs 2–4%) | 26/34 | 76.5 | fair |
| Validated objective | Savin et al., 1975 | Assess scratching during sleep | Cross-sectional study | Yes | Polysomnography | 15 patients with varied dermatological disorders (5 with, 5 with dermatitis herpetiformis, 3 with lichen planus, 1 with urticaria and 1 with psoriasis) | Scratching was found to be most prevalent in stage 1 for all participants, with its frequency decreasing through stages 2,3 and 4. | 16/30 | 53.3 | poor |
AHI—Apnoea Hypopnoea Index, BMI—Body Mass Index, BPI (30)–Brief Pain Inventory, ESS–Epworth Sleepiness Scale, FOSQ—Functional Outcomes of Sleep Questionnaire, GSDS–General Sleep Disturbance Scale, HRQoL/QoL—Health related quality of life/Quality of Life, ISI–Insomnia Severity Index, MCID–Minimal Clinically Important Difference, MOS-SS–Medical Outcomes Study Sleep Scale, OSA–Obstructive Sleep Apnoea, PSA–Psoriatic Arthritis, PSQI–Pittsburgh Sleep Quality Index, RCT—Randomized controlled trial, SD—Sleep duration, SE–Sleep Efficiency, SOL–Sleep Onset Latency, SQ–Sleep Quality, SQQ–Sleep Quality Questionnaire, TWT–Total Wake Time
Table 5. Information (i.e. aim, study design, primary focus of the study, measure of sleep used, sample, pertinent results relating to sleep) and quality scores for the qualitative included in the review.
| Type of measure of sleep | Study | Aim | Study Design | Primary focus on sleep | Measure of Sleep | Participants | Results pertaining to sleep | Quality Score | Quality Percentage | Quality Decision |
|---|---|---|---|---|---|---|---|---|---|---|
| Focus groups and interviews | Globe et al., 2009 | Develop a disease model of psoriasis to identify the most important domains to psoriasis patients through physician interviews and patient focus groups | Qualitative study | No | Focus group | 31 patients with psoriasis and 5 dermatologists | Patients reported that they experienced difficulty falling asleep, waking up, having non restorative sleep and sleeping less | 19/22 | 86.4 | good |
Table 4. Information (i.e. aim, study design, primary focus of the study, measure of sleep used, sample, pertinent results relating to sleep) and quality scores for each study using an unvalidated measure of sleep included in the review.
| Type of measure of sleep | Study | Aim | Study Design | Primary focus on sleep | Measure of Sleep | Participants | Results pertaining to sleep | Quality Score | Quality Percentage | Quality Decision |
|---|---|---|---|---|---|---|---|---|---|---|
| HRQoL (unvalidated subjective) | Takahasi et al., 2013 | Investigated the effect of various treatments on QoL and mental health of psoriasis patients | Cross-sectional study | No | GHQ-30 | 199 patients with psoriasis vulgaris | Biologics, other treatments and topical treatments all resulted in significant reductions in sleep disturbance from pre-to-post treatment, with biologics having the greatest effect (P < 0.05) | 21/32 | 65.6 | fair |
| HRQoL/Medical Record (unvalidated subjective) | Sanchez-Carazo et al., 2014 | Analyse the clinical profile of patients with moderate-to-severe psoriasis with regards to comorbid conditions and to establish its correlation with QoL | Cross-sectional survey | No | Medical history/SF-36 | 1022 patients with psoriasis | Moderate-severe psoriasis patients who possessed a diagnosis of a sleep disorder (12.2%) had significantly lower SF-36 scores (P < 0.05) | 26/30 | 86.7 | good |
| HRQoL (unvalidated subjective) | Kim et al., 2013 | Examine the relative effects of psoriasis and obesity on Chronic QoL by analysing the physical and psychological burden of the disease that accumulate across the lifespan | Cross-sectional survey | No | Modified DLQI (Chronic QoL) to include questions about social and psychological problems due to psoriasis | 114 patients with psoriasis | Sleep problems were significant across the lifetime (p<0.05) for those with higher BMI. | 24/30 | 80 | good |
| HRQoL (unvalidated subjective) | Oostveen et al., 2012 | Longitudinal assessment of QoL in juvenile psoriasis | Longitudinal study | No | CDLQI | 125 children with psoriasis | Sleep disturbance as measured by CDLQI reduced significantly from initial visit to follow up across all treatments (P < 0.05), with it having the greatest improvement along with itch. | 25/30 | 83.3 | good |
| Pain questionnaire (unvalidated subjective) | Ljosaa et al., 2010 | Describe the prevalence of skin pain and discomfort in psoriasis patients, whether skin pain/discomfort differed on demographic and clinical levels and to explore associated symptom characteristics | Cross-sectional study | No | BPI (30) | 139 patients with psoriasis | Sleep was the most severely disrupted function (P < 0.05) reported by 74/139 participants. | 27/30 | 90 | good |
| General Health (unvalidated subjective) | Sharma et al., 2001 | Evaluate psychiatric morbidity associated with psoriasis and vitiligo | Case-control study | No | GHQ-H | 30 patients with psoriasis or vitiligo | Sleep disturbance was the most common complaint, reported by 56.7% of psoriasis patients | 23/32 | 71.9 | fair |
| Pain questionnaire (unvalidated subjective) | Yosipovitch et al., 2000 | Assess the prevalence of itch in extensive psoriasis in an outpatient clinic and to assess its clinical pattern | Cross-sectional study | No | Questionnaire based on the McGill Pain Questionnaire | 101 patients with psoriasis | 69% of patients report that itch results in difficulty falling asleep, with 66% being woken up as a result of itch | 25/30 | 83.3 | good |
| Clinical Interview | Nyunt et al., 2013 | Determine the impact of psoriasis on HRQoL, examine the factors associated with HRQoL impairment and determine predictive factors of severe impact of psoriasis on HRQoL | Cross-sectional study | No | Clinical interview | 223 patients with psoriasis | Sleep disturbance as reported via clinical interview was significantly associated with severe reductions in DLQI score (P < 0.05) | 25/30 | 83.3 | good |
| Medical records | Tsai et al., 2011 | Describe the epidemiology of psoriasis and the prevalence of comorbidities in Taiwanese psoriasis patients | Cross-sectional study | No | Medical Records | 51,800 patients with psoriasis | Sleep disorders had a significantly increased prevalence ratio in psoriasis patients (3.89 [2.26, 6.71) (p<0.05.) | 23/38 | 82.14 | good |
| Medical records | Egeberg et al., 2016 | To examine the bidirectional impact of psoriasis and sleep apnoea | Cohort study | Yes | Medical Records | 66,523 patients with psoriasis | Psoriasis was associated with elevated risk of obstructive sleep apnoea even when adjusting for age, sex, alcohol, comorbidities and socioeconomic status. (mild psoriasis: IRR: 1.30, 95% CI: 1.17–1.44, severe psoriasis: IRR: 1.65, 95% CI:1.23–2.22) | 26/30 | 86.7 | good |
| Medical records | Chiu et al., 2016 | Investigate the association between cardiovascular risk and sleep disorders in psoriasis | Cohort study | Yes | Medical Records | 99,628 patients with psoriasis | Sleep disorders were significantly associated with increased cardiovascular risk (aHR: 1.25, 95% CI:1.22–1.38) and stroke (aHR: 1.24, 95% CI:1.1–1.33) | 23/26 | 88.5 | good |
| Unvalidated individual question | Duffin et al., 2009 | Determine what aspects of psoriasis and psoriatic arthritis are predictive of sleep disturbance using the National Psoriasis Foundation patient surveys | Cross-sectional study | Yes | Individual question ('In a typical month how many days did your disease interfere with your sleeping?') | 420 individuals with psoriasis | Psoriatic arthritis and itch were significant predictors of sleep disturbance (P < 0.05). | 21/30 | 70 | fair |
| proxy HRQoL (unvalidated subjective) | Hu et al., 2010 | Pilot a WTP instrument and evaluate its feasibility in measuring HRQoL domains within PsA | Cross-sectional study | No | Willingness-to-pay paradigm | 59 patients with psoriasis and PsA | The highest median amount of money individuals were willing to pay for a cure was applied to sleep ($10,000) | 23/28 | 82.1 | good |
| proxy HRQoL (unvalidated subjective) | Delfino et al., 2008 | Pilot a WTP instrument and evaluate its feasibility in measuring HRQoL domains within Psoriasis and to identify areas of HRQoL most severely affected by psoriasis | Cross-sectional study | No | Willingness-to-pay paradigm | 40 patients with psoriasis | Sleep was allocated the lowest median amount of money by patients ($625), but was reported by 22/40 participants as being present. | 22/28 | 78.6 | fair |
| Itch questionnaire (unvalidated subjective) | Amatya et al., 2008 | Characterize pruritus and its aggravating and relieving factors and to assess the effect of treatment and the impact of itch on QoL in psoriasis | Cross-sectional study | No | Pruritus questionnaire with one sleep item | 80 patients with psoriasis | 35% of individuals report itch as interfering with their sleep, and 65% report that good sleep improves itch | 23/30 | 76.7 | fair |
| Unvalidated sleep questionnaire | Gupta & Gupta., 1989 | Comparison of the dermatological and psychosocial factors of two psoriasis groups, both of whom report severe itch during wakefulness with and without frequent nocturnal awakenings from sleep | Case-control study | Yes | Sleep questionnaire assessing discomfort during sleep | 79 patients with psoriasis (46 with nocturnal awakenings and 33 without) | W group reported greater discomfort due to shedding (P < 0.05); heat intolerance (P < 0.05); cold intolerance (P < 0.05) and jerking of limbs during sleep (P < 0.05), and increased presence of depression than the without awakenings group. No significant differences relating to pruritus. | 22/30 | 73.3 | Fair |
| Unvalidated sleep question | Krueger et al., 2001 | Assess patient's views on the impact of psoriasis on their life and emotional wellbeing, along with obtaining their views and satisfaction of current treatments available | Cross-sectional survey | No | Single question asking what activities of daily living are impacted by psoriasis | 17,488 | Sleep was the second most disrupted activity of daily living, indicated by 20% of 18–34 year olds, 22% of 35–54 year olds and 22% of those >55. | 20/28 | 71.4 | Fair |
BMI—Body Mass Index, BPI (30)–Brief Pain Inventory, (C)DLQI—(Children’s) Dermatology Life Quality Index, GHQ-30 –General Health Questionnaire (30) HRQoL/QoL—Health related quality of life/Quality of Life, SF-36 –Short Form 36 Health Survey
Quality Evaluation
We adapted a standard quality scoring tool [34] for quantitative and qualitative study designs to include information about the quality of the sleep measure used, details of potential confounding variables and the sleep findings reported by each paper. Members of the review team (ALH, SB, CB, AC) tested the quality tool on a sample of four papers. The first author then reviewed all papers, discussing any problems with the fourth and sixth authors with amendments discussed and agreed upon during subsequent meetings until there was full agreement on the domains included in the tool. Subsequently ten randomly selected papers were coded by the third author (SB) independently with these scores compared to those of the first author (ALH). Disagreement in scores was found for one domain which was resolved following discussion with the rest of the team resulting in 100% agreement.
Fifteen domains were used for quantitative papers and seven for qualitative papers (supplementary data). Each quality evaluation domain had specific criteria that had to be fulfilled in order to obtain a score from 0 to 2. A standardised rating scale was used to score each item thus: a score of 2 indicates that criteria had been fulfilled, a score of 1 indicates partial fulfillment, and a score of 0 indicates no fulfillment of the criteria. Quantitative studies, depending on their design could score a maximum score of 40, whereas qualitative studies could score a maximum of 22 points.
Each paper's total quality score was then standardised as a percentage, obtained by dividing the study’s score by the total points available. Quality appraisal thresholds determined by the team were then applied to each study based upon its percentage score (good: ≥80%; fair: ≥61–79%; and poor: ≤60%).
Results
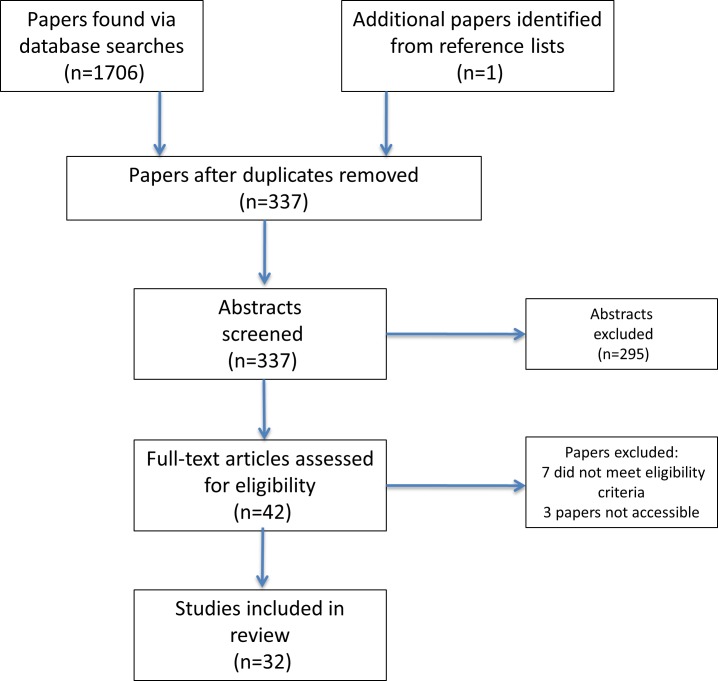
We identified 337 original papers. Abstracts were screened for eligibility and 42 full papers were obtained and scrutinised (Fig 1). In total, 32 papers were included in the final review. Thirty-one studies employed quantitative designs, and one study used a qualitative framework. We structure our review findings under the following category headings: study quality; sleep methodology and prevalence of sleep disruption; and factors associated with sleep disturbance.
Fig 1. PRISMA Flow diagram outlining the systematic review process.
Study Quality
Fewer than half of all studies (15/32; 46.9%) were rated as good quality (i.e. scoring ≥ 80%), over fair (15/32;46.9%) (scoring ≥ 61–79%), and the remainder (2/32; 6.25%) as poor (scoring ≤ 60%) (Tables 3–5). Overall quality scores were fair (mean % = 76.9), with a number of methodological limitations identified across studies: 27 studies failed to perform sample size calculations, 17 had inappropriately small or large sample sizes, 7 studies did not provide demographic data, 2 studies failed to report any data on central tendency or variability, and 5 studies reported this data for only one time point and 14 studies failed to control for confounding variables (S1 Table). Of the 14 studies using valid measures of sleep, the majority (9) scored in fair (scoring ≥ 61–79%), and poor (2) categories (scoring ≤ 60%) and only 3 were rated as good (scoring ≥80%).
Measurement and prevalence of sleep disturbance
Studies used a range of sleep measures but only in 14 of 31 (45.2%) had these been validated. Five studies used polysomnography (PSG), and focused on OSA [26,27,35,36] or itch [37], with one of these also using the Functional Outcomes of Sleep Questionnaire [26]. Four used the Pittsburgh Sleep Quality Index (PSQI) [23,24,38,39], one of which was conducted on a PsA sample [39] and one study used a condensed version of the PSQI [40]. One study used the Insomnia Severity Index (ISI) [23] while a further four studies used other validated self-report measures specifically, the General Sleep Disturbance Scale [41] and the Medical Outcomes Study Sleep Scale (MOS-SS) [28,42,43]. One study was qualitative, evaluating the impact of pruritus using interviews with physicians alongside patient focus groups [44].
The remaining 17 (54.8%) quantitative studies used non-validated sleep measures. Six studies used broad measures of health and functioning, which incorporated sleep items, such as measures health-related quality of life (HRQoL) (General Health Questionnaire, modified Dermatology Life Quality Index, Children’s Dermatology Life Quality Index, General Health Questionnaire Hindi) or other general health-related questionnaires (Brief Pain Inventory, McGill Pain Questionnaire) [45–50]. Four studies used non-validated questionnaires [25,51–53] three of which contained a single item pertaining to sleep disturbance [25,52,53] (e.g "in a typical month, how many days did your disease interfere with sleeping?”) [25]. Four studies solely used medical records [54,55,56,57], and another used a clinical diagnostic interview with a sleep disorder diagnosis [58]. In two validation studies HRQoL was assessed using a willingness-to-pay paradigm, where participants were asked to indicate how much money they would allocate to relieve themselves of a particular symptom [59,60]. Interestingly, in one of the studies using a PsA sample, sleep was allocated the highest median amount of money ($10,000) [60], whereas in the other study, with a psoriasis sample, sleep was the lowest ranked issue ($625) [59].
Of all studies reviewed, 11 (34.4%) had an a priori aim to assess sleep, however, only three (9.4%) had the primary objective of assessing sleep quality and quantifying sleep disturbance in psoriasis [23,24,37]. The other studies were not primarily focused on general sleep in psoriasis. Two studies investigated associations between PsA and sleep [25,39] while four studies assessed the link between OSA and psoriasis [26,27,35,36]. The remaining two studies measured the influence of itch on psoriasis [37,51], one of which used a mixed dermatological sample [37] with just 1 of 15 participants (6.67%) having psoriasis. The former [51] used a questionnaire and the latter [37] used PSG to assess sleep. One study aimed to assess the links between sleep disorders in psoriasis and cardiovascular risk [56].
Thirty (93.7%) of 32 studies, including one qualitative study, observed sleep disturbance. However, in studies where sleep was measured prospectively, values varied from 0.05% to 87.5% (Table 6). Two papers reported no sleep problems relative to healthy controls [24,59]. In one of these studies, whilst sleep disturbance was reported by the majority (55%) of participants, relative to other domains of life, sleep disturbance was the least bothersome [59].
Table 6. Rates of sleep disturbance found in studies where sleep was measured or quantified within the sample.
Studies where the whole sample had sleep disturbance or where the number of those experiencing sleep disturbance are not included in this table.
| Rates of sleep disruption (%) | Measures used to assess sleep | |
|---|---|---|
| Karaca et al. | 18/32 (Dx with OSA) (56.3) | Polysomnography |
| Papadavid et al. | 19/35 (Dx with OSA) (54.3) | Polysomnography |
| Buslau & Bentomane | 9/25 (Dx with OSA) (36) | Polysomnography |
| Gezer et al. | 35/41 (85.4) | PSQI |
| Shutty et al. | 81.80% | PSQI |
| Ljosaa et al. 2012 | 63% | GSDS |
| Sharma et al. | 17/30 (56.7) | GHQ-H |
| Ljosaa et al. 2010 | 74/139 (53.2) | BPI (30) |
| Hu et al. | 60/100 (60) | Willingness-to-pay (proxy HRQoL measure) |
| Delfino et al. | 22/40 (55) | Willingness-to-pay (proxy HRQoL measure) |
| Duffin et al. | 208/420 (49.5) | Individual question ('In a typical month how many days did your disease interfere with your sleeping?') |
| Nyunt et al | 91/223 (40.9) | Clinical interview |
| Sanchez-Carazo et al. | 125/1022 (12.2) | Medical History |
| Chiu et al. | 2,223/99,628 (2.23) | Sleep disorder diagnosis |
| Tsai et al. | 28/51,800 (0.05) | Sleep disorder diagnosis |
BPI–Brief Pain Inventory, Dx–Diagnosed, GHQ–General Health Questionnaire,
GSDS–General Sleep Disturbance Scale, HRQoL–Health Related Quality of Life,
OSA–Obstructive Sleep Apnea, PSG–Polysomnography, PSQI—Pittsburgh Sleep Quality Index
In contrast, another study reported sleep as being the second-most disrupted domain as a consequence of psoriasis, across young, middle aged and older-aged respondents; indicated by 20%, 22% and 22% respectively [53]. Sleep quality was worse in psoriasis patients (8.8±4.4 vs. 6.3±4.4) [23] and PsA patients (9.70±3.9 vs. 4.05±1.85) [39] relative to healthy controls in two out of three studies using PSQI with the third paper showing no difference between psoriasis patients and controls (5.56 vs. 5.13) [46]. The fourth paper using the PSQI showed that on two components of the measure scores were significantly worse in psoriasis subjects than healthy controls (subjective sleep quality; 1.48±0.17 vs. 1.02±0.13 and habitual sleep efficiency; 0.79±0.19 vs. 0.32±0.14) [38]. Nevertheless, they did not report data for one component nor did they provide global PSQI scores for both groups [38]. Furthermore, one of these studies showed significantly increased ISI scores in psoriasis patients (11.0±7.0) relative to healthy controls (6.3±6.0) [23], with psoriasis patients’ mean score at the threshold for clinical insomnia [61].
Four studies found high rates of OSA in psoriasis patients ranging from 36% to as high as 56.3% [27,35,36]. Moreover in a large epidemiological study, psoriasis patients had a significantly increased prevalence of sleep disorder diagnosis than non-psoriasis counterparts [57] and in another, psoriasis patients with a sleep disorder had elevated cardiovascular risk relative to those without a sleep disorder [56].
Factors associated with sleep disturbance
Sixteen of the 32 reviewed studies reported factors associated with sleep disturbance. Itch, depression, PsA and pain were the most commonly reported factors associated with sleep disturbance however others were reported across studies. Itch was reported to occur more frequently at night [50] and in six studies it was associated with disrupted sleep [25,37,42,44,50,52]. Itch predicted lower scores on the MOS-SS, indicating worse sleep [42]; increased scratching was associated with more awakenings during stages 1 and 2 of sleep [37]; and itch was reported by subjects as causing difficulty initiating and maintaining sleep as well as performing daily activities [44]. Additionally, in one study 65% of participants reported that sleep ameliorated itch [52].
The association between itch and sleep was documented further in one study finding the association between depressive symptoms and itch severity was partially mediated by sleep quality [40]. In another study daytime sleepiness was associated with depression [28]. Contrasting findings were indicated by one study in which itch levels were reported as equivalent across those with and without sleep disruption, whereas depression was significantly higher in those who experienced sleep disruption relative to those who did not [51]. This suggests that depression may have played a greater role in sleep disruption than itch. This is supported by another study showing that when controlling for depression variables, psoriasis patients are no more likely to experience poor sleep or have greater insomnia symptoms than controls [23], and another study showing that while PSQI scores were higher in psoriasis subjects, there were no differences in psychiatric symptoms relative to controls [38].
Pain appears to contribute to poorer sleep, and in one study over 85% of those reporting increased pain specified sleep as the most disrupted function [47]. Another study showed that sleep disturbance mediated the relationship between pain and reduced HRQoL [41]. Four studies showed associations between PsA and sleep [25,28,39,60]. PsA and generalized pain was associated with reduced PSQI scores relative to healthy controls [39], increased want for improved sleep [59] and predicted sleep disturbance [25]. Additionally sleep disturbance was associated with anxiety, enthesitis, levels of C-reactive protein and erythrocyte sedimentation rate [39].
Sleep disturbance was consistently associated with reduced QOL [28,47,54,58]. Moreover, in four treatment studies using biologics, improvements were observed in sleep quality, disturbance, daytime sleepiness and adequacy [26,41,43,46] suggesting that improvements in psoriasis disease processes may lead to concomitant improvements in sleep. Furthermore in a longitudinal cohort study sleep showed the greatest improvement from baseline to follow-up across varied psoriasis therapies (topicals and systemic) relative to other items on the Children’s Dermatology Life Quality Index [48].
Discussion
Key findings from the review
This systematic review examined the evidence for sleep disturbance in psoriasis and the measures and methodology used to assess sleep. The majority of studies showed sleep problems were associated with itch, low mood and pain. Itch appears to be associated with increased sleep fragmentation, disturbance and reduced quality of sleep. These findings are consistent with research in other pruritic conditions [62–64]. Low mood and pain were also implicated in poor sleep as previously shown in both healthy individuals and other illness populations [65, 66]. It is likely that mood, pain and itch interact disturb sleep; although the nature of this interaction requires examination within the context of purposively-designed studies.
Prevalence rates varied substantially and while studies were of fair-to-good quality, many demonstrated a number of methodological and statistical shortcomings. In particular, the lack of validated measures of sleep, coupled with limited theoretical work-up may have accounted for the variation in rates of sleep disturbance across studies. Nevertheless, prevalence clustered around the 50–60% mark; higher than current estimations of sleep pathology in the general population [67]. Additionally, rates of OSA appear to be higher in psoriasis (36–56.3%) relative to the general population (3–7%)[68]. Although the reasons for this are unclear; OSA is multifaceted, involving both inflammatory pathways and lifestyle factors including high BMI and physical inactivity [69]. OSA can have a significant impact on health and contribute to cardiovascular disease risk [70], which is already elevated in psoriasis [4]. Further research should identify mechanisms underpinning the link between OSA and psoriasis.
Lack of detailed assessments and incomplete data reporting have impeded further conclusions and provide a somewhat superficial picture of the true problem. For instance, studies using the PSQI often failed to report full data for the measure (sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medication and daytime dysfunction). Providing these data would facilitate a more thorough and itemised understanding of sleep quality.
Moreover, despite the ‘gold standard’ PSG being used in five studies, it provides limited information about the range of factors that may impact upon sleep [71, 72]. Comprehensive assessment of sleep involves the integration of objective and subjective measures, profiling of sleep-wake patterning over time, and the assessment of factors that may contribute to night-to-night variation in sleep quantity and quality [71,72]. We found no studies in this review that conformed to these standards and neither actigraphy nor sleep diaries have been applied in psoriasis populations. These data would support a more nuanced understanding of sleep disruption in psoriasis and help to design appropriate interventions [71,72].
Limitations
This review only included studies published in English and those that have undergone peer review. Therefore, it is possible that some relevant findings may have been missed. Further, due to the subjective nature of the quality scoring, there is greater opportunity for personal bias to influence scores. However we attempted to minimize this risk by the authors scoring papers independently and there was close to 100% agreement in paper scores following independent scoring.
Concluding remarks: Recommendations for future research
There is a need to systematically and consistently examine sleep in psoriasis populations, employing comprehensive and validated measures of sleep in specifically designed studies. Accurate prevalence rates of sleep disturbance must first be established; from here prospective studies can explore the relationship between sleep and psoriasis, focusing on potential precipitating and perpetuating factors that may drive and maintain poor sleep, (candidate targets: itch, mood, pain and OSA). Work should also explore sleep-wake variability in this population and assess any concomitant consequences on daytime functioning.
Obtaining a comprehensive understanding of sleep in psoriasis is of importance due to the implications of sleep disruption for health. Sleep disturbance may result in disruptions to immune and sympathetic nervous system functioning possibly leading to the maintenance and/or exacerbation of psoriasis, conferring risk for adverse psychological and medical outcomes. This has been suggested in a recent study which found that the presence of a sleep disorder alongside psoriasis confers increased risk for CVD morbidity [56].
The potential role of inflammation in the relationship between poor sleep and psoriasis has also been suggested. Four studies included in this review [26,41,43,46] showed improvements but not a mitigation of sleep problems following the administration of biologic medication. This suggests that reductions in systemic inflammation may confer sleep improvement possibly through a reduction in itch and associated skin discomfort. Further research is required to examine mediating pathways and treatment mechanisms in greater detail.
Supporting Information
(DOCX)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by R117541, www.psoriasis-association.org.uk. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583): 263–71. [DOI] [PubMed] [Google Scholar]
- 2.Gladman D, Antoni C, Mease P, Clegg D, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64(suppl 2): ii14–ii7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christophers E, Barker J, Griffiths C, Daudén E, Milligan G, Molta C, et al. The risk of psoriatic arthritis remains constant following initial diagnosis of psoriasis among patients seen in European dermatology clinics. JEADV. 2010;24(5): 548–54. 10.1111/j.1468-3083.2009.03463.x [DOI] [PubMed] [Google Scholar]
- 4.Parisi R, Rutter MK, Lunt M, Young HS, Symmons DP, Griffiths CE, et al. Psoriasis and the Risk of Myocardial Infarction: A Population-Based Cohort Study Using the Clinical Practice Research Datalink. Pharmacoepidemiol Dr S; 2014: 23(S1) [Google Scholar]
- 5.Cohen A, Dreiher J, Shapiro Y, Vidavsky L, Vardy D, Davidovici B, et al. Psoriasis and diabetes: a population‐based cross‐sectional study. JEADV. 2008;22(5): 585–9. 10.1111/j.1468-3083.2008.02636.x [DOI] [PubMed] [Google Scholar]
- 6.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3): 163–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson N, Dinges DF. Sleep and inflammation. Nutr Rev. 2007;65(suppl 3): S244–S52. [DOI] [PubMed] [Google Scholar]
- 8.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention? JAMA. 1989;262(11): 1479–84. [DOI] [PubMed] [Google Scholar]
- 9.Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Imm. 2002;16(5):503–12. [DOI] [PubMed] [Google Scholar]
- 10.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol; 2005; 25(1): 117–129 [DOI] [PubMed] [Google Scholar]
- 11.Beattie L, Kyle SD, Espie CA, Biello SM. Social interactions, emotion and sleep: A systematic review and research agenda. Sleep Med Rev. 2014. [DOI] [PubMed] [Google Scholar]
- 12.Kyle SD, Morgan K, & Espie CA. Insomnia and health-related quality of life. Sleep Med Rev. 2010;14(1):69–82.13. 10.1016/j.smrv.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 13.Kyle SD, Beattie L, Spiegelhalder K, Rogers Z, & Espie CA. Altered emotion perception in insomnia disorder. Sleep. 2014;37(4):775 10.5665/sleep.3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Int Med. 2005;165(8):863–7. [DOI] [PubMed] [Google Scholar]
- 15.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–92. 10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- 16.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the Risk of Acute Myocardial Infarction A Population Study. Circulation. 2011;124(19):2073–81. 10.1161/CIRCULATIONAHA.111.025858 [DOI] [PubMed] [Google Scholar]
- 17.Palagini L, Maria Bruno R, Gemignani A, Baglioni C, Ghiadoni L, Riemann D. Sleep loss and hypertension: a systematic review. Curr Pharm Des. 2013;19(13):2409–19. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, et al. Insomnia With Objective Short Sleep Duration and Incident Hypertension The Penn State Cohort. Hypertension. 2012;60(4):929–35. 10.1161/HYPERTENSIONAHA.112.193268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morin CM, Ware JC. Sleep and psychopathology. Appl Prev Psychol. 1996;5(4):211–24. [Google Scholar]
- 20.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1):10–9. [DOI] [PubMed] [Google Scholar]
- 21.Manber R, Chambers AS. Insomnia and depression: a multifaceted interplay. Current Psych Rep. 2009;11(6):437–42. [DOI] [PubMed] [Google Scholar]
- 22.Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psych Res. 2003;37(1):9–15. [DOI] [PubMed] [Google Scholar]
- 23.Shutty BG, West C, Huang KE, Landis E, Dabade T, Browder B, et al. Sleep disturbances in psoriasis. Dermatol Online J. 2013;19(1). [PubMed] [Google Scholar]
- 24.Stinco G, Trevisan G, Piccirillo F, Di Meo N, Nan K, Deroma L, et al. Psoriasis vulgaris does not adversely influence the quality of sleep. G Ital Dermatol Venereol. 2013;148(6):655–9. [PubMed] [Google Scholar]
- 25.Duffin KC, Wong B, Horn EJ, Krueger GG. Psoriatic arthritis is a strong predictor of sleep interference in patients with psoriasis. JAAD. 2009;60(4):604–8. [DOI] [PubMed] [Google Scholar]
- 26.Maari C, Bolduc C, Nigen S, Marchessault P, Bissonnette R. Effect of adalimumab on sleep parameters in patients with psoriasis and obstructive sleep apnea: a randomized controlled trial. J Dermatol Treat. 2014;25(1):57–60. [DOI] [PubMed] [Google Scholar]
- 27.Papadavid E, Vlami K, Dalamaga M, Giatrakou S, Theodoropoulos K, Gyftopoulos S, et al. Sleep apnea as a comorbidity in obese psoriasis patients: a cross-sectional study. Do psoriasis characteristics and metabolic parameters play a role? JEADV. 2013;27(7):820–6. 10.1111/j.1468-3083.2012.04580.x [DOI] [PubMed] [Google Scholar]
- 28.Strober BE, Sobell JM, Duffin KC, Bao Y, Guerin A, Yang H, et al. Sleep quality and other patient-reported outcomes improve after patients with psoriasis with suboptimal response to other systemic therapies are switched to adalimumab: results from PROGRESS, an open-label Phase IIIB trial. Brit J Dermatol. 2012;167(6):1374–81. [DOI] [PubMed] [Google Scholar]
- 29.Gowda S, Goldblum OM, McCall WV, & Feldman SR. Factors affecting sleep quality in patients with psoriasis. JAAD. 2010;63(1):114–123. [DOI] [PubMed] [Google Scholar]
- 30.Thorburn PT, Riha RL. Skin disorders and sleep in adults: where is the evidence? Sleep Med Rev. 2010;14(6):351–8. 10.1016/j.smrv.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 31.Gupta MA, Gupta AK. Sleep-wake disorders and dermatology. Clin Dermatol. 2013;31(1):118–26. 10.1016/j.clindermatol.2011.11.016 [DOI] [PubMed] [Google Scholar]
- 32.Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: A systematic Review. Sleep Med Rev. 2016;29:63–75 [DOI] [PubMed] [Google Scholar]
- 33.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W-65–W-94. [DOI] [PubMed] [Google Scholar]
- 34.Kmet L, Lee R, Cook L. Standard Quality Assessment Criteria for Evaluating Primary Research Papers From a Variety of Fields. 2004. Edmonton: Alberta Heritage Foundation for Medical Research; 2011. [Google Scholar]
- 35.Buslau M, Benotmane K. Cardiovascular complications of psoriasis: does obstructive sleep apnoea play a role? Acta Derm Venereol. 1999;79(3):234 [DOI] [PubMed] [Google Scholar]
- 36.Karaca S, Fidan F, Erkan F, Nural S, Pinarci T, Gunay E, et al. Might psoriasis be a risk factor for obstructive sleep apnea syndrome? Sleep & Breathing. 2013;17(1):275–80. [DOI] [PubMed] [Google Scholar]
- 37.Savin JA, Paterson WD, Oswald I, Adam K. Further studies of scratching during sleep. Brit J Dermatol. 1975;93(3):297–302. [DOI] [PubMed] [Google Scholar]
- 38.Balta U, Karadag AS, Selek S, Onder S, Kanbay A, Burakgazi-Yilmaz H. General psychiatric symptoms, quality of sleep and coping strategies in patients with psoriasis vulgaris. Int J Dermatol. 2015 [DOI] [PubMed] [Google Scholar]
- 39.Gezer O, Batmaz I, Sariyildiz MA, Sula B, Ucmak D, Bozkurt M, et al. Sleep quality in patients with psoriatic arthritis. International Journal of Rheum Dis. 2014. [DOI] [PubMed] [Google Scholar]
- 40.Zachariae R, Zachariae COC, Lei U, Pedersen AF. Affective and sensory dimensions of pruritus severity: Associations with psychological symptoms and quality of life in psoriasis patients. Acta Derm Venereol. 2008;88(2):121–7. 10.2340/00015555-0371 [DOI] [PubMed] [Google Scholar]
- 41.Ljosaa TM, Mork C, Stubhaug A, Moum T, Wahl AK. Skin pain and skin discomfort is associated with quality of life in patients with psoriasis. JEADV. 2012;26(1):29–35. 10.1111/j.1468-3083.2011.04000.x [DOI] [PubMed] [Google Scholar]
- 42.Mrowietz U, Chouela E, Mallbris L, Stefanidis D, Marino V, Pedersen R, et al. Pruritus and quality of life in moderate‐to‐severe plaque psoriasis: post hoc explorative analysis from the PRISTINE study. JEADV. 2014. [DOI] [PubMed] [Google Scholar]
- 43.Thaçi D, Galimberti R, Amaya-Guerra M, Rosenbach T, Robertson D, Pedersen R, et al. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: Results from the PRISTINE trial. JEADV. 2014;28(7):900–6. 10.1111/jdv.12207 [DOI] [PubMed] [Google Scholar]
- 44.Globe D, Bayliss MS, Harrison DJ. The impact of itch symptoms in psoriasis: results from physician interviews and patient focus groups. Health Qual Life Outcomes. 2009;7:62 10.1186/1477-7525-7-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi H, Iinuma S, Tsuji H, Honma M, Iizuka H. Biologics are more potent than other treatment modalities for improvement of quality of life in psoriasis patients. J Dermatol. 2014;41(8):686–9. 10.1111/1346-8138.12544 [DOI] [PubMed] [Google Scholar]
- 46.Kim GE, Seidler E, Kimball AB. The relative impact of psoriasis and obesity on socioeconomic and medical outcomes in psoriasis patients. JEADV. 2013. [DOI] [PubMed] [Google Scholar]
- 47.Ljosaa TM, Rustoen T, Mork C, Stubhaug A, Miaskowski C, Paul SM, et al. Skin pain and discomfort in psoriasis: an exploratory study of symptom prevalence and characteristics. Acta Derm Venereol. 2010;90(1):39–45. 10.2340/00015555-0764 [DOI] [PubMed] [Google Scholar]
- 48.Oostveen AM, De Jager MEA, Van De Kerkhof PCM, Donders ART, De Jong EMGJ, Seyger MMB. The influence of treatments in daily clinical practice on the Children's Dermatology Life Quality Index in juvenile psoriasis: A longitudinal study from the Child-CAPTURE patient registry. Brit J Dermatol. 2012;167(1):145–9. [DOI] [PubMed] [Google Scholar]
- 49.Sharma N, Koranne RV, Singh RK. Psychiatric morbidity in psoriasis and vitiligo: A comparative study. J Dermatol. 2001;28(8):419–23. [DOI] [PubMed] [Google Scholar]
- 50.Yosipovitch G, Goon A, Wee J, Chan Y, Goh C. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Brit J Dermatol. 2000;143(5):969–73. [DOI] [PubMed] [Google Scholar]
- 51.Gupta MA, Gupta AK, Kirkby S, Schork NJ, Weiner HK, Ellis CN, et al. Pruritus associated with nocturnal wakenings: organic or psychogenic? JAAD. 1989;21(3 Pt 1):479–84. [DOI] [PubMed] [Google Scholar]
- 52.Amatya B, Wennersten G, Nordlind K. Patients' perspective of pruritus in chronic plaque psoriasis: a questionnaire-based study. JEADV. 2008;22(7):822–6. 10.1111/j.1468-3083.2008.02591.x [DOI] [PubMed] [Google Scholar]
- 53.Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137(3):280–4. [PubMed] [Google Scholar]
- 54.Sanchez-Carazo JL, Lopez-Estebaranz JL, Guisado C. Comorbidities and health-related quality of life in Spanish patients with moderate to severe psoriasis: a cross-sectional study (Arizona study). J Dermatol. 2014;41(8):673–8. 10.1111/1346-8138.12465 [DOI] [PubMed] [Google Scholar]
- 55.Tsai TF, Wang TS, Hung ST, Tsai PI, Schenkel B, Zhang M, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. 2011;63(1):40–6. 10.1016/j.jdermsci.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 56.Chiu HY, Hsieh CF, Chiang YT, Tsai YW, Huang WF, Li CY, Wang TS, Tsai TF. Concomitant Sleep Disorders Significantly Increase the Risk of Cardiovascular Disease in Patients with Psoriasis. PloS one. 2016. January 1;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egeberg A, Khalid U, Gislason GH, Mallbris L, Skov L, & Hansen PR. Psoriasis and sleep apnea: a Danish nationwide cohort study. Journal of Clinical Sleep Medicine. 2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nyunt WW, Low WY, Ismail R, Sockalingam S, Min AK. Determinants of Health-Related Quality of Life in Psoriasis Patients in Malaysia. Asia Pac J Public Health. 2013. [DOI] [PubMed] [Google Scholar]
- 59.Delfino M Jr., Holt EW, Taylor CR, Wittenberg E, Qureshi AA. Willingness-to-pay stated preferences for 8 health-related quality-of-life domains in psoriasis: a pilot study. JAAD. 2008;59(3):439–47. [DOI] [PubMed] [Google Scholar]
- 60.Hu SW, Holt EW, Husni ME, Qureshi AA. Willingness-to-pay stated preferences for 8 health-related quality-of-life domains in psoriatic arthritis: a pilot study. Semin Arthritis Rheum. 2010;39(5):384–97. 10.1016/j.semarthrit.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 61.Morin CM, Belleville G, Bélanger L, & Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang YS, Chou YT, Lee JH, Dai YS, Sun C, Lin YT et al. Atopic dermatitis, melatonin and sleep disturbance. Pediatrics. 2014:134(2):e397–e405 10.1542/peds.2014-0376 [DOI] [PubMed] [Google Scholar]
- 63.Sherry HY, Attarian H, Zee P, & Silverberg JI. Burden of Sleep and Fatigue in US Adults with Atopic Dermatitis. Dermatitis. 2016;27(2):50–58 10.1097/DER.0000000000000161 [DOI] [PubMed] [Google Scholar]
- 64.O'Neill JL, Chan YH, Rapp SR & Yosipovitch G. Differences in itch characteristics between psoriasis and atopic dermatitis patients: results of a web-based questionnaire. Acta Dermato-venereologica. 2011:91(5):537–540 10.2340/00015555-1126 [DOI] [PubMed] [Google Scholar]
- 65.Tang NK, Goodchild CE, Webster LR. Sleep and chronic pain In Treatment of Chronic Pain by Integrative Approaches 2015. (pp. 203–217). Springer; New York. [Google Scholar]
- 66.Bower B, Bylsma LM, Morris BH, Rottenberg J. Poor reported sleep quality predicts low positive affect in daily life among healthy and mood‐disordered persons. Journal of sleep research. 2010. June 1;19(2):323–32. 10.1111/j.1365-2869.2009.00816.x [DOI] [PubMed] [Google Scholar]
- 67.Leger D, Poursain B, Neubauer D, Uchiyama M. An international survey of sleeping problems in the general population. Curr Med Res Opin. 2008;24(1):307–17 [DOI] [PubMed] [Google Scholar]
- 68.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society 2008;5(2):136–43. 10.1513/pats.200709-155MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirotsu C, Noqueira H, Albuquerque R, et al. The bidirectional interactions between psoriasis and obstructive sleep apnea. International Journal of Dermatology 2015;54(12):1352–58 10.1111/ijd.13026 [DOI] [PubMed] [Google Scholar]
- 70.McNicholas W, Bonsignore M, B26 MCoECA. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. European Respiratory Journal 2007;29(1):156–78. [DOI] [PubMed] [Google Scholar]
- 71.Kyle SD, Henry AL, Miller CB. Measurement and disorders of sleep. In Horton C et al, editors. Sleep and Cognition. 2015. In press.
- 72.Buysse DJ, Ancoli-lsrael S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.