Abstract
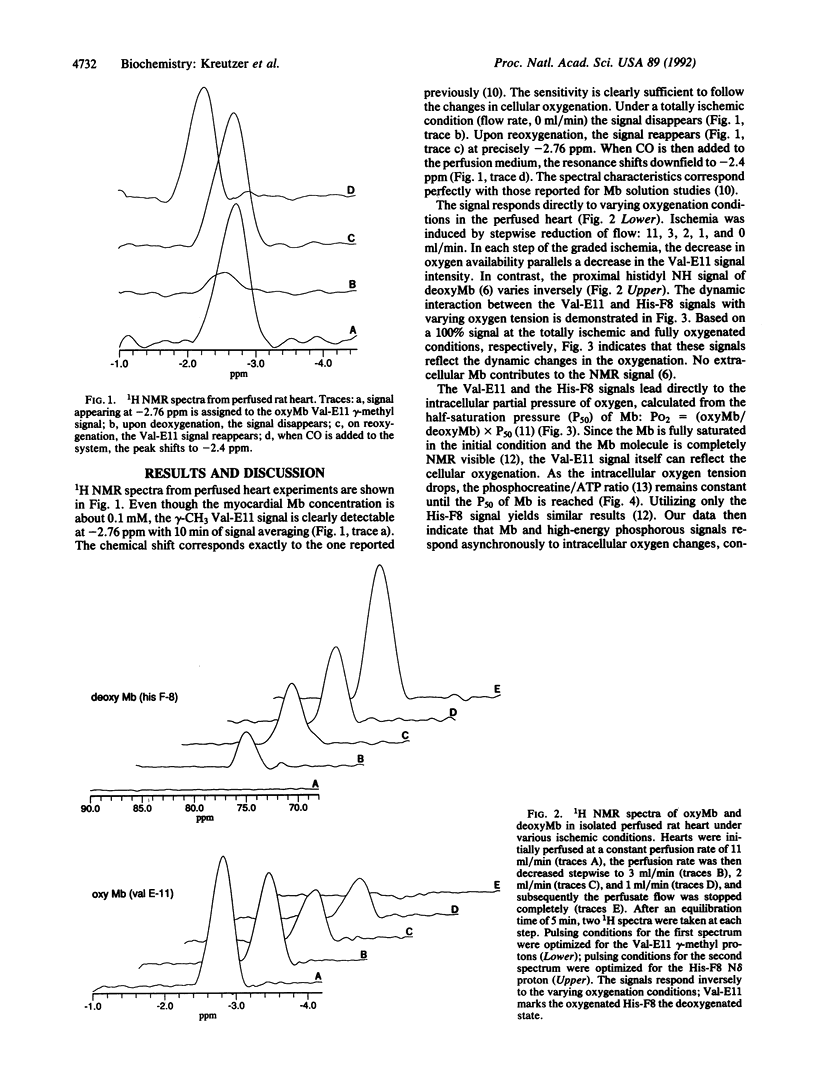
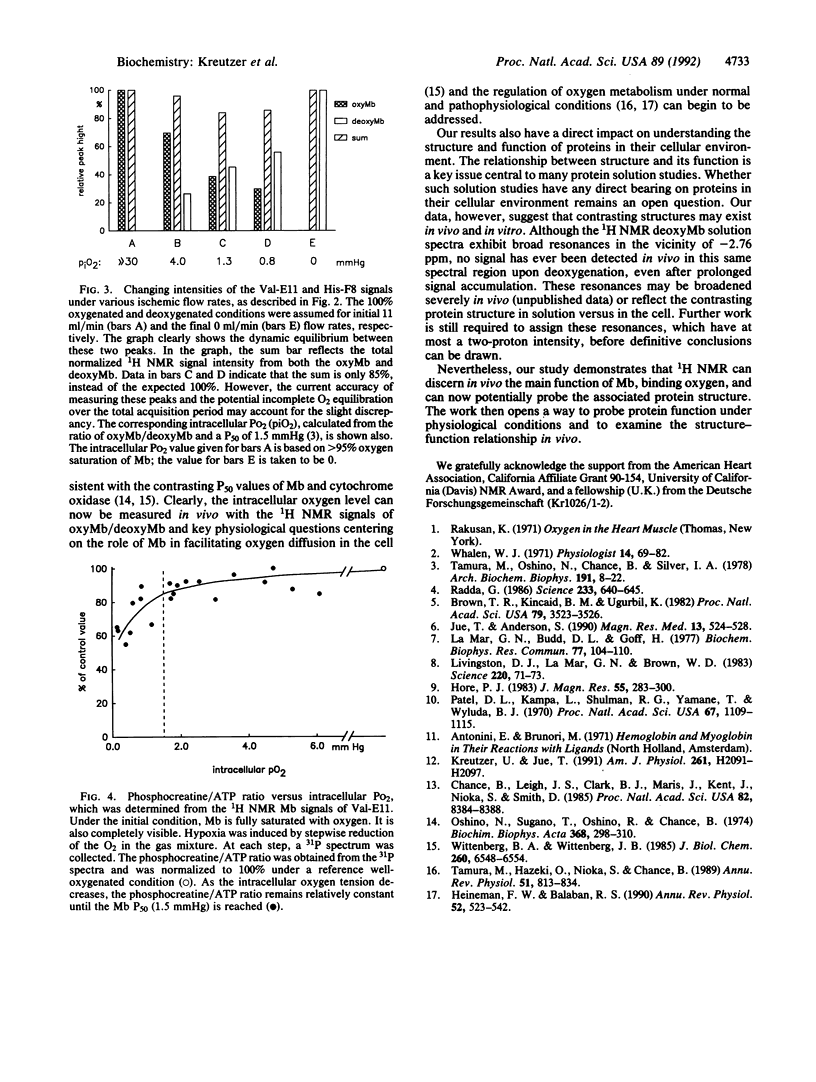
The 1H NMR signal from oxymyoglobin, a low-concentration diamagnetic protein, is visible in myocardial tissue. The methyl group of the Val-E11 resonates in a clear spectral region at -2.76 ppm and responds to dynamic changes in cellular oxygenation. With CO, the signal shifts to -2.4 ppm. The Val-E11 peak assignment and its response to oxygen and CO agree perfectly with previous myoglobin solution studies. Intracellular oxygen level can now be determined in vivo with the signal intensity ratio of oxymyoglobin/deoxymyoglobin, reflected by the Val-E11 and His-F8 peaks in the 1H NMR spectra. Moreover, protein structure-function relationship in vivo can now be probed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T. R., Kincaid B. M., Ugurbil K. NMR chemical shift imaging in three dimensions. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3523–3526. doi: 10.1073/pnas.79.11.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr, Clark B. J., Maris J., Kent J., Nioka S., Smith D. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8384–8388. doi: 10.1073/pnas.82.24.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineman F. W., Balaban R. S. Control of mitochondrial respiration in the heart in vivo. Annu Rev Physiol. 1990;52:523–542. doi: 10.1146/annurev.ph.52.030190.002515. [DOI] [PubMed] [Google Scholar]
- Jue T., Anderson S. 1H NMR observation of tissue myoglobin: an indicator of cellular oxygenation in vivo. Magn Reson Med. 1990 Mar;13(3):524–528. doi: 10.1002/mrm.1910130322. [DOI] [PubMed] [Google Scholar]
- Kreutzer U., Jue T. 1H-nuclear magnetic resonance deoxymyoglobin signal as indicator of intracellular oxygenation in myocardium. Am J Physiol. 1991 Dec;261(6 Pt 2):H2091–H2097. doi: 10.1152/ajpheart.1991.261.6.H2091. [DOI] [PubMed] [Google Scholar]
- La Mar G. N., Budd D. L., Goff H. Assignment of proximal histidine proton NMR peaks in myoglobin and hemoglobin. Biochem Biophys Res Commun. 1977 Jul 11;77(1):104–110. doi: 10.1016/s0006-291x(77)80170-8. [DOI] [PubMed] [Google Scholar]
- Livingston D. J., La Mar G. N., Brown W. D. Myoglobin diffusion in bovine heart muscle. Science. 1983 Apr 1;220(4592):71–73. doi: 10.1126/science.6828881. [DOI] [PubMed] [Google Scholar]
- Oshino N., Sugano T., Oshino R., Chance B. Mitochondrial function under hypoxic conditions: the steady states of cytochrome alpha+alpha3 and their relation to mitochondrial energy states. Biochim Biophys Acta. 1974 Dec 19;368(3):298–310. doi: 10.1016/0005-2728(74)90176-5. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kampa L., Shulman R. G., Yamane T., Wyluda B. J. Proton nuclear magnetic resonance studies of myoglobin in H2O. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1109–1115. doi: 10.1073/pnas.67.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radda G. K. The use of NMR spectroscopy for the understanding of disease. Science. 1986 Aug 8;233(4764):640–645. doi: 10.1126/science.3726553. [DOI] [PubMed] [Google Scholar]
- Tamura M., Hazeki O., Nioka S., Chance B. In vivo study of tissue oxygen metabolism using optical and nuclear magnetic resonance spectroscopies. Annu Rev Physiol. 1989;51:813–834. doi: 10.1146/annurev.ph.51.030189.004121. [DOI] [PubMed] [Google Scholar]
- Tamura M., Oshino N., Chance B., Silver I. A. Optical measurements of intracellular oxygen concentration of rat heart in vitro. Arch Biochem Biophys. 1978 Nov;191(1):8–22. doi: 10.1016/0003-9861(78)90062-0. [DOI] [PubMed] [Google Scholar]
- Whalen W. J. Intracellular PO2 in heart and skeletal muscle. Physiologist. 1971 May;14(2):69–82. [PubMed] [Google Scholar]
- Wittenberg B. A., Wittenberg J. B. Oxygen pressure gradients in isolated cardiac myocytes. J Biol Chem. 1985 Jun 10;260(11):6548–6554. [PubMed] [Google Scholar]



