Abstract
The majority of brain tumors arising in children are low-grade gliomas. Although historically categorized together as pediatric low-grade gliomas (PLGGs), there is significant histologic and genetic diversity within this group. In general, prognosis for PLGGs is excellent, and limitation of sequelae from tumor and treatment is paramount. Advances in high-throughput genetic sequencing and gene expression profiling are fundamentally changing the way PLGGs are classified and managed. Here, we review the histologic subtypes and highlight how recent advances in elucidating the molecular pathogenesis of these tumors have refined diagnosis and prognostication. Additionally, we discuss how characterizing specific genetic alterations has paved the way for the rational use of targeted therapies that are currently in various phase clinical trials.
KEYWORDS : pediatric low-grade glioma, pediatrics, targeted therapy
Pediatric low-grade glioma (PLGG) represents the most common brain tumor of childhood [1]. For the majority of children with PLGG these tumors are primarily managed with surgery, with gross total resection often leading to excellent durable disease control [2]. However, tumors residing in critical locations where complete resection is not safe may pose a threat to neurologic function and survival. For instance, about three-quarters of patients with cerebral and cerebellar hemisphere tumors are able to undergo gross total resection, while less than a quarter of children with chiasmic-hypothalamic and midline tumors are able to have a complete resection. For subtotal resections, the volume of residual tumor is predictive of disease progression [2]. Furthermore, some histologic and molecular subtypes of PLGG may have a propensity to recur even after gross total resection. Radiation and cytotoxic or cytostatic chemotherapies have classically been used to improve progression-free survival rates among children with incompletely resected or recurrent tumors. With these treatments, event-free survival has been promising, up to 74% at 10 years [3]. However, concerns over long-term toxicities have led to efforts to reduce the use or dose of radiation and chemotherapy among children at lower risk for progression or at higher risk for toxicity. Conformal radiation is able to better spare normal tissue to limit toxicity [4], though children of younger age still experience significant IQ decline at 5 years after intracranial irradiation [5]. Single or polychemotherapy regimens have been used to delay or obviate the need for radiation therapy. However, chemotherapeutic agents themselves carry risks of significant long term toxicities such as peripheral neuropathies and secondary leukemia [6]. Fortunately, there has been substantial progress in understanding the molecular pathogenesis underlying low-grade glioma subtypes, and targeted agents are being developed and tested in clinical trials with the hope of improving progression-free survival while limiting long-term toxicity.
Histologic classifications & molecular refinements
Histologically, PLGGs are defined as WHO grade I or II, with the major histologic subtypes including low-grade astrocytomas, oligodendrogliomas and glioneuronal tumors. We will discuss these histologic subtypes here, highlighting the detection of genetic alterations that provides greater clinicopathologic specificity for children diagnosed with PLGG.
• Low-grade astrocytomas
Low-grade astrocytomas are the most common PLGG, and within this group are pilocytic astrocytoma, pilomyxoid astrocytoma, pleomorphic xanthroastrocytoma, diffuse astrocytoma and subependymal giant cell astrocytoma (SEGA). Low-grade astrocytomas can occur anywhere within the central nervous system but are most commonly found in the posterior fossa, followed by the cerebral hemispheres, midline structures, brainstem and least commonly the spinal cord [7].
Pilocytic astrocytoma, WHO grade I, is the most common of the low-grade astrocytomas. Pilocytic astrocytomas are classically well-circumscribed cystic lesions that contain an enhancing mural nodule on T1-weighted MRI. These tumors generally have an excellent prognosis and are usually not associated with recurrence after complete surgical resection. Very rarely, however, pilocytic astrocytomas may recur after complete resection, disseminate throughout the CSF or undergo malignant transformation [8], highlighting the need to better understand the genetic and molecular underpinnings of typically benign gliomas.
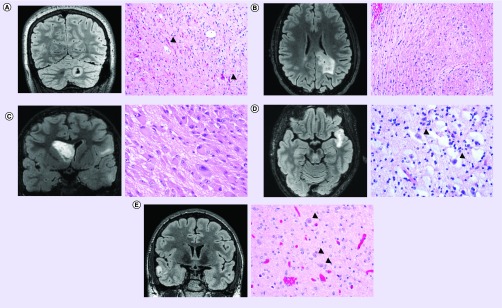
Histologic sections usually demonstrate a neoplastic proliferation of piloid astrocytes in an alternating loose and compact stroma, often containing Rosenthal fibers and/or eosinophilic granular bodies (Figure 1A).
Figure 1. . Representative imaging and histology from pediatric low-grade gliomas.
(A) Pilocytic astrocytoma, WHO grade I, with KIAA1549–BRAF gene fusion. Coronal T2-weighted fluid-attenuated inversion recovery (FLAIR) MRI (left) demonstrating a solid and cystic lesion centered in the cerebellar hemisphere. Hematoxylin and eosin (H&E) stained section (right) demonstrating a compact area of the neoplasm composed of piloid astrocytes containing numerous Rosenthal fibers (examples of which are highlighted by black arrowheads). Molecular analysis (not shown) demonstrated duplication of the 3′ portion of the BRAF gene along with fusion to the KIAA1549 locus. (B) Diffuse astrocytoma, WHO grade II, with MYB gene rearrangement. Axial T2-weighted FLAIR MRI (left) demonstrating an infiltrative hyperintense lesion within the deep white matter of the parietal lobe and infiltrating into the splenium of the corpus callosum. H&E stained section (right) demonstrating infiltrating neoplastic astrocytes with elongate nuclei and coarse chromatin in a background of densely fibrillar glial processes. FISH (not shown) demonstrated rearrangement of the MYB gene in this tumor. (C) Subependymal giant cell astrocytoma, WHO grade I, in a tuberous sclerosis patient. Coronal T2-weighted FLAIR MRI (left) demonstrating an intraventricular mass lesion arising from the caudate nucleus. Also seen are multiple regions of FLAIR-hyperintensity within cerebral cortex and subcortical white matter consistent with cortical tubers. H&E stained section (right) demonstrating a solid neoplasm composed of large epithelioid astrocytes with abundant eosinophilic cytoplasm within a densely fibrillar background. (D) Dysembryoplastic neuroepithelial tumor, WHO grade I. Axial T2-weighted FLAIR MRI (left) demonstrating a well circumscribed, cortically based mass lesion in the temporal lobe with internal nodularity. H&E stained section (right) demonstrating a neoplasm of round oligodendrocyte-like cells arranged in linear columns along capillaries and neuronal processes within a mucin-rich stroma containing ‘floating’ neurons (examples of which are highlighted by black arrowheads). (E) Ganglioglioma, WHO grade I, with BRAF V600E mutation. Coronal T2-weighted FLAIR MRI (left) demonstrating a well circumscribed, cortically based solid and cystic mass lesion in the temporal lobe. H&E stained section (right) demonstrating a biphasic tumor composed of neoplastic glial cells admixed with large dysmorphic ganglion cells (highlighted by black arrowheads). Molecular testing (not shown) demonstrated the presence of BRAF V600E mutation in this tumor.
Overall, pilocytic astrocytomas account for approximately 35% of posterior fossa and optic pathway tumors in children [7]. A subset of pilocytic astrocytomas arise in patients with neurofibromatosis type 1 (NF-1) due to germline NF1 mutation that is accompanied by loss of heterozygosity of the remaining wild-type NF1 allele in the tumor. Optic pathway pilocytic astrocytomas occur more commonly among children with NF-1, with a third to a half of patients with optic pathway gliomas being present in NF-1 patients [7]. On the other hand, the vast majority of sporadic pilocytic astrocytomas arising in the posterior fossa/cerebellum of non-NF-1 patients harbor a duplication of the 3′ portion of the BRAF gene encoding the C-terminal kinase domain. This kinase domain is often fused in-frame with the downstream KIAA1549 gene to produce a KIAA1549–BRAF fusion transcript lacking the N-terminal regulatory domain of the BRAF protein. Other less common fusion partners for the duplicated BRAF kinase domain have been described including FAM131B, RNF130, CLCN6 and GNAI1 [9]. Outside of the posterior fossa BRAF duplication and gene fusion is less common, being found in approximately half of cases centered in the diencephalon and cerebral hemispheres. Pilocytic astrocytomas lacking BRAF duplication and gene fusion occasionally harbor the BRAF V600E activating missense mutation (6%), somatic mutations in NF1 (3%) or PTPN11 (2%), activating mutations within the kinase domain of FGFR1 (6%) and gene fusions involving NTRK2 (3%). Indeed, it appears that virtually all pilocytic astrocytomas harbor genetic alterations that activate the Ras–Raf–MEK–ERK signaling pathway [9]. BRAF mutations and the resultant signaling aberrations will be discussed in greater detail below.
Pilomyxoid astrocytoma, WHO grade II, is recognized as a distinct variant of pilocytic astrocytoma with unique histological features and a more aggressive clinical course [10,11]. Similar to pilocytic astrocytoma, pilomyxoid astrocytomas are often solid and cystic in appearance radiographically, but often demonstrate a more prominent solid component [12]. Histologically, pilomyxoid astrocytomas are composed of a neoplastic proliferation of piloid astrocytes in a myxoid stroma and show prominent perivascular arrangement of the tumor cells, while typically lacking Rosenthal fibers and eosinophilic granular bodies [10]. The same KIAA1549–BRAF fusion found in pilocytic astrocytomas has also been identified in a subset of pilomyxoid astrocytomas, albeit less commonly, suggesting that this might be a histologic variant of pilocytic astrocytoma rather than a distinct entity [13].
Pleomorphic xanthoastrocytoma, WHO grade II, is a rare but distinct glial neoplasm with a predilection for arising in the superficial areas of the cerebral cortex, particularly in the temporal lobe. Histologic sections typically show a solid, noninfiltrative glial neoplasm composed of markedly pleomorphic astrocytes with occasional cells containing lipidized cytoplasm along with scattered eosinophilic granular bodies. Genetic analysis has shown that BRAF V600E mutations are very common in these tumors, as are CDKN2A homozygous deletions [13–15]. Radiographically, pleomorphic xanthoastrocytomas are classically peripherally located, well-circumscribed and partially cystic neoplasms, often with enhancing mural nodules [16].
Diffuse astrocytomas, WHO grade II, are infiltrative glial neoplasms in contradistinction to the solid/circumscribed glial tumors described above. Histologically, these tumors are characterized by infiltrating neoplastic astrocytes with elongated and hyperchromatic nuclei with fibrillary glial processes (Figure 1B). Mitotic figures are rare to absent, otherwise a diagnosis of anaplastic astrocytoma (WHO grade III) may be warranted. Diffuse astrocytomas in children appear similar in radiographic appearance to their counterpart diffuse astrocytomas in adult patients, being infiltrative hyperintense lesions on T2/FLAIR MRI. Pediatric-type diffuse astrocytomas are genetically distinct from diffuse astrocytomas arising in adult patients [17], though older teenagers with diffuse astrocytomas arising within the cerebral hemispheres may sometimes have genetic alterations similar to those found in adults [18]. The vast majority of grade II and III infiltrative gliomas arising in adult patients harbor an IDH1 or IDH2 mutation (most commonly the R132H substitution in IDH1), thought to be an early transforming event during gliomagenesis. However, IDH mutations are rare in pediatric low-grade astrocytomas, highlighting one of the distinct differences in pathobiology between these adult and pediatric gliomas [13,19].
Recent genetic analyses of diffuse astrocytomas arising in the cerebral hemispheres of pediatric patients have found rearrangement of MYB or MYBL1 genes, BRAFV600E mutation, and FGFR1 alterations including missense mutations, duplications of the kinase domain, and gene fusions. MYB and MYBL1 encode transcriptional activator proteins, and the rearrangements of these genes in pediatric gliomas typically lead to truncation of their C-terminal negative regulatory domains causing constitutive activation and altered gene transcription [13,20]. The rearrangements of MYB and MYBL1 genes have only been found in PLGGs within the cerebral hemispheres and have not been found in pediatric high-grade gliomas [13].
As compared with infiltrative gliomas arising in the cerebral hemispheres, those arising within midline structures (e.g., thalamus, pons and spinal cord) frequently harbor missense mutations at codon 27 in either of the H3F3A or HIST1H3B genes, encoding the histone H3 variants, H3.3 and H3.1, respectively [21–26]. These missense mutations cause a lysine to methionine substitution, altering a critical site of post-translational modification within these histone H3 variants that leads to altered gene-expression profiles thought to contribute to tumorigenesis [27,28]. A mutant-specific antibody for histone H3-K27M mutant protein has been developed for immunohistochemical use and is now routinely used in surgical neuropathology for the identification of the diffuse midline gliomas with this important molecular alteration [29–31].
Though only 2% of PLGG as a whole harbor the histone H3-K27M mutation [13], this alteration occurs in a significant subset of low-grade and high-grade diffuse gliomas arising in midline structures where it has significant prognostic implications. In a recent study of diffuse midline gliomas, seven of the nine (78%) pediatric cases that displayed low-grade histologic features at time of initial biopsy were found to have the histone H3-K27M mutation [31]. These seven cases included five diffuse intrinsic pontine gliomas, one thalamic glioma and one third ventricular glioma. The patient with thalamic glioma had a subsequent biopsy six months later that demonstrated high-grade histologic features and was classified as WHO grade III. The patient with third ventricular glioma had a subsequent resection 1 month later that demonstrated high-grade histologic features of glioblastoma and was classified as WHO grade IV. Among the five patients with K27M+ pontine gliomas that displayed only low-grade histologic features, all experienced disease course typical of diffuse intrinsic pontine glioma (i.e., progression and death) despite aggressive therapeutic regimens including radiation, chemotherapy and various targeted small molecule therapies. In at least some of these cases, it is hypothesized that the small nature of the biopsy from these tumors, that are located in deep or critical structures within the midline, failed to capture tissue within the tumor containing high-grade histologic features. Alternatively, these biopsies may have occurred early within the course of tumor progression before development of any high-grade histologic features. With the exception of a couple rare case reports of children with K27M+ gliomas with indolent behavior [32,33], the vast majority of K27M+ gliomas in children have aggressive clinical course regardless of the grade of histologic features observed in biopsy specimens. Multiple large studies have corroborated this finding [34,35]. This is in contrast to K27M+ gliomas located in the thalamus of adult patients, where histone H3 status does not uniformly appear to portend worse prognosis [26,32].
Finally, SEGA, WHO grade I, is seen as a solidly enhancing mass within the lateral ventricles without invasion of adjacent brain parenchyma on T1-weighted MRI sequence. Hematoxylin and eosin stain shows these solid neoplasms to be composed of large epithelioid astrocytes containing abundant eosinophilic cytoplasm accompanied by a densely fibrillar background (Figure 1C). SEGA is virtually always associated with the genetic syndrome tuberous sclerosis, resulting from germline mutations in TSC1 or TSC2, and up to 20% of children with tuberous sclerosis develop SEGAs [36].
• Low-grade oligodendrogliomas
Pediatric oligodendrogliomas are infiltrative tumors that may be low-grade (WHO grade II) or anaplastic (WHO grade III). Histologically and radiographically, pediatric oligodendrogliomas are quite similar to the adult type. A recent case series of 50 pediatric oligodendrogliomas reported histologic features of uniform round cells with perinuclear halos and secondary structures such as perineuronal satellitosis in all cases, with a subset of tumors containing calcifications and/or microcysts. However, the genetic profile of pediatric oligodendroglioma appears to be distinct from those oligodendrogliomas arising in adult patients. Whereas virtually all oligodendrogliomas in adult patients have mutation of the IDH1 or IDH2 genes, codeletion of chromosomes 1p and 19q, TERT promoter mutation and mutations in CIC or FUBP1, these alterations are uncommon in their pediatric counterpart, being only present in tumors arising in older teenagers [37]. Whole-genome sequencing has found a duplication of the 3′ portion of the FGFR1 gene encoding the intracellular kinase domain portion of the protein in three of five pediatric oligodendrogliomas [13]. Other smaller series corroborate the lack of 1p19q codeletion in pediatric-type oligodendroglioma, with the presence of 1p19q codeletion occurring only in the ‘adult-type’ usually in older teenagers and young adults [13,38–40]. In contrast to the adult-type, the prognostication by molecular subtype of pediatric oligodendroglioma has been quite difficult given the rarity of the tumor and small patient series present in the literature [37].
• Glioneuronal tumors
Glioneuronal tumors, WHO grade I, are mixed tumors composed of both neoplastic glial and neuronal components. They most commonly arise within the cerebral hemispheres, usually within the temporal lobes, and also have predilection for the cervicomedullary junction. Subtypes of glioneuronal tumors include dysembryoplastic neuroepithelial tumor (DNT), ganglioglioma and desmoplastic infantile ganglioglioma (DIG).
Histologically, DNT is composed of round oligodendrocyte-like cells arranged in linear columns along neuronal processes and capillaries, surrounded by mucin-rich stroma with ‘floating’ neurons. (Figure 1D). The BRAF V600E mutation is present in 15–51% of DNT [41], and FGFR1 alterations are present in 58–82% [14,42]. Radiographically these tumors usually are not associated with mass effect or peritumoral edema. DNT tumors have low or no gadolinium enhancement, and they appear bright on T2-weighted imaging [43].
Ganglioglioma is a biphasic tumor made up of large dysmorphic ganglion cells admixed with neoplastic astrocytes (Figure 1E). The frequency of the BRAF V600E mutation ranges between 18 and 58% among low-grade glioneuronal tumor series [15,44–45]. On MRI, ganglioglioma tumors may be of low intensity on T1-weighted images and hyperintense on T2-weighed images. However, imaging characteristics are largely nonspecific to gangliogliomas, and diagnosis is usually established by histology.
Other neuroepithelial tumors
Other very rare glial neoplasms that may occasionally arise in pediatric patients include astroblastoma and angiocentric glioma. Astroblastomas typically occur in children and adolescents and are usually located within the cerebral hemispheres. They are solid, noninfiltrative neoplasms characterized by glial cells radially arranged vessels with extensive vascular sclerosis and lacking the perivascular fibrillarity seen in ependymomas. The genetic alterations that drive astroblastomas are unknown. Given the rarity of this entity, the biologic behaviors of these tumors are not well understood, and astroblastomas were thus not assigned a grade in the 2007 WHO Classification.
Angiocentric glioma, a WHO grade I tumor, mainly occurs in children and young adults (mean age at diagnosis is 17 years) [46]. Angiocentric glioma was first reported in 2005 [47] and recognized as a distinct clinicopathologic entity in the 2007 WHO classification [46]. Angiocentric glioma is a stable or slow growing cerebral pediatric tumor for which surgical resection is generally curative. Angiocentric gliomas are epileptogenic lesions; most patients have a several year history of presurgical epilepsy. Histologically, this tumor is characterized by an angiocentric pattern of growth, monomorphous bipolar cells, and features of ependymal differentiation. Superficial cerebrocortical location is typical, and on MR imaging angiocentric gliomas are well-delineated solid, T2-hyperintense, nonenhancing cortical lesions that usually extend into the subcortical white matter. There is usually focal enlargement of the affected cortical gyrus, and calcifications are rare. Recently, the MYB–QKI gene fusion was found to be a specific genetic alteration in angiocentric gliomas and was demonstrated to be the single genetic driver of these rare glial tumors [48].
Genetic mutations & cellular signaling aberrations
Though histomorphology has historically guided the diagnosis of PLGG subtypes, integration of histopathology with emerging genomic data is helping to refine PLGG subtypes to provide meaningful prognostic information. Some of the first insights into the molecular pathobiology came from the genetic syndromes NF-1 and tuberous sclerosis. We have come to understand that PLGGs are genetically distinct from low-grade gliomas in adult patients, particularly the infiltrative gliomas. Thus we will focus our discussion here on some of the most important genetic alterations and signaling pathway alterations in the pediatric-type low-grade gliomas. The strong association between the genetic syndromes NF-1 and tuberous sclerosis with PLGG, as well as the fact that early insights into the molecular pathogenesis of PLGG came from understanding these syndromes, merit a discussion of neurofibromatosis and tuberous sclerosis.
• NF-1 & Ras–Raf–MAPK pathway
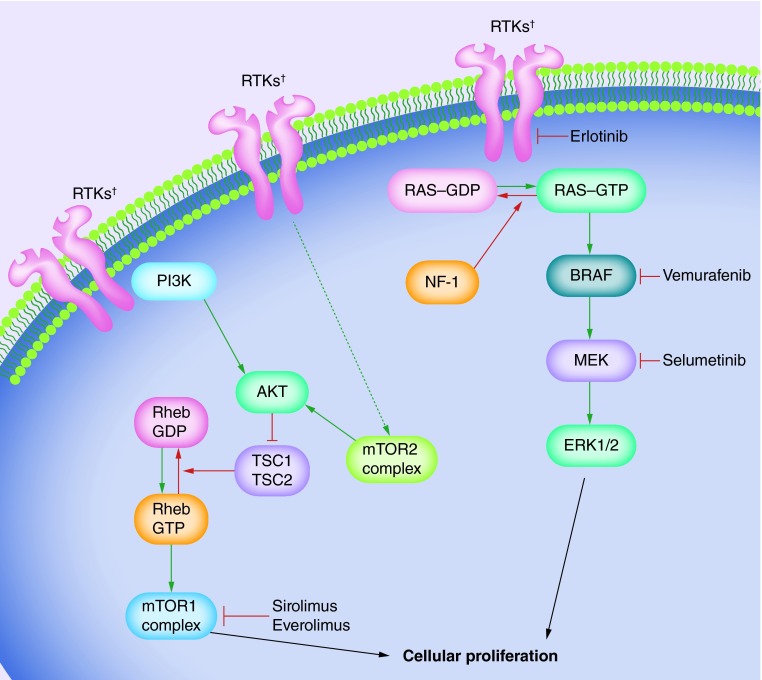
NF-1 is inherited as an autosomal dominant syndrome, characterized by the development of neurofibromas and astrocytomas. The association between low-grade gliomas and NF-1 is strong, with up to 15% of children with NF-1 developing a PLGG before adulthood; the most common being pilocytic astrocytomas and diffuse astrocytomas [49]. The NF-1 syndrome results from mutation of NF1, a tumor suppressor gene residing on chromosome 17q. The majority of NF1 mutations result in protein truncation, causing disruption of its functional domain, Ras–GAP related-domain (Ras–GRD). Ras–GRD accelerates the conversion of the active GTP-bound Ras into its inactive GDP-bound form, downregulating the Raf and PI3K transduction pathways (Figure 2). Truncation of NF-1 and disruption of Ras–GRD results in dysregulation of the Raf and PI3K pathways and promotion of cellular proliferation [50,51].
Figure 2. . BRAF and mTOR signaling pathways shown with targeted therapies.
Green arrows represent activating steps that ultimately lead to cellular proliferation. The dashed green arrow represents indirect activation. Red arrows represent de-activating steps that ultimately inhibit cellular proliferation. Blind-ended arrows represent inhibitory interactions.
†RTKs include EGFR, PDGFRA, NTRK2 and FGFR1.
RTK: Receptor tyrosine kinase.
Indeed, dysregulation of the Ras–Raf–MAP kinase pathway plays an important role in the molecular pathogenesis of PLGG. Within the Ras-Raf-MAP kinase pathway, Raf regulates the MEK/MAP kinase cascade, itself a regulator of cellular differentiation and proliferation (Figure 2) [1,17]. Notably, there has been much attention over the last decade on a specific member of the Raf family, BRAF, that is now recognized as one of the most commonly mutated genes in both pediatric and adult cancers [1,43,52]. There have been two major BRAF genomic alterations characterized in PLGG, the BRAF V600E missense mutation and BRAF gene duplication/fusions.
The BRAF V600E mutation results from replacement of valine by glutamic acid within the activation loop of the enzyme. This substitution mimics phosphorylation of the active cite causing constitutive activation of BRAF kinase domain [43], thus leading to dis-inhibition of the MEK/MAP kinase cascade (Figure 2). The BRAF V600E mutation is sufficient for NIH3T3 fibroblast transformation in vitro [53]. Interestingly, BRAF V600E also promotes proliferative transformation of human neural stem cells followed by senescence, and it has been hypothesized that this ‘oncogene-induced senescence’ may be one underlying mechanism for the low-grade pathogenesis of pilocytic astrocytomas [53,54]. In the whole-genome sequencing study by Zhang and colleagues, BRAF V600E mutations were detected in 70% of pleomorphic xanthoastrocytomas, 23% of diffuse astrocytomas, 33% of gangliogliomas and 6% of pilocytic astrocytomas [13].
In addition to the BRAF V600E missense mutation, genetic duplication/fusion mutations are common in PLGG. The gain of chromosomal region 7q34, that contains the BRAF locus, is the most common copy number alteration in sporadic (non-NF-1 related) PLGG. Tandem insertion of this locus is frequently at the KIAA1549 gene [43,55–56]], and the KIAA1549–BRAF fusion gene codes for a BRAF protein that lacks its auto-inhibitory domain and is thus constitutively active [57]. Over 90% of pilocytic astrocytomas arising in the cerebellum in children without NF-1 have KIAA1549–BRAF gene fusions, whereas approximately half of pilocytic astrocytomas arising outside the cerebellum have the KIAA1549–BRAF fusion [9,13]. The other BRAF fusion transcripts that have been characterized, including GNA11, MACF1, MKRN1, CLCN6, SRGAP3, FAM131B and RNF130, all result in loss of the N-terminal inhibitory domain of BRAF [9,13,58–61], resulting in constitutive activation of the BRAF kinase domain and dysregulation of the downstream MAPK signaling pathway [43].
• Tuberous sclerosis & the mTOR pathway
Tuberous sclerosis results from germline mutations in either of the genes hamartin (TSC1) or tuberin (TSC2) [62–64], and SEGA is strongly associated with tuberous sclerosis. Both TSC1 and TSC2 function together as a tumor suppressor protein complex within the mTOR signaling pathway. The TSC1–TSC2 complex converts active GTP-bound Rheb into its inactive GDP-bound form (Figure 2) [62,65]. Mutations in TSC1 or TSC2 can result in loss of function of the protein complex, resulting in unopposed activation of Rheb-GTP. This disinhibited activation of the mTOR signaling cascade promotes the development of hamartomatous lesions and helps drive the tumorigenesis of SEGAs [62,64].
Sporadic mutations within the mTOR signaling pathway in children without tuberous sclerosis have also been shown to be important in the pathogenesis in PLGG. The PI3K–Akt–mTOR signaling pathway normally integrates both extracellular and intracellular signals to integrate cellular metabolism, proliferation and survival [66]. mTOR is a multiprotein serine–threonine kinase, that itself is composed of two protein complexes, mTORC1 and mTORC2.
In high nutritional states, mTOR undergoes a conformational change that facilitates mTORC1 activation by Rheb. Activated mTORC1 then activates p70S6 kinase that results in formation of phospho-S6 and phospho-4EBP1, driving translation and cellular proliferation (Figure 2). Approximately half of PLGG show enhanced expression of phospho-S6 and phospho-EBP1 [1], and expression of these two proteins is associated with worse progression-free survival [67].
Like mTORC1, the mTORC2 component also responds to cellular nutritional status as well as redox states. As a regulator of cellular proliferation, mTORC2 activates Akt. Akt is a serine–threonine kinase that plays a critical role in cellular metabolism and proliferation, and Akt has been implicated in numerous human cancers. Akt phosphorylation is associated with a more clinically aggressive pilocytic astrocytoma [68].
Both the Ras–Raf–MAPK and mTOR pathways are affected by alterations of the FGFR1 gene that encodes the transmembrane receptor tyrosine kinase FGFR1. A variety of FGFR1 alterations have been found in PLGG including somatic missense mutations, duplication of the 3′ portion of the gene encoding the kinase domain and rearrangement usually involving fusion with TACC genes. These alterations in FGFR1 lead to its constitutive activation of downstream signaling pathways including both Ras–Raf–MEK–ERK and PI3K–Akt–mTOR [13].
• Targeted systemic agents
Elucidation of oncogenic mutations within the Ras–Raf–MAPK and PI3K–AKT–mTOR pathways has led to the development of agents that specifically target oncogenic proteins within these pathways for the treatment of pediatric gliomas. As described above, the BRAF V600E mutation is prevalent among pleomorphic xanthoastrocytomas, diffuse astrocytomas, gangliogliomas and pilocytic astrocytomas. The enzyme inhibitor vemurafenib specifically inhibits BRAF V600E from activating MEK, and has been shown to have strong clinical activity in BRAF V600E-positive melanoma. The clinical success in melanoma has led to great interest in using vemurafenib in other BRAF V600E-positive cancers. A multicenter trial under the auspices of the Pacific Pediatric Neuro-Oncology Consortium (PNOC002) is enrolling children with recurrent or refractory BRAF V600E gliomas to evaluate the safety and pharmacokinetic characteristics of vemurafenib. Dabrafenib is a selective ATP-competitive inhibitor of the BRAF V600E kinase, approved in unresectable or metastatic melanoma with the BRAF V600E mutation. NCT01677741 is currently enrolling children with BRAF V600E-positive relapsed or refractory solid tumors, including high-grade and low-grade gliomas (Table 1). Preliminary results of NCT01677741 demonstrating good tolerability and manageable toxicity were recently presented [69].
Table 1. . Clinical trials for pediatric low-grade gliomas.
| Trial Identifier | Title | Sponsor |
|---|---|---|
|
NCT01748149 (enrolling) |
PNOC002: Safety, Phase 0, and Pilot Efficacy Study of Vemurafenib, an Oral Inhibitor of BRAFV600E, in Children With Recurrent/Refractory BRAFV600E-mutant Gliomas |
Pacific Pediatric Neuro-Oncology Consortium |
|
NCT01089101 (enrolling) |
A Phase 1 and Phase II and Retreatment Study of AZD6244 (PBTC-029B) for Recurrent or Refractory Pediatric Low Grade Glioma |
Pediatric Brain Tumor Consortium/National Cancer Institute |
|
NCT01734512 (enrolling) |
PNOC 001: Phase II Study of Everolimus for Recurrent or Progressive Low-grade Gliomas in Children |
Pacific Pediatric Neuro-Oncology Consortium |
|
NCT01158651 (ongoing, enrollment complete) |
A Phase II Study of RAD001 (Everolimus) for Children With NeurF1 and Chemotherapy-Refractory Radiographic Progressive Low Grade Gliomas |
Pediatric Oncology Experimental Therapeutics Investigators' Consortium |
|
NCT01677741 (enrolling) |
A Study to Determine Safety, Tolerability and Pharmacokinetics of Oral Dabrafenib In Children and Adolescent Subjects |
GlaxoSmithKline |
| NCT00901849 (completed) | Phase 1 Study of Tarceva and Rapamycin For Recurrent Low-Grade Gliomas in Children With or Without Neurofibromatosis Type 1 (NF1) | Children's Research Institute |
Enthusiasm over targeted agents in general should be met with some degree of caution. A Phase II trial of sorafenib, a multikinase inhibitor targeting BRAF, VEGFR, PDGFR and c-kit, was terminated early because of a rapid and unexpectedly high rate of progression in children with PLGGs [70]. In vitro studies suggest this finding may be due to paradoxical activation of ERK by sorafenib. A proportion of BRAF mutated tumors have BRAF mutations other than the V600E missense mutation, including alternative missense mutations, duplications, fusions and deletions that have been shown to decrease the efficacy of BRAF V600E-targeted inhibition. For instance, in cells expressing KIAA1549–BRAF, these fusion kinases function as homodimers that are resistant to PLX4720 (a research analog of vemurafenib) and PLX4720 leads to paradoxical activation of MEK and ERK [71].
However, some tumors harboring BRAF alteration do have sensitivity to MEK inhibition [72]. Trametinib is a MEK inhibitor shown to have clinical activity against melanoma, colorectal, hepatocellular and non-small-cell lung cancers. Selumetinib (AZD6244), another MEK inhibitor, has been shown to have activity against a pilocytic astrocytoma xenograft harboring the BRAF V600E mutation [73]. A Phase I study of AZD6244 by the Pediatric Brain Tumor Consortium (PBTC-029B) has been completed [74], and a Phase II study is currently underway (Table 1). Furthermore, the maximal tolerated dose of selumetinib has been evaluated in children with histologically confirmed recurrent or refractory PLGG under the auspices of the Pediatric Brain Tumor Consortium. In addition, the National Cancer Institute is sponsoring a Phase II trial of selumetinib for children with recurrent or refractory PLGGs (Table 1).
There has also been progress in targeting the mTOR pathway. Sirolimus is an allosteric inhibitor of mTORC1, and the binding of mTORC1 with sirolimus interferes with mTORC1 activation of S6 kinase, itself a regulator of translation. A clinical response to sirolimus in a tuberous sclerosis child with SEGA harboring a TSC2 gene mutation was first reported in 2008 [75]. Everolimus is a derivative of sirolimus and has been used for multiple cancer types in adults [76]. The efficacy of sirolimus was first reported in SEGA in 2006 [77]. Among children with tuberous sclerosis and progressive SEGA tumors, 75% of tumors respond to everolimus [78] and everolimus is now US FDA approved for the treatment of SEGA in children with tuberous sclerosis. Kieran and colleagues reported the results of a prospective Phase II study of everolimus for children with recurrent PLGG after initial treatment with carboplatin-containing chemotherapy regimens. Of the 23 children enrolled, four patients had a partial response (greater than 50% decrease on MRI), 13 had stable disease and six children had progressive disease within 1 year. This trial met the goal of greater than 25% response rate defined a priori for everolimus to be considered a promising regimen for further study [79].
Sirolimus has also been evaluated in combination with erlotinib, an EGF receptor inhibitor. In this feasibility and efficacy study, 19 children with recurrent PLGG received the two-drug regimen. Of these children there was one partial response, five were stable and ten had progressive disease during the planned 1 year of therapy, and three children discontinued therapy due to toxicity or compliance issues. There was tumor stabilization for at least 12 months in six children, and two children experienced tumor control for over 1 year after therapy completion [80].
Kaul and colleagues recently showed that the KIAA1549–BRAF fusion is sufficient to induce glioma-like lesions in vivo in a cell type-specific and mTOR-dependent manner, and mTOR inhibition blocks KIAA1549–BRAF fusion induced S6 activation and proliferation in neural stem cells. These data also provide preclinical evidence for use of mTOR inhibitors for sporadic PLGGs [81]. Overall, there is strong biologic evidence supporting the notion that molecular markers will define PLGG subgroups most likely to respond to mTOR inhibition, and clinical evidence is still being gathered. A PNOC Phase II study of everolimus is enrolling children with recurrent or progressive PLGGs with the aim of seeking a molecular signature that predicts responses to mTOR inhibition (Table 1).
Though not directly compared in clinical trial, BRAF, MEK and mTOR inhibitors appear to have favorable toxicity profiles compared with the chemotherapies commonly used for progressive or recurrent PLGGs. Some of the major toxicity profiles of chemotherapy, including neurotoxicity (vincristine), hypersensitivity (carboplatin), cytopenia (temozolomide, vinblastine, platinum agents), infertility (alkylating agents) and secondary malignancies [82], appear distinct from the targeted agents. In a Phase II trial of everolimus, two patients had to discontinue treatment due to oral sores [79]. Children who received erlotinib combined with sirolimus on a Phase I–II study most commonly experienced grade 1–2 rash (58%), oral apthous ulcers (47%) and diarrhea (37%). No children required removal from this trial due to toxicity [80]. In a Phase I study of dabrafenib, among 29 patients, one child had a dose limiting grade 3 maculopapular rash. The serious adverse events reported as related to dabrafenib included hypotension (one patient), disseminated intravascular coagulation (one patient), fever (one patient) and arthralgia (one patient) [69]. In a Phase I study of the MEK inhibitor, selumetinib, the most common toxicity was rash. Dose-limiting toxicities were headache, rash, mucositis and elevation of amylase and lipase [74].
Conclusion & future perspective
The histologic subtypes of PLGGs are diverse, and the work in high-throughput genetic sequencing and gene expression profiling is adding both clarity and complexity to the way these childhood tumors are being diagnosed, prognosticated and treated. Fortunately, PLGG have excellent prognoses in general, though tumors progressive or recurrent after surgical resection pose significant challenges in management.
Going forward, it will continue to be a fine balance between the benefits of aggressive treatment with minimization of long-term toxicities. Targeted agents, including those that act within the Ras–Raf–MAPKand PI3K–AKT–mTOR pathways, may provide durable disease control for tumors at risk for progression. By augmenting and perhaps replacing chemotherapies and radiotherapy, which both have considerable toxicities, molecularly targeted agents will hopefully transform progressive and incurable PLGG into a chronic manageable disease. The results of current and future clinical trials will be met with anticipation.
EXECUTIVE SUMMARY.
Introduction & histologic classifications & molecular refinements
Pediatric low-grade gliomas (PLGG) are defined as WHO grade I or II. Major histologic subtypes include low-grade astrocytomas, oligodendrogliomas and glioneuronal tumors.
Prognosis of PLGG is generally excellent, though long-term sequelae from tumor, surgery or cytotoxic therapies can be significant.
Low-grade astrocytomas
Pilocytic astrocytomas, well circumscribed cystic tumors that can be cured surgically, are the most common low-grade astrocytoma.
Sporadic pilocytic astrocytomas arising in the posterior fossa harbor BRAF duplication and gene fusion.
Outside the posterior fossa sporadic pilocytic astrocytomas lacking BRAF duplication and gene fusion may harbor the BRAF V600E missense mutation, NF1 or PTPN11 somatic mutations, activating FGFR1 mutations and gene fusions involving NTRK2.
Virtually all pilocytic astrocytomas harbor genetic alterations that activate the Ras–Raf–MEK–ERK signaling pathway.
Diffuse astrocytomas are infiltrative gliomas. Pediatric-type diffuse astrocytomas are genetically distinct from the adult-type, lacking IDH mutations.
Immunohistochemistry for histone H3-K27M mutant protein plays an important role in the diagnosis of midline diffuse gliomas, as this mutation is associated with a poor prognosis.
Low-grade oligodendrogliomas
Pediatric oligodendrogliomas are infiltrative tumors that are histologically and radiographically similar to the adult type.
The genetic profile of pediatric oligodendroglioma is distinct from the adult type.
IDH1/IDH2 mutations, codeletion of chromosomes 1p and 19q, TERT promoter mutation and mutations in CIC or FUBP1 are rare in children.
Glioneuronal tumors
Up to 60% of ganglioglioma tumors harbor the BRAF V600E mutation.
Genetic mutations & cellular signaling aberrations
Integrating histopathology with genomic data is helping to refine pediatric-type low-grade glioma subtypes.
Neurofibromatosis 1 & Ras–Raf–MAPK pathway
Neurofibromatosis 1 (NF-1) is an inherited autosomal dominant syndrome. Up to 15% of NF-1 children develop PLGG.
Majority of NF1 mutations result in protein truncation, causing disruption in its ability to regulate the Ras–Raf–MEK–ERK signaling pathway.
A Raf family member, BRAF, is one of the most commonly mutated genes in human malignancy.
The major BRAF alterations known are the BRAF V600E missense mutation and BRAF gene duplication/fusions. Both are implicated in the pathogenesis of PLGG.
Tuberous sclerosis & the mTOR pathway
Tuberous sclerosis results from germline mutations in TSC1 or TSC2.
Mutations in TSC1 or TSC2 lead to disinhibited activation of the PI3K–Akt–mTOR signaling cascade, promoting SEGA tumorigenesis.
mTORC2 activates Akt, which plays an important role in cell proliferation.
Targeted systemic agents
Agents that target the Ras–Raf–MEK–ERK and PI3K–AKT–mTOR pathways are being developed and tested for use in PLGG.
Vemurafenib specifically inhibits BRAFV600E from activating MEK. Vemurafenib is being evaluated in the multicenter trial PNOC002 for recurrent BRAF V600E-positive PLGG.
Everolimus is an inhibitor of mTORC1 and has resulted in clinical responses in SEGA tumors. Phase II studies are evaluating everolimus for refractory PLGG.
Conclusion & future perspective
Genome sequencing and identification of genetic alterations in PLGG subtypes are changing the way these tumors are diagnosed, prognosticated and managed.
In general PLGG have excellent prognoses, though progressive or recurrent tumors after resection pose significant challenges.
Minimizing long-term sequelae of tumor and treatment is paramount.
Characterizing genetic alterations has led to the rational use of molecular targeted therapies that are in various phase clinical trials.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Dasgupta T, Haas-Kogan DA. The combination of novel targeted molecular agents and radiation in the treatment of pediatric gliomas. Front. Oncol. 2013;3:110. doi: 10.3389/fonc.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wisoff JH, Sanford RA, Heier LA, et al. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children's Oncology Group. Neurosurgery. 2011;68(6):1548–1555. doi: 10.1227/NEU.0b013e318214a66e. [DOI] [PubMed] [Google Scholar]
- 3.Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children's Oncology Group. J. Clin. Oncol. 2012;30(21):2641–2647. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus KJ, Goumnerova L, Billett AL, et al. Stereotactic radiotherapy for localized low-grade gliomas in children: final results of a prospective trial. Int. J. Radiat. Oncol. Biol. Phys. 2005;61(2):374–379. doi: 10.1016/j.ijrobp.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J. Clin. Oncol. 2009;27(22):3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Deley M-C, Vassal G, Taïbi A, Shamsaldin A, Leblanc T, Hartmann O. High cumulative rate of secondary leukemia after continuous etoposide treatment for solid tumors in children and young adults. Pediatr. Blood Cancer. 2005;45(1):25–31. doi: 10.1002/pbc.20380. [DOI] [PubMed] [Google Scholar]
- 7.Gupta N, Banerjee A, Haas-Kogan D. Pediatric CNS Tumors (2nd Edition). Springer Berlin Heidelberg; Berlin, Heidelberg, Germany: 2010. [Google Scholar]
- 8.Coelho J, Nunes S, Salgado D. Spontaneous malignant transformation of a pilocytic astrocytoma of cerebellum case report. Child Neurol. Open. 2015;2(1) doi: 10.1177/2329048X14566813. 2329048X14566813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones DTW, Hutter B, Jäger N, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat. Genet. 2013;45(8):927–932. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Whole-genome sequencing study of 96 pilocytic astrocytomas conducted by the International Cancer Genome Consortium PedBrain Tumor Project.
- 10.Tihan T, Fisher PG, Kepner JL, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J. Neuropathol. Exp. Neurol. 1999;58(10):1061–1068. doi: 10.1097/00005072-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ceppa EP, Bouffet E, Griebel R, Robinson C, Tihan T. The pilomyxoid astrocytoma and its relationship to pilocytic astrocytoma: report of a case and a critical review of the entity. J. Neurooncol. 2007;81(2):191–196. doi: 10.1007/s11060-006-9216-z. [DOI] [PubMed] [Google Scholar]
- 12.Arslanoglu A, Cirak B, Horska A, et al. MR imaging characteristics of pilomyxoid astrocytomas. AJNR Am. J. Neuroradiol. 2003;24(9):1906–1908. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat. Genet. 2013;45(6):602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Whole-genome sequencing study of a large series of PLGG, identifying new gene alterations in BRAF, RAF1, FGFR1, MYB, MYBL1, H3F3A and ATRX?.
- 14.Qaddoumi I, Orisme W, Wen J, et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016 doi: 10.1007/s00401-016-1539-z. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 16.Rippe DJ, Boyko OB, Radi M, Worth R, Fuller GN. MRI of temporal lobe pleomorphic xanthoastrocytoma. J. Comput. Assist. Tomogr. 1992;16(6):856–859. doi: 10.1097/00004728-199211000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Gilheeney SW, Kieran MW. Differences in molecular genetics between pediatric and adult malignant astrocytomas: age matters. Future Oncol. 2012;8(5):549–558. doi: 10.2217/fon.12.51. [DOI] [PubMed] [Google Scholar]; • Review of important molecular differences between pediatric and adult gliomas.
- 18.Cancer Genome Atlas Research Network. Brat DJ, Verhaak RGW, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 2015;372(26):2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 20.Ramkissoon LA, Horowitz PM, Craig JM, et al. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc. Natl Acad. Sci. USA. 2013;110(20):8188–8193. doi: 10.1073/pnas.1300252110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartzentruber J, Korshunov A, Liu X-Y, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 22.Khuong-Quang D-A, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–447. doi: 10.1007/s00401-012-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Gielen GH, Gessi M, Hammes J, Kramm CM, Waha A, Pietsch T. H3F3A K27M mutation in pediatric CNS tumors: a marker for diffuse high-grade astrocytomas. Am. J. Clin. Pathol. 2013;139(3):345–349. doi: 10.1309/AJCPABOHBC33FVMO. [DOI] [PubMed] [Google Scholar]
- 25.Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat. Genet. 2014;46(5):444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aihara K, Mukasa A, Gotoh K, et al. H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro Oncol. 2014;16(1):140–146. doi: 10.1093/neuonc/not144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender S, Tang Y, Lindroth AM, et al. Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell. 2013;24(5):660–672. doi: 10.1016/j.ccr.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Chan K-M, Fang D, Gan H, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27(9):985–990. doi: 10.1101/gad.217778.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bechet D, Gielen GGH, Korshunov A, et al. Specific detection of methionine 27 mutation in histone 3 variants (H3K27M) in fixed tissue from high-grade astrocytomas. Acta Neuropathol. 2014;128(5):733–741. doi: 10.1007/s00401-014-1337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venneti S, Santi M, Felicella MM, et al. A sensitive and specific histopathologic prognostic marker for H3F3A K27M mutant pediatric glioblastomas. Acta Neuropathol. 2014;128(5):743–753. doi: 10.1007/s00401-014-1338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon DA, Wood MD, Tihan T, et al. Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 2015 doi: 10.1111/bpa.12336. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Pathology series describing morphologic spectrum of diffuse midline gliomas with H3-K27M mutation.
- 32.Feng J, Hao S, Pan C, et al. The H3.3 K27M mutation results in a poorer prognosis in brainstem gliomas than thalamic gliomas in adults. Hum. Pathol. 2015;46(11):1626–1632. doi: 10.1016/j.humpath.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen AT, Colin C, Nanni-Metellus I, et al. Evidence for BRAF V600E and H3F3A K27M double mutations in paediatric glial and glioneuronal tumours. Neuropathol. Appl. Neurobiol. 2015;41(3):403–408. doi: 10.1111/nan.12196. [DOI] [PubMed] [Google Scholar]
- 34.Korshunov A, Ryzhova M, Hovestadt V, et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015;129(5):669–678. doi: 10.1007/s00401-015-1405-4. [DOI] [PubMed] [Google Scholar]
- 35.Buczkowicz P, Bartels U, Bouffet E, Becher O, Hawkins C. Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol. 2014;128(4):573–581. doi: 10.1007/s00401-014-1319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adriaensen MEAPM, Schaefer-Prokop CM, Stijnen T, Duyndam DAC, Zonnenberg BA, Prokop M. Prevalence of subependymal giant cell tumors in patients with tuberous sclerosis and a review of the literature. Eur. J. Neurol. 2009;16(6):691–696. doi: 10.1111/j.1468-1331.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez FJ, Tihan T, Lin D, et al. Clinicopathologic features of pediatric oligodendrogliomas: a series of 50 patients. Am. J. Surg. Pathol. 2014;38(8):1058–1070. doi: 10.1097/PAS.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Series that demonstrated oligodendrogliomas with classic histology occur in children but often lack 1p19q codeletion and IDH1 R132H mutations.
- 38.Suri V, Jha P, Agarwal S, et al. Molecular profile of oligodendrogliomas in young patients. Neuro Oncol. 2011;13(10):1099–1106. doi: 10.1093/neuonc/nor146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreiger PA, Okada Y, Simon S, Rorke LB, Louis DN, Golden JA. Losses of chromosomes 1p and 19q are rare in pediatric oligodendrogliomas. Acta Neuropathol. 2005;109(4):387–392. doi: 10.1007/s00401-004-0976-2. [DOI] [PubMed] [Google Scholar]
- 40.Myal Y, Del Bigio MR, Rhodes RH. Age-related differences in 1p and 19q deletions in oligodendrogliomas. BMC Clin. Pathol. 2003;3(1):6. doi: 10.1186/1472-6890-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee D, Cho YH, Kang SY, Yoon N, Sung CO, Suh Y-L. BRAF V600E mutations are frequent in dysembryoplastic neuroepithelial tumors and subependymal giant cell astrocytomas. J. Surg. Oncol. 2015;111(3):359–364. doi: 10.1002/jso.23822. [DOI] [PubMed] [Google Scholar]
- 42.Rivera B, Gayden T, Carrot-Zhang J, et al. Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors. Acta Neuropathol. 2016:1–17. doi: 10.1007/s00401-016-1549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergthold G, Bandopadhayay P, Bi WL, et al. Pediatric low-grade gliomas: how modern biology reshapes the clinical field. Biochim. Biophys. Acta. 2014;1845(2):294–307. doi: 10.1016/j.bbcan.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dahiya S, Haydon DH, Alvarado D, Gurnett CA, Gutmann DH, Leonard JR. BRAF V600E mutation is a negative prognosticator in pediatric ganglioglioma. Acta Neuropathol. 2013;125(6):901–910. doi: 10.1007/s00401-013-1120-y. [DOI] [PubMed] [Google Scholar]
- 45.Koelsche C, Wöhrer A, Jeibmann A, et al. Mutant BRAF V600E protein in ganglioglioma is predominantly expressed by neuronal tumor cells. Acta Neuropathol. 2013;125(6):891–900. doi: 10.1007/s00401-013-1100-2. [DOI] [PubMed] [Google Scholar]
- 46.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang M, Tihan T, Rojiani AM, et al. Monomorphous angiocentric glioma: a distinctive epileptogenic neoplasm with features of infiltrating astrocytoma and ependymoma. J. Neuropathol. Exp. Neurol. 2005;64(10):875–881. doi: 10.1097/01.jnen.0000182981.02355.10. [DOI] [PubMed] [Google Scholar]
- 48.Bandopadhayay P, Ramkissoon LA, Jain P, et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat. Genet. 2016;48(3):273–282. doi: 10.1038/ng.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hernáiz Driever P, Hornstein von S, Pietsch T, et al. Natural history and management of low-grade glioma in NF-1 children. J. Neurooncol. 2010;100(2):199–207. doi: 10.1007/s11060-010-0159-z. [DOI] [PubMed] [Google Scholar]
- 50.Le LQ, Parada LF. Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene. 2007;26(32):4609–4616. doi: 10.1038/sj.onc.1210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costa RM, Federov NB, Kogan JH, et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415(6871):526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- 52.Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505(7484):495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raabe EH, Lim KS, Kim JM, et al. BRAF activation induces transformation and then senescence in human neural stem cells: a pilocytic astrocytoma model. 2011;17(11):3590–3599. doi: 10.1158/1078-0432.CCR-10-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacob K, Quang-Khuong D-A, Jones DTW, et al. Genetic aberrations leading to MAPK pathway activation mediate oncogene-induced senescence in sporadic pilocytic astrocytomas. 2011;17(14):4650–4660. doi: 10.1158/1078-0432.CCR-11-0127. [DOI] [PubMed] [Google Scholar]
- 55.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl Acad. Sci. USA. 2003;100(25):15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacob K, Albrecht S, Sollier C, et al. Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours. Br. J. Cancer. 2009;101(4):722–733. doi: 10.1038/sj.bjc.6605179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones DTW, Kocialkowski S, Liu L, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forshew T, Tatevossian RG, Lawson ARJ, et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J. Pathol. 2009;218(2):172–181. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 59.Lin A, Rodriguez FJ, Karajannis MA, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549:BRAF fusion variants. J. Neuropathol. Exp. Neurol. 2012;71(1):66–72. doi: 10.1097/NEN.0b013e31823f2cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones DTW, Kocialkowski S, Liu L, Pearson DM, Ichimura K, Collins VP. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene. 2009;28(20):2119–2123. doi: 10.1038/onc.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cin H, Meyer C, Herr R, et al. Oncogenic FAM131B–BRAF fusion resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway activation in pilocytic astrocytoma. Acta Neuropathol. 2011;121(6):763–774. doi: 10.1007/s00401-011-0817-z. [DOI] [PubMed] [Google Scholar]
- 62.Hargrave D. Paediatric high and low grade glioma: the impact of tumour biology on current and future therapy. Br. J. Neurosurg. 2009;23(4):351–363. doi: 10.1080/02688690903158809. [DOI] [PubMed] [Google Scholar]
- 63.van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277(5327):805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 64.Reuss D, Deimling von A. Hereditary tumor syndromes and gliomas (chapter 5) In: Deimling von A, editor. Gliomas. Springer Berlin Heidelberg; Berlin, Heidelberg, Germany: 2009. pp. 83–102. [Google Scholar]
- 65.Rosner M, Hanneder M, Siegel N, Valli A, Hengstschläger M. The tuberous sclerosis gene products hamartin and tuberin are multifunctional proteins with a wide spectrum of interacting partners. Mutat. Res. 2008;658(3):234–246. doi: 10.1016/j.mrrev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Hassan B, Akcakanat A, Holder AM, Meric-Bernstam F. Targeting the PI3-kinase/Akt/mTOR signaling pathway. Surg. Oncol. Clin. N. Am. 2013;22(4):641–664. doi: 10.1016/j.soc.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int. J. Mol. Sci. 2012;13(2):1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodriguez EF, Scheithauer BW, Giannini C, et al. PI3K/AKT pathway alterations are associated with clinically aggressive and histologically anaplastic subsets of pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):407–420. doi: 10.1007/s00401-010-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kieran MW, Hargrave DR. American Society of Clinical Oncology Annual Meeting. Chicago, IL, USA: 29 May–2 June 2015. Phase 1 study of dabrafenib in pediatric patients with relapsed or refractory BRAF V600E high- and low-grade gliomas, Langerhans cell histiocytosis, and other solid tumors. Presented at. [Google Scholar]
- 70.Karajannis MA, Legault G, Fisher MJ, et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol. 2014;16(10):1408–1416. doi: 10.1093/neuonc/nou059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sievert AJ, Lang S-S, Boucher KL, et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc. Natl Acad. Sci. USA. 2013;110(15):5957–5962. doi: 10.1073/pnas.1219232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 2012;367(18):1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kolb EA, Gorlick R, Houghton PJ, et al. Initial testing (stage 1) of AZD6244 (ARRY-142886) by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer. 2010;55(4):668–677. doi: 10.1002/pbc.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Banerjee A, Jakacki R, Onar-Thomas A, et al. American Society of Clinical Oncology Annual Meeting. Chicago, IL, USA: 30 May–3 June 2014. A Phase 1 study of AZD6244 in children with recurrent or refractory low-grade gliomas: a Pediatric Brain Tumor Consortium report. Presented at. [Google Scholar]
- 75.Koenig MK, Butler IJ, Northrup H. Regression of subependymal giant cell astrocytoma with rapamycin in tuberous sclerosis complex. J. Child Neurol. 2008;23(10):1238–1239. doi: 10.1177/0883073808321764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled Phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 77.Franz DN, Leonard J, Tudor C, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann. Neurol. 2006;59(3):490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 78.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N. Engl. J. Med. 2010;363(19):1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]; •• Phase I–II study demonstrating significant response of SEGA to everolimus.
- 79.Kieran MW, Yao X, Macy M, et al. Final results of a prospective multi-institutional phase II study of everolimus (RAD001), an mTOR inhibitor, in pediatric patients with recurrent or progressive low-grade glioma. A POETIC Consortium Trial. Neuro Oncol. 2014;16(Suppl. 3):iii27–iii27. [Google Scholar]
- 80.Yalon M, Rood B, MacDonald TJ, et al. A feasibility and efficacy study of rapamycin and erlotinib for recurrent pediatric low-grade glioma (LGG) Pediatr. Blood Cancer. 2013;60(1):71–76. doi: 10.1002/pbc.24142. [DOI] [PubMed] [Google Scholar]
- 81.Kaul A, Chen Y-H, Emnett RJ, Dahiya S, Gutmann DH. Pediatric glioma-associated KIAA1549:BRAF expression regulates neuroglial cell growth in a cell type-specific and mTOR-dependent manner. Genes Dev. 2012;26(23):2561–2566. doi: 10.1101/gad.200907.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nageswara Rao AA, Packer RJ. Advances in the management of low-grade gliomas. Curr. Oncol. Rep. 2014;16(8):398–398. doi: 10.1007/s11912-014-0398-9. [DOI] [PubMed] [Google Scholar]