Supplemental Digital Content is Available in the Text.
Key Words: human, human immunodeficiency virus, Mycobacterium tuberculosis, interferon-gamma release assay, tuberculosis-associated immune reconstitution inflammatory syndrome, latent tuberculosis, cytokines/chemokines
Abstract
Objectives:
To investigate whether mycobacterial antigen–induced cytokine secretions are helpful in detecting Mycobacterium tuberculosis (Mtb) infection in a cohort of HIV-infected patients living in a country with a high burden of Mtb and HIV infections, and to determine their predictive value for the development of tuberculosis (TB)-associated immune reconstitution inflammatory syndrome.
Design:
A total of 352 HIV-infected patients (186 with active TB) were prospectively enrolled when initiating antiretroviral therapy (ART). Sequential blood samples were collected during the first 6 months of ART. Eighty-three HIV-uninfected subjects (39 with active TB) were enrolled as controls.
Methods:
The concentrations of 13 cytokines were measured in supernatants from blood mononuclear cells in vitro stimulated with purified protein derivative (PPD), heparin-binding hemagglutinin (HBHA) or early secreted antigen-6 (ESAT-6) and culture filtrate protein-10 (CFP-10), and results were compared with those of tuberculin skin tests (TST).
Results:
The best detection of Mtb infection was achieved by ESAT-6/CFP-10–induced interferon-γ concentrations, but results were often negative for patients with CD4+ T-cell counts <50 per cubic millimeters. Patients with active TB were identified by high ESAT-6/CFP-10–induced interleukin-6. Conversions of interferon-γ-release assays (IGRA) and TST occurred under ART, and combined TB and antiretroviral treatments of coinfected patients resulted in a decrease of ESAT-6/CFP-10–induced and an increase of HBHA-induced interferon-γ responses. No Mtb antigen–induced cytokines allowed us to predict TB–immune reconstitution inflammatory syndrome or ART-associated TB.
Conclusions:
In Uganda, ESAT-6/CFP-10–IGRA is better in detecting Mtb infection than TST and, when combined with an HBHA–IGRA, could help to evaluate anti-TB treatment success.
INTRODUCTION
Tuberculosis (TB) is a global leading cause of morbidity and mortality due to infection, second only to HIV/AIDS.1 Most Mycobacterium tuberculosis (Mtb)–infected individuals remain asymptomatic, ie, have latent tuberculosis infection (LTBI), whereas 5%–10% of them develop active clinical TB.2 This proportion is higher in HIV-infected persons, HIV infection being an important risk factor for the development of active TB (aTB) among subjects with LTBI.3 Furthermore, a major complication of dual HIV–Mtb infection is the development of an immune reconstitution inflammatory syndrome (IRIS).4 The World Health Organization estimates that 13% of deaths among patients with AIDS worldwide are related to TB.1
Detection of LTBI among HIV-infected people for preventive therapy is one of the strategies that has been implemented because it reduces the risk of progression to aTB.1,5 However, this strategy is poorly implemented,6 mainly as a consequence of the lack of a gold-standard LTBI diagnostic tool, risks related to treatment toxicity, and interference with antiretroviral treatment (ART).
The tuberculin skin test (TST) is the established method for LTBI detection, but it performs poorly in immune-compromised patients.7 Short-term interferon-gamma release assays (IGRA) have been approved in many countries as alternative methods to TST, because both T-SPOT.TB (Oxford Immunotec Global PLC, Marlborough, MA) and QuantiFERON-TB Gold In-Tube (Qiagen, Hilden, Germany) assays were reported to provide at least equal sensitivities, but improved specificities over TST for LTBI diagnosis in immune-competent persons.8,9 However, available data have not yet clearly demonstrated that IGRA have a clear across-the-board advantage over TST in immune-compromised patients.10 More studies in these populations, as well as the development of new and improved assays are needed. Longer incubation time IGRA11,12 and/or IGRA in response to additional/alternative Mtb antigens provides potential alternatives. We previously reported promising results of an in-house IGRA based on heparin-binding hemagglutinin (HBHA)–induced interferon-gamma (IFN-γ) secretions (HBHA–IGRA) to detect subjects with LTBI among immune-competent adults living in a low-TB-incidence country.13,14 We further reported on the added value of this test to detect in the same settings LTBI among immune-suppressed patients under hemodialysis for end-stage renal disease15 and HIV-infected subjects.16
The aim of the present study was to evaluate, for the first time in a high-TB-incidence setting, the potential contribution of an HBHA-based assay to LTBI screening in HIV-infected patients. A 3-day incubation time was chosen to get optimal sensitivity, and results obtained in response to HBHA were compared with those in response to other mycobacterial antigens [purified protein derivative (PPD), early secreted antigen-6 (ESAT-6), culture filtrate protein-10 (CFP-10)], and with the TST results. Cytokine/chemokine analyses were further performed on the culture supernatants of these IGRA to identify alternative read-outs other than IFN-γ that might increase the sensitivity of the test. The performance of the different IGRA for predicting TB-associated IRIS (TB–IRIS) and ART-associated TB (AATB) was also evaluated, because this might be useful to decide whether to delay ART initiation in order to avoid potentially lethal TB–IRIS.
METHODS
Ethical Approval
It was obtained from the Infectious Disease Institute Scientific Review Committee, Makerere Faculty of Medicine Ethics Committee, the Uganda National Council on Science and Technology, the ethics committees of Mulago Hospital (Kampala, Uganda) and ULB-Hôpital Erasme (Brussels, Belgium). Informed consent was obtained from all study participants.
Study Population
A total of 352 HIV-infected patients were prospectively included in this study. Cohort characteristics were previously described17–20 because patients were part of an observational study conducted at the Mulago Hospital and at the Infectious Disease Institute, where they were screened for study eligibility to be included for ART initiation. The patients were divided according to their TB infection status (with or without aTB, respectively, 186 and 166 patients). In case of aTB, they were further subdivided into 2 groups according to the duration of anti-TB therapy at enrollment (more or less than 5 days, for 151 and 35 patients, respectively). Patients were followed up for at least 6 months to monitor paradoxical TB–IRIS or AATB, unless the patient died before the end of the study. HIV-uninfected subjects with or without aTB (respectively, 39 and 44 patients) were also included in this study. Patients' baseline characteristics are summarized in Figure 1 and detailed in Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A799. For HIV-infected patients, a follow-up IGRA was performed for most of them at 1, 2, and 6 months after ART initiation. TST was also performed at enrollment and during follow-up if initially negative with a positivity cutoff of ≥5 mm induration. For HIV-uninfected subjects, LTBI diagnosis was based on TST positivity (≥10 mm induration) with no symptoms and normal chest radiography, whereas diagnosis of aTB was based on positive Mtb culture.
FIGURE 1.
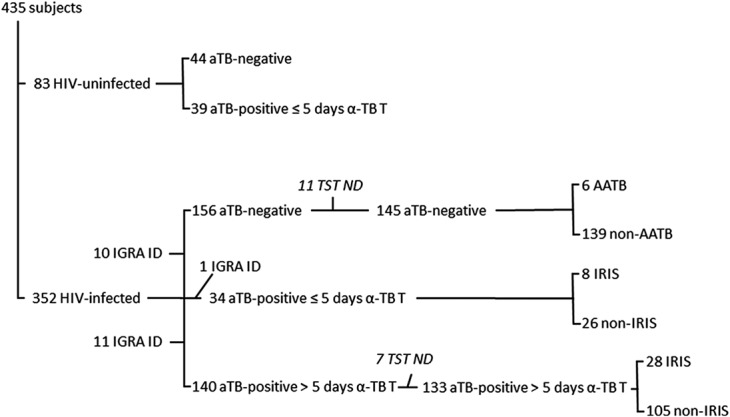
Flow chart of the patient's enrollment and clinical classification. ID, indeterminate; ND, not done; α-TB T, anti-TB treatment.
Interferon-γ Release Assays
IGRA were performed as previously described13 with slight modifications (see Methods 1, Supplemental Digital Content, http://links.lww.com/QAI/A799). Results were considered indeterminate in case of low phytohemagglutinin (PHA)-induced IFN-γ concentrations (<100 pg/mL), or of high IFN-γ secretion in the unstimulated condition.
Multiplex Analysis
For selected subjects, multiple analytes [interleukin (IL)-1β, IL-1RA, IL-2, IL-6, IL-7, IL-10, IL-12p70, IL-13, IL-17, IP-10, tumor necrosis factor-α, and monocyte chemotactic protein (MCP)-1] were quantified in the IGRA supernatants using MILLIPLEX MAP panels (Merck Millipore, Darmstadt, Germany) following the manufacturers' instructions. Results were analyzed with a Luminex 200 system (Luminex, MV's Hertogenbosch, The Netherlands) and Bio-Plex Manager Software (Bio-Rad Laboratories, Nazareth Eke, Belgium). When detectable, the analyte concentrations of the antigen-free conditions were subtracted from those obtained with antigen stimulations.
Statistical Methods
Results were analyzed using GraphPad Prism version 5.04. To compare continuous variables between groups, a Mann–Whitney U Test or Kruskal–Wallis test was used. The degree of agreement between categorical variables was quantified using kappa test, while correlations were analyzed by the Spearman test. A P value < 0.05 was considered significant.
RESULTS
Detection of LTBI and aTB in HIV-Uninfected Subjects
Results from IGRA performed on blood samples from 44 HIV-uninfected subjects were compared with those of the TST. TST identified 30/44 (68%) subjects as having LTBI (Fig. 2A, and Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A799). These subjects with LTBI were characterized by higher PPD-induced and ESAT-6/CFP-10–induced IFN-γ concentrations compared with non–Mtb-infected TST-negative subjects (P ≤ 0.0001), whereas no significant difference between the 2 groups was found for the HBHA-induced IFN-γ concentrations. Only half of the subjects with LTBI responded to HBHA (Fig. 2B). However, as the results of the PPD–IGRA are influenced by previous bacille Calmette-Guérin vaccination21 in contrast to those of ESAT-6/CFP-10–IGRA and HBHA–IGRA,13,21,22 we further focused on these 2 IGRA. Receiver operating characteristic (ROC) curves analyses comparing results obtained for TST-positive and TST-negative subjects provided sensitivities of 78% and 47%, and specificities of 86% and 93% for the ESAT-6/CFP-10–IGRA and the HBHA–IGRA, respectively, for the selected cutoffs (see Table S2, Supplemental Digital Content, http://links.lww.com/QAI/A799). Combining the results of these 2 IGRA allowed us to detect 25/30 (83%) LTBI TST-positive subjects (Fig. 2A). Five TST-positive subjects were IGRA-negative, 4 of them being bacille Calmette-Guérin vaccinated, whereas 2/14 TST-negative subjects were IGRA-positive.
FIGURE 2.
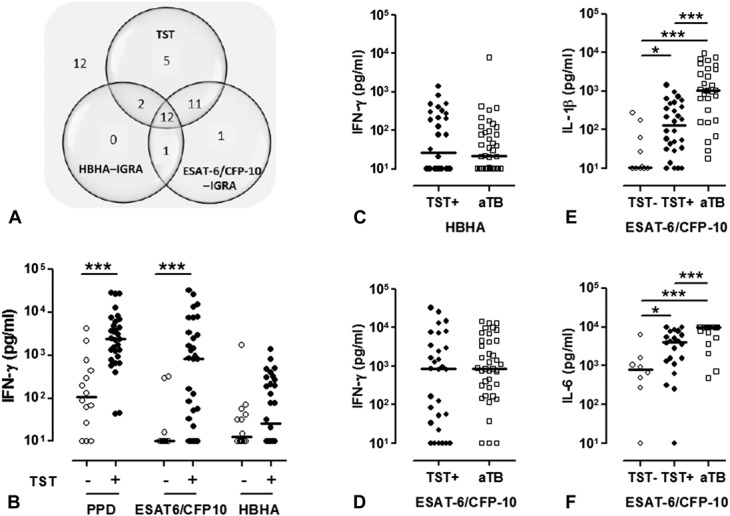
TST and mycobacterial antigens–induced cytokine secretions in the HIV-uninfected control groups. A, HIV-uninfected subjects without aTB (n = 44) were classified according to positive TST, HBHA–IGRA, or ESAT-6/CFP-10–IGRA results. For each test, the number of positive subjects is noted in the corresponding circle of the Venn diagram, with overlaps representing matching results. Number of subjects with negative results for the 3 tests is indicated outside the diagram. B, PPD-induced, a mixture of ESAT-6–induced and CFP-10–induced, and HBHA-induced IFN-γ concentrations were measured in the 72-hour cell culture supernatants, and the results were compared between TST-negative and TST-positive HIV-uninfected subjects without aTB. White dots represent TST-negative subjects (n = 14) and black dots TST-positive subjects (n = 30). C, HBHA-induced and (D) ESAT-6/CFP-10–induced IFN-γ concentrations were measured in the 72-hour cell culture supernatants from HIV-uninfected TST-positive subjects without active TB (black dots, n = 30) and from HIV-uninfected patients with aTB (white squares, n = 39). ESAT-6/CFP-10–induced (E) IL-1β or (F) IL-6 concentrations were measured in the 72-hour cell culture supernatants from HIV-uninfected patients without active TB who were TST-negative (white dots, n = 14) or TST-positive (black dots, n = 25) and from HIV-uninfected patients with aTB (white squares, n = 29) patients. Individual background cytokine secretions were subtracted before being represented on the figures. Horizontal lines represent the medians of cytokine concentrations. *P ≤ 0.05; ***P ≤ 0.001.
To evaluate the diagnostic performance of in-house IGRA to identify patients with aTB, 39 HIV-uninfected patients with untreated pulmonary aTB were included (Fig. 1 and Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A799). TST testing was not performed, but 92% of these patients had at least one positive IGRA. The only patient with a negative IGRA also had a low IFN-γ response to PHA, and 2 patients had indeterminate results. Measuring the Mtb antigens–induced IFN-γ concentrations did not allow us to differentiate subjects with aTB from subjects with LTBI (Figs. 2C, D). We therefore further evaluated the potential added value of 12 additional cytokines. High ESAT-6/CFP-10–induced IL-1β and IL-6 concentrations were found to be specific for untreated aTB, allowing us to differentiate them from both Mtb-uninfected and Mtb-infected subjects with 97% and 93% specificity for IL-1β and IL-6, respectively (Figs. 2E, F, and Table S2, Supplemental Digital Content, http://links.lww.com/QAI/A799). Positive predictive values for aTB reached 94.4% for IL-1β and 90.5% for IL-6, with sensitivities of 59% for IL-1β and 73% for IL-6. However, even though the ESAT-6/CFP-10–induced IL-1β and IL-6 concentrations were higher for TST-positive compared with TST-negative subjects, they did not improve the detection of LTBI over the IFN-γ concentrations. Similarly, no ESAT-6/CFP-10–induced or HBHA-induced cytokines other than IFN-γ were helpful (see Table S3, Supplemental Digital Content, http://links.lww.com/QAI/A799).
Detection of Mtb Infection in HIV-Infected, aTB-Treated Patients
We next compared IGRA with TST results in HIV-infected patients treated for aTB (median and range 22 and 6–163 days, respectively), a population whose infection returned to a quiescent phase.23 ESAT-6/CFP-10–IGRA and HBHA–IGRA were performed for 133 patients who also underwent TST (Fig. 1 and Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A799). TST and both IGRA were negative for 59 patients (44.4%) (Fig. 3A). TST was positive for 31.6% (42/133) of the patients, whereas 41.3% (55/133) of them were detected with the ESAT-6/CFP-10–IGRA and 21.8% (29/133) with the HBHA–IGRA. Combining the results of both IGRA allowed us to detect 51.9% (69/133) of the HIV-infected patients treated for aTB, among which 53.6% (37/69) also had a positive TST. No correlation was found between the TST size and the HBHA-induced or ESAT-6/CFP-10–induced IFN-γ concentrations (r = 0.44 for both IGRA). No significant difference was found between patients with pulmonary (n = 109) and extrapulmonary aTB (n = 20) because similar proportions of patients were detected as Mtb-infected in both groups (P = 0.65 and 0.68 for HBHA–IGRA and ESAT-6/CFP-10–IGRA, respectively).
FIGURE 3.
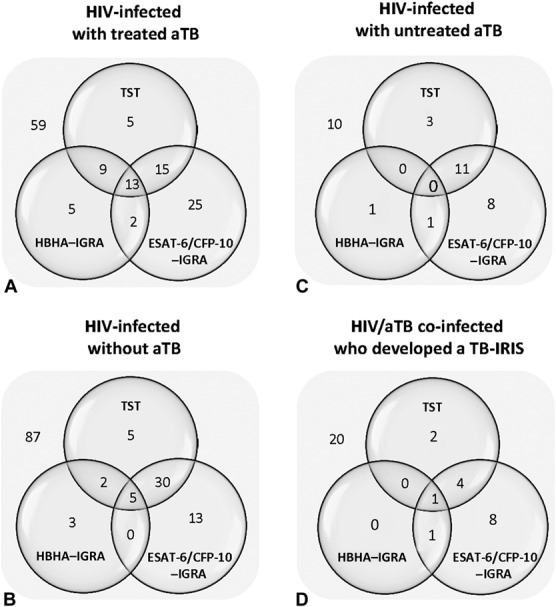
TST and IGRA characteristics of HIV-infected subgroups. Venn diagrams illustrated the numbers of TST and/or IGRA responders among (A) HIV-infected patients with treated aTB, (B) HIV-infected patients without aTB, (C) HIV-infected with untreated aTB, (D) and HIV/aTB coinfected patients who developed TB–IRIS. For each test, the number of subjects with a positive result is noted in the corresponding circle of the Venn diagram, with overlaps representing matching results. Number of subjects with negative results for the 3 tests is indicated outside the diagram.
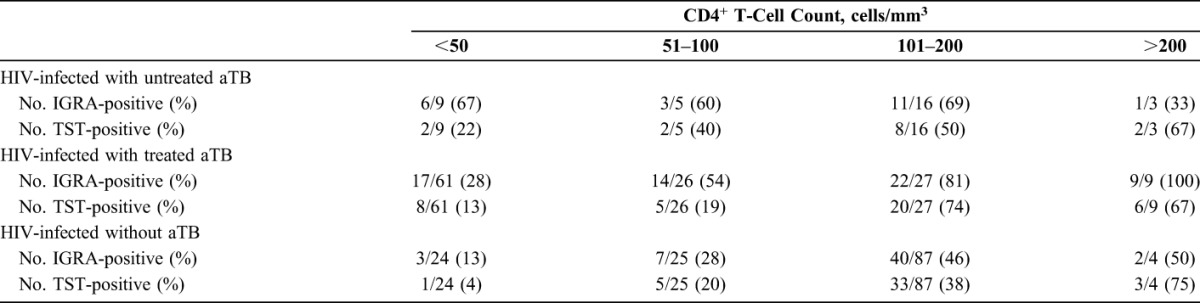
Negative IGRA were essentially found in the patients with CD4+ T-cell counts <50 cells per cubic millimeter (Table 1), and 10/11 indeterminate results were equally found in this patient group. Similarly, higher proportion of negative TST was found in HIV-infected patients with low CD4+ T-cell counts (<100/mm3) (Table 1).
TABLE 1.
Positive Results of TST and IGRA According to CD4+ T-Cell Counts
Detection of LTBI in HIV-Infected Subjects
The results of the TST and both IGRA were available for 145 of the 166 included patients who did not present clinical signs of aTB at baseline. Sixty percent (87/145) had negative TST and IGRA, whereas 40% (58/145) had at least one positive test. Only 5 individuals (3.5%) were positive for all 3 tests (Fig. 3B).
TST was only positive for 42 subjects (29%), whereas combining the results of both IGRA allowed us to detect 53 subjects as Mtb-infected (36.5%), 16 of them being TST-negative (Fig. 3B). TST and IGRA results were in agreement for 124/145 subjects with a kappa coefficient of 0.67, but poor to fair correlations were noted between the TST size and the HBHA-induced and ESAT-6/CFP-10–induced IFN-γ levels (r = 0.32 for HBHA–IGRA and 0.66 for ESAT-6/CFP-10–IGRA). When CD4+ T-cell counts were classified by ordinal variables, numbers of IGRA-positive and TST-positive individuals increased for higher CD4+ T-cell numbers (Table 1).
Most patients underwent a clinical follow-up at 1, 2, and/or 6 months after the start of ART.19 TST and IGRA were repeated for 71/87 patients who were initially TST-negative and IGRA-negative. TST conversion was noted for 9 (12.7%) of them, whereas 27/71 (38%) patients, including 4 of the TST converters, became IGRA-positive 1 or 2 months after ART initiation. These TST and/or IGRA conversions were not related to the significant increase of CD4+ T-cell counts (P < 0.01) that occurred after 6 months of ART for the whole group. Among the 13/16 subjects who were TST-negative and IGRA-positive at enrollment, TST conversion occurred for 5 of them. Finally, for 4 of the 5 subjects who were initially TST-positive and IGRA-negative, the IGRA became positive 1 or 2 months later. Considering these conversions, 69.8% of the HIV-infected subjects may be considered as having LTBI because they were positive at least for 1 of the 3 tests at month 6 post-ART.
As 87/145 (60%) subjects were classified as not having LTBI (negative IGRA and TST) at enrollment before starting ART, and as the follow-up indicated that 32 of them in fact had LTBI, other cytokine/chemokine concentrations were further measured in the IGRA supernatants collected at enrollment from 8 of them and compared with results obtained for 22 subjects with LTBI as defined by a positive TST (IGRA-positive for 19 of them) and with results for 10 subjects with LTBI as defined by a positive IGRA (and TST-negative). TNF-α (Fig. 4B), IL-1β, IL-1RA, IL-6, IL-10, and MCP-1 (data not shown), in contrast to IFN-γ (Fig. 4A), were detectable at enrollment, in response to ESAT-6/CFP-10, in most supernatants from subjects with LTBI not detected with the IGRA and/or TST, with similar concentrations as those obtained for TST-positive or IGRA-positive subjects.
FIGURE 4.
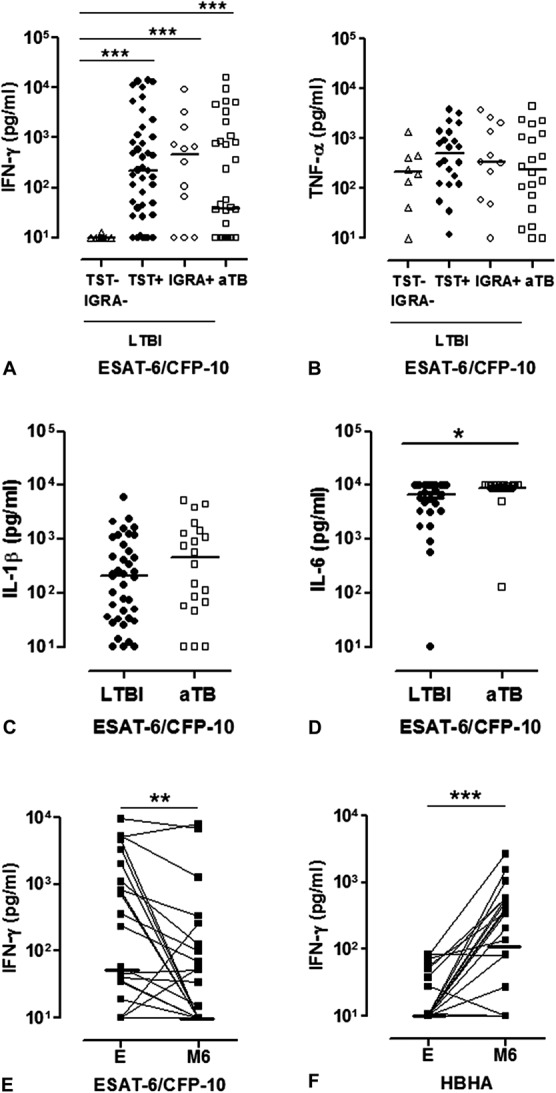
Mycobacterial antigen–induced cytokine secretions by the PBMC from HIV-infected subjects at enrollment and after 6 months of anti-TB and ART. ESAT-6/CFP-10–induced (A) IFN-γ and (B) tumor necrosis factor-α concentrations were measured in the 72-hour cell culture supernatants, and results were compared between patients classified by the TST and IGRA results as Mtb-uninfected at enrollment, but with TST and/or IGRA conversion during follow-up (white triangles); patients classified as LTBI based on the TST (black dots) or on the IGRA (white dots) results at enrollment; and those identified as having aTB (white squares). ESAT-6/CFP-10–induced (C) IL-1β and (D) IL-6 concentrations were measured in 72-hour cell culture supernatants and compared between subjects with LTBI (TST and/or IGRA-positive at enrollment or during follow-up, black dots) and patients with untreated aTB (white squares). E, ESAT-6/CFP-10–induced and (F) HBHA-induced IFN-γ concentrations were measured in the 72-hour cell culture supernatants of HIV-infected patients with untreated aTB (black squares) at enrollment (E) and after 6 months of anti-TB therapy and ART (M6). Values obtained at enrollment are connected with those obtained at month 6. Horizontal lines represent the medians of cytokine concentrations. Individual background cytokine secretions were subtracted before being represented on the figures. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Detection of Untreated aTB in HIV-Infected Subjects
Thirty-four HIV-infected patients with aTB were included before starting anti-TB treatment or during the first 5 days of treatment. Ten of them (29.4%) remained undetected by both TST and IGRA, whereas 14 (41.2%) were TST-positive, and 21 (61.8%) had a positive IGRA (Fig. 3C). Only 3 patients with aTB were TST-positive IGRA-negative.
ESAT-6/CFP-10–induced IFN-γ levels were poorly correlated with TST size (r = 0.35), and no association was noted between IFN-γ levels and CD4+ T-cell counts (r = 0.16). ESAT-6/CFP-10–IGRA detected patients with aTB even with low CD4+ T-cell counts, whereas a higher proportion of TST-negative patients was found in this patient subgroup (Table 1).
Follow-up IGRA results were available for 28 patients after 6 months of ART and anti-TB treatment. ESAT-6/CFP-10–induced IFN-γ responses were significantly lower upon treatment (P = 0.0045, Fig. 4E), whereas HBHA-induced IFN-γ concentrations were significantly higher than at enrollment (P = 0.0004, Fig. 4F).
Even though approximately two thirds of HIV-infected patients with untreated aTB had a positive IGRA, the IFN-γ concentrations did not allow us to differentiate them from HIV-infected subjects with LTBI (medians of positive ESAT-6/CFP-10–IGRA results: 781 and 534 pg/ml for HIV-infected patients with aTB and HIV-infected patients without aTB, respectively) (Fig. 4A). Similar to HIV-uninfected patients with aTB, IL-6 concentrations were significantly higher in aTB compared with patients with LTBI (P = 0.0478, Fig. 4D), but the positive predictive value of high IL-6 concentrations remained poor (53.8%). IL-1β concentration was in contrast not helpful to differentiate HIV-infected patients with aTB from those with LTBI (Fig. 4C).
Detection of IRIS and AATB
During the follow-up period, 53/254 ART-treated HIV-infected patients, also treated for aTB, developed TB–IRIS.18 TST and IGRA were performed at enrollment for 43 of them, with IGRA results available only for 36 patients (7 indeterminate results). Only 16 of them (44.4%) had a positive TST and/or IGRA at enrollment, with only 2 patients with a single TST-positive result (Fig. 3D). The ESAT-6/CFP-10–induced IFN-γ concentrations were not different between patients who did or did not develop TB–IRIS (medians of 162 and 142 pg/ml, respectively).
During the follow-up period, 8/219 ART-treated HIV-infected patients with no clinical sign of aTB at baseline developed AATB. Three of them were detected at enrollment as having LTBI (1 TST-positive, 2 ESAT-6/CFP-10–IGRA-positive), whereas 3 were TST-negative IGRA-negative, and 2 had indeterminate IGRA results.
Other analytes were measured in the IGRA supernatants to evaluate their possible value as a biomarker for TB–IRIS or AATB. At enrollment, no cytokine/chemokine concentration provided a predictive value for developing TB–IRIS or AATB (data not shown). Similarly, results obtained at the IRIS time point were not statistically different from those obtained for patients with aTB after 1 month of both ART and anti-TB treatment.
DISCUSSION
According to the Centers for Disease Control recommendations,24 all individuals with HIV should be tested for LTBI at the time of HIV diagnosis considering the increased rates of progression to aTB in this group25 and the high risk of developing AATB19,26 or TB–IRIS within 3 months of starting ART.18,26–28 LTBI is usually diagnosed by TST and/or commercialized IGRA, which are known to perform poorly in immune-compromised individuals and cannot be used to rule out Mtb infection. In addition, they have a low predictive value for progression to aTB.10,29,30
The present study evaluated the potential added value of long-term in-house IGRA in response to the major proteins present as peptide pools in the commercial IGRA, ESAT-6/CFP-10, and in response to the latency antigen HBHA, in a high-TB-incidence country. We previously reported that a combination of both tests was more optimal to detect most subjects with LTBI in a low-TB-incidence country, both among healthy subjects13,31 and immune-compromised patients.15 In this study, we first evaluated the diagnostic potential of these tests in HIV-uninfected subjects living in Uganda, and in HIV-infected patients known to be Mtb-infected and treated. We then screened a large cohort of HIV-infected subjects for LTBI.
The results obtained in healthy HIV-uninfected subjects were globally in agreement with those obtained in a low-TB-incidence country, ie, a similar classification of the subjects as being noninfected or having LTBI based on the TST results or on the combined results of 2 in-house IGRA. However, in contrast to results obtained in low-TB-incidence countries, the proportions of subjects with LTBI with a positive ESAT-6/CFP-10–IGRA were high compared with only ∼50% of subjects with a positive HBHA–IGRA. The rationale for combining an IGRA in response to antigens expressed by actively replicating bacteria (ESAT-6/CFP-10) and an IGRA in response to an antigen overproduced during the quiescent phase of the infection (HBHA) was to allow the identification of the various stages of the latent TB spectrum.32–35 The hypothesis is that, compared with high-TB-incidence countries, most subjects with LTBI in low-TB-incidence countries, once exposed to a TB index case are much less likely to be re-exposed at a later date and therefore evolve to a stable latency stage characterized by high IFN-γ responses to HBHA and absent/low IFN-γ responses to ESAT-6. In contrast, people living in Uganda are frequently re-exposed to Mtb, and their immune system may therefore be constantly exposed to the ESAT-6/CFP-10, which would explain the high IFN-γ response to these antigens. In accordance with previous reports,36,37 ESAT-6/CFP-10–IFN-γ concentrations did not allow us to differentiate aTB from LTBI. We further evaluated the potential added value of measuring other cytokines in the cell culture supernatants. Both ESAT-6/CFP-10–induced IL-1β and IL-6 concentrations were significantly higher in subjects with aTB compared with those with LTBI, suggesting their potential value as biomarkers of aTB, as previously reported.38,39
In contrast to results obtained in LTBI HIV-uninfected subjects, the diagnostic performance of IGRA in aTB HIV-infected subjects was better than that of TST. As TST is known to be falsely negative in these patients,10 HIV-infected patients treated for aTB were evaluated as control group, and results from IGRA were compared with those of TST. TST detected only 31.6% of the treated patients, whereas combined IGRA were positive for 51.9% of the patients. Accordingly, further screening of HIV-infected subjects for LTBI was performed using both IGRA and TST.
Results of this combined screening strategy indicated that, among HIV-infected subjects with no clinical symptoms of aTB before ART initiation, 40% were positive for LTBI. However, in contrast to low-TB-incidence country, the utility of HBHA–IGRA for the detection of LTBI is questionable because only 3 subjects were single positive for HBHA–IGRA. Follow-up of the patients during 6 months confirmed the added value of combining IGRA and TST for the detection of LTBI, because 4/5 TST-positive only subjects became IGRA-positive and 5/13 IGRA-positive only converted their TST. These conversions during follow-up also confirmed the specificity of the initially single positive tests. Combination of follow-up results with those of initial screening allowed us to detect LTBI in 70% of HIV-infected subjects. Although high, this percentage is probably still an underestimation because 5/8 subjects who developed an AATB during follow-up remained undetected. Measurement of cytokines other than IFN-γ in the cell culture supernatants had no added value.
As diagnosis of aTB is not always easy to perform in Uganda,19 we further evaluated the possible value of IGRA for the detection of aTB. Two thirds of untreated patients with aTB had a positive ESAT-6/CFP-10–IGRA, compared with only 1/5 with a positive TST. IGRA allowed detection of aTB even in patients with very low CD4+ T-cell counts. IFN-γ concentrations were not significantly different in aTB compared with LTBI, but, as with HIV-uninfected subjects, higher ESAT-6/CFP-10–induced IL-6 concentrations were detected in aTB compared with LTBI, suggesting a possible added value of these tests to detect aTB in Uganda.
In addition to the added value of IGRA for the identification of aTB in HIV-infected patients, we demonstrated the utility of these assays for follow-up and monitoring of the response to antibiotic treatment. In this case, the HBHA–IGRA was useful because the HBHA-induced IFN-γ concentrations rose, whereas those induced by ESAT-6/CFP-10 decreased during treatment. In contrast, but in concordance with previous reports,40–42 among the 254 aTB-treated patients who were followed up during ART treatment, IGRA results did not allow the identification of those patients at risk for developing TB–IRIS. Of the 53 patients diagnosed with IRIS during follow-up, only 14/36 had a positive ESAT-6/CFP-10–IGRA, and the IFN-γ concentrations were not different from those of patients who did not develop TB–IRIS. According to the complexity of the mechanism underlying TB–IRIS, involving both innate and adaptive immunities (review in Ref. 43), a single biomarker is probably not sufficient to accurately diagnose the syndrome.
This study performed in Uganda on 352 HIV-infected patients demonstrates the clear added value of ESAT-6/CFP-10–IGRA to TST to identify subjects with LTBI who might benefit, after careful elimination of a possible aTB, from a prophylactic anti-TB treatment. This could help to avoid the development of AATB in these patients by close monitoring. Measuring IL-6 concentrations in the IGRA supernatants could further help to identify patients with aTB, and combined follow-up of the HBHA–IGRA and the ESAT-6/CFP-10–IGRA could help to monitor the efficacy of the anti-TB treatment.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank E. Nabulime, J. Tresnie, G. Pottier, and M. Libin for their technical help as well as the local study participants and the study team in particular: H. Mayanya-Kizza, I. Nankya, D. Mazakpwe, K. Luzinda, P. Lwanga, M. Nakuya, C. O. Namujju, C. Ahimbisibwe, J. Namaganda, A. Andama, E. Bazze, B. Kirenga, and H. Kisembo. We thank N. Pakker and the data staff of the Infectious Diseases Network for treatment and Research in Africa (INTERACT) for assistance with data monitoring and management. We also thank L. Aerts for critically reading the manuscript.
Lead author of the TB-IRIS study group: L. Kestens, Institute of Tropical Medicine, Antwerp, Belgium. Other members of the TB-IRIS study group are as follows: Institute of Tropical Medicine, Antwerp, Belgium: R. Colebunders and M. Massinga Loembé; Infectious Disease Institute, Kampala, Uganda: H. Mayanja and W. Worodria; Joint Clinical Research Centre: H. Mayanja; Université Libre de Bruxelles, Belgium: F. Mascart; VIB, Brussels, Belgium, and Vrije Universiteit Brussel, Brussels, Belgium: R. van den Bergh; Institut Pasteur de Lille, France: C. Locht; Academic Medical Centre, Department of Global Health and Amsterdam Institute for Global Health and Development, Amsterdam, The Netherlands: P. Reiss, F. Cobelens, P. Ondoa, and N. Pakker; INTERACT, Kampala, Uganda: R. Mugerwa, H. Mayanja, N. Pakker, and W. Worodria.
Footnotes
Supported by the European Commission FP6 Specific Targeted Research Project Grant LSHP-CT-2007-037659-TBIRIS.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
Conceived and designed the experiments: V.D., K.S., and F.M. Performed the experiments: V.D., K.S., and M.M.-L. Provided the antigens: M.S. and C.L. Analyzed the data: V.D. and F.M. Wrote the manuscript: V.D. and F.M. Project management: M.M.L., W.W., R.C., and L.K. Study physicians: W.W. and R.C.
REFERENCES
- 1.WHO | Global Tuberculosis Report 2014. WHO. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed July 20, 2015. [Google Scholar]
- 2.CDC | TB | Basic TB Facts. Available at: http://www.cdc.gov/tb/topic/basics/. Accessed July 20, 2015. [Google Scholar]
- 3.Walker NF, Meintjes G, Wilkinson RJ. HIV-1 and the immune response to TB. Future Virol. 2013;8:57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller M, Wandel S, Colebunders R, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010:CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Getahun H, Granich R, Sculier D, et al. Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. AIDS. 2010;24(suppl 5):S57–S65. [DOI] [PubMed] [Google Scholar]
- 7.Huebner RE, Schein MF, Bass JB. The tuberculin skin test. Clin Infect Dis Off Publ Infect Dis Soc Am. 1993;17:968–975. [DOI] [PubMed] [Google Scholar]
- 8.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007;146:340–354. [DOI] [PubMed] [Google Scholar]
- 9.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redelman-Sidi G, Sepkowitz KA. IFN-γ release assays in the diagnosis of latent tuberculosis infection among immunocompromised adults. Am J Respir Crit Care Med. 2013;188:422–431. [DOI] [PubMed] [Google Scholar]
- 11.Leyten EMS, Arend SM, Prins C, et al. Discrepancy between Mycobacterium tuberculosis-specific gamma interferon release assays using short and prolonged in vitro incubation. Clin Vaccine Immunol. 2007;14:880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butera O, Chiacchio T, Carrara S, et al. New tools for detecting latent tuberculosis infection: evaluation of RD1-specific long-term response. BMC Infect Dis. 2009;9:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hougardy JM, Schepers K, Place S, et al. Heparin-binding-hemagglutinin-induced IFN-gamma release as a diagnostic tool for latent tuberculosis. PLoS One. 2007;2:e926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyndham-Thomas C, Corbière V, Dirix V, et al. Key role of effector memory CD4+ T lymphocytes in a short-incubation heparin-binding hemagglutinin gamma interferon release assay for the detection of latent tuberculosis. Clin Vaccine Immunol. 2014;21:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dessein R, Corbière V, Nortier J, et al. Heparin-binding haemagglutinin, a new tool for the detection of latent Mycobacterium tuberculosis infection in hemodialysis patients. PLoS One. 2013;8:e71088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyndham-Thomas C, Dirix V, Schepers K, et al. Contribution of a heparin-binding haemagglutinin interferon-gamma release assay to the detection of Mycobacterium tuberculosis infection in HIV-infected patients: comparison with the tuberculin skin test and the QuantiFERON-TB Gold In-tube. BMC Infect Dis. 2015;15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Worodria W, Massinga-Loembe M, Mazakpwe D, et al. Incidence and predictors of mortality and the effect of tuberculosis immune reconstitution inflammatory syndrome in a cohort of TB/HIV patients commencing antiretroviral therapy. J Acquir Immune Defic Syndr. 2011;58:32–37. [DOI] [PubMed] [Google Scholar]
- 18.Worodria W, Menten J, Massinga-Loembe M, et al. Clinical spectrum, risk factors and outcome of immune reconstitution inflammatory syndrome in patients with tuberculosis-HIV coinfection. Antivir Ther. 2012;17:841–848. [DOI] [PubMed] [Google Scholar]
- 19.Worodria W, Massinga-Loembe M, Mayanja-Kizza H, et al. Antiretroviral treatment-associated tuberculosis in a prospective cohort of HIV-infected patients starting ART. Clin Dev Immunol. 2011;2011:758350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirenga BJ, Worodria W, Massinga-Loembe M, et al. Tuberculin skin test conversion among HIV patients on antiretroviral therapy in Uganda. Int J Tuberc Lung Dis. 2013;17:336–341. [DOI] [PubMed] [Google Scholar]
- 21.Andersen P, Munk ME, Pollock JM, et al. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099–1104. [DOI] [PubMed] [Google Scholar]
- 22.Delogu G, Chiacchio T, Vanini V, et al. Methylated HBHA produced in M. smegmatis discriminates between active and non-active tuberculosis disease among RD1-responders. PLoS One. 2011;6:e18315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walzl G, Ronacher K, Hanekom W, et al. Immunological biomarkers of tuberculosis. Nat Rev Immunol. 2011;11:343–354. [DOI] [PubMed] [Google Scholar]
- 24.Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-infected Adults and Adolescents Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5804a1.htm. Accessed July 20, 2015. [PubMed] [Google Scholar]
- 25.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. [DOI] [PubMed] [Google Scholar]
- 26.Karmakar S, Sharma SK, Vashishtha R, et al. Clinical characteristics of tuberculosis-associated immune reconstitution inflammatory syndrome in North Indian population of HIV/AIDS patients receiving HAART. Clin Dev Immunol. 2011;2011:239021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawn SD, Myer L, Bekker LG, et al. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–341. [DOI] [PubMed] [Google Scholar]
- 28.Manosuthi W, Van Tieu H, Mankatitham W, et al. Clinical case definition and manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2009;23:2467–2471. [DOI] [PubMed] [Google Scholar]
- 29.Santin M, Muñoz L, Rigau D. Interferon-γ release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis. PLoS One. 2012;7:e32482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corbière V, Pottier G, Bonkain F, et al. Risk stratification of latent tuberculosis defined by combined interferon gamma release assays. PLoS One. 2012;7:e43285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barry CE, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends Microbiol. 2009;17:183–188. [DOI] [PubMed] [Google Scholar]
- 34.O'Garra A, Redford PS, McNab FW, et al. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. [DOI] [PubMed] [Google Scholar]
- 35.Delogu G, Goletti D. The spectrum of tuberculosis infection: new perspectives in the era of biologics. J Rheumatol Suppl. 2014;91:11–16. [DOI] [PubMed] [Google Scholar]
- 36.Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-γ release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis. J Infect Dis. 2011;204(suppl 4):S1120–S1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sester M, Sotgiu G, Lange C, et al. Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2011;37:100–111. [DOI] [PubMed] [Google Scholar]
- 38.Prabhavathi M, Kabeer BSA, Deenadayalan A, et al. Role of QuantiFERON-TB Gold antigen-specific IL-1β in diagnosis of active tuberculosis. Med Microbiol Immunol (Berl). 2015;204:567–574. [DOI] [PubMed] [Google Scholar]
- 39.Nemeth J, Winkler HM, Boeck L, et al. Specific cytokine patterns of pulmonary tuberculosis in Central Africa. Clin Immunol. 2011;138:50–59. [DOI] [PubMed] [Google Scholar]
- 40.Goovaerts O, Jennes W, Massinga-Loembé M, et al. Antigen-specific interferon-gamma responses and innate cytokine balance in TB-IRIS. PLoS One. 2014;9:e113101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tieu HV, Ananworanich J, Avihingsanon A, et al. Immunologic markers as predictors of tuberculosis-associated immune reconstitution inflammatory syndrome in HIV and tuberculosis coinfected persons in Thailand. AIDS Res Hum Retroviruses. 2009;25:1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elliott JH, Vohith K, Saramony S, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J Infect Dis. 2009;200:1736–1745. [DOI] [PubMed] [Google Scholar]
- 43.Lai RP, Meintjes G, Wilkinson RJ. HIV-1 tuberculosis-associated immune reconstitution inflammatory syndrome. Semin Immunopathol. 2016;38:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


