ABSTRACT
OBJECTIVE:
The objective of this retrospective study was to evaluate the efficacy of the authors’ combination therapy protocol for keloid treatment.
DESIGN:
Retrospective.
SETTING:
Plastic surgery office-based outpatient setting in New York City.
PATIENTS:
Forty patients with 44 keloid scars requiring surgical excision.
INTERVENTIONS:
Keloid scars were treated using surgical excision, platelet-rich plasma, and postoperative in-office superficial photon X-ray radiation therapy. Intralesional triamcinolone injections were administered once to 4 patients with poor results on scar scale assessment. Patient follow-up visits ranged from 3 to 11 months to assess for evidence of recurrence and adverse effects.
MAIN OUTCOME MEASURE(S):
For the purpose of this study, recurrence was defined as any sign of extraordinary erythema, induration, and hypertrophy beyond the site of excision.
MAIN RESULTS:
In the 16 keloids treated with 2 fractions, there was no evidence of recurrence. One of 25 keloids treated with 3 fractions demonstrated evidence of recurrence. One of 3 keloids treated with a single fraction displayed signs of recurrence. Postirradiation hyperpigmentation was noted in all patients.
CONCLUSIONS:
Surgical excision combined with platelet-rich plasma and postoperative in-office superficial radiation therapy achieved a 95.5% nonrecurrence rate at 1- to 3-month follow-up. This protocol appears to be a safe and viable option in the management of keloids and merits further randomized controlled study of its comparative efficacy.
KEYWORDS: keloid, platelet-rich plasma, superficial photon X-ray radiation, surgical incision
INTRODUCTION
Keloids are fibrous lesions that form at a site of injury due to irregular production of types I and III collagen.1 Unlike hypertrophic scars, keloids continue to grow outside the original wound margins and fail to resolve over time.2 They itch and become painful.2 High-tension areas, such as the chest, trunk, and back, as well as commonly pierced areas, such as the ears, are some of the more common sites for keloid formation.3 Although there is limited understanding on the cause(s) of keloids, they are known to occur more frequently in persons aged 10 to 30 years and in females (likely the result of more frequent body piercing).1 Although documented in all races, keloids are more prevalent in individuals with darker skin (African American, Asian, Hispanic), with a positive correlation to skin pigmentation. Most keloids appear in those with darker skin types, and there are no reported incidents in albinos.2 Keloids often mature into unsightly lesions affecting self-esteem and quality of life. Depending on the location and size of the keloid, range of motion may be impaired.3
With limited knowledge about the actual cause(s) of keloids, determining the most effective treatment for keloids has proven challenging. It is agreed, however, that combination therapies are necessary to reach lower recurrence rates. No single therapeutic modality alone has proven effective. Treatments such as surgical excision, postoperative radiotherapy, triamcinolone injections, pulsed dye laser, cryotherapy, and silicone sheeting are among the more popular treatments used.3 Many combinations of these therapies have been used, with results varying drastically.3 In some studies, surgical excision alone results in a recurrence rate of 65% to 99%.4 Other studies reported that surgical excision combined with other adjuvant therapies, such as cryotherapy, pressure treatment, intralesional steroids, and silicone gel sheeting had a recurrence rate of greater than 50%; surgical excision followed by radiation therapy proved most effective with a recurrence rate of nearly 20%.5 Multimodality therapy involving surgical excision, postoperative radiotherapy, and intralesional steroid injections yielded up to an 89% cure rate in 1 study.6
Although not studied in the treatment of keloids, platelet-rich plasma (PRP) has shown favorable wound-healing properties, demonstrating organized collagen deposition.7 As a result, patients were treated using surgical excision, PRP, and in-office superficial photon radiation therapy.
PATIENTS AND METHODS
Study Population
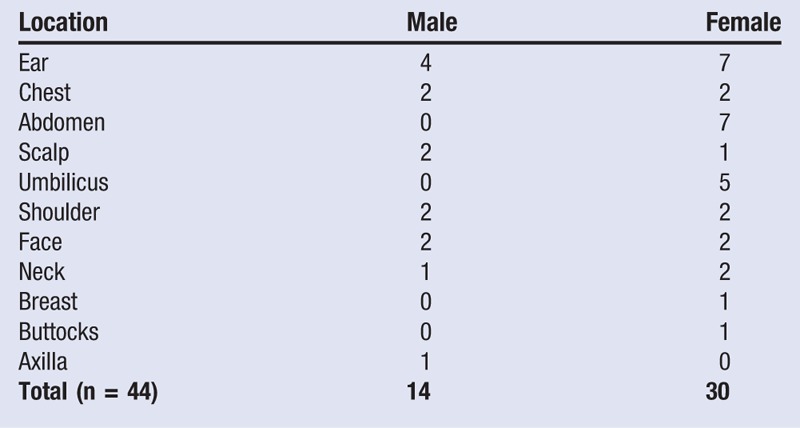
From November 2013 to July 2014 at a plastic surgery office in New York, New York, the authors treated keloids with extralesional surgical excision, PRP, and postoperative superficial radiation therapy. A total of 44 keloid scars were treated in 40 patients. Patients in this study were assessed at a minimum postoperative period of 3 months and a maximum period of 11 months. Of the 44 keloids, 70.5% belonged to African Americans, 68.2% were in females, and the ear was the most common treatment area, comprising 11 of 44 lesions (25%) treated (Table 1). Patient ages ranged from 16 to 67 years, with a mean age of 41.75 years.
Table 1.
Keloid Area: Male Versus Female
Surgical Procedure
Informed consent was obtained for surgery and PRP on the morning of surgery. Using the Harvest PRP Procedure Pack (Harvest Technologies, Lakewood, Colorado), a preoperative blood draw was performed in order to obtain a sample for PRP. After a 14-minute centrifuge cycle, the platelet concentrate was aspirated and ready for use. All keloids were extralesionally excised under either intravenous sedation or local anesthesia. Undermining was performed to mobilize the flaps for minimal skin tension closures. Two to 3 cm3 of PRP were applied to the wound bed and under the skin flaps. Drains were placed as needed. Absorbable monofilament sutures secured the deep dermal layer while the skin was approximated using a running subcuticular monofilament polypropylene suture. The PRP was also applied to the skin and incision site and allowed to dry. Dermabond (2-octyl cyanoacrylate; Ethicon, Somerville, New Jersey) was applied to the incisions and allowed to begin drying for 30 to 45 seconds. Nexcare Steri-strips (3M, St Paul, Minnesota) were then applied in a linear fashion along the length of the incision, while the Dermabond (2-octyl cyanoacrylate) finished polymerizing.
Radiation Protocol
Irradiation simulation was performed prior to excision and verified again after the operation. Informed consent was obtained prior to the start of the procedure, using a customized SRT 100 consent form. In-office superficial photon X-ray radiation treatment using the FDA–approved SRT-100 (Sensus Healthcare, Boca Raton, Florida) was delivered within 72 hours of excision. External and internal lead shields, along with a custom lead cutout, were strategically placed to maximize exposure to the affected area and minimize exposure to the surrounding normal tissue. Custom-made lead shields were designed to treat keloids with a 5-mm clear margin. Ears were treated with 70 kV, the chin was treated with 50 kV, and all other sites were treated with 100 kV. Based on patient availability, they were treated on a schedule of either 13, 16, or 18 Gy. Cumulative radiation doses ranged from 13 to 18 Gy.
From November 2013 to February 2014, 16 keloids were treated using 2 fractions for a cumulative radiation dose of approximately 16 Gy. With the delivery of 2 fractions and 16 Gy, it was noted that patients developed significant hyperpigmentation in the treatment field. To reduce the postinflammatory response and hyperpigmentation, treatments were increased to 3 fractions. From March 2014 to July 2014, 25 keloids were treated using 3 fractions for a cumulative radiation dose of approximately 18 Gy. Because of personal limitations, the remaining 3 keloids were treated using 1 fraction for a cumulative radiation dose of approximately 13 Gy. Regardless the fraction scheme, all keloids were treated with 100 kV.
Clinical Follow-up
Patients were instructed to follow up at 10 days and at 1, 3, 6, and 9 months. For personal scheduling limitations, some patients were seen outside the suggested follow-up appointments. All patients were instructed to use Keloid Care (Scar Shield, LLC, New York, New York), a proprietary scar cream with antioxidants, botanical moisturizers, silicone, vitamin E, and 0.5% hydrocortisone, twice daily for the first 3 months. Recurrence was determined by examination and photo documentation. Any sign of extraordinary erythema, induration, and hypertrophy of the scar beyond the site of excision was determined to constitute a recurrence. Triamcinolone injections were administered once postoperatively to 4 patients who fell into the “poor” category of the scar assessment scale displaying induration and/or hypertrophy.
Assessment
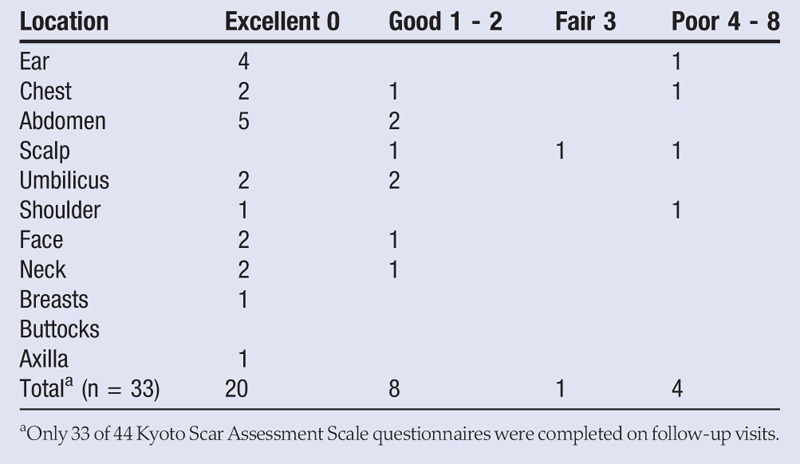
The Kyoto Scar Assessment Scale was developed by the Department of Plastic and Reconstructive Surgery at Kyoto University Graduate School of Medicine in Kyoto City, Japan.6 This scale measures objective symptoms of redness, elevation, and hardness, and subjective symptoms of itching and pain. The scale grades the objective symptoms into 3 levels and the subjective symptoms into 2 levels. Adding the points together determined the overall score for each scar (Table 2). Four categories were determined: excellent (0), good (1–2), fair (3), and poor (4–8).
Table 2.
Kyoto Scar Assessment Scale Scores
RESULTS
In the 16 patients treated with 2 fractions, there was no evidence of recurrence. In addition, 1 of 3 keloids treated with a single fraction displayed evidence of recurrence at 7 months’ postoperative, and 1 of 25 keloids treated with 3 fractions showed signs of recurrence at 3 months’ postoperative. This resulted in a nonrecurrence rate of 95.5% (42/44) at 3 to 11 months’ postoperative. Radiation-induced hyperpigmentation was noted in all patients (Figures 1 and 2). However, hyperpigmentation decreased when treatment increased from 2 to 3 fractions of radiation. Thirty-three postoperative scars were evaluated by the Kyoto scar scale: 61% (excellent), 24% (good), 3% (fair), and 12% (poor) (Table 2).
Figure 1.
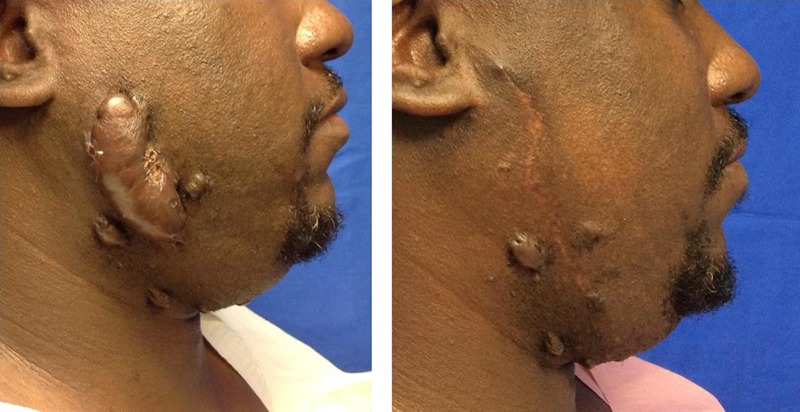
MAN WITH FACIAL KELOID
Left, A 38-year-old African American man with a 15 x 5-cm right facial keloid underwent extralesional excision, followed by 3 fractions of superficial photon X-ray radiation therapy for a cumulative dose of 18 Gy. Right, Eight months following the procedure, the patient demonstrates no evidence of recurrence and minimal hyperpigmentation. Satellite lesions will be excised during a second procedure.
Figure 2.
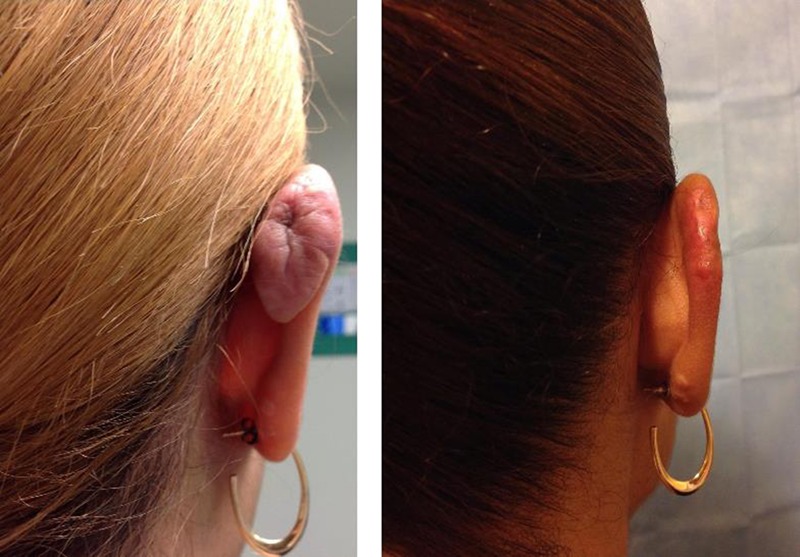
WOMAN WITH POSTERIOR EAR KELOID
Left, A 29-year-old Hispanic woman with a 2 x 3-cm right posterior ear keloid who underwent extralesional excision, followed by 3 fractions of superficial photon X-ray radiation therapy for a cumulative dose of 18 Gy. Right, Six months following the procedure, the patient demonstrates no evidence of recurrence and minimal hyperpigmentation.
DISCUSSION
Keloids are benign, yet locally invasive, lesions related to complex cellular, environmental, and genetic interactions4 and are more common among people with darker skin.8 Although there are a multitude of treatment modalities for keloids, none are satisfactory. The use of silicone sheets, cryotherapy, laser treatments, and pressure dressings are among the conservative treatments recommended, but the effect of these modalities is limited.1 The use of intralesional steroid injections, either prior to or after surgical excision, has yielded less than optimal results.1 Multimodality therapy involving surgical excision, postoperative radiotherapy, and intralesional steroid injections has yielded up to 89% cure rate in 1 study.6 In this study, the authors present a new and innovative in-office treatment protocol for keloids with an early nonrecurrence rate of 95.5%.
Radiation
This is the first study, to the authors’ knowledge, to report on the use of in-office superficial photon X-ray radiation in the treatment of keloids. This low-energy therapy may be advantageous over more traditional radiation machines that utilize high-energy electron beams. The device focuses the photon X-rays into the skin only, where the pathology is located, and does not penetrate and impact the underlying tissue.9 Because of the rapid fall-off of radiation, exposure to surrounding tissues is limited.9 Electron-beam radiotherapy has the ability to penetrate beyond the surface of the skin, impacting underlying structures.9 Furthermore, the superficial radiation therapy was easier for the authors to coordinate with the surgical excision, because both occurred in the same office.
In the treatment of keloids, irradiation dose, fraction, and period from surgical excision to irradiation are important. There currently is no consensus on the ideal radiation dose to be administered; a dose of 15 to 20 Gy has been used in many protocols.6 In the authors’ study, patients were treated with 13 to 18 Gy over 1 to 3 fractions with a biological effective dose of 30 Gy.10 It is commonly accepted that radiation treatment should be started within 72 hours of the surgical excision.11 In the authors’ study, all patients were treated within 72 hours of surgical excision.
Recurrence
For the purpose of this study, recurrence was defined as any sign of extraordinary erythema, induration, and hypertrophy beyond the site of excision. Two of the 44 lesions showed signs of recurrence. One of the recurrences in the authors’ study occurred in a patient who had only 1 fraction administered at 13 Gy. The recurrences occurred along the scar at areas where the radiation dose may have been minimized because of feathering of the radiation shielding used. The other recurrence occurred in a white woman with a chest keloid. In this case, the wound dehisced at 10 days and healed by secondary intention (Figure 3). It is worth noting that both recurrences occurred in high-tension areas.
Figure 3.
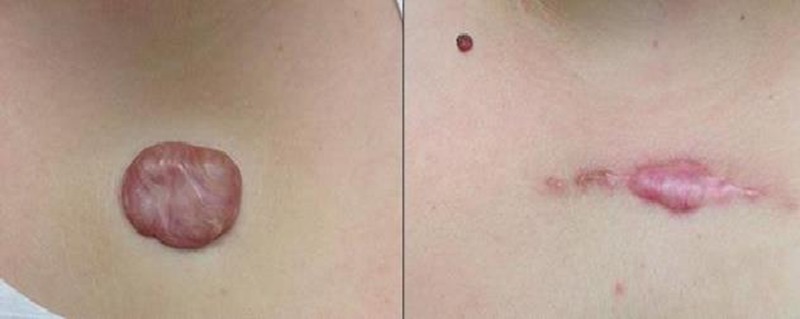
WOMAN WITH MIDCHEST KELOID
Left, A 25-year-old white woman with a 3 x 5 x 2-cm midchest keloid underwent extralesional excision, followed by 3 fractions of superficial photon X-ray radiation therapy for a cumulative dose of 18 Gy. Right, Three months following the procedure, the patient displays extraordinary redness, hypertrophy, and hardness on palpation. This was classified as a recurrence. No signs of hyperpigmentation.
Postirradiation Hyperpigmentation
Most patients reported overall satisfaction with their treatment outcomes. Postirradiation hyperpigmentation was the most disliked adverse effect of radiotherapy. Because high-dose radiation has been reported to damage melanocytes and decrease hyperpigmentation, the authors’ protocol was changed from 2 to 3 fractions.11 The increase in fractions allowed for the delivery of high-dose radiation. Patients were also allowed to bathe with a mild cleanser and water and use topical steroids, which have been proven to lessen the radiation-induced skin reactions.12,13 After the change of protocol, a marked difference was noted in the severity of hyperpigmentation in the radiation field (Figure 4).
Figure 4.
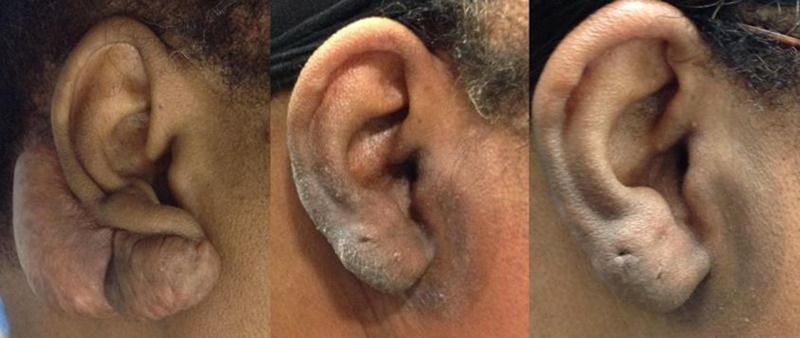
WOMAN WITH KELIOD ON RIGHT EAR
Left, A 67-year-old African American woman with a 6 x 7-cm keloid on the right ear underwent surgical excision followed by 2 fractions of superficial photon X-ray radiation. Middle, Two months following the procedure, the patient demonstrates no evidence of recurrence; however, there is marked hyperpigmentation. Right, At 11 months’ postoperative, there are still no signs of recurrence, but the hyperpigmentation persists.
Triamcinolone
Triamcinolone is widely used in the treatment of keloids and hypertrophic scars. Studies have shown decreased keloid fibroblast proliferation, degradation of mature collagen, and enhanced organization of existing collagen bundles in lesions treated with triamcinolone.4 All 3 findings are critical in the treatment of keloids and hypertrophic scars. Intralesional triamcinolone injections were administered once to all 4 lesions, with “poor” results on the Kyoto scar scale due to hypertrophy and induration. Intralesional triamcinolone injections were administered to the following lesions: a posterior scalp and ear lesion with elevation and hardness on palpation, as well as a left shoulder and midchest lesions, which were classified as recurrences. All of these lesions were classified as “poor” using the Kyoto Scar Assessment Scale.
Platelet-Rich Plasma
Platelet-rich plasma is an autologous platelet concentrate shown to stimulate tissue repair.14 In addition to accelerating wound healing, PRP has been shown to decrease pain and disability in the treated area.15,16 Furthermore, research has shown decreased inflammation and collagen deposition, as well as more organized collagen structures in injured vocal cords treated with PRP.7 In the authors’ practice, evidence of improved wound healing with lower keloid recurrence was noted anecdotally with surgical excision and PRP alone, prior to the use of in-office radiation therapy. As a result, all patients received PRP to the surgical site. Future controlled prospective studies should look at the efficacy of surgery and PRP versus surgery, PRP and photon X-ray therapy versus surgery, and photon X-ray therapy alone in the treatment of keloids.
Kyoto Scar Assessment Scale
The Kyoto Scar Assessment Scale was used to qualify scars during postoperative visits. As pain and itching are expected after surgery, the scores were negatively impacted in the early phases of wound healing. Of the 44 keloids treated (33 completed the assessment), 20 qualified as excellent, 8 were good, 1 was fair, and 4 were poor. The most common qualifying symptom was partial hardness (8/33). Normal early postsurgical inflammation can contribute to hardness or induration along the incision. Signs of recurrence will be closely monitored.
It should be noted that, to the authors’ knowledge, the Kyoto Scar Assessment Scale has not been validated with other peer-reviewed studies. It was chosen because of the measurement of objective and subjective symptoms commonly associated with keloid lesions. Other scar assessments often incorporate hyperpigmentation into the assessment, which is a common finding in patients after radiation. Furthermore, the use of the assessment was limited in that not all patients had the scar assessment performed because of either patient refusal or inconsistent usage of the assessment tool during follow-up visits. Future studies should look to validate the Kyoto Scar Assessment Scale in determining keloid recurrence.
Short Follow-up
The short follow-up period is a limitation of this study. At maximum, patients were followed up at 11 months’ postoperative. Keloids are widely considered cured after 3 to 5 years without recurrence. Ongoing monitoring will continue.
CONCLUSIONS
Surgical excision of keloids combined with intraoperative PRP and postoperative in-office superficial radiation therapy achieved a preliminary 95.5% nonrecurrence rate at 3- to 11-month follow-up. Hyperpigmentation was noted in all patients. The study was limited by the short follow-up period, the nonstandardized radiation protocol, variable use of triamcinolone, and inconsistent use of the Kyoto Scar Assessment Scale.
An additional limitation to this study includes the lack of per-patient analysis. Data were reported per keloid, as opposed to per patient, for easy identification of lesions that respond well or may require additional treatment. Because different keloids are exposed to known triggers, such as the shoulders (high tension) and ears (piercing), it was ideal to report based on keloid lesions independently. Reporting this information provides the audience with the most accurate information on how types of lesions respond, and it allows for more customized, preventive measures to be implemented. Early results of this study demonstrate that this protocol appears to be a safe and viable option in the management of keloids and merits further randomized controlled study of its comparative efficacy.
Footnotes
The authors have disclosed that they have no financial relationships related to this article.
REFERENCES
- 1.Viera MH, Caperton CV, Berman B. Advances in the treatment of keloids. J Drugs Dermatol 2011;10:468-80. [PubMed] [Google Scholar]
- 2.Viera MH, Vivas AC, Berman B. Update on keloid management: clinical and basic science advances. Adv Wound Care (New Rochelle) 2012;1:200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg 2010;125:557-68. [DOI] [PubMed] [Google Scholar]
- 4.Al-Attar A, Mess S, Thomassen JM, Kauffman CL, Davison SP. Keloid pathogenesis and treatment. Plast Reconstr Surg 2006;117:286-300. [DOI] [PubMed] [Google Scholar]
- 5.Song C, Wu H, Chang H, Kim H, Ha SW. Adjuvant single-fraction radiotherapy is safe and effective for intractable keloids. J Radiat Res 2014;55:912-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamawaki S, Naitoh M, Ishiko T, Muneuchi G, Suzuki S. Keloids can be forced into remission with surgical excision and radiation, followed by adjuvant therapy. Ann Plast Surg 2011;67:402-6. [DOI] [PubMed] [Google Scholar]
- 7.Woo SH, Jeong H, Kim JP, et al. Favorable vocal fold wound healing induced by platelet-rich plasma injection. Clin Exp Otorhinolaryngol 2014;7:47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nassiri M, Woolery-Lloyd H, Ramos S, et al. Gene expression profiling reveals alteration of caspase 6 and 14 transcripts in normal skin of keloid-prone patients. Arch Dermatol Res 2009;301:183-8. [DOI] [PubMed] [Google Scholar]
- 9.Bodner WR, Hilaris BS, Alagheband M, Safai B, Mastoras CA, Saraf S. Use of low-energy x-rays in the treatment of superficial nonmelanomatous skin cancers. Cancer Invest 2003;21:355-62. [DOI] [PubMed] [Google Scholar]
- 10.Kal HB, Veen RE. Biologically effective doses of postoperative radiotherapy in the prevention of keloids. Strahlenther Onkol 2005;23:717-23. [DOI] [PubMed] [Google Scholar]
- 11.Panizzon RG. Radiation therapy of benign dermatoses. Keloids. In: Panizzon RG, Cooper JS, eds. Radiation Treatment and Radiation Reactions in Dermatology. New York, NY: Springer-Verlag; 2004. [Google Scholar]
- 12.Chan RJ, Webster J, Chung B, Marquart L, Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2014;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer 2013;21:2933-48. [DOI] [PubMed] [Google Scholar]
- 14.Klosova H, Stetinksy J, Bryjova I, Hledik S, Klein L. Objective evaluation of the effect of autologous platelet concentrate on post-operative scarring in deep burns. Burns 2013;39:1263-76. [DOI] [PubMed] [Google Scholar]
- 15.Obolenskiy VN, Ermolova DA, Laberko LA, Semenova TV. Efficacy of platelet rich plasma for the treatment of chronic wounds. EWMA J 2014:14:37-41. [Google Scholar]
- 16.Rodriguez R. Corticosteroid versus platelet-rich plasma injection in epicondylitis. Orthop Nurs 2014;33:257-65. [DOI] [PubMed] [Google Scholar]


