Abstract
Recent advances in neuroscience challenge the old dogma that neurogenesis occurs only during embryonic development. Mounting evidence suggests that functional neurogenesis occurs throughout adulthood. This review article discusses molecular factors that affect adult neurogenesis, including morphogens, growth factors, neurotransmitters, transcription factors, and epigenetic factors. Furthermore, we summarize and compare current evidence of associations between adult neurogenesis and human brain diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and brain tumors.
Keywords: adult neurogenesis, molecular factors, human brain diseases
Introduction
According to traditional belief, mammals do not produce new neurons from precursors (neurogenesis) into adulthood. In 1965, Altman and Das published groundbreaking anatomical evidence indicating the existence of adult neurogenesis in rats.1 Since then, the research field of adult neurogenesis has exploded. It is now widely accepted that neurogenesis occurs in limited regions such as the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) and the subventricular zone (SVZ) of the lateral ventricle/striatum in adult humans and rodents.2–5 These adult-born neurons function and integrate into the rest of the brain circuit.6 Furthermore, the number of newborn neurons has been qualified and demonstrated to be significant.7 The physiological functions of adult neurogenesis include learning, emotions, and memory such as pattern separation, temporal separation, high-resolution memory, fear conditioning, and synaptic plasticity.8,9 Abnormal adult neurogenesis has also been linked to diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), demyelinating disease, stroke, epilepsy, and depression.8,10 This review article discusses the molecular factors that affect adult neurogenesis and current evidence of associations between adult neurogenesis and human brain diseases.
Molecular Mechanisms of Adult Neurogenesis
Adult neurogenesis generally includes the following four key stages:
Maintenance and proliferation of quiescent adult neural stem cells (NSCs).
Fate specification.
Differentiation, maturation, and survival of the immature neurons.
Integration into the existing brain circuit.
Here, the quiescent NSCs are slow-growing, multipotent cells with unlimited self-renewal. After NSCs become activated, they divide asymmetrically and produce transit amplifying cells (TACs) in the SVZ and transient intermediate progenitors (TIPs) in the SGZ. The TACs and TIPs are rapidly dividing cells with the potential to differentiate into neurons with limited ability for self-renewal. After a limited number of cell divisions, the TACs and TIPs give rise to the neuroblasts. The proliferating neuroblasts then exit the cell cycle, and a subpopulation survives and differentiates into newborn neurons that will then be integrated into the neuronal network in the brain (Fig. 1).11–13 Various molecular players were found to regulate specific stages of adult neurogenesis in mammals. In this section, we focus on five groups of molecular players that play critical roles in adult neurogenesis: morphogens, growth factors, neurotransmitters, transcription factors, and epigenetic factors.
Figure 1.
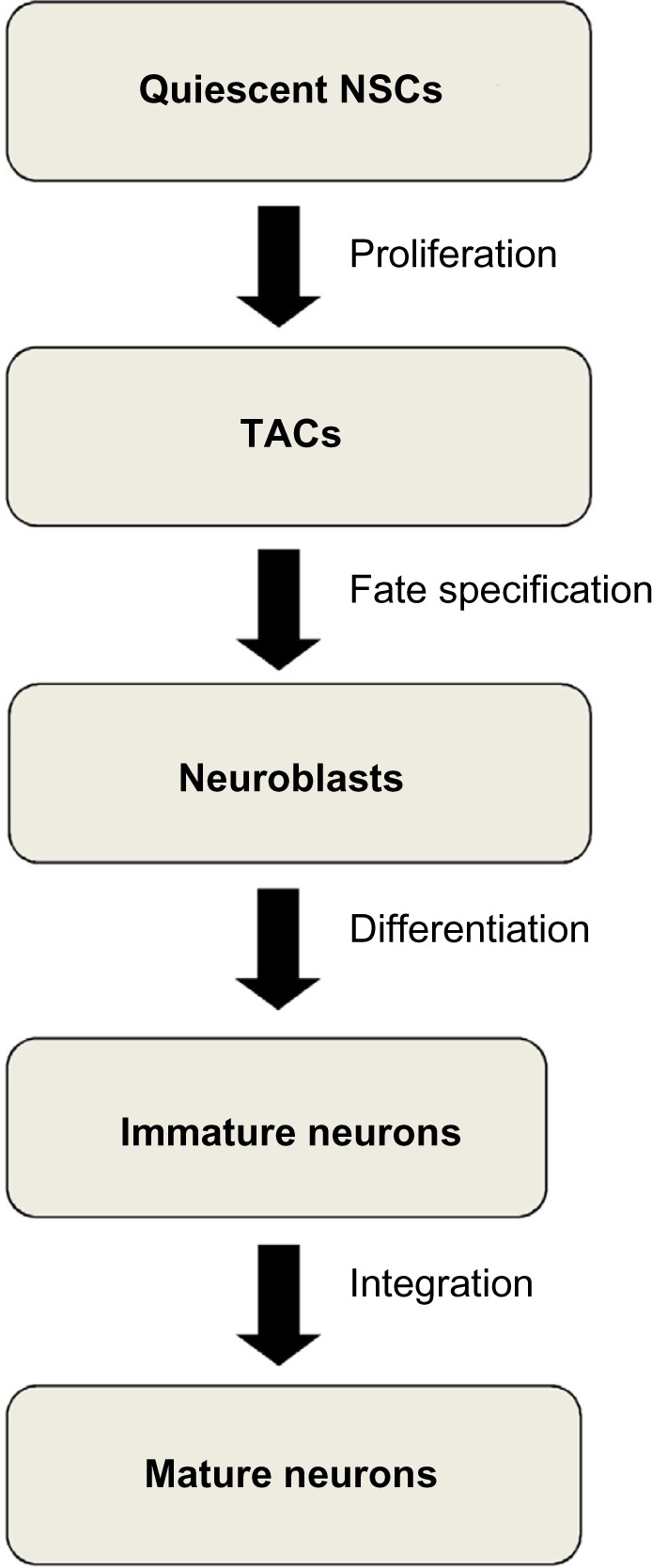
Key stages of adult neurogenesis. The quiescent neural stem cells (NSCs) start to proliferate to generate the transit amplifying cells (TACs). The TACs undergo fate specification and give rise to the neuroblasts. The neuroblasts differentiate into immature neurons. The immature neurons migrate and get integrated into the brain circuit to become fully mature neurons.
Morphogens
Morphogens are extracellular signaling molecules well known for their roles in embryonic patterning and axis formation during development.14 In adult neurogenesis, a number of morphogens were found to be critical in establishing/regulating the stem cell niche, including Notch, sonic hedgehog (Shh), Wnts, and bone morphogenetic proteins (BMPs).
Notch signaling has been reported to regulate NSC maintenance, neurogenic niche, and newborn neuron survival and maturation in postnatal life. Inducible Notch1 loss-of-function mice had increased progenitors exiting the cell cycle, while the mice overexpressing the intracellular portion of the Notch receptor (Notch intracellular domain) had decreased progenitors exiting the cell cycle in the adult hippocampus.15 Inactivation of the Notch ligand Jagged1 during adult SGZ neurogenesis resulted in defective neural stem cell maintenance and proliferation in mice.16 Deletions of RBPj (recombination signal binding protein for immunoglobulin kappa J region), a downstream mediator of all Notch receptors, resulted in loss of neurogenesis accompanied by depletion of neural precursors in both SVZ and SGZ.17,18 In the lateral ventricular walls of adult mouse brain, Notch via its downstream target EphB2 was implicated in maintaining the identity and plasticity of neurogenic niche cells.19 Notch signaling is also implicated in the later stages of neurogenesis such as dendrite morphology15 and synaptic plasticity20 in newborn neurons.
Wnt/β-catenin signaling uses both paracrine and autocrine canonical mechanisms and regulates adult hippocampal neurogenesis in vitro and in vivo.21–23 Their actions span multiple steps of neurogenesis, including maintaining multipotency of neural stem cells, enhancing neuroblast proliferation, and promoting neuronal fate specification.24,25 Most importantly, their actions on these biological processes are linked to the functions of the adult hippocampus. Blocking Wnt signaling in the DG impaired spatial and object recognition memory in adult rats.26
BMPs are necessary for maintaining the quiescence of NSCs through BMPR-IA27 in the adult DG as well as the differentiation and maturation of granule cells through BMPR-II.28
Growth factors
Growth factors are extracellular peptides that function as stimulants during tissue growth and development.29 Lines of evidence support the critical roles for adult neurogenesis of the following growth factors: brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1), and fibroblast growth factor 2 (FGF-2).
BDNF overexpression via various methods resulted in increased neurogenesis in adult DG30 and SVZ.31,32 Conditional loss of TrkB, the membrane receptor of BDNF, resulted in decreased proliferation of NSCs and impaired neurogenesis in adult DG.33 Moreover, BDNF/TrkB signaling is required for survival, dendritic arborization, and integration of newborn neurons in the adult DG.34 Research demonstrates that an enriched environment enhances neurogenesis in the adult hippocampus. Interestingly, this enhancement was not observed in BDNF heterozygous knockout mice.35
IGF-1 regulates various processes during adult neurogenesis including progenitor cell proliferation, neuronal differentiation, and maturation.36–40 Peripheral infusion of IGF1 increased progenitor cell proliferation and increased production of new neurons in the adult rat hippocampus.40 IGF-1 overexpression in NSCs in transgenic mice increased NSC proliferation in the SGZ and SVZ via MEK/ERK pathway, and at the same time it induced differentiation of NSCs via the PI3K/Akt pathway.36 Blocking IGF-1 using antibodies in adult NSC cultures inhibited differentiation into neurons.38 Interestingly, an in vitro study showed that the diverse actions of IGF-1 could be dosage dependent. At high dose (100 ng/mL), it increased adult rat hippocampal progenitor cell proliferation and decreased differentiation, while at low dose (1 ng/mL), it stimulated differentiation.39 The expression of endogenous IGF-1 in the adult hippo campus naturally decreases with age and the rate of neurogenesis. Research demonstrates that restoration of IGF-1 levels by intracerebroventricular infusion increased the rate of neurogenesis by approximately threefold. This suggests that changes in endogenous IGF-1 levels may underline age-related decline of neurogenesis.37 A recent study identified another IGF – IGF2 – as a novel adult neurogenesis regulator. Transcriptome analysis showed that IGF2 was expressed in the DG NSCs at a significantly higher level than in immature neurons in adult mice, and it governs NSC proliferation in vitro and in vivo via AKT-dependent pathway.41
Conditional overexpression of an activated FGF receptor in adult neural precursor cells increased the production of new neurons, whereas conditional deletion of the FGF receptors in these cells decreased NSCs, progenitor cells, and immature neurons in mice. Interestingly, overexpression of the activated FGF receptor in older mice was able to restore the age-related decline in neurogenesis.42 Similarly, intracerebroventricular FGF-2 infusion increased production of new dentate granule cells and their dendritic growth in the hippocampus in middle-aged rats.43
Neurotransmitters
A number of neurotransmitters are involved in adult neurogenesis including gamma-aminobutyric acid (GABA), dopamine, glutamate, and serotonin.44
GABA, an inhibitory neurotransmitter, was shown to be a critical niche signal regulating activation and proliferation of quiescent adult NSCs, granule cell maturation, and migration. It is secreted from neuroblasts, and its receptor GABAA ion channel is present on NSCs and their progeny. The neuroblast-derived GABA inhibits NSC proliferation, forming a negative-feedback mechanism in maintaining neurogenesis homeostasis.45,46 Similarly, GABA derived from local parvalbumin-expressing interneurons in the DG restored quiescence of NSCs following proproliferative neuronal activities such as social isolation.47 GABAA receptor loss-of-function caused NSCs to rapidly exit from quiescence and start symmetrical self-renewal.47 In the anterior SVZ, GABA was also released from astrocyte-like cells, which surround the migrating neuroblasts. Here, it slowed down neuroblast migration en route to the olfactory bulb (OB) through the same receptor.48
Transcription factors
A number of transcription factors including sex-determining region Y-box 2 (Sox2), Orphan nuclear receptor TLX, forkhead box O proteins (FoxOs), prospero homeobox 1 (Prox1), neuronal differentiation (NeuroD), Kruppel-like factor 9, paired box protein (Pax6), and neurogenin 2 (Neurog2) were found to regulate adult neurogenesis.44
TLX is required for NSCs to self-renew and maintain undifferentiated state in both adult SVZ49 and hippocampus50 through the canonical Wnt pathway.51 Furthermore, deletion of TLX resulted in impaired spatial learning in adult mice.52
Sox2 regulates different stages of adult neurogenesis including precursor cell proliferation, neuronal maturation, and migration. Sox2 null mutant mice had impaired precursor cell proliferation with a decreased production of new neurons.53 Sox2 knockdown mutant mice had impaired neuronal maturation, abnormal morphology and migration, and diseased number of GABAergic neurons.54
Epigenetic factors
Epigenetic factors are molecules that modify gene expression via mechanisms such as DNA methylation and histone modification. Those modifications are heritable but do not involve DNA mutations and are therefore termed epigenetic.53 Various epigenetic factors were reported to regulate adult neurogenesis including methyl-CpG-binding domain protein 1 (Mbd1), MYST family histone acetyltransferase Querkopf (Qkf), mixed-lineage leukemia 1 (Mll1), polycomb complex protein (Bmi-1), histone deacetylase 2 (HDAC2), and microRNAs (miR124, 137, 184, 185, and 491-3p).44,55–57
Mbd1 is critical for adult hippocampal neurogenesis and spatial learning by inhibiting proliferation and promoting differentiation.58–60 Mbd1 functions through at least two arms. It directly binds to the FGF-2 promoter and induces its methylation. As a result, the mitogen FGF-2 expression was downregulated allowing for differentiation to occur.60 The second arm involves the downregulation of miR-184 by Mbd1. miR-184 is a microRNA that promotes proliferation and inhibits differentiation by inhibiting the expression of Numb1 (Numblike 1, a regulator of brain development). When miR-184 is downregulated by Mbd1, it results in inhibition of proliferation.59
Qkf is a MYST family histone acetyltransferase expressed in the SVZ of the adult brain. The Qkf-deficient mice had reduced adult neurogenesis. The number of interneurons in the OB decreased, accompanied by a reduction in the number of NSCs and migrating neuroblasts in the rostral migratory stream. Furthermore, NSCs isolated from Qkf-deficient mice exhibited reduced both self-renewal and the ability to differentiate into neurons.61
Adult Neurogenesis in Brain Diseases
The association between adult neurogenesis and brain diseases is studied in both human and animal models. This section focuses on reports directly related to human, either by immunohistochemical labeling of neurogenic markers in postmortem brain tissues or by magnetic resonance imaging (MRI).
Alzheimer’s disease
AD is characterized by widespread neurodegeneration throughout the basal forebrain, the cortex, and the limbic system. The hallmarks of AD include deposition of amyloid plaque and formation of neurofibrillary tangles.62,63 Some studies have found reduction of neuronal progenitor proliferation with bromodeoxyuridine labeling in aging rats64 and AD mouse models.65,66 However, to date, studies on postmortem brain tissues of AD patients using immunohistochemical staining against neurogenic markers showed alternations in neurogenesis with inconsistent patterns. Elevated hippocampal expressions of neurogenic marker proteins, such as DCX, PSA-NCAM, TUC-4, and NeuroD, were found in particular in the granule cell layer of SGZ,67 suggesting higher level of neurogenesis in AD. In another study, both Nestin and PSA-NCAM showed significantly higher immunoreactivities in patients, and the increase correlated with the progression of the disease.68,69 However, the neurogenic marker Musashi-1 (Msi1) immunoreactivities were significantly lower in the AD patients,68,69 as well as choline-acetyltransferase, which is an indicator of a reduction of cholinergic activity.68 Another study showed that neurogenic markers Sox 2 and DCX were downregulated accompanied by an increase in NSC quiescence regulator BMP6.70 These discrepancies might be due to the differential expression of certain biomarkers but not others (for example, Msi1 vs. Nestin).63 The differences reflect particular stages of AD progression or glial and vascular-associated changes independent of modulation of neurogenesis.71,72 Furthermore, a recent study identified proliferating cell nuclear antigen (PCNA)-positive cells within the CA regions that lacked amyloid beta pathology. These cells were not colabeled with astrocyte maker glial fibrillary acidic protein (GFAP) but with Iba1, a microglial marker, in the CA regions as well as the DG and SGZ.73 Iba1-positive cells often formed a concentric ring around amyloid plaques and were found in proximity of plaque pathology.73
In general, microglia are activated by brain damage to release cytokines such as toll-like receptors (TLR-2 and TLR-4), tumor necrosis factor α, and interleukin 1 beta.74,75 These cytokines activate astrogliosis.76,77 During AD progression, degradation of astrocytes and the migration and congregation of microglia within neuritic and dense-core plaques are commonly seen.78 The proliferation of microglia is likely to be a critical step to initiate these changes.79 In fact, the microglial activity could regulate neurogenesis in its own right.79,80
Parkinson’s disease
PD is a progressive, chronic neurodegenerative disorder that is associated with the degeneration of dopaminergic neurons of the substantia nigra located in the midbrain. The pathological hallmarks of PD are accumulation of alpha-synuclein and intracellular deposits to form inclusion bodies called Lewy bodies and filamentary Lewy neurites.81,82 PD affects the neuronal activities at various regions of the brain such as the amygdala, hippocampus, and OB.83–85 With a limited number of studies using postmortem brain tissues of PD patients, the involvement of neurogenesis is largely unclear due to inconsistent results from different reports. In two studies, the number of cells expressing epidermal growth factor receptor (EGFR)86 and PCNA87 in the SVZ of PD patients significantly decreased compared with age-matched controls. However, another study with older patients and shorter postmortem time observed a higher variation in the number of NSCs at SVG. The number of cells expressing PCNA or the mitotic marker phosphohistone H3 did not show significant differences in patients with PD pathology, with or without dopamine replacement therapy, compared to age- and sex-matched controls.88 In particular, hyposmia, a reduced ability to smell and to detect odors, is one of the most prevalent symptoms of PD.89,90 Correspondingly, the total number of tyrosine hydroxylase-immunoreactive neurons in the OB was twice as high in the PD patients.91,92 However, the number of neural precursor cells decreased,87 with the OB volume unchanged in some studies83,84 but reduced in others.85,93,94 Similar to AD, recent evidence also indicates that the proliferating cells in the hippocampus of PD patients are predominantly microglia,95,96 probably due to the neuroinflammatory response to developing PD pathology.
Huntington’s disease
HD is a progressive neurodegenerative genetic disorder caused by autosomal-dominant mutations in the form of CAG repeats in the huntingtin gene located on chromosome 4.97,98 Increased cell proliferation defined by expression of PCNA in SVZ was identified in postmortem brains of HD patients, and the proliferating cells were colabeled by a neuronal marker beta III-tubulin or GFAP, suggesting increased neurogenesis.35 The degree of cell proliferation correlated with the disease severity and with the number of CAG repeats in the huntingtin gene.99 The thickness of SVZ increased with a 2.6-fold increase in the number of new neurons in SVZ.100,101 Some PCNA-positive cells also expressed cannabinoid CB1 receptors, which are preferentially lost in HD, but not with neuronal, glial, microglial, or oligodendrocyte markers, in SVZ of both adult normal and HD brains.102
Brain tumors
The multipotency and self-renewal ability of neural stem cells are very similar to brain tumor stem cells in human brain tumors. It is hypothesized and supported by accumulating research that NSCs within the SVZ transform give rise to brain tumors.103 Glioblastoma multiforme (GBM), the most common and most aggressive malignant primary brain tumor in humans, has been the focus of many studies. A study showed that in 93% of cases of GBM, the lesions contacted at least one region of the lateral ventricular wall where adult neurogenesis occurs.104 Multiple MRI analyses of GBM cases showed that the subtypes in contact with the SVZ and involving the cortex were most likely to be multifocal at the time of initial diagnosis.105 These patients are also more likely to have recurrent tumors at locations distant to the initial lesion,105 with more rapid progression,106 and have decreased overall survival rate.106,107
Conclusion
Adult neurogenesis is regulated by both extracellular factors (morphogens, growth factors, and neurotransmitters) and intracellular factors (transcription factors and epigenetic factors). Their functions have been identified and related to specific stages of adult neurogenesis. As our understanding on the molecular mechanism of adult neurogenesis deepens, its association with a number of human diseases begins to emerge. Accumulating evidence has shown that adult neurogenesis is altered in various brain diseases such as AD, PD, HD, and brain tumors. However, the findings are highly variable and sometimes contradicting. This is most likely due to the use of different approaches, different markers, or the examinations of different disease stages. More efforts with standardized methods and specific stages of the diseases are necessary to elucidate the associations between adult neurogenesis and these brain diseases.
Acknowledgments
We thank Katherine Hanna, Grace Liu Anderson, and Stephanie Blackstone (Registered Nurse and president of Treasures of the Heart Inc. 501(c)(3) charity) for proofreading the manuscript.
Footnotes
ACADEMIC EDITOR: Alexander Rotenburg, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,826 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Wrote the first draft of the manuscript: HL, NS. Contributed to the writing of the manuscript: HL, NS. Agree with manuscript results and conclusions: HL, NS. Jointly developed the structure and arguments for the paper: HL, NS. Made critical revisions and approved final version: HL, NS. Both authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–35. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla A, García-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22(3):629–34. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kempermann G, Gage FH. Neurogenesis in the adult hippocampus. Novartis Found Symp. 2000;231:220–35. discussion 235–41, 302–6. [PubMed] [Google Scholar]
- 4.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 5.Ernst A, Alkass K, Bernard S, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156(5):1072–83. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 6.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spalding KL, Bergmann O, Alkass K, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–27. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun SMG, Jessberger S. Adult neurogenesis: mechanisms and functional significance. Development. 2014;141(10):1983–6. doi: 10.1242/dev.104596. [DOI] [PubMed] [Google Scholar]
- 9.Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev. 2014;94(4):991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron HA, Glover LR. Adult neurogenesis: beyond learning and memory. Annu Rev Psychol. 2015;66(1):53–81. doi: 10.1146/annurev-psych-010814-015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh J. Orchestrating transcriptional control of adult neurogenesis. Genes Dev. 2012;26(10):1010–21. doi: 10.1101/gad.187336.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vishwakarma SK, Bardia A, Tiwari SK, Paspala SAB, Khan AA. Current concept in neural regeneration research: NSCs isolation, characterization and transplantation in various neurodegenerative diseases and stroke: a review. J Adv Res. 2014;5(3):277–94. doi: 10.1016/j.jare.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaidi HA, Kosztowski T, DiMeco F, Quiñones-Hinojosa A. Origins and clinical implications of the brain tumor stem cell hypothesis. J Neurooncol. 2009;93(1):49–60. doi: 10.1007/s11060-009-9856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabata T, Takei Y. Morphogens, their identification and regulation. Development. 2004;131(4):703–12. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- 15.Breunig JJ, Silbereis J, Vaccarino FM, Šestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(51):20558–63. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavado A, Oliver G. Jagged1 is necessary for postnatal and adult neurogenesis in the dentate gyrus. Dev Biol. 2014;388(1):11–21. doi: 10.1016/j.ydbio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30(9):3489–98. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierfelice T, Alberi L, Gaiano N. Notch in the vertebrate nervous system: an old dog with new tricks. Neuron. 2011;69(5):840–55. doi: 10.1016/j.neuron.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Nomura T, Göritz C, Catchpole T, Henkemeyer M, Frisén J. EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell Stem Cell. 2010;7(6):730–43. doi: 10.1016/j.stem.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberi L, Liu S, Wang Y, et al. Activity-induced notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron. 2011;69(3):437–44. doi: 10.1016/j.neuron.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Ferrari GV, Chacón MA, Barría MI, et al. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol Psychiatry. 2003;8(2):195–208. doi: 10.1038/sj.mp.4001208. [DOI] [PubMed] [Google Scholar]
- 22.Inestrosa NC, Montecinos-Oliva C, Fuenzalida M. Wnt signaling: role in Alzheimer disease and schizophrenia. J Neuroimmune Pharmacol. 2012;7(4):788–807. doi: 10.1007/s11481-012-9417-5. [DOI] [PubMed] [Google Scholar]
- 23.Inestrosa NC, Varela-Nallar L. Wnt signalling in neuronal differentiation and development. Cell Tissue Res. 2015;359(1):215–23. doi: 10.1007/s00441-014-1996-4. [DOI] [PubMed] [Google Scholar]
- 24.Lie DC, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–5. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 25.Wexler EM, Paucer A, Kornblum HI, Palmer TD, Geschwind DH. Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells. 2009;27(5):1130–41. doi: 10.1002/stem.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jessberger S, Clark RE, Broadbent NJ, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16(2):147–54. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mira H, Andreu Z, Suh H, et al. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7(1):78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Bond AM, Peng CY, Meyers EA, McGuire T, Ewaleifoh O, Kessler JA. BMP signaling regulates the tempo of adult hippocampal progenitor maturation at multiple stages of the lineage. Stem Cells. 2014;32(8):2201–14. doi: 10.1002/stem.1688. [DOI] [PubMed] [Google Scholar]
- 29.McGeachie J, Tennant M. Growth factors and their implications for clinicians: a brief review. Aust Dent J. 1997;42(6):375–80. doi: 10.1111/j.1834-7819.1997.tb06081.x. [DOI] [PubMed] [Google Scholar]
- 30.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192(2):348–56. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Zigova T, Pencea V, Wiegand SJ, Luskin MB. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci. 1998;11(4):234–45. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]
- 32.Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21(17):6718–31. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Luikart BW, Birnbaum S, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergami M, Rimondini R, Santi S, Blum R, Götz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105(40):15570–5. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi C, Angelucci A, Costantin L, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24(7):1850–6. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 36.Yuan H, Chen R, Wu L, et al. The regulatory mechanism of neurogenesis by IGF-1 in adult mice. Mol Neurobiol. 2015;51(2):512–22. doi: 10.1007/s12035-014-8717-6. [DOI] [PubMed] [Google Scholar]
- 37.Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107(4):603–13. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 38.Brooker GJ, Kalloniatis M, Russo VC, Murphy M, Werther GA, Bartlett PF. Endogenous IGF-1 regulates the neuronal differentiation of adult stem cells. J Neurosci Res. 2000;59(3):332–41. doi: 10.1002/(sici)1097-4547(20000201)59:3<332::aid-jnr6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Aberg MAI, Aberg ND, Palmer TD, et al. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci. 2003;24(1):23–40. doi: 10.1016/s1044-7431(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 40.Åberg MAI, Åberg ND, Hedbäcker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20(8):2896–903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bracko O, Singer T, Aigner S, et al. Gene expression profiling of neural stem cells and their neuronal progeny reveals IGF2 as a regulator of adult hippocampal neurogenesis. J Neurosci. 2012;32(10):3376–87. doi: 10.1523/JNEUROSCI.4248-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang W, Hébert JM. FGF signaling is necessary for neurogenesis in young mice and sufficient to reverse its decline in old mice. J Neurosci. 2015;35(28):10217–23. doi: 10.1523/JNEUROSCI.1469-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rai KS, Hattiangady B, Shetty AK. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur J Neurosci. 2007;26(7):1765–79. doi: 10.1111/j.1460-9568.2007.05820.x. [DOI] [PubMed] [Google Scholar]
- 44.Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. 2013;1830(2):2435–48. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls GFAP-expressing progenitor proliferation. Nat Neurosci. 2005;8(9):1179–87. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giachino C, Barz M, Tchorz JS, et al. GABA suppresses neurogenesis in the adult hippocampus through GABAB receptors. Development. 2014;141(1):83–90. doi: 10.1242/dev.102608. [DOI] [PubMed] [Google Scholar]
- 47.Song J, Zhong C, Bonaguidi MA, et al. Neuronal circuitry mechanism regulating adult quiescent neural stem cell fate decision. Nature. 2012;489(7414):150–4. doi: 10.1038/nature11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24(35):7623–31. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu HK, Belz T, Bock D, et al. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 2008;22(18):2473–8. doi: 10.1101/gad.479308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi Y, Chichung Lie D, Taupin P, et al. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427(6969):78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 51.Qu Q, Sun G, Li W, et al. Orphan nuclear receptor TLX activates Wnt/β-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol. 2010;12(1):31–9. doi: 10.1038/ncb2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451(7181):1004–7. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 53.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 54.Cavallaro M, Mariani J, Lancini C, et al. Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development. 2008;135(3):541–57. doi: 10.1242/dev.010801. [DOI] [PubMed] [Google Scholar]
- 55.Fitzsimons CP, van Bodegraven E, Schouten M, et al. Epigenetic regulation of adult neural stem cells: implications for Alzheimer’s disease. Mol Neurodegener. 2014;9:25. doi: 10.1186/1750-1326-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serafini G, Pompili M, Hansen KF, et al. The involvement of microRNAs in major depression, suicidal behavior, and related disorders: a focus on miR-185 and miR-491-3p. Cell Mol Neurobiol. 2014;34(1):17–30. doi: 10.1007/s10571-013-9997-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serafini G, Pompili M, Innamorati M, et al. The role of microRNAs in synaptic plasticity, major affective disorders and suicidal behavior. Neurosci Res. 2012;73(3):179–90. doi: 10.1016/j.neures.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Zhao X, Ueba T, Christie BR, et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc Natl Acad Sci U S A. 2003;100(11):6777–82. doi: 10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu C, Teng ZQ, Santistevan NJ, et al. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6(5):433–44. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, Barkho BZ, Luo Y, et al. Epigenetic regulation of the stem cell mitogen Fgf-2 by Mbd1 in adult neural stem/progenitor cells. J Biol Chem. 2008;283(41):27644–52. doi: 10.1074/jbc.M804899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merson TD, Dixon MP, Collin C, et al. The transcriptional coactivator Querkopf controls adult neurogenesis. J Neurosci. 2006;26(44):11359–70. doi: 10.1523/JNEUROSCI.2247-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 63.De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. Alzheimer’s disease. Subcell Biochem. 2012;65:329–52. doi: 10.1007/978-94-007-5416-4_14. [DOI] [PubMed] [Google Scholar]
- 64.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghosal K, Stathopoulos A, Pimplikar SW. APP intracellular domain impairs adult neurogenesis in transgenic mice by inducing neuroinflammation. PLoS One. 2010;5(7):e11866. doi: 10.1371/journal.pone.0011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li G, Bien-Ly N, Andrews-Zwilling Y, et al. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell. 2009;5(6):634–45. doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin K, Peel AL, Mao XO, et al. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101(1):343–7. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perry EK, Johnson M, Ekonomou A, Perry RH, Ballard C, Attems J. Neurogenic abnormalities in Alzheimer’s disease differ between stages of neurogenesis and are partly related to cholinergic pathology. Neurobiol Dis. 2012;47(2):155–62. doi: 10.1016/j.nbd.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ziabreva I, Perry E, Perry R, et al. Altered neurogenesis in Alzheimer’s disease. J Psychosom Res. 2006;61(3):311–6. doi: 10.1016/j.jpsychores.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 70.Crews L, Adame A, Patrick C, et al. Increased BMP6 levels in the brains of Alzheimer’s disease patients and APP transgenic mice are accompanied by impaired neurogenesis. J Neurosci. 2010;30(37):12252–62. doi: 10.1523/JNEUROSCI.1305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arisi I, D’Onofrio M, Brandi R, et al. Gene expression biomarkers in the brain of a mouse model for Alzheimer’s disease: mining of microarray data by logic classification and feature selection. J Alzheimers Dis. 2011;24(4):721–38. doi: 10.3233/JAD-2011-101881. [DOI] [PubMed] [Google Scholar]
- 72.Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006;24(1):1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 73.Marlatt MW, Bauer J, Aronica E, et al. Proliferation in the Alzheimer hippocampus is due to microglia, not astroglia, and occurs at sites of amyloid deposition. Neural Plast. 2014;2014:693851. doi: 10.1155/2014/693851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoogland ICM, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993;150(7):2659–67. [PubMed] [Google Scholar]
- 76.Chen SH, Oyarzabal EA, Sung YF, et al. Microglial regulation of immunological and neuroprotective functions of astroglia. Glia. 2015;63(1):118–31. doi: 10.1002/glia.22738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giulian D, Li J, Li X, George J, Rutecki PA. The impact of microglia-derived cytokines upon gliosis in the CNS. Dev Neurosci. 1994;16(3–4):128–36. doi: 10.1159/000112099. [DOI] [PubMed] [Google Scholar]
- 78.Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang K-C, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging. 2004;25(5):663–74. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 79.Gemma C, Bachstetter AD. The role of microglia in adult hippocampal neurogenesis. Front Cell Neurosci. 2013;7:229. doi: 10.3389/fncel.2013.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ji R, Tian S, Lu HJ, et al. TAM receptors affect adult brain neurogenesis by negative regulation of microglial cell activation. J Immunol. 2013;191(12):6165–77. doi: 10.4049/jimmunol.1302229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stefanis L. α-Synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(2):a009399. doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Irvine GB, El-Agnaf OM, Shankar GM, Walsh DM. Protein aggregation in the brain: the molecular basis for Alzheimer’s and Parkinson’s diseases. Mol Med. 2008;14(7–8):451–64. doi: 10.2119/2007-00100.Irvine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hummel T, Witt M, Reichmann H, Welge-Luessen A, Haehner A. Immunohistochemical, volumetric, and functional neuroimaging studies in patients with idiopathic Parkinson’s disease. J Neurol Sci. 2010;289(1–2):119–22. doi: 10.1016/j.jns.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 84.Mueller A, Abolmaali ND, Hakimi AR, et al. Olfactory bulb volumes in patients with idiopathic Parkinson’s disease a pilot study. J Neural Transm (Vienna) 2005;112(10):1363–70. doi: 10.1007/s00702-005-0280-x. [DOI] [PubMed] [Google Scholar]
- 85.Chen S, Tan H, Wu Z, et al. Imaging of olfactory bulb and gray matter volumes in brain areas associated with olfactory function in patients with Parkinson’s disease and multiple system atrophy. Eur J Radiol. 2014;83(3):564–70. doi: 10.1016/j.ejrad.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 86.O’Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci U S A. 2009;106(21):8754–9. doi: 10.1073/pnas.0803955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Höglinger GU, Rizk P, Muriel MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7(7):726–35. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 88.van den Berge SA, van Strien ME, Korecka JA, et al. The proliferative capacity of the subventricular zone is maintained in the parkinsonian brain. Brain. 2011;134(pt 11):3249–63. doi: 10.1093/brain/awr256. [DOI] [PubMed] [Google Scholar]
- 89.Berendse HW, Ponsen MM. Detection of preclinical Parkinson’s disease along the olfactory trac(t) J Neural Transm Suppl. 2006;70:321–5. doi: 10.1007/978-3-211-45295-0_48. [DOI] [PubMed] [Google Scholar]
- 90.Hoyles K, Sharma JC. Olfactory loss as a supporting feature in the diagnosis of Parkinson’s disease: a pragmatic approach. J Neurol. 2013;260(12):2951–8. doi: 10.1007/s00415-013-6848-8. [DOI] [PubMed] [Google Scholar]
- 91.Huisman E, Uylings HBM, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov Disord. 2004;19(6):687–92. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- 92.Huisman E, Uylings HBM, Hoogland PV. Gender-related changes in increase of dopaminergic neurons in the olfactory bulb of Parkinson’s disease patients. Mov Disord. 2008;23(10):1407–13. doi: 10.1002/mds.22009. [DOI] [PubMed] [Google Scholar]
- 93.Brodoehl S, Klingner C, Volk GF, Bitter T, Witte OW, Redecker C. Decreased olfactory bulb volume in idiopathic Parkinson’s disease detected by 3.0-tesla magnetic resonance imaging. Mov Disord. 2012;27(8):1019–25. doi: 10.1002/mds.25087. [DOI] [PubMed] [Google Scholar]
- 94.Wu X, Yu C, Fan F, et al. Correlation between progressive changes in piriform cortex and olfactory performance in early Parkinson’s disease. Eur Neurol. 2011;66(2):98–105. doi: 10.1159/000329371. [DOI] [PubMed] [Google Scholar]
- 95.Doorn KJ, Drukarch B, van Dam AM, Lucassen PJ. Hippocampal proliferation is increased in presymptomatic Parkinson’s disease and due to microglia. Neural Plast. 2014;2014:959154. doi: 10.1155/2014/959154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doorn KJ, Moors T, Drukarch B, van de Berg WD, Lucassen PJ, van Dam AM. Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol Commun. 2014;2:90. doi: 10.1186/s40478-014-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Agostinho LA, Dos Santos SR, Alvarenga RMP, Paiva CLA. A systematic review of the intergenerational aspects and the diverse genetic profiles of Huntington’s disease. Genet Mol Res. 2013;12(2):1974–81. doi: 10.4238/2013.June.13.6. [DOI] [PubMed] [Google Scholar]
- 98.Walker FO. Huntington’s Disease. Semin Neurol. 2007;27(2):143–50. doi: 10.1055/s-2007-971176. [DOI] [PubMed] [Google Scholar]
- 99.Curtis MA, Penney EB, Pearson AG, et al. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc Natl Acad Sci U S A. 2003;100(15):9023–7. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Curtis MA, Penney EB, Pearson J, Dragunow M, Connor B, Faull RLM. The distribution of progenitor cells in the subependymal layer of the lateral ventricle in the normal and Huntington’s disease human brain. Neuroscience. 2005;132(3):777–88. doi: 10.1016/j.neuroscience.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 101.Curtis MA, Waldvogel HJ, Synek B, Faull RLM. A histochemical and immunohistochemical analysis of the subependymal layer in the normal and Huntington’s disease brain. J Chem Neuroanat. 2005;30(1):55–66. doi: 10.1016/j.jchemneu.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 102.Curtis MA, Faull RLM, Glass M. A novel population of progenitor cells expressing cannabinoid receptors in the subependymal layer of the adult normal and Huntington’s disease human brain. J Chem Neuroanat. 2006;31(3):210–5. doi: 10.1016/j.jchemneu.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 103.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353(8):811–22. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 104.Barami K, Sloan AE, Rojiani A, Schell MJ, Staller A, Brem S. Relationship of gliomas to the ventricular walls. J Clin Neurosci. 2009;16(2):195–201. doi: 10.1016/j.jocn.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 105.Lim DA, Cha S, Mayo MC, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007;9(4):424–9. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jafri NF, Clarke JL, Weinberg V, Barani IJ, Cha S. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro Oncol. 2013;15(1):91–6. doi: 10.1093/neuonc/nos268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sonoda Y, Saito R, Kanamori M, Kumabe T, Uenohara H, Tominaga T. The association of subventricular zone involvement at recurrence with survival after repeat surgery in patients with recurrent glioblastoma. Neurol Med Chir (Tokyo) 2014;54(4):302–9. doi: 10.2176/nmc.oa.2013-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]


