Abstract
Myelodysplastic syndromes (MDS) and myeloproliferative neoplasms (MPN) are hematologically diverse stem cell malignancies sharing phenotypic features of both myelodysplastic syndromes and myeloproliferative neoplasms. There are currently no standard treatment recommendations for most adult patients with MDS/MPN. To optimize efforts to improve the management and disease outcomes, it is essential to identify meaningful clinical and biologic end points and standardized response criteria for clinical trials. The dual dysplastic and proliferative features in these stem cell malignancies define their uniqueness and challenges. We propose response assessment guidelines to harmonize future clinical trials with the principal objective of establishing suitable treatment algorithms. An international panel comprising laboratory and clinical experts in MDS/MPN was established involving 3 independent academic MDS/MPN workshops (March 2013, December 2013, and June 2014). These recommendations are the result of this collaborative project sponsored by the MDS Foundation.
Introduction
Myelodysplastic/myeloproliferative neoplasms (MDS/MPN) comprise a World Health Organization (WHO) category of hematopoietic stem cell malignancies sharing morphologic and hematologic features of both myelodysplastic syndromes and myeloproliferative neoplasms.1 As characterized by the WHO in 2008, these disorders include chronic myelomonocytic leukemia (CMML), juvenile myelomonocytic leukemia (JMML), atypical BCR-ABL1 negative chronic myeloid leukemia (aCML), myelodysplastic/myeloproliferative neoplasm unclassifiable (MDS/MPN-U), and a provisional entity named refractory anemia with ring sideroblasts and thrombocytosis (RARS-T).2 Although CMML, the most frequent subgroup of MDS/MPN, is heterogeneous in presentation, it differs from other MDS/MPN in adults because of the presence of sustained monocytosis defined as a monocyte count >1000/μL that comprises at least 10% of the white blood cell (WBC) differential. aCML is characterized by left-shifted leukocytosis with severe granulocytic dysplasia but lacks monocytosis or the BCR-ABL1 fusion characteristic of chronic myeloid leukemia (CML). MDS/MPN-U patients are phenotypically heterogeneous, displaying a combination of dysplastic and myeloproliferative features that do not fulfill criteria for assignment to any other MDS/MPN subtype.3 The provisional entity of RARS-T—which shares features of anemia, dyserythropoiesis, and >15% bone marrow ring sideroblasts similar to refractory anemia with ring sideroblasts—is distinguished by sustained thrombocytosis (≥450 × 109/L), megakaryocytes with myeloproliferative cytological features, and, similar to other MDS/MPN or MPN, moderate splenomegaly.3,4 The molecular, diagnostic, and clinical features of MDS/MPN have been reviewed by this group elsewhere.5
Currently, few evidence-based recommendations can be made for managing patients with MDS/MPN. Overall survival is variable, measured in years for many RARS-T or low-risk CMML patients and months for many aCML or high-risk CMML patients. The molecular and clinical heterogeneity and absence of uniform response criteria by which to assess meaningful therapeutic benefit make developing and comparing new therapies a challenge. Novel agents that target biological features important in MDS/MPN are in development; testing the effectiveness of these agents requires a harmonized assessment approach designed specifically for MDS/MPN.
Challenges in diagnosing MDS/MPN and current response criteria for MDS and MPN
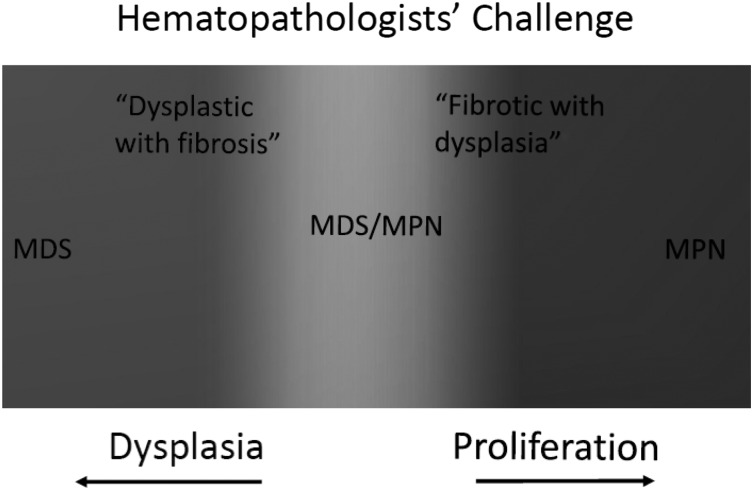
The WHO 2008 diagnostic criteria afford considerable latitude to individual hematopathologic interpretation. MDS/MPN include a spectrum of stem cell malignancies that harbor a phenotype with elements of both groups of disorders (Figure 1). Likewise, there is considerable heterogeneity within subtypes of MDS/MPN. Thus, the diagnostic criteria are imperfect for the subjective interpretation of morphology and the considerable heterogeneity of the diseases described. For example, the elevation of monocytes (>1 × 109/L sustained ≥3 months) is a diagnostic criterion for CMML. Admittedly, there are “proliferative-phase” myelofibrosis (MF) patients with considerable leukocytosis who fulfill these criteria,4,6 and conversely, severely dysplastic CMML patients with WBC ≤1 × 109/L with as much as 80% monocytosis who fail to meet criteria for CMML definition. Likewise, there is clearly a temporal aspect involved because the composition and plasticity of bone marrow changes in these diseases vary considerably over time.
Figure 1.
Classically, chronic myeloid malignancies are either proliferative or dysplastic. MDS/MPN that harbor hybrid characteristics of both MDS and MPN (overlap syndromes), represent a spectrum of different disorders.
To gauge the clinical benefits of emerging therapy, it is essential to develop meaningful clinically relevant and biologic end points with standardized response criteria. Following the discovery of the JAK2 V617F mutation in myelofibrosis, the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) proposed MF response criteria in 2006 that were revised in 2013. The metrics for measuring complete response (CR) and partial response in treating MF included quantification of changes in bone marrow fibrosis, composition of the peripheral blood, and resolution of disease-associated symptoms and clinical features of disease, including hepatosplenomegaly. Clinical improvement requires ≥50% reduction in splenomegaly, improvement in cytopenias (2.0 g/dL increase in hemoglobin (Hgb); 100% increase in platelets or neutrophils to reach greater than 50 × 109/L and 0.5 × 109/L, respectively), or transfusion independence for transfusion-dependent patients with pretreatment Hgb ≤10 g/dL.7 Interestingly, the most successful therapeutic clinical trials in MF, Controlled Myelofibrosis Study With Oral Janus-associated Kinase (JAK) Inhibitor Treatment (COMFORT) I and COMFORT II (testing ruxolitinib vs placebo, and ruxolitinib vs best available therapy, respectively), suggested improvement in survival and meaningful clinical benefit without significantly affecting rates of complete or partial response as defined by the IWG-MRT.8-10 Although it is possible that long-term therapy with ruxolitinib may reverse fibrosis in some patients,11 most patients in the COMFORT trials had little or no documented change in bone marrow findings to fulfill IWG-MRT remission criteria and no discernable reduction in the rate of leukemic transformation. Reduction in splenomegaly and improvement in quality of life, however, led to approval of ruxolitinib for patients with MF by the Food and Drug Administration, and may be harbingers for altering the natural history of the disease.12
Similarly, the IWG response criteria for MDS measure changes in marrow myeloblast percentage, cytogenetics, amelioration of cytopenias, and magnitude and duration of hematologic improvement.13 Reduction in the number of transfusions by 4 transfusions per 8 weeks compared with pretreatment transfusion frequency or Hgb increase by ≥1.5 g/dL constitutes erythroid hematologic improvement. An absolute increase in platelets of ≥30 × 109/L for patients with thrombocytopenia ≥20 × 109/L or an increase from ≤20 × 109/L to ≥20 × 109/L and at least 100% improvement defines platelet hematologic improvement. Only MDS patients with pretreatment neutropenia <1 × 109/L qualify for neutrophil hematologic improvement, accompanied by an increase of at least 100% with absolute rise ≥0.5 × 109/L.13 The advanced age and comorbidities of many patients with MDS obfuscate the need to achieve conventional CRs, whereas priority is given to symptom control and quality of life. Thus, recent clinical trials have been designed to capture hematologic improvement as a primary end point in MDS. In some studies, correlation of those clinically meaningful responses (eg, clinical benefit) with an outcome such as overall survival or leukemia transformation had been demonstrated. For example, in the AZA-001 study in higher risk MDS, patients who achieved hematologic improvement had a survival benefit that was comparable to patients achieving a partial response or CR.14 Further, the significance of other response criteria such as marrow CR13 in MDS is less clear because reduction in blasts without improvements in other hematologic parameters has not yet translated to patient benefit. Likewise, the erythroid response criterion of reduction in transfusion needs as opposed to transfusion independence has no relationship to outcome, but perhaps impacts quality of life. Practically, this is a cumbersome assessment in clinical trials, and regulatory authorities favor more meaningful, clinically relevant end points that reflect clear benefit to the patient.
Future clinical trials may benefit by harmonizing metrics established in MF IWG-MRT and MDS IWG response criteria by measuring effectiveness via evidence for reduction in spleen size, reduction in bone marrow fibrosis, improvement in cytopenias, and reduction of symptoms as measured by a validated measurement tool such as the total symptom score (TSS).15 In addition, reduction in bone marrow or peripheral blasts and rate of transformation to leukemia may be incorporated. Other features of the disease, such as resolution of leukocytosis or thrombocytosis, in patients with MDS/MPN must also be considered. If the patient has more “proliferative” disease, for example, should reduction in spleen size take precedence? If the patient has predominantly dysplastic and ineffective hematopoiesis, should improvement in cytopenias be prioritized?
In prospective trials for patients with MDS/MPN, several approaches have been applied to assess response. Historically, some MDS trials including patients with CMML have measured response via the IWG response criteria for MDS because dysplastic cases of CMML clinically behave similarly to myelodysplasia with primarily hematopoietic insufficiency and without organomegaly or severe constitutional symptoms. For example, a phase 2 decitabine trial involving 38 CMML patients reported a CR rate of 10%, and a 21% marrow CR, with a corresponding 2-year survival of 48%.16 However, the effects of the therapy on the myeloproliferative aspects of the disease were not captured with this approach because more proliferative cases of CMML are characterized by myeloproliferative features of leukocytosis, organomegaly, and considerable constitutional symptoms—clinical features gauged less discernibly with IWG criteria for MDS. The first published attempt to prospectively employ specific response criteria for patients with MDS/MPN was the Thalidomide, Arsenic Trioxide, Dexamethasone, and Ascorbic Acid trial published in 2012. In this prospective study, thalidomide, arsenic, dexamethasone, and ascorbic acid were administered over 12-week cycles to MF and MDS/MPN patients. Bejanyan et al measured response using the MF IWG-MRT criteria for all patients, and then applied the IWG MDS response criteria with respect to hematologic improvement for the 54% of patients in this trial with a diagnosis of MDS/MPN-U.17 This composite response measurement captured by both sets of criteria yielded a 21% overall response rate. That said, nonsynchronous responses with this approach introduce added complexity to measuring the benefit of a given therapy. For example, if a patient experienced a reduction in spleen size by 50% accompanied by a reduction in Hgb ≥ 2 g/dL, that patient would have “clinical improvement” per the MF IWG-MRT criteria, yet no improvement per the MDS IWG criteria. This scenario might reflect a disease-modifying effect, but lead to therapy discontinuation because of no improvement, and greater therapy-induced anemia because the mechanism of action of the therapy such as JAK2 inhibition may account for some reduction in Hgb. In similar attempts to capture responses and potential clinical benefit as accurately as possible for patients with CMML, the Italian Society of Hematology adopted the MDS criteria for specific dysplastic CMML and MF criteria for proliferative CMML.18
Additional candidate metrics for measuring response
The translation of symptom improvement to possible survival advantage and the subsequent approval of ruxolitinib for MF after phase 3 randomized trials have left symptom management as an important focus with particular relevance to MDS/MPN. Symptom burden in MF has been extensively investigated and both spleen-related and nonsplenic symptomatology are meaningful end points in clinical practice, and as in the case of ruxolitinib in MF, potential early surrogates for survival.8-10 Traditionally, the symptom burden in MDS was largely attributed to cytopenias, but evolving data belie this presumption. For example, fatigue poorly correlated with Hgb level in a large quality-of-life survey of MDS patients.19 Regardless, the symptom burden in MDS is underevaluated, and given the improvement in quality-of-life tools and success in quantifying improvement in other myeloid diseases,15 we strongly recommend incorporation of symptom assessment tools in the response criteria for MDS/MPN but realize that any such approach will require validation (ongoing).
Biologic end points could be considered for evaluating response and may be helpful to interpret impact on disease. This is particularly true when employing myelosuppressive therapies that may make discerning drug-related cytopenias from disease-related progression difficult. Karyotype analysis provides a well-studied standardized methodology that has proven clinically relevant in MDS/MPN.20,21 Chromosomal abnormalities can be identified in many cases that provide informative prognostic data and represent a marker of disease burden that may be followed over time. Serum levels of interleukin-8 and other cytokines have also been independently associated with disease progression in MF,22 and treatment with JAK inhibitors has led to downregulation in several genes involved in the inflammatory response in myeloproliferative neoplasms.23 Still, biologic end points may be misleading and there are a lack of standardized assays to measure most biomarkers. For example, the JAK2 V617F allele burden is not necessarily concordant with response for patients receiving JAK2 inhibitor therapy.8,10,24 Whereas the BCR-ABL tyrosine kinase is the hallmark of chronic myelogenous leukemia, there are no singular driving genetic aberrations in any of the MDS/MPN in adults, but, rather recurrent molecular abnormalities. The best example of genotype–phenotype linkage in MDS/MPN is the aberrant regulation of the RAS-RAF pathway in 90% of JMML with largely mutually exclusive germ line mutations involving PTPN11, KRAS, NRAS, NF1, or other related genes.25-27 This is discussed, expertly, elsewhere.28 Recurrent molecular aberrations at several loci have recently been identified in MDS/MPN, and ultimately, these may lead to clearer distinction between the disorders and means by which outcomes can be assessed.29-37 Improved molecular characterization will likely enhance treatment opportunities and development of therapy. Table 1 outlines current understanding of the distribution of molecular aberrations in MDS/MPN.
Table 1.
Known frequency of genetic mutations seen in MDS/MPN (% mutated)
| Gene | CMML21,30-32,45,55-57 | JMML26,27,58-61 | RARS-T29,36,62-64 | aCML35,54,65,66 | MDS/MPN-U46,53 | |
|---|---|---|---|---|---|---|
| Cell signaling | ||||||
| JAK2 V617F | 5-10 | — | 58.7 | 7 | — | |
| JAK3 | <1 | 9 | — | — | — | |
| CALR | — | — | 13 | — | — | |
| MPL | 0 | — | 2 | — | — | |
| NRAS | 4-10 | 12 | — | 8-35 | 2-14 | |
| KRAS | 7-10 | 12 | — | 2 | 0 | |
| PTPN11 | 2 | 40 | — | — | — | |
| NF1 | 1 | 11 | — | — | — | |
| FLT3 | <5 | — | — | — | — | |
| CSF3R | <5 | — | — | <10 | — | |
| CBL | 10-14 | 14 | — | 7 | 2 | |
| KIT | <1 | — | — | — | — | |
| Epigenetic regulators | ||||||
| TET2 | 50-60 | — | 9-26 | 25 | 18 | |
| ASXL1 | 35-40 | 4 | 10 | 25 | 14 | |
| DNMT3A | <5 | — | 17 | — | 3 | |
| IDH1 | <1 | — | — | — | 0 | |
| IDH2 | <1 | — | — | — | 0 | |
| UTX | 8 | — | — | — | — | |
| EZH2 | 5-13 | 0 | 25 | 13-15 | 6-10 | |
| SETBP1 | 5-10 | 7 | — | 25 | — | |
| RNA splicing | ||||||
| SF3B1 | 5-10 | — | 72 | — | 1 | |
| U2AF1 | 5 | — | — | — | 1 | |
| SRSF2 | 50 | — | — | — | 2 | |
| Other | ||||||
| NPM1 | <1-3 | — | — | — | — | |
| TP53 | 5 | — | — | — | 4 | |
| RUNX1 | 15 | — | — | 2 | — |
Risk stratification models
Large MDS and MF patient registries informed creation of the International Prognostic Scoring System, the revised International Prognostic Scoring System, the WHO-based Prognostic Scoring System, and the Dynamic IPSS, which have greatly improved prognostication, and, as a consequence, management strategy. Currently, available outcome data for patients with MDS/MPN are predominantly restricted to CMML, for which there are at least 8 published scoring tools, many of which that have been externally validated. Unfortunately, the CMML models have not been systematically compared, making it difficult to recommend any 1 CMML-specific prognostic tool over another.20,21,38-46
Based on the data acquired from these models, new CMML specific models have been developed that represent advances in risk stratification of the disease. The Spanish MDS group developed a CMML prognostic model from a data set including 558 patients that identified chromosome abnormalities, red blood cell transfusion dependence (or anemia), bone marrow blast count, and elevated WBC count as independent covariates for overall survival.40 This model, for the first time, highlighted the importance of chromosomal abnormalities in CMML, analogous to their prognostic role in MDS. In an analysis of 226 CMML patients from the Mayo clinic, increased peripheral monocyte count, leukoerythroblastosis, anemia, and thrombocytopenia were identified as independent prognostic factors for survival. This model highlights the prognostic impact of monocyte count that was externally validated in an independent CMML cohort.45 The most recent model, proposed by Itzykson et al, merged both clinical parameters and emerging genetic mutations from a cohort of 312 patients.43 This is the first CMML-specific model to incorporate gene mutations into risk analysis that identified ASXL1 mutations, age ≥65 years, leukocytosis, thrombocytopenia, and anemia as independent prognostic variables. The prognostic relevance of gene mutations may differ among MDS/MPN. For instance, SF3B1 mutations have been found to be important prognostically in the RARS-T subgroup,29,47 whereas their impact on the outcome of CMML patients remains to be investigated. How these aberrations may later be incorporated into risk models for these diseases remains unclear, and clarifying this information to properly incorporate these aberrations in prognostication is a priority.
Although these efforts have led to some validated refinements for gauging behavior of the most common MDS/MPN—ie, CMML—prognostic models for MDS/MPN remain an unmet challenge. A recent early attempt to develop a specific risk stratification score in MDS/MPN-U revealed that an absolute neutrophil count ≥8.5 × 103/μL, presence of peripheral blood blasts, Hgb ≤11.5 g/dL, lactate dehydrogenase ≥550 U/L, and age ≥65 as prognostic variables for survival.46 Still, there is a need for uniform risk stratification to complement response criteria so that treatment goals may be tailored according to the disease risk.
Methods for developing candidate criteria
IWG response criteria for MDS and IWG-MRT criteria for MPN were designed based on consensus recommendations. To address the topical issues in MDS/MPN, a panel comprising laboratory and clinical experts in MDS/MPN assembled in early 2013, with 3 independent MDS/MPN workshops held on March 9, 2013, in Miami, Florida; on December 6, 2013, in New Orleans, Louisiana; and June 13, 2014, in Milan, Italy. During this time, members submitted candidate measures of response assessment (eg, bone marrow, symptoms, laboratory measures). The proposed measures were annotated and discussed, and additional members were added at each subsequent meeting to provide complementary perspective and expertise. From these discussions, 2 questionnaires were designed to refine and develop objective consensus for each potential response criterion. Panelists were asked to prioritize each criterion by assigning a weighted rank, allowing for quantitative assessment while achieving 100% participation. Questionnaire results were compiled and further collaborative teleconferences and meetings were held to finalize the criteria. These consensus recommendations of uniform response criteria for adult MDS/MPN are the result of this collaborative project, which was endorsed and supported by the MDS Foundation.
Proposed criteria for measurement of treatment response in MDS/MPN
While recognizing the limitations of current response criteria, the risk of dyssynchronous response, and the difficulty in capturing response within such a heterogeneous group as MDS/MPN, we propose a model of disease assessment that combines the strengths and familiarity of prior assessment tools for MDS and MF and suggests additions to account for clinically relevant end points. As outlined in Table 2, the proposed criteria for CR in MDS/MPN are consistent with previously published response criteria with respect to histologic remission, given the correlation between bone marrow remodeling after allogeneic stem cell transplantation and survival in myelofibrosis39-42 and morphologic remission and survival in MDS.14 Likewise, spleen size, peripheral blood counts, and related symptoms consistent with changes in the natural history of disease are included. The resolution of symptoms should ultimately represent a similar requirement of symptoms in a CR for myelofibrosis as assessed by the IWG-MRT criteria48; however, validation of this threshold is ongoing, so the provisional category of “CR with resolution of symptoms” has been added but is not required to obtain CR.
Table 2.
Proposed criteria for measurement of treatment response in adult MDS/MPN
| CR (presence of all of the following improvements)* |
| Bone marrow: ≤5% myeloblasts (including monocytic blast equivalent in case of CMML) with normal maturation of all cell lines and return to normal cellularity* |
| Osteomyelofibrosis absent or equal to “mild reticulin fibrosis” (≤grade 1 fibrosis)† |
| Peripheral blood‡ |
| WBC ≤10 × 109 cells/L |
| Hgb ≥11 g/dL |
| Platelets ≥100 × 109/L; ≤450 × 109/L |
| Neutrophils ≥1.0 × 109/L |
| Blasts 0% |
| Neutrophil precursors reduced to ≤ 2% |
| Monocytes ≤1 × 109/L |
| Extramedullary disease: Complete resolution of extramedullary disease present before therapy (eg, cutaneous disease, disease-related serous effusions), including palpable hepatosplenomegaly |
| Provisional category of CR with resolution of symptoms:‡ CR as described above, and complete resolution of disease-related symptoms as noted by the MPN-SAF TSS |
| Persistent low-level dysplasia is permitted given subjectivity of assignment of dysplasia* |
| Complete cytogenetic remission |
| Resolution of previously present chromosomal abnormality (known to be associated with myelodysplastic, syndrome myeloproliferative neoplasms, or MDS/MPN), as seen on classic karyotyping with minimal of 20 metaphases or FISH§ |
| Partial remission |
| Normalization of peripheral counts and hepatosplenomegaly with bone marrow blasts (and blast equivalents) reduced by 50%, but remaining >5% of cellularity except in cases of MDS/MPN with ≤5% bone marrow blasts at baseline |
| Marrow response |
| Optimal marrow response: Presence of all marrow criteria necessary for CR without normalization of peripheral blood indices as presented above. |
| Partial marrow response: Bone marrow blasts (and blast equivalents) reduced by 50%, but remaining >5% of cellularity, or reduction in grading of reticulin fibrosis from baseline on at least 2 bone marrow evaluations spaced at least 2 mo apart |
| Clinical benefit |
| Requires 1 of the following in the absence of progression or CR/partial response and independent of marrow response (cord blood response must be verified at ≥8 wk) to be considered a clinical benefit |
| Erythroid response |
| Hgb increase by ≥2.0 g/dL |
| TI for ≥ 8 wk for patients requiring at least 4 packed red blood cell transfusions in the previous 8 wk |
| Only red blood cell transfusions given based on physician’s judgment for a pretreatment Hgb of ≤8.5 g/dL will count in the red blood cell TI response evaluation|| |
| Platelet response |
| Transfusion independence when previously requiring platelet transfusions of at least a rate of 4 platelet transfusions in the previous 8 wk |
| Pretreatment ≤20 × 109/L: increase from <20 × 109/L to >20 × 109/L and by at least 100% |
| Pretreatment >20 × 109/L but ≤ 100 × 109/L: absolute increase of ≥30 × 109/L|| |
| Neutrophil response |
| Pretreatment ≤0.5 × 109/L at least 100% increase and an absolute increase ≥0.5 × 109/L |
| Pretreatment, >0.5 × 109/L and ≤1.0 × 109/L At least 50% increase and an absolute increase ≥0.5 × 109/L|| |
| Spleen response |
| Either a minimum 50% reduction in palpable splenomegaly of a spleen that is at least 10 cm at baseline or a spleen that is palpable at more than 5 cm at baseline becomes not palpable |
| Symptom response |
| Improvement in symptoms as noted by decrease of ≥50% as per the MPN-SAF TSS scoring <20 were not considered eligible for measuring clinical benefit.¶ |
Presence of dysplastic changes, which may be interpreted within the scope of normal range of dysplastic changes, may still exist in the presence of CR as allowed in MDS IWG. Marrow should exhibit age-adjusted normocellularity in CR.
If there is no significant fibrosis present on the initial bone marrow biopsy, a second biopsy is not required to prove resolution of fibrosis. Grading of fibrosis in measurement of treatment response should be according to the European Consensus System.67
Given the current lack of a validated tool to assess complete resolution of symptoms in MDS/MPN, “CR with resolution of symptoms” (a complete resolution of disease-related symptoms as noted by the MPN-SAF TSS in presence of CR) will be a provisional category of disease response.
Loss of cytogenetic burden of disease by (via FISH or classic karyotyping) known to adversely affect prognosis is required to reach complete cytogenetic remission. Decrease in the cytogenetic burden of disease must be by ≥50% (via FISH or classic karyotyping) to be indicative of a partial cytogenetic response. Given variability of fluorescent probes used in FISH, cytogenetic normalization via FISH will depend on the performance characteristics of the specific probes used.
Resolution of abnormal peripheral blood counts must persist for at least 2 separate analyses over at least 8 wk. In the case of proliferative MDS/MPN, CR will include resolution of thrombocytosis to a normal platelet count (150-450 × 109/L) and resolution of leukocytosis to WBC ≤10 × 109 cells/L but ≥1.5 × 109/L. Hgb should be maintained >11 g/dL and platelets ≥100 × 109/L without the support of transfusions. Clinical benefit may occur when these changes occur in absence of other changes required for CR or marrow response. Platelet and packed red blood cell TI would be considered for clinical benefit, and duration of TI should be monitored. Reduction in myeloid precursors (promyelocytes, myelocytes, metamyelocytes, nucleated red blood cells) to less than appreciable levels (≤2-3%) and/or 1 × 109/L monocytosis in the absence of infection, cytokine treatment, or other reactive causes.
MPN-SAF TSS validation among patients with MDS/MPN is currently under way (R.A. Mesa, personal communication, 2014).
The assessment of CR must be confirmed by a minimum of 2 bone marrow assessments only to confirm improvement in fibrosis.13 In the event that marrow fibrosis is not present on the first response assessment marrow, and all other marrow criteria for CR are achieved as noted on follow-up bone marrow aspirate, further bone marrow biopsies are not required for designation of CR. Corresponding improvements in the peripheral blood should be maintained over a minimum of 8 weeks. In recognizing considerable debate over the use of transfusion reduction in the measurement of response to MDS, MF, or MDS/MPN, we have chosen to include only transfusion independence (TI), as defined by freedom from dependence of ≥4 units/8 weeks for packed red blood cells or platelets, and not transfusion reduction, in these proposed criteria., Although TI beyond 8 weeks is not required in these criteria, we do recognize the importance of including duration of response/duration of TI in clinical trials.
Reduction of blast percentage or partial resolution in fibrosis in the marrow carry inherent value, but the relationship between these bone marrow changes and impact on the disease’s natural history or survival in MDS/MPN is unknown. Likewise, the value of these changes in the setting of continued cytopenias and symptomology are in doubt. Despite this, we recommend the inclusion of a provisional category of “marrow response” to capture improvement in marrow fibrosis or blast count in the absence of CR or partial response, which could be assessed for a relationship to established measures of clinical benefit. The use of marrow response in the absence of clinical benefit or establishment of remission is controversial, and the relationship between these responses and survival must be thoroughly evaluated. Future database analyses and clinical trials should bring clarity to the value of independent marrow changes on survival. We propose 2 subcategories of marrow response that include (1) optimal marrow response, which indicates achievement of all bone marrow changes required for CR without the extramedullary changes; and (2) partial marrow response in which bone marrow blasts (and blast equivalents) are reduced by 50% but a remaining >5% cellularity OR reduction in reticulin fibrosis grade from baseline on at least 2 bone marrow evaluations at least 2 months apart.
The category of clinical benefit expands upon previously established metrics of hematologic improvement to include blood cell counts, spleen size reduction, and improved functional status as meaningful patient outcomes, which, as mentioned previously, may translate to survival benefit or improved quality of life. Measurement of functional status has been shown to be reproducible with use of a symptom assessment tool. Although a symptom assessment instrument that is specific for MDS/MPN has not been developed, the MPN-SAF TSS has been used in clinical trials and adopted by some centers15 and is currently being validated in MDS/MPN (R.A. Mesa, personal communication, 2014). This instrument has demonstrated the ability to capture symptoms from the spectrum of MPN patients including cytopenia-based, spleen-based, and cytokine-based symptoms and should prove responsive to diverse phenotypes of the MDS/MPN patient base. Additionally, 50% reduction of TSS was correlated with improved quality of life and change in patients’ global impression of change in myelofibrosis trials.49 This working group believes this approach provides the best potential for accurately capturing changes in symptoms in MDS/MPN patients with the threshold of requiring a 50% reduction to equate a clinical benefit chosen to be consistent with both IWG-MRT/ELN criteria in MPN.
Proposed criteria for measurement of progressive disease in MDS/MPN
Accurate measurement of disease progression is challenging but can be accomplished by using a system that employs major and minor criteria to define changes in disease, and thus, failure of a tested therapy. In this example, progression could be defined as disease-related mortality, transformation to acute leukemia, or a combination of criteria as noted in Table 3. Major criteria include significant growth in spleen size or rise in blast percentage, whereas minor criteria include meaningful increases in transfusion needs or disease-related symptoms, verifiable loss of improvement in cytopenias—not from therapy, but rather disease progression—or cytogenetic evidence of clonal evolution. Table 3 outlines proposed criteria for progression of disease. There is unanimous agreement on inclusion of cytogenetic clonal evolution or rise in blast count as major criteria, whereas there was some disagreement within the group as to whether to include splenomegaly as a major criterion. Nevertheless, this adaptive approach using major and minor criteria to define progression can provide guidance for trials involving MDS/MPN patients with expected heterogeneous clinical features, while accounting for nonsynchronous signs of progression. Progression based upon changes in the peripheral blood should be considered after serial blood counts over 8 weeks. Transient or persistent cytopenias despite marrow response or decreased spleen size are often expected with successful azanucleoside therapy in MDS,14 tyrosine-kinase inhibitors in CML,50-52 or ruxolitinib in MF,8-10 so cytopenias alone cannot constitute attribution of disease progression.
Table 3.
Proposed criteria for measurement of disease progression in adult MDS/MPN
| Combination of 2 major criteria, 1 major and 2 minor criteria, or 3 minor criteria from list |
|---|
| Major criteria |
| Increase in blast count* |
| <5% blasts: ≥50% increase and to >5% blasts |
| 5-10% blasts: ≥50% increase and to >10% blasts |
| 10-20% blasts: ≥50% increase and to >20% blasts |
| 20-30% blasts: ≥50% increase and to >30% blasts† |
| Evidence of cytogenetic evolution‡ |
| Appearance of a previously present or new cytogenetic abnormality in complete cytogenetic remission via FISH or classic karyotyping |
| Increase in cytogenetic burden of disease by ≥50% in partial cytogenetic remission via FISH or classic karyotyping |
| New extramedullary disease |
| Worsening splenomegaly |
| Progressive splenomegaly that is defined by IWG-MRT: the appearance of a previously absent splenomegaly that is palpable at >5 cm below the left costal margin or a minimum 100% increase in palpable distance for baseline splenomegaly of 5-10 cm or a minimum 50% increase in palpable distance for baseline splenomegaly of >10 cm |
| Extramedullary disease outside of the spleen |
| To include new/worsening hepatomegaly, granulocytic sarcoma, skin lesions, etc. |
| Minor criteria |
| Transfusion dependence§ |
| Significant loss of maximal response on cytopenias ≥50% decrement from maximum remission/response in granulocytes or platelets |
| Reduction in Hgb by ≥1.5g/dL from best response or from baseline as noted on complete blood count |
| Increasing symptoms as noted by increase in ≥50% as per the MPN-SAF TSS|| |
| Evidence of clonal evolution (molecular)¶ |
Blasts as measured from the bone marrow.
Patients with development of acute myeloid leukemia from MDS/MPN; 20-30% blasts may be allowed on some clinical trials for patients with MDS/MPN.
Increase in cytogenetic burden of disease by ≥50% (via FISH or classic karyotyping). Given variability of fluorescent probes used in FISH, cytogenetic normalization via FISH will depend on specific probes used.
Transfusion dependency is defined by a history of at least 2 U of red blood cell transfusions in the past month for a hemoglobin level <8.5 g/dL that was not associated with clinically overt bleeding. Cytopenias resulting from therapy should not be considered in assessment of progression.
MPN-SAF TSS validation among patients with MDS/MPN is currently under way (R.A. Mesa, personal communication, 2014).
The identification of new abnormalities using single nucleotide polymorphism arrays or sequencing or a clearly significant increase in mutational burden of a previously detected abnormality. Precise criteria for defining new abnormalities and what exactly constitutes a significant increase in mutational burden are open to interpretation; we suggest that this criterion should be used conservatively based on current evidence.
Gene mutation testing has begun to change the way we diagnose and risk stratify MDS/MPN. For example, the vast majority of these disorders harbor 1 or more gene mutations that are more common in specific subtypes of MDS/MPN such as SETBP1 in atypical CML, SF3B1 and JAK2 in RARS-T, and SRSF2 and c-CBL in CMML.29,32,35,36,47 It is still unclear how and which molecular or cytogenetic changes most directly influence progression in each MDS/MPN subtype and the potential value of therapies that can reduce allelic burden. Cytogenetic remission in the setting of morphologic CR implies resolution of previous chromosomal abnormalities identified by metaphase karyotyping, or fluorescence in situ hybridization (FISH). Cytogenetic and molecular relapse are listed as minor criteria for progression (Table 2) if the clonal burden of a previously present cytogenetic abnormality increases by 50%. Presence of +8, abnormalities of chromosome 7, complex karyotype, or mutations in ASXL1, SETBP1, and EZH2 are clonal findings that adversely influence disease prognosis in MDS/MPN.21,30,35,53 Additional mutations that adversely affect prognosis will surely be validated in the near future. Likewise, new methods to assay prognostic findings will be more widely available; for example, in the absence of sufficient metaphases for traditional karyotype analysis, single nucleotide polymorphism arrays have proven utility in MDS/MPN, and next-generation sequencing-based approaches are likely to become a routine tool in the clinical workup of MDS/MPN.54 Although a progressive change in burden of mutant alleles might in the future be used as a criterion for progressive disease or molecular response, we have intentionally deemphasized molecular monitoring in this proposal because of current uncertainties about the significance of changes in clonal and subclonal architecture. We recommend that definitions of molecular remission should only be made in the context of morphological and cytogenetic remission at this time. We strongly encourage sequential molecular monitoring in future clinical trials involving MDS/MPN patients in the hope that this brings clarity to the manner by which these data are used in assessing response to therapy.
Conclusion
The dual findings of dysplastic and proliferative features in these stem cell malignancies define the unique challenges of the MDS/MPNs. Properly conducted clinical trials will assess response to new agents and influence management strategies to extend survival or improve quality of life. It is anticipated that these initial recommendations will require further refinement as our understanding of the disease biology improves. Though we recognize the heterogeneity of these disease phenotypes, we hope to validate these criteria in all MDS/MPN in adults (not JMML). We look forward to standardizing outcome evaluation and reporting in clinical trials and fostering reliable comparisons of the impact of new therapies for MDS/MPN with the response assessment guidelines proposed herein.
Acknowledgments
The authors thank the MDS Foundation and Alpine Oncology Foundation (Switzerland) for logistical support, and Incyte Corporation and Celgene Corporation for providing unrestricted grant funding. All funding was provided without further input into the work conduct, results interpretation, and the writing of the manuscript.
Footnotes
There is an Inside Blood Commentary on this article in this issue.
Authorship
Contribution: M.R.S., T.I.M., and A.F.L. developed the concept; M.R.S., T.I.M., A.F.L., N.C.P.C., L.M., R.K., R.V.T., A.O., J.-J.K., J.M., E.P., E.S., R.T., R.M., and G.G.-M. drafted initial response criteria; M.S., T.I.M., A.F.L., N.C.P.C., L.M., R.K., R.V.T., A.O., J.-J.K., J.M., E.P., E.S., R.T., R.M., G.G.-M., R.I., M.C., P.F., A.G., G.S., C.M.N., U.G., and F.C. edited the response criteria; M.R.S., T.I.M., A.F.L., N.C.P.C., L.M., R.K., R.V.T., A.O., J.-J.K., J.M., E.P., E.S., R.T., R.M., G.G.-M., R.I., M.C., P.F., A.G., G.S., C.M.N., U.G., and F.C. analyzed data; M.R.S., T.I.M., A.F.L., N.C.P.C., L.M., R.K., R.V.T., and J.M. wrote the paper.
Conflict-of-interest disclosure: M.R.S. sits on advisory boards for Celgene, Incyte, Karyopharm and data and safety monitoring boards for Celgene and Gilead; receives research funding from TG Therapeutics; and provides consulting for Karyopharm Therapeutics and honorarium from Incyte. R.K. sits on advisory boards for Incyte and Celgene and receives research funding from Celgene and Incyte. R.V.T. sits on advisory board for Incyte and speaker bureaus for Incyte, Novartis, and BMS. T.M. provides consulting for Incyte. A.O. sits on an advisory board for Novartis and receives research funding from GlaxoSmithKline. J.J.K. sits on an advisory board for Novartis, receives research funding from Novartis, AOP Orphan, and provides consulting for Novartis and Shire. E.P. sits on a speaker bureau for Incyte and Novartis and receives research funding from Incyte, Cell Therapeutics, and Forma. R.I. sits on an advisory board for Novartis. R.M. receives research funding from Genentech, Incyte, Gilead, NS pHarma, CTI, and Promedior and provides consulting for Novartis. J.M. sits on an advisory board for Alexxion and speaker bureaus for Alexxion, Celgene, and Incyte, and receives research funding from Celgene and Alexxion. P.F. receives research funding from Celgene, Novartis, Janssen, and GlaxoSmithKline. C.N. provides consulting to Celgene. F.C. sits on advisory board and speaker bureau for Novartis. N.C. sits on advisory board and speaker bureau for Novartis. A.F.L. sits on advisory board for CTI and Tetrologic, and data and safety monitoring boards for Celgene and Amgen and provides consulting to Celgene. The remaining authors declare no competing interests.
Correspondence: Michael R. Savona, Division of Hematology/Oncology, Department of Internal Medicine, Vanderbilt University Medical Center, 2220 Pierce Ave, 777 PRB, Nashville, TN 37232-6307; e-mail: michael.savona@vanderbilt.edu.
References
- 1.Orazi A, Germing U. The myelodysplastic/myeloproliferative neoplasms: myeloproliferative diseases with dysplastic features. Leukemia. 2008;22(7):1308–1319. doi: 10.1038/leu.2008.119. [DOI] [PubMed] [Google Scholar]
- 2.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 3.Foucar K. Myelodysplastic/myeloproliferative neoplasms. Am J Clin Pathol. 2009;132(2):281–289. doi: 10.1309/AJCPJ71PTVIKGEVT. [DOI] [PubMed] [Google Scholar]
- 4.Elliott MA, Verstovsek S, Dingli D, et al. Monocytosis is an adverse prognostic factor for survival in younger patients with primary myelofibrosis. Leuk Res. 2007;31(11):1503–1509. doi: 10.1016/j.leukres.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 5.Mughal TCN, Padron E, Savona M, et al. Myelodysplastic/myeloproliferative neoplasms (MDS/MPN): an international perspective and recommendations on molecular pathogenesis and clinical characterization. Haematologica. doi: 10.3324/haematol.2014.114660. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boiocchi L, Espinal-Witter R, Geyer JT, et al. Development of monocytosis in patients with primary myelofibrosis indicates an accelerated phase of the disease. Mod Pathol. 2013;26(2):204–212. doi: 10.1038/modpathol.2012.165. [DOI] [PubMed] [Google Scholar]
- 7.Tefferi A, Barosi G, Mesa RA, et al. IWG for Myelofibrosis Research and Treatment (IWG-MRT) International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood. 2006;108(5):1497–1503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- 8.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 9.Verstovsek S, Kantarjian HM, Estrov Z, et al. Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: survival advantage in comparison to matched historical controls. Blood. 2012;120(6):1202–1209. doi: 10.1182/blood-2012-02-414631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kvasnicka H-M, Thiele J, Bueso-Ramos CE, et al. Exploratory analysis of the effect of ruxolitinib on bone marrow morphology in patients with myelofibrosis. J Clin Oncol. 2013;31(suppl) abstract 7030. [Google Scholar]
- 12.Savona MR. Are we altering the natural history of primary myelofibrosis? Leuk Res. 2014;38(9):1004–1012. doi: 10.1016/j.leukres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 14.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. International Vidaza High-Risk MDS Survival Study Group. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs [published correction appears in J Clin Oncol. 2012;30(36):4590]. J Clin Oncol. 2012;30(33):4098–4103. doi: 10.1200/JCO.2012.42.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun T, Itzykson R, Renneville A, et al. Groupe Francophone des Myélodysplasies. Molecular predictors of response to decitabine in advanced chronic myelomonocytic leukemia: a phase 2 trial. Blood. 2011;118(14):3824–3831. doi: 10.1182/blood-2011-05-352039. [DOI] [PubMed] [Google Scholar]
- 17.Bejanyan N, Tiu RV, Raza A, et al. A phase 2 trial of combination therapy with thalidomide, arsenic trioxide, dexamethasone, and ascorbic acid (TADA) in patients with overlap myelodysplastic/myeloproliferative neoplasms (MDS/MPN) or primary myelofibrosis (PMF). Cancer. 2012;118(16):3968–3976. doi: 10.1002/cncr.26741. [DOI] [PubMed] [Google Scholar]
- 18.Onida F, Barosi G, Leone G, et al. Management recommendations for chronic myelomonocytic leukemia: consensus statements from the SIE, SIES, GITMO groups. Haematologica. 2013;98(9):1344–1352. doi: 10.3324/haematol.2013.084020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steensma DP, Heptinstall KV, Johnson VM, et al. Common troublesome symptoms and their impact on quality of life in patients with myelodysplastic syndromes (MDS): results of a large internet-based survey. Leuk Res. 2008;32(5):691–698. doi: 10.1016/j.leukres.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Kantarjian H, O’Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113(6):1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Such E, Germing U, Malcovati L, et al. Development and validation of a prognostic scoring system for patients with chronic myelomonocytic leukemia. Blood. 2013;121(15):3005–3015. doi: 10.1182/blood-2012-08-452938. [DOI] [PubMed] [Google Scholar]
- 22.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29(10):1356–1363. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- 23.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamini O, Jain P, Estrov Z, Kantarjian HM, Verstovsek S. Therapeutic effects of ruxolitinib in patients with myelofibrosis without clinically significant splenomegaly. Blood. 2012;120(13):2768–2769. doi: 10.1182/blood-2012-07-446849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauchle JO, Braun BS, Loh ML, Shannon K. Inherited predispositions and hyperactive Ras in myeloid leukemogenesis. Pediatr Blood Cancer. 2006;46(5):579–585. doi: 10.1002/pbc.20644. [DOI] [PubMed] [Google Scholar]
- 26.Niemeyer CM, Kratz CP. Paediatric myelodysplastic syndromes and juvenile myelomonocytic leukaemia: molecular classification and treatment options. Br J Haematol. 2008;140(6):610–624. doi: 10.1111/j.1365-2141.2007.06958.x. [DOI] [PubMed] [Google Scholar]
- 27.Tartaglia M, Mehler EL, Goldberg R, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29(4):465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 28.Niemeyer CM, Loh ML, Cseh A, Cooper T, Dvorak CC, Chan R. Criteria for evaluating response and outcome in clinical trials for children with juvenile myelomonocytic leukemia. Haematologica. 2015;100(1):17–22. doi: 10.3324/haematol.2014.109892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broséus J, Alpermann T, Wulfert M, et al. MPN and MPNr-EuroNet (COST Action BM0902) Age, JAK2(V617F) and SF3B1 mutations are the main predicting factors for survival in refractory anaemia with ring sideroblasts and marked thrombocytosis. Leukemia. 2013;27(9):1826–1831. doi: 10.1038/leu.2013.120. [DOI] [PubMed] [Google Scholar]
- 30.Itzykson R, Kosmider O, Renneville A, et al. Clonal architecture of chronic myelomonocytic leukemias. Blood. 2013;121(12):2186–2198. doi: 10.1182/blood-2012-06-440347. [DOI] [PubMed] [Google Scholar]
- 31.Jankowska AM, Makishima H, Tiu RV, et al. Mutational spectrum analysis of chronic myelomonocytic leukemia includes genes associated with epigenetic regulation: UTX, EZH2, and DNMT3A. Blood. 2011;118(14):3932–3941. doi: 10.1182/blood-2010-10-311019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makishima H, Yoshida K, Nguyen N, et al. Somatic SETBP1 mutations in myeloid malignancies. Nat Genet. 2013;45(8):942–946. doi: 10.1038/ng.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maxson JE, Gotlib J, Pollyea DA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013;368(19):1781–1790. doi: 10.1056/NEJMoa1214514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meggendorfer M, Roller A, Haferlach T, et al. SRSF2 mutations in 275 cases with chronic myelomonocytic leukemia (CMML). Blood. 2012;120(15):3080–3088. doi: 10.1182/blood-2012-01-404863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piazza R, Valletta S, Winkelmann N, et al. Recurrent SETBP1 mutations in atypical chronic myeloid leukemia. Nat Genet. 2013;45(1):18–24. doi: 10.1038/ng.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visconte V, Makishima H, Jankowska A, et al. SF3B1, a splicing factor is frequently mutated in refractory anemia with ring sideroblasts. Leukemia. 2012;26(3):542–545. doi: 10.1038/leu.2011.232. [DOI] [PubMed] [Google Scholar]
- 37.Wang SA, Hasserjian RP, Fox PS, et al. Atypical chronic myeloid leukemia is clinically distinct from unclassifiable myelodysplastic/myeloproliferative neoplasms. Blood. 2014;123(17):2645–2651. doi: 10.1182/blood-2014-02-553800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Germing U, Gattermann N, Minning H, Heyll A, Aul C. Problems in the classification of CMML—dysplastic versus proliferative type. Leuk Res. 1998;22(10):871–878. doi: 10.1016/s0145-2126(97)00192-6. [DOI] [PubMed] [Google Scholar]
- 39.Germing U, Kündgen A, Gattermann N. Risk assessment in chronic myelomonocytic leukemia (CMML). Leuk Lymphoma. 2004;45(7):1311–1318. doi: 10.1080/1042819042000207271. [DOI] [PubMed] [Google Scholar]
- 40.González-Medina I, Bueno J, Torrequebrada A, López A, Vallespí T, Massagué I. Two groups of chronic myelomonocytic leukaemia: myelodysplastic and myeloproliferative. Prognostic implications in a series of a single center. Leuk Res. 2002;26(9):821–824. doi: 10.1016/s0145-2126(02)00021-8. [DOI] [PubMed] [Google Scholar]
- 41.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. [PubMed] [Google Scholar]
- 42.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Itzykson R, Kosmider O, Renneville A, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol. 2013;31(19):2428–2436. doi: 10.1200/JCO.2012.47.3314. [DOI] [PubMed] [Google Scholar]
- 44.Onida F, Kantarjian HM, Smith TL, et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood. 2002;99(3):840–849. doi: 10.1182/blood.v99.3.840. [DOI] [PubMed] [Google Scholar]
- 45.Patnaik MM, Padron E, LaBorde RR, et al. Mayo prognostic model for WHO-defined chronic myelomonocytic leukemia: ASXL1 and spliceosome component mutations and outcomes [published correction appears in Leukemia. 2013;27(10):2112]. Leukemia. 2013;27(7):1504–1510. doi: 10.1038/leu.2013.88. [DOI] [PubMed] [Google Scholar]
- 46.Liu YTA, Visconte V, Visconte V, et al. A prognostic scoring system for unclassifiable MDS and MDS/MPN. Blood. 2012;120. Abstract 1701. [Google Scholar]
- 47.Malcovati L, Papaemmanuil E, Ambaglio I, et al. Driver somatic mutations identify distinct disease entities within myeloid neoplasms with myelodysplasia. Blood. 2014;124(9):1513–1521. doi: 10.1182/blood-2014-03-560227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122(8):1395–1398. doi: 10.1182/blood-2013-03-488098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mesa RA, Gotlib J, Gupta V, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2013;31(10):1285–1292. doi: 10.1200/JCO.2012.44.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 51.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 52.Saglio G, Kim DW, Issaragrisil S, et al. ENESTnd Investigators. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 53.Ernst T, Chase AJ, Score J, et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet. 2010;42(8):722–726. doi: 10.1038/ng.621. [DOI] [PubMed] [Google Scholar]
- 54.Tiu RV, Gondek LP, O’Keefe CL, et al. Prognostic impact of SNP array karyotyping in myelodysplastic syndromes and related myeloid malignancies. Blood. 2011;117(17):4552–4560. doi: 10.1182/blood-2010-07-295857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Score J, Hidalgo-Curtis C, Jones AV, et al. Inactivation of polycomb repressive complex 2 components in myeloproliferative and myelodysplastic/myeloproliferative neoplasms. Blood. 2012;119(5):1208–1213. doi: 10.1182/blood-2011-07-367243. [DOI] [PubMed] [Google Scholar]
- 56.Jankowska AM, Szpurka H, Tiu RV, et al. Loss of heterozygosity 4q24 and TET2 mutations associated with myelodysplastic/myeloproliferative neoplasms. Blood. 2009;113(25):6403–6410. doi: 10.1182/blood-2009-02-205690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kar SA, Jankowska A, Makishima H, et al. Spliceosomal gene mutations are frequent events in the diverse mutational spectrum of chronic myelomonocytic leukemia but largely absent in juvenile myelomonocytic leukemia. Haematologica. 2013;98(1):107–113. doi: 10.3324/haematol.2012.064048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loh ML, Sakai DS, Flotho C, et al. Mutations in CBL occur frequently in juvenile myelomonocytic leukemia. Blood. 2009;114(9):1859–1863. doi: 10.1182/blood-2009-01-198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muramatsu H, Makishima H, Jankowska AM, et al. Mutations of an E3 ubiquitin ligase c-Cbl but not TET2 mutations are pathogenic in juvenile myelomonocytic leukemia. Blood. 2010;115(10):1969–1975. doi: 10.1182/blood-2009-06-226340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakaguchi H, Okuno Y, Muramatsu H, et al. Exome sequencing identifies secondary mutations of SETBP1 and JAK3 in juvenile myelomonocytic leukemia. Nat Genet. 2013;45(8):937–941. doi: 10.1038/ng.2698. [DOI] [PubMed] [Google Scholar]
- 61.Sugimoto Y, Muramatsu H, Makishima H, et al. Spectrum of molecular defects in juvenile myelomonocytic leukaemia includes ASXL1 mutations. Br J Haematol. 2010;150(1):83–87. doi: 10.1111/j.1365-2141.2010.08196.x. [DOI] [PubMed] [Google Scholar]
- 62.Jeromin S, Eder C, Meggendorfer M, et al. RARS-T patients harbor SF3B1 mutations in 90.2% and can be characterized by mutations in ASXL1 and other spliceosome genes in most of the remaining cases. Blood. 2013;122(21) [Google Scholar]
- 63.Szpurka H, Jankowska AM, Makishima H, et al. Spectrum of mutations in RARS-T patients includes TET2 and ASXL1 mutations. Leuk Res. 2010;34(8):969–973. doi: 10.1016/j.leukres.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szpurka H, Tiu R, Murugesan G, et al. Refractory anemia with ringed sideroblasts associated with marked thrombocytosis (RARS-T), another myeloproliferative condition characterized by JAK2 V617F mutation. Blood. 2006;108(7):2173–2181. doi: 10.1182/blood-2006-02-005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gotlib J, Maxson JE, George TI, Tyner JW. The new genetics of chronic neutrophilic leukemia and atypical CML: implications for diagnosis and treatment. Blood. 2013;122(10):1707–1711. doi: 10.1182/blood-2013-05-500959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Makishima H, Jankowska AM, Tiu RV, et al. Novel homo- and hemizygous mutations in EZH2 in myeloid malignancies. Leukemia. 2010;24(10):1799–1804. doi: 10.1038/leu.2010.167. [DOI] [PubMed] [Google Scholar]
- 67.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90(8):1128–1132. [PubMed] [Google Scholar]