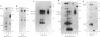Abstract
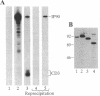
In the endoplasmic reticulum (ER), newly synthesized subunits of the T-cell antigen receptor (TCR), membrane-bound immunoglobulin (mIg), and major histocompatibility complex (MHC) class I antigens must fold correctly and assemble completely into multimeric protein complexes prior to transport to the cell surface. Although folding and assembly may occur spontaneously, the concept that molecular chaperones facilitate these events is emerging. Here, an intracellular protein of 90-kDa apparent molecular mass, denoted IP90, was shown to be an ER resident protein that associated with partial complexes of the TCR, mIg, and MHC class I proteins but was absent from fully assembled complexes. We speculate that IP90 might participate in folding and assembly processes of these and other multisubunit protein complexes during their transit through the ER.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Anderson P., Blue M. L., O'Brien C., Schlossman S. F. Monoclonal antibodies reactive with the T cell receptor zeta chain: production and characterization using a new method. J Immunol. 1989 Sep 15;143(6):1899–1904. [PubMed] [Google Scholar]
- Ashwell J. D., Klusner R. D. Genetic and mutational analysis of the T-cell antigen receptor. Annu Rev Immunol. 1990;8:139–167. doi: 10.1146/annurev.iy.08.040190.001035. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Warnke R. A., Jones N., Strominger J. L. Characterization and expression of the human alpha beta T cell receptor by using a framework monoclonal antibody. J Immunol. 1987 Mar 1;138(5):1502–1509. [PubMed] [Google Scholar]
- Brenner M. B., Trowbridge I. S., Strominger J. L. Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell. 1985 Jan;40(1):183–190. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]
- Campbell K. S., Cambier J. C. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990 Feb;9(2):441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Degen E., Williams D. B. Participation of a novel 88-kD protein in the biogenesis of murine class I histocompatibility molecules. J Cell Biol. 1991 Mar;112(6):1099–1115. doi: 10.1083/jcb.112.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. Proteins as molecular chaperones. 1987 Jul 30-Aug 5Nature. 328(6129):378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- Geisler C., Schøller J., Wahi M. A., Rubin B., Weiss A. Association of the human CD3-zeta chain with the alpha beta-T cell receptor/CD3 complex. Clues from a T cell variant with a mutated T cell receptor-alpha chain. J Immunol. 1990 Sep 15;145(6):1761–1767. [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Hochstenbach F., Brenner M. B. T-cell receptor delta-chain can substitute for alpha to form a beta delta heterodimer. Nature. 1989 Aug 17;340(6234):562–565. doi: 10.1038/340562a0. [DOI] [PubMed] [Google Scholar]
- Krangel M. S., Orr H. T., Strominger J. L. Assembly and maturation of HLA-A and HLA-B antigens in vivo. Cell. 1979 Dec;18(4):979–991. doi: 10.1016/0092-8674(79)90210-1. [DOI] [PubMed] [Google Scholar]
- Krissansen G. W., Owen M. J., Verbi W., Crumpton M. J. Primary structure of the T3 gamma subunit of the T3/T cell antigen receptor complex deduced from cDNA sequences: evolution of the T3 gamma and delta subunits. EMBO J. 1986 Aug;5(8):1799–1808. doi: 10.1002/j.1460-2075.1986.tb04429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarella R. A., Green M. ERp99, an abundant, conserved glycoprotein of the endoplasmic reticulum, is homologous to the 90-kDa heat shock protein (hsp90) and the 94-kDa glucose regulated protein (GRP94). J Biol Chem. 1987 Jun 25;262(18):8875–8883. [PubMed] [Google Scholar]
- Minami Y., Weissman A. M., Samelson L. E., Klausner R. D. Building a multichain receptor: synthesis, degradation, and assembly of the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1987 May;84(9):2688–2692. doi: 10.1073/pnas.84.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Saito T., Weiss A., Gunter K. C., Shevach E. M., Germain R. N. Cell surface T3 expression requires the presence of both alpha- and beta-chains of the T cell receptor. J Immunol. 1987 Jul 15;139(2):625–628. [PubMed] [Google Scholar]
- Seong R. H., Clayberger C. A., Krensky A. M., Parnes J. R. Rescue of Daudi cell HLA expression by transfection of the mouse beta 2-microglobulin gene. J Exp Med. 1988 Feb 1;167(2):288–299. doi: 10.1084/jem.167.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam N. J., Spits H., Ploegh H. L. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986 Oct 1;137(7):2299–2306. [PubMed] [Google Scholar]
- Watkins S. C., Samuel J. L., Marotte F., Bertier-Savalle B., Rappaport L. Microtubules and desmin filaments during onset of heart hypertrophy in rat: a double immunoelectron microscope study. Circ Res. 1987 Mar;60(3):327–336. doi: 10.1161/01.res.60.3.327. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Purification of the major mammalian heat shock proteins. J Biol Chem. 1982 Dec 25;257(24):14949–14959. [PubMed] [Google Scholar]
- Wienands J., Hombach J., Radbruch A., Riesterer C., Reth M. Molecular components of the B cell antigen receptor complex of class IgD differ partly from those of IgM. EMBO J. 1990 Feb;9(2):449–455. doi: 10.1002/j.1460-2075.1990.tb08130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Word C. J., Kuehl W. M. Expression of surface and secreted IgG2a by a murine B-lymphoma before and after hybridization to myeloma cells. Mol Immunol. 1981 Apr;18(4):311–322. doi: 10.1016/0161-5890(81)90055-9. [DOI] [PubMed] [Google Scholar]