Key Points
Adult patients with ETP-ALL/LBL have poor long-term outcomes.
Novel therapies are urgently needed for adult patients with ETP-ALL/LBL.
Abstract
Early T-cell precursor (ETP) acute lymphoblastic leukemia/lymphoma (ALL/LBL) is a recently recognized high-risk T lymphoblastic leukemia/lymphoma (T-ALL/LBL) subgroup. The optimal therapeutic approaches to adult patients with ETP-ALL/LBL are poorly characterized. In this study, we compared the outcomes of adults with ETP-ALL/LBL who received treatment on frontline regimens with those of patients with other T-ALL/LBL immunophenotypic subtypes. Patients with newly diagnosed T-ALL/LBL who received frontline chemotherapy between the years 2000 and 2014 at The University of Texas MD Anderson Cancer Center were identified and immunophenotypically categorized into early, thymic, and mature per the World Health Organization (WHO) classification using CD1a and surface CD3 status. Patients with ETP-ALL/LBL were identified on the basis of the following immunophenotypes: CD1a−, CD8−, CD5− (dim), and positivity for 1 or more stem cell or myeloid antigens. A total of 111 patients with T-ALL/LBL (68% T-ALL; 32% T-LBL) with adequate immunophenotype data were identified. The median age was 30 years (range, 13-79). There was no difference in the outcomes of patients based on the WHO subtypes. Nineteen patients (17%) had ETP-ALL/LBL. The complete remission rate /complete remission with incomplete platelet recovery rate in patients with ETP-ALL/LBL was significantly lower than that of non–ETP-ALL/LBL patients (73% vs 91%; P = .03). The median overall survival for patients with ETP-ALL/LBL was 20 months vs not reached for the non–ETP-ALL/LBL patients (P = .008). ETP-ALL/LBL represents a high-risk disease subtype of adult ALL. Novel treatment strategies are needed to improve treatment outcomes in this T-ALL/LBL subset.
Introduction
T-cell acute lymphoblastic leukemia/lymphoma (referred as T-ALL/LBL in this manuscript) is a malignant neoplasm of immature T cells. Immunophenotypic subtypes of T-ALL/LBL that correspond to T-cell maturation stages have been recognized.1-4 Recently, a subtype of T-ALL/LBL derived from thymic cells at the early T-cell precursor (ETP) differentiation stage has been recognized.5,6 ETPs are recent immigrants from the bone marrow (BM) to the thymus, derived from hematopoietic stem cells, which retain a certain level of multilineage pluripotency.7,8 By gene expression profiling, ETP cells share similarities with hematopoietic stem cells and myeloid progenitor cells.5 The definition of ETP-ALL/LBL is based on the immunophenotype of the leukemic cells, which are typically CD1a−, CD8−, CD5− (dim), and positive for 1 or more stem cell or myeloid antigens.5 In the World Health Organization (WHO) classification, ETP-ALL/LBL falls within the early T-ALL/LBL category. ETP ALL has been reported in 11% to 12% of childhood T-ALL/LBL5,9 and in 7.4% of adult T-ALL/LBL.9 ETP-ALL/LBL is also characterized by a distinct molecular profile with a lower incidence of NOTCH1 mutations and frequent presence of FLT3 and DNMT3A mutations.6,10-12 Importantly, ETP-ALL/LBL is associated with a significantly worse outcome in children and young adults compared with other T-ALL/LBL subtypes.5,13
Combination chemotherapy has been the cornerstone of T-ALL/LBL treatment. Yet, despite an overall complete response (CR) rate of 90% to 95%, approximately one-third of patients experience disease relapse, and the 5-year overall survival (OS) rate for adults is approximately 50% to 55%.14-17 These outcomes highlight the need for alternate therapeutic approaches, ideally based on a prognostication strategy that identifies T-ALL/LBL patients at a high risk for relapse.
The aim of this study is to characterize the clinical features of patients with ETP-ALL/LBL seen at our institution and treated on frontline regimens for T-ALL/LBL and to compare their outcomes to those with other T-ALL/LBL subtypes.
Methods
Study group
Patients with newly diagnosed T-ALL/LBL who received frontline chemotherapy between the years 2000 and 2014 at The University of Texas MD Anderson Cancer Center (MDACC) were identified. All patients fulfilled the diagnostic criteria of T-ALL/LBL according to the WHO classification.4 The diagnosis of T-ALL/LBL was based on the identification of a neoplastic proliferation of small- to medium-sized blasts in an extramedullary mass, peripheral blood, and/or BM. A cutoff of <20% BM blasts was used to define LBL. Blasts expressed T-cell lineage markers (CD2, cytoplasmic CD3, surface CD3 (sCD3), CD4, CD5, CD7, and/or CD8) as well as markers of precursor T lymphoblasts (CD1a, CD34, CD99, and/or terminal deoxynucleotidyl transferase [TdT]). The immunophenotype was assessed by review of multiparameter flow cytometry analysis scatter plots and/or quantitative immunophenotyping data on record. The immunophenotype was preferentially based on pretreatment peripheral blood, BM, or extramedullary tumor material. In a minor subset of patients (n = 4), the immunophenotype was based in part on immunohistochemical analysis. All the latter cases were LBL with a thymic immunophenotype; none was ETP-ALL/LBL. Minimum requirements for immunophenotypic markers included the following: CD1a, CD2, CD3 (surface vs cytoplasmic), CD4, CD5, CD7, CD8, CD13, CD19, CD33, CD34, CD117, HLA–antigen D related (HLA-DR), TdT, and myeloperoxidase. Patients with mixed-phenotype acute leukemia such as T/myeloid leukemia as defined in the WHO classification were excluded.4 Of 127 T-ALL/LBL patients identified, 16 were excluded because of insufficient data for immunophenotypic subtyping. Patients in the study group (n = 111) were subtyped based on the expression of CD1a and sCD3 as early (CD1a−, sCD3−), thymic (CD1a+, sCD3−), or mature (CD1a−, sCD3+) T-ALL/LBL. Patients were also characterized by detailed immunophenotype per WHO criteria (see supplemental Table 1, available on the Blood Web site).4 The ETP-ALL/LBL immunophenotype was defined as follows: (1) absent (<5% positive cells) CD1a and CD8 expression, (2) absent or dim (<75% positive cells) CD5 expression, and (3) expression (>25% positive cells) of 1 or more myeloid (CD11b, CD13, CD33, CD117) or stem cell (CD34, HLA-DR) markers.5 Minimal residual disease (MRD) was assessed by multiparameter flow cytometry with a sensitivity of 0.01% in the remission BM sample for patients who presented with ALL as described previously.18 Briefly, MRD analysis was performed using a panel of markers that included, in most cases, CD1a, CD2, CD3 (surface and cytoplasmic), CD4, CD5, CD7, CD8, CD10, CD13, CD33, CD34, CD45, CD56, HLA-DR, and TdT assessed on 200 000 aspirated nucleated BM cells.
Patients received frontline treatment with the following chemotherapy regimens: hyper-cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride, and dexamethasone (hyper-CVAD) alone (n = 43), hyper-CVAD + nelarabine (n = 44), or augmented Berlin-Frankfurt-Munster (BFM) regimen (n = 24). The details of these chemotherapy regimens were published previously.17,19,20 Decision among the 3 regimens was based on a variety of factors: time period (hyper-CVAD: year 2000 onwards; hyper-CVAD + nelarabine: year 2007 onwards; augmented BFM: years 2006-2012); age (18 years and older for hyper-CVAD–based regimens; 40 years and younger for augmented BFM); clinical trial eligibility; insurance coverage of clinical trials; and patient or physician preference. CR was defined as disappearance of all clinical and/or radiologic evidence of disease, normal BM differential (≤5% blasts), no extramedullary leukemia, neutrophil count ≥1.0 × 109/L, and platelet count ≥100 × 109/L. CR with incomplete platelet recovery (CRp) was defined as meeting all criteria for CR except platelets <100 × 109/L. Response in LBL was assessed by computed tomography imaging studies. Response assessment was performed after induction and every 1 to 2 months thereafter (until the best response was achieved). Routine allogeneic-stem cell transplantation (allo-SCT) in first clinical remission (CR1) was not recommended. This study was approved by the MDACC Institutional Review Board and carried out in accordance with the Declaration of Helsinki.
Statistical analysis
Fisher’s exact test and Mann-Whitney U test were used to assess categorical and continuous variables, respectively. The Spearman rank method was used to assess correlations. The Kaplan-Meier method was used to asses OS and event-free survival (EFS) using the log-rank (Mantel-Cox) test. The former was calculated as the time from the date of diagnosis to the date of last follow-up or death of any cause. The EFS was calculated from the beginning of treatment until an event (relapse, treatment failure, death during induction, or death during complete remission). Patients who received allo-SCT were censored at the time of transplant. Univariate and multivariate analyses were performed to identify potential prognostic factors (age, gender, diagnosis, white blood cell [WBC] count ≥50.0 × 109/L, hemoglobin <10 g/dL, platelet <100 × 109/L, lactate dehydrogenase [LDH] ≥600 IU/L, central nervous system [CNS] involvement at diagnosis, WHO classification [early, thymic, mature], ETP-ALL/LBL, treatment received) associated with OS. For multivariate analyses, we used the Cox proportional hazards model for OS. A P value <.05 (2-tailed) was considered statistically significant.
Results
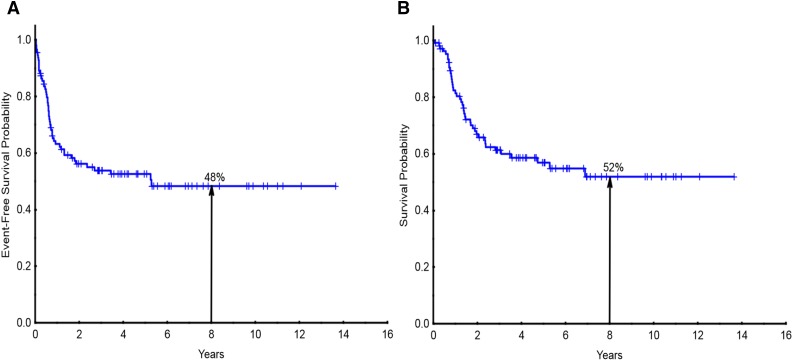
A total of 111 patients with T-ALL/LBL who received frontline chemotherapy at MDACC and with adequate immunophenotype data were identified. The median age was 30 years (range, 13-79) at the time of diagnosis, with 4 patients younger than age 18 years. Twenty-six percent were women. Patient demographics are detailed in Table 1. Overall, 88% of the patients achieved CR or CRp. The median follow-up was 55 months. The 8-year EFS and OS were 48% and 52%, respectively (Figure 1A-B). A total of 32% (35 of the 111 patients) patients presented as LBL (baseline characteristics of LBL vs ALL are detailed in supplemental Table 2). Patients who presented with ALL had an 8-year OS of 44% compared with 70% for LBL (supplemental Figure 1); however, this was not statistically significant. The presentation for LBL included mediastinal adenopathy ± systemic adenopathy (n = 22), systemic adenopathy (n = 10), skin involvement (n = 1), supraclavicular mass (n = 1), and tonsillar mass (n = 1). Seven patients (5 early, 1 thymic, 1 mature) underwent allo-SCT in CR1 for high-risk disease features such as presence of MRD.
Table 1.
Baseline characteristics of T-ALL/LBL patients stratified by WHO immunophenotypic categories
| WHO categories | |||||
|---|---|---|---|---|---|
| Total | Early | Thymic | Mature | P value | |
| n | 111 | 44 | 48 | 19 | |
| Diagnosis | |||||
| ALL | 76 (68) | 34 (78) | 27 (56) | 15 (79) | |
| LBL | 35 (32) | 10 (22) | 21 (44) | 4 (21) | .05 |
| Age | 30 (13-79) | 29 (13-75) | 31 (17-79) | 28 (17-64) | .14 |
| Gender | |||||
| Female | 29 (26) | 9 (20) | 15 (31) | 5 (26) | |
| Male | 82 (74) | 35 (80) | 33 (69) | 14 (74) | .5 |
| Cytogenetics (n = 105) | |||||
| Diploid | 71 (68) | 24 (57) | 34 (74) | 13 (76) | .2* |
| Hyperdiploid | 5 (5) | 2 (5) | 2 (4) | 1 (6) | |
| Hypodiploid | 2 (2) | 2 (5) | |||
| Miscellaneous | 27 (25) | 14 (33) | 10 (22) | 3 (18) | |
| Presenting laboratory values | |||||
| WBC (×109/L) | 8.0 (0.4-292.3) | 5.5 (0.4-151.2) | 8.1 (1.8-292.3) | 8.6 (2.7-134.9) | .28 |
| WBC ≥100 (×109/L) | 8 (7) | 2 (5) | 5 (10) | 1 (5) | .52 |
| Platelet count (×109/L) | 127 (10-488) | 82 (10-391) | 197 (10-488) | 148 (22-402) | .14 |
| Hemoglobin (g/dL) | 11.4 (6.8-16.7) | 10.3 (6.8-16.7) | 11.3 (7.2-15.2) | 11.9 (8.8-16.1) | .21 |
| LDH (IU/L) | 938 (209-32 029) | 879 (209-11 300) | 1298 (285-32 029) | 834 (284-20 795) | .08 |
| CNS involvement at diagnosis | 6 (5) | 4 (9) | 1 (2) | 1 (5) | .33 |
| Treatment received | |||||
| Hyper-CVAD | 43 (39) | 15 (34) | 18 (38) | 10 (53) | |
| Hyper-CVAD + nelarabine | 44 (40) | 19 (43) | 19 (39) | 6 (31) | |
| Augmented BFM | 24 (21) | 10 (23) | 11 (23) | 3 (16) | |
Cytogenetics either not done or insufficient metaphases recovered for 6 patients. Miscellaneous cytogenetics included del(5q), del(6q), del(9p), and others.
Diploid vs other.
Figure 1.
Survival for the entire study population. (A) Event-free survival and (B) overall survival for the entire study population of patients with T-ALL/LBL (n = 111).
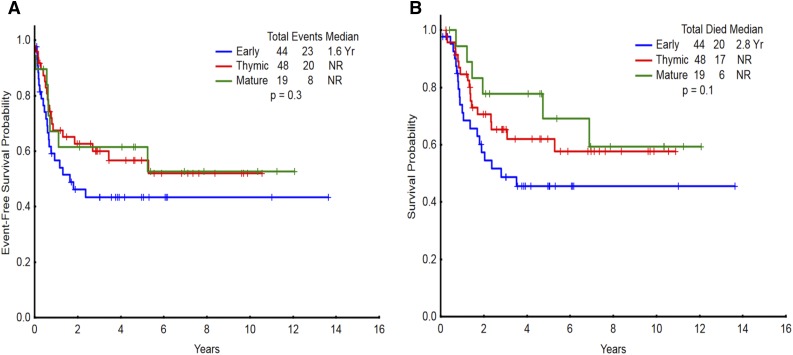
When grouped by WHO criteria based on CD1a and sCD3 status, the most common T-ALL/LBL subtype was thymic (43%), followed by early (40%) and mature (17%) (demographic details in Table 1). Patients with thymic subtype were more likely to present with LBL. There was no difference in the achievement of CR/CRp among patients with early, thymic, or mature T-ALL/LBL subtypes (82%, 94%, and 89%, respectively; P = .2). There was no difference in EFS (Figure 2A, P = .3) or OS (Figure 2B, P = .1) between WHO subtypes. Comparing early subtype vs thymic/mature subtypes, the P values for EFS and OS were .16 and .08, respectively. Similarly, there was no difference in OS when only hyper-CVAD–based regimens (hyper-CVAD alone or in combination with nelarabine) were considered (supplemental Figure 2). Of the 19 patients with mature T-ALL/LBL, only 1 patient underwent allo-SCT in CR1. In this disease subtype, 5-year EFS and OS were 63% and 70%, respectively. Of the 111 patients, only 49 could be subtyped into pro-T, pre-T, cortical T, and medullary T subtypes, largely because of the discordant expression of differentiation markers that do not conform to this immunophenotypic classification scheme that recapitulates normal T-cell differentiation stages. There was no difference in EFS and OS between these immunophenotypic subtypes (supplemental Figures 3 and 4, respectively).
Figure 2.
Survival for the entire study population categorized by immunophenotype per WHO classification. (A) Event-free survival and (B) overall survival of patients with T-ALL/LBL (n = 111) categorized as early, thymic, and mature per WHO classification. NR, not reached.
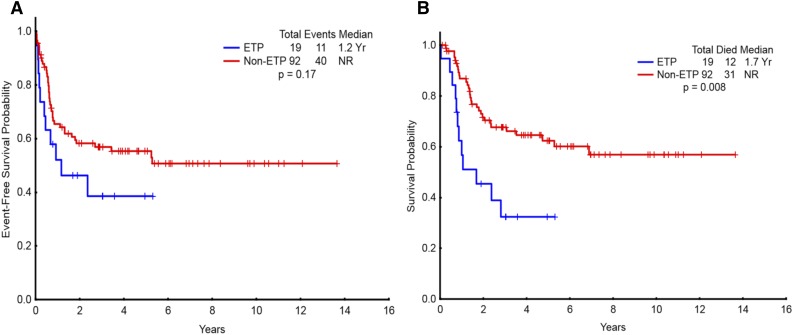
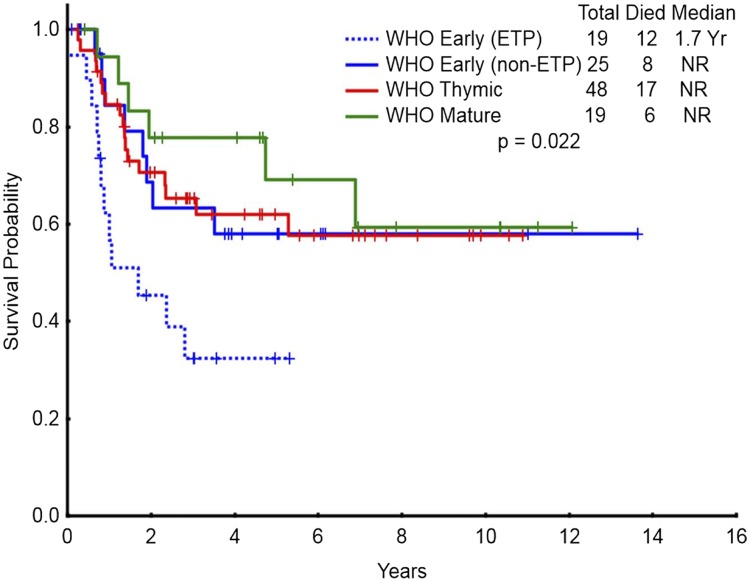
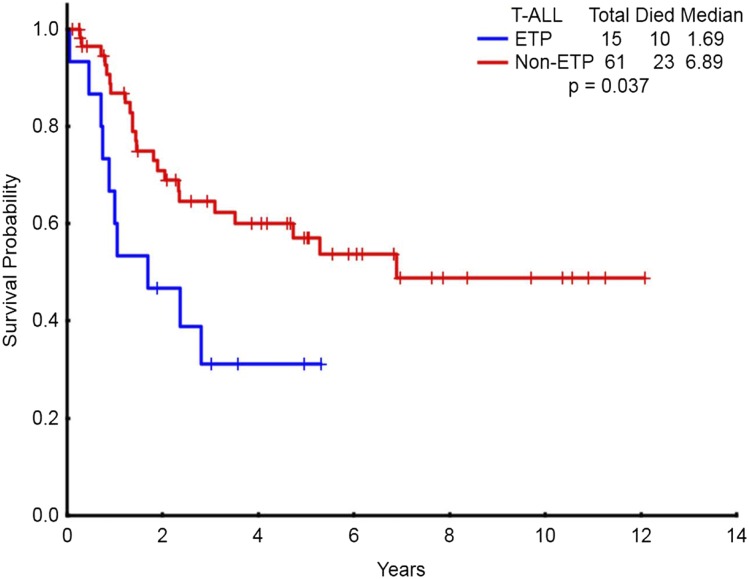
Overall, 17% (19/111) of the study population had ETP-ALL/LBL. The demographic characteristics of patients with ETP-ALL/LBL are detailed in Table 2. Of the 17 ETP patients in whom sufficient immunophenotype data were available, 8 were categorized as pro-T and 9 as pre-T subtype. Patients with ETP-ALL/LBL were less likely to have diploid karyotype, had higher BM blast count (75% vs 43%; P = .03), and higher risk of CNS involvement at the time of diagnosis compared with non–ETP-ALL/LBL. The CR/CRp rate in patients with ETP-ALL/LBL (68% CR, 5% CRp) was significantly lower than that of non–ETP-ALL/LBL patients (73% vs 91%; P = .03). The median EFS for ETP-ALL/LBL patients was 14 months vs not reached for the non–ETP-ALL patients (P = .17) (Figure 3A). The median OS for ETP-ALL/LBL patients was 20 months vs not reached for the non–ETP-ALL/LBL patients (P = .008) (Figure 3B). Figure 4 shows OS of WHO early patients subcategorized as ETP vs non-ETP, WHO thymic, and WHO mature. Within the WHO early category, ETP-ALL/LBL remained a poor subgroup. The baseline characteristics of ETP patients compared with WHO early non-ETP patients is detailed in supplemental Table 3. Patients with ETP presenting as ALL (n = 15) had significantly worse OS compared with non-ETP patients with ALL presentation (n = 61) (Figure 5, P = .037). There was no difference in EFS (supplemental Figure 5, P = .337). For patients presenting as LBL, there was no difference in outcomes of ETP (n = 4) vs non-ETP (n = 31), likely because of small numbers (supplemental Figures 6 and 7). Three patients with ETP-ALL/LBL underwent allo-SCT in first remission (1 patient has been in ongoing CR for more than 6 years after allo-SCT; 1 patient relapsed 3 years after allo-SCT and has achieved second clinical remission with plans for a second allo-SCT; and 1 patient relapsed 1 year after allo-SCT and is receiving salvage therapy).
Table 2.
Baseline characteristics of ETP-ALL/LBL patients
| Categories | ||||
|---|---|---|---|---|
| Total | ETP ALL | Non-ETP ALL | P value | |
| n | 111 | 19 | 92 | |
| Diagnosis | ||||
| ALL | 76 (68) | 15 (79) | 61 (66) | |
| LBL | 35 (32) | 4 (21) | 31 (34) | .28 |
| Age | 30 (13-79) | 37 (19-75) | 30 (13-79) | .26 |
| Gender | ||||
| Female | 29 (26) | 4 (21) | 25 (27) | |
| Male | 82 (74) | 15 (79) | 67 (73) | .6 |
| Cytogenetics (n = 105) | ||||
| Diploid | 71 (68) | 7 (37) | 64 (75) | .002* |
| Hyperdiploid | 5 (5) | 2 (11) | 3 (3) | |
| Hypodiploid | 2 (2) | 2 (2) | ||
| Miscellaneous | 27 (25) | 10 (52) | 17 (20) | |
| Presenting laboratory values | ||||
| WBC (×109/L) | 8.0 (0.4-292.3) | 13.4 (0.8-87.4) | 7.8 (0.4-292.3) | .72 |
| WBC ≥100 (×109/L) | 8 (7) | 0 | 8 (9) | .18 |
| Platelet count (×109/L) | 127 (10-488) | 110 (10-391) | 150 (10-488) | .27 |
| Hemoglobin (g/dL) | 11.4 (6.8-16.7) | 10.3 (6.8-15) | 11.4 (7.2-16.7) | .15 |
| LDH (IU/L) | 938 (209-32 029) | 850 (209-4675) | 959 (236-32 029) | .2 |
| CNS involvement at diagnosis | 6 (5) | 3 (16) | 3 (3) | .03 |
| Treatment received | ||||
| Hyper-CVAD | 43 (39) | 6 (31) | 37 (40) | |
| Hyper-CVAD + nelarabine | 44 (40) | 9 (48) | 35 (38) | |
| Augmented BFM | 24 (21) | 4 (21) | 20 (22) | |
Cytogenetics either not done or insufficient metaphases recovered for 6 patients.
Diploid vs other.
Figure 3.
Survival for the entire study population categorized as ETP vs non-ETP. (A) Event-free survival and (B) overall survival of patients with ETP ALL (n = 19) compared with non-ETP ALL (n = 92). NR, not reached.
Figure 4.
Survival for the entire study population categorized by ETP and WHO classification. Overall survival of patients with the WHO early classification subcategorized as ETP vs non-ETP, WHO thymic, and WHO mature (n = 111). NR, not reached.
Figure 5.
Survival for the patients presenting as ALL categorized as ETP vs non-ETP. Overall survival of ETP subtype (presenting as ALL, n = 15) vs non-ETP subtype (presenting as ALL, n = 61).
The prognostic significance of ETP-ALL/LBL was maintained when only hyper-CVAD–based chemotherapy regimens were considered (supplemental Figure 8). Because it was difficult to assess for MRD in patients presenting with LBL because of a lack of remission tumor biopsy, MRD analysis was restricted to patients presenting with ALL. A total of 54 patients who presented with ALL had MRD data available at the time of remission. There was no difference in the MRD rate in patients with ETP-ALL (7/10 [70%]) vs non–ETP-ALL (27/44 [61%]; P = .61). MRD negativity did not influence the outcomes for patients with ETP-ALL; however, the patient numbers were small. By univariate analysis, the following variables were significant for survival: age, WBC count (<50 vs ≥50 ×109/L), platelet count (<100 vs ≥100 ×109/L), LDH (<600 vs ≥600 IU/L), and ETP-ALL/LBL (Table 3). By multivariate analysis, only age (hazard ratio [HR], 2.862; 95% confidence interval [CI], 1.140-7.183; P = .025) and ETP-ALL/LBL (HR, 2.275; 95% CI, 1.117-4.631; P = .023) were significant (Table 3).
Table 3.
Univariate and multivariate analysis for survival
| Parameter | Survival | ||||
|---|---|---|---|---|---|
| n | UVA | MVA | |||
| P | P | HR | 95% CI | ||
| Age ≥60 y | 8 | .013 | .025 | 2.862 | 1.140-7.183 |
| <60 y | 103 | ||||
| Gender: F | 29 | .24 | — | — | — |
| M | 82 | ||||
| Diagnosis: ALL | 76 | .13 | — | — | — |
| LBL | 35 | ||||
| WBC ≥50.0 (×109/L) | 18 | .009 | — | — | — |
| <50.0 (×109/L) | 93 | ||||
| Hemoglobin <10 (g/dL) | 31 | .36 | — | — | — |
| ≥10 (g/dL) | 80 | ||||
| Platelet <100 (×109/L) | 47 | .036 | — | — | — |
| ≥100 (×109/L) | 64 | ||||
| LDH ≥600 (IU/L) | 79 | .045 | — | — | — |
| <600 (IU/L) | 32 | ||||
| CNS involvement at diagnosis: yes | 6 | .18 | — | — | — |
| No | 105 | ||||
| WHO classification | 111 | .101 | — | — | — |
| ETP-ALL/LBL: yes | 19 | .008 | .023 | 2.275 | 1.117-4.631 |
| No | 92 | ||||
| Treatment received | 111 | .43 | — | — | — |
Diagnosis = ALL vs LBL; treatment received = hyper-CVAD, hyper-CVAD + nelarabine, augmented BFM.
MVA, multivariate analysis; UVA, univariate analysis.
A subset of patients with early T-ALL/LBL had an immunophenotype that resembled that of ETP-ALL/LBL except for having ≥75% CD5 expression (ETP + CD5).9 The OS of patients with ETP + CD5 (n = 19) was similar to that of non–ETP-ALL/LBL patients and differed from that of ETP-ALL/LBL patients (P = .059) (supplemental Figure 9).
Discussion
We report here one of the largest series of newly diagnosed adult patients with ETP-ALL/LBL with long-term clinical outcomes. All patients received induction chemotherapy at our institution with either hyper-CVAD chemotherapy or augmented BFM chemotherapy.17,19,20 Our findings show (1) significantly poorer long-term outcomes for ETP-ALL/LBL patients compared with non–ETP-ALL/LBL patients, (2) lack of the prognostic significance of the WHO-defined immunophenotypic T-ALL/LBL categories, and (3) favorable long-term outcomes of mature T-ALL/LBL without allo-SCT.
Immunophenotypic subtyping of T-ALL/LBL is commonly used to stratify patients.1,4 The German Multicenter ALL Working Group (GMALL) reported subtype-oriented treatment results in a large group of 744 patients with T-ALL/LBL (ages 15 to 55 years) who were treated on the sequential GMALL clinical trials (05/93, 06/99, 07/03).16 In their series, thymic was the most common subtype (56%), followed by early (23%), and mature (21%).16 In the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) ALL study reported by Vitale et al,21 early T-ALL/LBL was the most common subtype (51%), followed by thymic (39%) and mature (10%). In the present study, thymic (43%) is the most common T-ALL/LBL subtype, followed by early (40%) and mature (17%). The prognostic impact of this immunophenotypic classification has been limited. Marks et al reported outcomes of 356 adult patients with T-ALL treated on the UK Medical Research Council Acute Lymphoblastic Leukaemia Trial XII/Eastern Cooperative Oncology Group (UKALLXII/ECOG) 2993 study.15 With the exception of CD1a expression, which was associated with a more favorable 5-year OS (64% vs 39%; P = .01), none of the other WHO-defined immunophenotypic categories affected clinical outcomes. They also noted that expression of myeloid markers such as CD13 was associated with a worse 5-year OS (35% vs 61%; P < .001). In retrospect, it could be postulated that these findings reflect the impact of admixed ETP-ALL/LBL cases. In the present report of newly diagnosed patients with T-ALL/LBL, we were unable to confirm the prognostic significance of any of the WHO-defined T-ALL/LBL subtypes.
The GMALL group has reported a beneficial effect of allo-SCT for patients with early and mature T immunophenotype.14,16 The beneficial effect of the transplant was especially pronounced in patients with mature T-ALL/LBL, in which the 10-year OS was only 25% without an allo-SCT vs 59% for those who had an allo-SCT. In the present report, only 7 patients underwent allo-SCT in CR1 because routine use of allo-SCT in CR1 was not recommended. Of the 19 patients with mature T-ALL/LBL, only 1 underwent allo-SCT in CR1. Despite this, we report a 5-year EFS and OS of 63% and 70%, respectively, in the mature T-ALL/LBL subtype. Therefore, we believe that allo-SCT in CR1 should not be considered standard for patients with mature T-ALL/LBL.
A total of 32% patients presented as LBL, with the most common presentation being a mediastinal mass. Patients presenting with LBL were more likely to have diploid karyotype compared with patients with ALL. Patients who presented with LBL appeared to have an improved survival compared with those presenting with ALL with an 8-year OS of 70% vs 44%, respectively; however, this was not statistically significant. This is consistent with other published series.22,23
ETP-ALL/LBL represents a disease subtype whereby blasts are derived from the earliest T-cell precursors in the thymus and have multilineage pluripotency.5,7,8 In children, ETP-ALL/LBL is associated with older age and a lower presenting WBC count than seen in the non–ETP-ALL/LBL patients.5,9,24,25 In our series, there was no major difference in the baseline characteristics of ETP-ALL/LBL patients compared with non–ETP-ALL/LBL patients, with the exception of the former having a higher likelihood of having clonal cytogenetic abnormalities, elevated BM blast counts, and a higher risk of CNS involvement at presentation. Children with ETP-ALL/LBL are less likely to be morphological early responders and are more likely to have minimal residual disease at the end of induction therapy.5,9,24,25 In the original report from the St. Jude Children’s Research Hospital group, patients with ETP-ALL/LBL had a significantly worse 10-year OS (19% vs 84%; P < .0001) and 10-year EFS (22% vs 69%; P < .0001) compared with other patients with T-ALL/LBL.5 The 10-year cumulative incidence of relapse was also significantly higher in patients with ETP-ALL/LBL (72% vs 10%; P < .0001).5 However, the Children’s Oncology Group recently reported that despite a higher likelihood of a positive MRD assessment at the end of induction in patients with ETP-ALL/LBL, their long-term outcomes were similar to the outcomes of the non-ETP patients, with 5-year EFS and OS of 87% and 93%, respectively.9 This was likely due to treatment intensification of MRD-positive patients. Patrick et al reported similar data for the Medical Research Council Working Party on Leukaemia in Children UK National Acute Lymphoblastic Leukaemia 2003 trial where children with ETP-ALL/LBL had relatively favorable long-term outcomes despite having high rates of rates of MRD at the end of induction.25 The reason for relatively favorable long-term outcomes of ETP-ALL/LBL patients in the Children’s Oncology Group and Medical Research Council Working Party on Leukaemia in Children UK National Acute Lymphoblastic Leukaemia 2003 study is not clear, but it is likely that treatment intensification for MRD-positive patients may have abrogated the negative prognostic impact of ETP-ALL/LBL. In our series, we report significantly poor outcomes in patients with ETP-ALL/LBL with a lower CR rate and a lower 5-year OS. The negative prognostic impact of ETP-ALL/LBL persisted when hyper-CVAD (an intensive regimen)–treated patients were analyzed separately. In addition, 9 of the 19 patients with ETP-ALL/LBL were treated on the hyper-CVAD + nelarabine clinical trial.17 The addition of nelarabine does not appear to have improved the outcomes of ETP-ALL/LBL in this small number of patients (supplemental Figures 10 and 11). Only limited data are available from other studies of adult patients with ETP-ALL/LBL.10,26 Vlierberghe et al stratified adult patients with T-ALL/LBL by gene expression profiling as opposed to the surface immunophenotype into early immature T-ALL/LBL and cortical/mature T-ALL/LBL.26 The early immature group had a lower OS compared with that seen in the cortical/mature group (5-year OS 34% vs 62%, respectively; P = .01). Neumann et al reported outcomes of WHO early T-ALL/LBL with a 5-year survival of 35% to 40%, and further categorization into ETP vs non-ETP did not influence clinical outcomes.10 In contrast, we report that even within the WHO early group, ETP-ALL/LBL retained prognostic significance. The prognostic impact of ETP subtype was maintained when only patients presenting with ALL were considered (Figure 5); for patients presenting as LBL, there was no difference in outcomes of ETP vs non-ETP, likely because of the small number of patients.
Several recent studies have characterized the molecular and genetic makeup of ETP-ALL/LBL.6,11,12,26 Several mutations that are common in acute myeloid leukemia such as FLT3 internal tandem duplication, FLT3 D835, and IDH1/IDH2 have been described in patients with ETP-ALL/LBL.26 This is not unexpected given that ETP cells are early hematopoietic stem cell derivatives that retain myeloid potential. These mutations are clinically actionable and thereby can provide a treatment strategy for a subset of patients with ETP-ALL/LBL. Chonghaile et al recently reported that ETP-ALL/LBL is dependent on Bcl2 and therefore Bcl2 antagonists such as ABT-199 may offer a potential therapeutic strategy.27,28 Maude et al reported aberrant activation of the Janus kinase/signal transducer and activator of transcription pathway in ETP-ALL/LBL and showed in vivo efficacy of the Jak2 inhibitor ruxolitinib.29
In summary, ETP-ALL/LBL represents a high-risk disease subtype of adult ALL. Novel treatment strategies are needed to improve treatment outcomes.
Acknowledgments
The authors thank the Anderson clinical flow cytometry laboratory team, especially Marian Kersh, for help with flow cytometry data. The authors also thank Leiloni Gilbert for administrative assistance.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: N.J., H.M.K., and J.D.K designed the study, collected, reviewed, and analyzed data, and wrote the paper; A.V.L. collected, reviewed, and analyzed data; S.O., F.R., M.K., E.J., S.P., D.T., M.R., G.B., T.K., and J.C. enrolled patients, interpreted data, and revised the manuscript; Z.Z. provided statistical expertise, analyses, and data interpretation; and J.J. and P.L. contributed to the data collection and critiqued the manuscript. All authors made substantial contributions to the concept and design of the study, were involved in the writing and critical revision of the manuscript, and gave final approval for the revision to be submitted.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nitin Jain, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: njain@mdanderson.org; and Joseph D. Khoury, Department of Hematopathology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: jkhoury@mdanderson.org.
References
- 1.Bene MC, Castoldi G, Knapp W, et al. European Group for the Immunological Characterization of Leukemias (EGIL) Proposals for the immunological classification of acute leukemias. Leukemia. 1995;9(10):1783–1786. [PubMed] [Google Scholar]
- 2.Ludwig WD, Raghavachar A, Thiel E. Immunophenotypic classification of acute lymphoblastic leukaemia. Baillieres Clin Haematol. 1994;7(2):235–262. doi: 10.1016/s0950-3536(05)80201-x. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig WD, Reiter A, Löffler H, et al. Immunophenotypic features of childhood and adult acute lymphoblastic leukemia (ALL): experience of the German Multicentre Trials ALL-BFM and GMALL. Leuk Lymphoma. 1994;13(Suppl 1):71–76. doi: 10.3109/10428199409052679. [DOI] [PubMed] [Google Scholar]
- 4.Borowitz MJ, Chan JKC. T Lymphoblastic Leukemia/Lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. pp. 176–178. [Google Scholar]
- 5.Coustan-Smith E, Mullighan CG, Onciu M, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10(2):147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Ding L, Holmfeldt L, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452(7188):764–767. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- 8.Wada H, Masuda K, Satoh R, et al. Adult T-cell progenitors retain myeloid potential. Nature. 2008;452(7188):768–772. doi: 10.1038/nature06839. [DOI] [PubMed] [Google Scholar]
- 9.Wood BL, Winter SS, Dunsmore KP, et al. T-lymphoblastic leukemia (T-ALL) shows excellent outcome, lack of significance of the early thymic precursor (ETP) immunophenotype, and validation of the prognostic value of end-induction minimal residual disease (MRD) in Children’s Oncology Group (COG) Study AALL0434. Available at: https://ash.confex.com/ash/2014/webprogram/Paper68699.html. Accessed January 12, 2016. [Google Scholar]
- 10.Neumann M, Heesch S, Gökbuget N, et al. Clinical and molecular characterization of early T-cell precursor leukemia: a high-risk subgroup in adult T-ALL with a high frequency of FLT3 mutations. Blood Cancer J. 2012;2(1):e55. doi: 10.1038/bcj.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann M, Coskun E, Fransecky L, et al. FLT3 mutations in early T-cell precursor ALL characterize a stem cell like leukemia and imply the clinical use of tyrosine kinase inhibitors. PLoS One. 2013;8(1):e53190. doi: 10.1371/journal.pone.0053190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann M, Heesch S, Schlee C, et al. Whole-exome sequencing in adult ETP-ALL reveals a high rate of DNMT3A mutations. Blood. 2013;121(23):4749–4752. doi: 10.1182/blood-2012-11-465138. [DOI] [PubMed] [Google Scholar]
- 13.Inukai T, Kiyokawa N, Campana D, et al. Clinical significance of early T-cell precursor acute lymphoblastic leukaemia: results of the Tokyo Children’s Cancer Study Group Study L99-15. Br J Haematol. 2012;156(3):358–365. doi: 10.1111/j.1365-2141.2011.08955.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoelzer D, Gökbuget N. T-cell lymphoblastic lymphoma and T-cell acute lymphoblastic leukemia: a separate entity? Clin Lymphoma Myeloma. 2009;9(Suppl 3):S214–S221. doi: 10.3816/CLM.2009.s.015. [DOI] [PubMed] [Google Scholar]
- 15.Marks DI, Paietta EM, Moorman AV, et al. T-cell acute lymphoblastic leukemia in adults: clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALL XII/ECOG 2993). Blood. 2009;114(25):5136–5145. doi: 10.1182/blood-2009-08-231217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoelzer D, Thiel E, Arnold R, et al. Successful subtype oriented treatment strategies in adult T-All; results of 744 patients treated in three consecutive GMALL studies. Available at: https://ash.confex.com/ash/2009/webprogram/Paper23425.html. Accessed January 12, 2016. [Google Scholar]
- 17.Jain P, Kantarjian H, Ravandi F, et al. The combination of hyper-CVAD plus nelarabine as frontline therapy in adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma: MD Anderson Cancer Center experience. Leukemia. 2014;28(4):973–975. doi: 10.1038/leu.2013.312. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Slack R, Jorgensen JL, et al. The effect of peritransplant minimal residual disease in adults with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk. 2014;14(4):319–326. doi: 10.1016/j.clml.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantarjian H, Thomas D, O’Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 20.Rytting ME, Thomas DA, O’Brien SM, et al. Augmented Berlin-Frankfurt-Münster therapy in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL). Cancer. 2014;120(23):3660–3668. doi: 10.1002/cncr.28930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitale A, Guarini A, Ariola C, et al. Adult T-cell acute lymphoblastic leukemia: biologic profile at presentation and correlation with response to induction treatment in patients enrolled in the GIMEMA LAL 0496 protocol. Blood. 2006;107(2):473–479. doi: 10.1182/blood-2005-04-1754. [DOI] [PubMed] [Google Scholar]
- 22.Gökbuget N, Wolf A, Stelljes M, et al. Favorable outcome in a large cohort of prospectively treated adult patients with T-lymphoblastic lymphoma (T-LBL) despite slowly evolving complete remission assessed by conventional radiography. Available at: https://ash.confex.com/ash/2014/webprogram/Paper67802.html. Accessed January 12, 2016. [Google Scholar]
- 23.Lepretre S, Touzart A, Vermeulin T, et al. Pediatric-Like Acute Lymphoblastic Leukemia Therapy in Adults With Lymphoblastic Lymphoma: The GRAALL-LYSA LL03 study [published online ahead of print December 7, 2015.]. J Clin Oncol. doi: 10.1200/JCO.2015.61.5385. doi:10.1200/JCO.2015.61.5385. [DOI] [PubMed] [Google Scholar]
- 24.Wood B, Winter S, Dunsmore K, et al. Patients with early T-cell precursor (ETP) acute lymphoblastic leukemia (ALL) have high levels of minimal residual disease (MRD) at the end of induction–A Children’s Oncology Group (COG) Study. Available at: https://ash.confex.com/ash/2009/webprogram/Paper25398.html. Accessed January 12, 2016. [Google Scholar]
- 25.Patrick K, Wade R, Goulden N, et al. Outcome for children and young people with Early T-cell precursor acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2014;166(3):421–424. doi: 10.1111/bjh.12882. [DOI] [PubMed] [Google Scholar]
- 26.Van Vlierberghe P, Ambesi-Impiombato A, De Keersmaecker K, et al. Prognostic relevance of integrated genetic profiling in adult T-cell acute lymphoblastic leukemia. Blood. 2013;122(1):74–82. doi: 10.1182/blood-2013-03-491092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chonghaile TN, Roderick JE, Glenfield C, et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov. 2014;4(9):1074–1087. doi: 10.1158/2159-8290.CD-14-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peirs S, Matthijssens F, Goossens S, et al. ABT-199 mediated inhibition of BCL-2 as a novel therapeutic strategy in T-cell acute lymphoblastic leukemia. Blood. 2014;124(25):3738–3747. doi: 10.1182/blood-2014-05-574566. [DOI] [PubMed] [Google Scholar]
- 29.Maude SL, Dolai S, Delgado-Martin C, et al. Efficacy of JAK/STAT pathway inhibition in murine xenograft models of early T-cell precursor (ETP) acute lymphoblastic leukemia. Blood. 2015;125(11):1759–1767. doi: 10.1182/blood-2014-06-580480. [DOI] [PMC free article] [PubMed] [Google Scholar]