Abstract
By the year 2020, potentially one half million hematopoietic cell transplant (HCT) recipients will need long-term follow up care to address not only chronic GvHD but also multiple other late consequences of transplant. Despite this increase in patients, there will not be a concomitant increase in the HCT workforce. Thus the future of long-term patient management will require a new “next-generation” clinical model that utilizes technological solutions to make the care of the HCT patient efficient, safe, and cost-effective. Guideline-based decision support will be embedded in clinical workflows. Documentation requirements will be reduced as automated data collection from electronic medical records (EMRs) will populate registries and provide feedback for a rapid learning health system. Interoperable EMRs will disseminate treatment protocols to multiple care providers in a distributed long-term clinic model, such that providers outside of the transplant center can provide services closer to the patient. Patients will increase their participatory role through patient portals and mobile devices. At Vanderbilt, we have responded so some of these future challenges by embedding guideline-based decision support, structuring clinical documentation, and being early adopters of communication technology. This manuscript describes the current state of some of these innovations, and a vision for the future of the long-term transplant clinic.
Keywords: late effects, long term transplant follow-up, health information technology
Introduction
With the increasing number of HCTs performed yearly worldwide, and the improvement of survival after transplantation, the number of patients surviving two years and longer after transplantation is continuously increasing. By 2020, there may be worldwide up to half a million long-term survivors after HCT, constituting a 250% increase over the number of survivors from 2009 (1, 2). Recent data suggest that early transplant mortality has improved and is due to better patient selection, use of reduced toxicity regimens, and better supportive care (2–4). The challenges in HCT extend beyond day 100, with an increasing incidence of chronic GvHD and transplant-related organ dysfunction (5). For patients surviving more than 25 years after transplantation there is still a two-fold excess of death rate (2, 4). Even three decades after HCT, the cumulative incidence for secondary solid tumors or non-malignant complications continues to increase, with no indication of a plateau developing (2, 6–8). The transition from the acute phase HCT to ongoing follow-up post-treatment care is critical to maintain long-term health. This involves not only the transplantation center and the patient but also their caregivers and their other physicians (2, 9).The long-term transplant patient needs to be followed closely for risks of secondary malignancy, osteoporosis, endocrine abnormalities, and other organ dysfunctions. Therefore, there should be a lifelong commitment for post-transplant survivorship care for an increasing number of patients.
HCT recipients beyond day 100 require attention to their underlying disease, infectious issues, immunosuppression, and multiple monitoring parameters. Health care providers must assess disparate organ systems for potential transplant-related dysfunctions while also fulfiling multiple documentation and data entry requirements imposed by the institution, payers, and regulatory agencies - all within the time constraints of a brief “return” clinic visit(10). This challenge is the subject of a national study where a pre-designed plan of care is being compared to the standard of care approach in a clinical trial (“Randomized Study of Individualized Care Plans for Hematopoietic Cell Transplant Survivors”, NCT02200133).
HCT recipients require multidisciplinary care and the transmission and coordination of information flow would be central to such a “next gen” clinic. In addition to an increasing number of survivors of HCT, future care of these patients will likely require an increasing number of monitoring parameters, multi-system evaluations, and other data elements to diagnose and treat the long-term complications of HCT(1, 11, 12). The information requirements of increasing numbers of patients multiplied by increasing monitoring complexity necessitates a new model of the long-term transplant clinic. However, while the number and complexity of long-term transplant patients is increasing; the number of transplant physicians will not keep pace (13). The growth in number of transplants has for many years outpaced the growth of the physician workforce to care for these patients (14). To address the future challenges of a long term transplant clinic (LTTC) we propose utilizing advances in health information technology (Health IT) to enable seeing patients more efficiently and with less errors of omission. This manuscript describes the current thought processes and groundwork that will be needed to make such “next gen” clinics a reality.
Long Term Transplant Guidelines: Current Challenges
A typical LTTC setup requires the HCT survivors to visit the clinic at least annually. A common outpatient HCT clinic combines acute care and long-term follow-up, allowing continuity of care. However, this arrangement faces the risk that the particular needs of long-term survivors are left behind the daily care of the immediate post-transplant patients. In contrast, separating acute and long term clinics risks losing the continuity of care as patients transition from one to the other.
The optimal care of the HCT recipient has evolved based on best institutional practices, consensus criteria and recommendations from national and international guidelines. While published guidelines attempt to standardize treatment plans, provider biases make decision making complex, leading to non-standardized care models (15–18). Post-transplant care guidelines were updated in 2012 with expanded recommendations for screening for chronic GvHD, post-transplant organ dysfunction screening, and vaccinations (2, 12). The growth in monitoring parameters from 31 in 2006 to 59 in 2012 demonstrates the increasing complexity of caring for long term transplant patients (11, 12). In consultation with several leading transplant organizations, the National Marrow Donor Program (NMDP) has created a website with guidelines for post-transplant care (www.marrow.org/HCT-guide). These guidelines provide recommendations post-transplant screening, vaccination and GvHD and have even been operationalized as a smartphone “app.” However, the penetration of these practices in the clinic and thus impact on patient care remains unmeasured (18).
Health Information Technology (HIT) for the Transplant Provider
Evolving Role of the Electronic Medical Record (EMR)
The use of information technology has revolutionized many fields over the past half-century(19). In healthcare the EMR is the primary tool of information technology. In the future the EMR and related software solutions will be needed to improve the efficiency of long-term transplant clinics as volume and complexity of care increases(20). However, the history of the EMR is not reassuring in its ability to improve efficiency, as some studies have noted an overall loss of productivity following EMR implementation (21–24). However, if the evolution of the EMR is compared to that of the digital camera it is possible to view the future more positively. Initial versions of digital cameras in the mid-1990s were laughably inferior to film cameras and significantly more expensive; however, with iterative improvements over two decades, digital photography displaced film photography to the extent that 35-mm film is nearly extinct. Digital format matched film in quality, but disrupted photography by offering superior cost advantages, sharing abilities, and customizability. With EMRs, many have only “digitized paper”, but the future holds the promise of improving care in ways that could not be accomplished without them. EMRs have the ability to provide guideline-based decision support, automatically aggregate standardized data, transmit data to registries, fluidly incorporate patient reported outcome measures, automate documentation, and make the increased patient volumes of a future long-term transplant clinic feasible (25–27). With the progressive increase in long-term transplant patients and the constraints of the provider workforce, a promising solution is to use information technology to improve clinical efficiency and distribute some of the workload outside the transplant clinic.
Increased Plan Sharing
The early transplant model is an excellent example of providing care in a “bundled case rate”. However, after day 100, these models are typically not applicable. The care of the patient transitions from the transplant center and starts including the primary care provider along with the referring oncologist. Thus, a seamless information transfer among EMR system will be needed to effectively distribute the care of the patient.
Three secular trends are in the favor of a distributed long-term transplant clinic model that distributes and coordinates care among providers outside the transplant center: 1) consolidation of health systems, 2) consolidation of EMR vendors, and 3) regulatory pressure for greater interoperability of health care data. These trends are described in turn:
Most transplant programs are located within large academic cancer centers; however, many large centers are partnering with other regional hospitals and clinics to create larger healthcare networks (28–30). These networks may be integrated with technological underpinnings and health information sharing. While the degree of interoperability will vary by the structure of the healthcare network, these networks will help to facilitate closer communication for transplant physicians and PCPs.
As EMRs have now saturated the market and are no longer a growth field, there are moves towards vendor consolidation (31). Fewer vendors increases, but does not guarantee, the likelihood of interoperable systems between a transplant center and the primary care provider closer to a patient’s home(32). Additionally, as future vendors compete based on usability and innovation, EMRs may become less burdensome to providers(33).
A key driver of health IT has been the “Meaningful Use” regulations which began as part of the American Recovery and Reinvestment Act of 2009 (34). Future federal mandates and regulations are likely to emphasize information interoperability of EMR data. The most recent proposal for stage 3 of the Meaningful Use program incorporates requirements for clinical document sharing(35). Recent congressional legislature entitled the “21st Century Cures Act” proposes to further reduce the barriers to health data interoperability. These regulations, and the increasing use of secure communication protocols (36), suggest that information exchange will become easier. In a recent editorial accompanying a special issue on biomedical data standards of the Journal of the American Medical Informatics Association, Dr. Doug Fridsma (President and CEO of AMIA) put forth the challenge thusly: “While the use of biomedical data standards is important for many reasons, none seem more timely than supporting interoperability across different health information technology applications(37).” Future versions of EMRs will likely demonstrate a greater ability for physicians to share treatment plans for common patients and create a LTTC where some of the plan of care is clearly distributed to non-transplant physicians outside the transplant center.
Data analytics for guideline operationalization and rapid learning systems
As mentioned previously, transplant care plans are based on institutional best practices, guidelines, and recommendations from national and international transplant organizations. However, it is unclear if these guidelines have improved outcomes. When practices are changed or adapted, it can be difficult to measure the impact of the change on patient outcomes, unless data is manually extracted and analyzed. Transplant programs are monitored by multiple agencies with an emerging emphasis on quality management and outcomes are reviewed by payers. Thus having structured data would be ideal to measure multiple aspects of transplant programs.
Starting with the first transplant registries in the 1970s, the field of transplantation relied on “big data” long before it was a technology buzzword (38, 39). Data collected on over 370,000 patients and donors is stored within the Center for International Blood and Marrow Transplant Research (CIBMTR), providing the basis for powerful data analytics. The collection of this data can be tedious and difficult, but the analysis has yielded significant results, such as the elucidation of differences in GvHD rates between ethnic populations (40). Despite this valuable resource, there are significant shortcomings – in particular the manual data extraction process and the lack of data targeting late effects beyond the one year survival landmark.
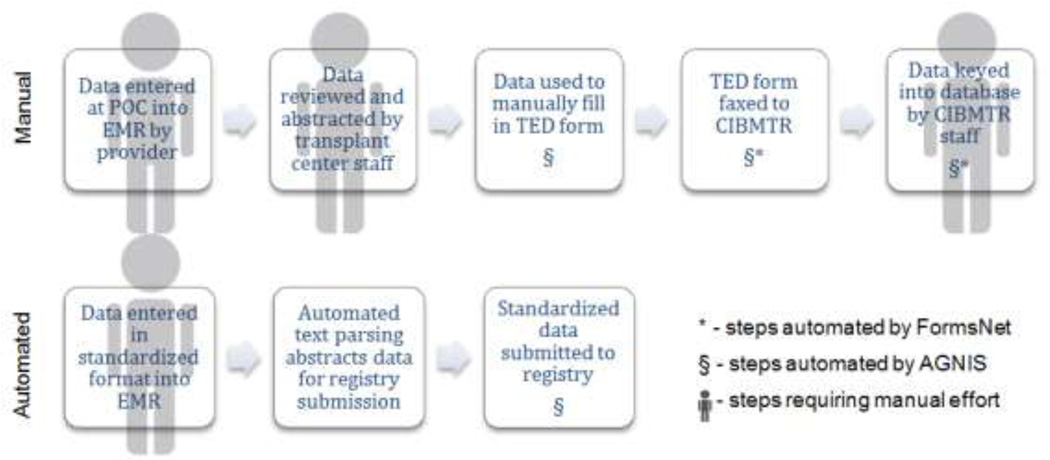
Much of the success of the CIBMTR data to improve transplant outcomes up to the one year mark comes from the inter-institution analysis of standardized data. The standardized Transplant Essential Data (TED) documents that comprise the input to the CIBMTR databases are supported by rigorous and well-maintained data standards (41). However, although computable data element structure exists, many transplant centers rely on manual data extraction from their EMR for reporting. This is unfortunate as converting computable data from the EMR into computable data for storage via TED forms by hand is expensive, tedious and prone to error (Figure 1). Additionally agreement between manual abstractors can be poor, even when subject matter experts are extracting data elements.(42). By automating standardized data extraction from the EMR and harmonizing data elements for registry reporting, quality monitoring, and payer requirements, documentation efficiency will improve and manual data extraction will decrease.
Figure 1.
Current manual data submission practices are tedious and inefficient. The process of documenting clinical data for submission to CIBMTR requires multiple people through multiple steps and several transitions from computable data to manual data and back. The process is inefficient and introduces greater possibilities for error. Partial solutions are FormsNet which allows electronic form submission, thus obviating a manual re-entry of data by CIBMTR staff; and A Growable Network Information System (AGNIS) which goes one step further and abstracts data from forms in secondary transplant software for submission to CIBMTR. In the future, standardized documentation will allow automated document parsing and data abstraction which will be automatically transmitted to reporting and registry databases.
A possible means for addressing data collection and guideline analysis for late term transplant effects is the “rapid learning system” concept (43). In 2010 the Institute of Medicine released a Workshop Summary entitled “A Foundation for Evidence-Driven Practice: A Rapid Learning System for Cancer Care (44).” In this summary, a rapid learning system is defined as system that collects data at the point of care to generate evidence that iteratively bridges the clinic and research and feeds evidence back into clinical practice(45–47). This could be utilized in a LTTC when data such as transplant history, laboratory values, exam findings, imaging results are structured in the EMR and stored automatically in a database. These data are then available for comparative effectiveness studies and pragmatic clinical trials to assess the impact of practice variations on clinical outcomes. The evidence from this analysis can then be codified in guidelines that can go on to additional assessment and evaluation with future patients. Implicit in this system is the reliance on health IT in general and EMRs in particular (48). While a rapid learning system will not supplant the registry concept, the field of marrow transplantation could exemplify how it could be utilized to achieve process improvement.
Another promise of a rapid learning system is the capability to formulate and assess machine-computable trustworthy clinical practice guidelines (49). Similar to the logic that is demonstrated in the NMDP transplant follow-up smartphone app, computable guidelines can be embedded into electronic systems to offer automated decision support to providers during patient care. However, as has been demonstrated in multiple studies the changes in clinical outcomes of these decision support systems are difficult to conclusively prove(50, 51). In topics where the standard of care is not well-established and includes a wide diversity of options (such as the management of chronic GvHD), disparate practices can be compared and evaluated to determine the best guidelines. The process of updating guidelines is amenable to the continuous evaluation of a rapid learning system where a first level of evaluation would be adherence to suggested guidelines and additional analysis of whether adherence to guidelines improved patient outcomes(52). Those guidelines that demonstrate improved outcomes can then be integrated back into EMRs to provide further clinical decision support and additional analysis, thus bringing the lessons from the rapid learning system back to the patient-provider interaction.
Vanderbilt Approach-Informatics
Vanderbilt University Medical Center (VUMC) is a large academic medical center similar to many others that host HCT programs. Vanderbilt has seen firsthand some of the constraints to the future of the long-term transplant clinic (Table 1). The VUMC transplant program is unique in its use of a “home-grown” EMR system, StarPanel (53, 54). StarPanel was designed and built by VUMC software architects and allows a degree of customization not typically allowed with most vendor systems(55).
Table 1.
Challenges and Potential Solutions from the Vanderbilt Experience
| Challenge | Potential Solution |
|---|---|
| Increased patient volumes without concomitant increase in provider capacity |
Well-trained nurse practitioners, closely supervised by transplant physicians, working through standardized documentation with embedded guideline-based decision support |
| Increased regulatory and reporting requirements |
Mapping documentation elements to required reporting elements for automated extraction |
| Long distances between many patients and transplant center |
Embracing communication solutions such as portal messaging |
The transplant program has created note templates aligned with FACT guidelines. Thus, quality metrics such as immunization reminders are embedded within the routine clinical work-flow. Notes are customized to the phase of HCT (pre-HCT, stem cell mobilization, autologous HCT, allogeneic HCT, LTTC initial visit and follow-up visits). These note templates allow for structured collection of practice data. The templates provide mid-level practitioners an outline of their encounters with patients, as well as a general timeline of their transplant course. Electronic forms for accurate grading and staging of acute and chronic graft-versus-host disease are built within the EMR and notes are updated as to when the next evaluation is needed. Clinical trials open for relevant patients are added to the templates, and serve as an ongoing reminder for encouraging enrollment in clinical trials. A section of the notes for the long-term transplant patient is dedicated to late effects. This section along with laboratories and imaging, and intake data (allergies, medicine reconciliation, pertinent past history) are auto-imported into the clinic documentation. In this way, these custom notes provide passive guideline decision support and also help to organize care. This clinical documentation structure helps to contribute to improvement in outcomes (56) and helps to organize data for retrospective research by the Vanderbilt program.
Online patient-provider messaging
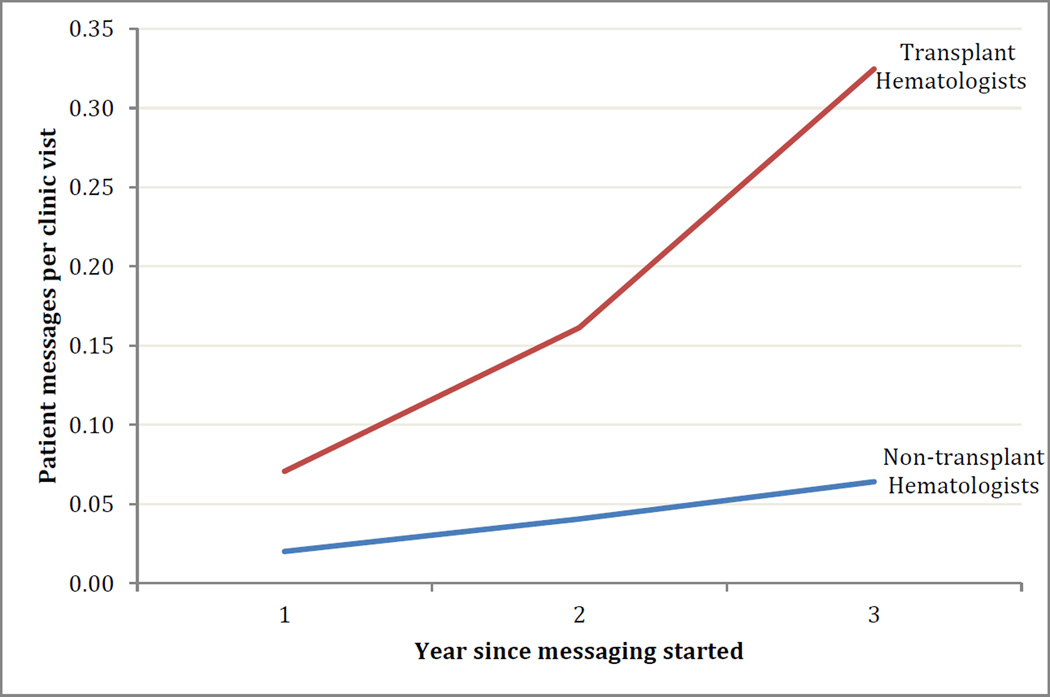
The VUMC transplant program was an early adopter of online patient portal technology called MyHealth@Vanderbilt. (57). The online portal sends lab results, clinic summaries, and prescription information to patients. This portal can also support sharing of clinical documentation with patients in the “Open Notes” concept, which is beginning to be widely embraced (58, 59). Current work on MHAV is focused on “closing the loop” by allowing patients to report non-emergent symptoms and ambulatory device measurements directly into the EMR. MHAV also contains a Meaningful-Use mandated secure messaging portal by which patients and providers can exchange non-emergent communications. Analysis of the message threads demonstrates that hematologists who perform HCT were early adopters and utilize portal messaging substantially more than non-transplant hematologists when accounting for number of providers and patient volumes (Figure 2). This indicates a willingness on the part of HCT patients and providers to embrace technology to improve communication and clinical care.
Figure 2.
Transplant patients and providers are early adopters of online messaging technology. In the first three years following the implementation of online messaging, HCT providers sent more messages per clinic encounter (normalized for number of providers and patient volume) than hematologists who do not perform HCT. Reasons for this could be greater technological literacy of patients, more acceptances among transplant providers, higher acuity, or socio-demographic differences. Regardless, these data corroborates an opinion that HCT patients are willing to utilize technological interventions and could be early adopters of other interventions such as mHealth tools.
Data warehousing
Embedded behind the VUMC EMR is a data warehouse that stores nearly all clinical data. The warehouse allows the creation of analytic data dashboards for ongoing operations, quality, and research endeavors (54, 60). In parallel to this identified data warehouse is an automatically de-identified data source linked to a large-scale de-identified DNA biobank that is used for GWAS and pharmacogenomic projects (61). The value of such informatics tools derived from clinical data has been described previously (62). Within the transplant program, standardized outcome measures are curated from the structured clinical documentation for additional investigative projects.
The as patient volume and regulatory reporting increase in the future, the work for providers and transplant data managers will increase exponentially. If the workforce for this task will exist is questionable, and even if it is the cost will be daunting for most transplant programs. Despite the ability to create custom data collection forms automated data extraction and submission is lacking at most centers, Vanderbilt included. Future efforts at Vanderbilt will include mapping structured data elements in notes to reporting data required by regulatory agencies.
Five Years into the Future
The growth of the long term HCT population in the future is not going to be met with a commensurate increase in the number of HCT physicians. Adding further strain on the operation of a long-term transplant clinic are the increasing numbers of metrics and regulatory data that must be extracted, curated, and submitted. A new, more efficient clinical model of the care of the transplant patient is needed to address these challenges (Table 2). The future of the long term transplant clinic will incorporate multiple information technology solutions to make the care of transplant patients safer, more efficient, and more cost effective.
Table 2.
Areas and goals of changes to bring about the next-gen long term transplant clinic. Action items are the authors’ proposals for starting to bring about those changes.
| Area | Goal | Action Item |
|---|---|---|
| mHealth | A comprehensive mobile app for patients |
Define minimum functional, interoperable and security requirements |
| Sponsor design challenge for app designers with winner receiving official designation by transplant bodies | ||
| Interoperability | Transplant plans of care accessible and actionable across all care locations |
Define standards of documentation that are comprehensible across providers |
| Partner with PCORnet and health information exchanges (HIE) to provide transplant data as use cases for (HIE) development | ||
| Clinical decision support (CDS) |
Real-time standard of care CDS integrated into LTTC clinical workflows |
Convert all ASBMT standard of care guidelines into computable logic for integration into EMR CDS systems |
| Develop API or webserver interface for NMDP guidelines to be used by EMR developers | ||
| Documentation Efficiency |
Streamlined efficient documentation saves providers time and improves accuracy |
Develop a standardized modular documentation design that can be implemented across EMRs |
| In conjunction with mHealth efforts and CDS efforts, increase automatic documentation of PROs as well as standard of care interventions | ||
| Data collection Efficiency |
Automated collection and transmission of standardized registry data |
Develop a timeline for requiring electronic TED form submission |
| Develop FHIR®-standardized TED forms that can be created and submitted by native EMRs. | ||
| Develop CIBMTR FHIR® receiver for all TED forms |
Driven by increased marketplace competition, future EMR designs will strive to appreciate the subtleties of the human-computer interface and display a more streamlined experience for providers, rather than the jarring alerts, pop-ups, and overrides too familiar to most practicing clinicians(63–65). Speech recognition and smart form completion can reduce documentation time. Automated guideline-based treatment plans will maintain a patient’s place in time in the course of their care, and provide decision support to providers. With improvements in decision support, some standard of care options will become automatic or opt-out thus reducing work and making the correct care easier to provide. Thus, the future care of the transplant patient will move towards smart algorithms, based on continually-evaluated guidelines, that provide reflexive decision support for providers (26).
Increased interoperability will allow a distributed long-term transplant clinic model that will move more care out of the transplant center. Efforts in allied oncology fields have already demonstrated the feasibility of a shared treatment plan approach across multiple providers(66). As the ability to communicate and share specific plans across EMRs increases, more routine care (such as bone density screening, thyroid function testing, and diabetes testing) can be provided closer to patients’ homes by their local physicians. Smart algorithms can provide decision support in the transplant clinic and could also determine which care would be best delivered locally and then communicate those recommendations to both the transplant physician and the local primary care physician.
Smart phones and mHealth
The future of computing is on mobile platforms with approximately 1 in 3 persons living in the United States now has a “smartphone” and this number is increasing(67). This pervasive computing power is an opportunity for involving patients in mobile health “mHealth” and to a greater extent in their care (68). In the future, mobile apps will be able to link outpatients to the transplant clinic for reporting of symptoms, medication compliance and clinical messaging. The PRO-CTCAE, which is now being translated into multiple languages, will provide a standardized starting point for application developers to create an interoperable system of symptoms reporting (69).
The EMR will increase efficiency of data extraction. With an increasing number of outcomes to track and report, EMRs and clinical data warehouses will operate behind the scenes of clinical care to unobtrusively complete outcome-reporting metrics. Additionally, solutions specific to HCT will integrate with international registries, quality reporting agencies, and payers will reduce the administrative needs of transplant programs. This automated data collection will serve as the basis for ongoing data analytics regarding best practices and standard of care which will feed back into a robust rapid learning system that can then implement best practices.
Evaluating the impact of technological changes can be difficult, as has been described in the literature (reviewed in (50)). However, potential outcome metrics that would indicate the success of these metrics would be decreased provider documentation time, improved guideline adherence, or greater fidelity in registry reporting. Economic outcomes could be measured such as reduced cost of registry reporting, reduced duplication of tests, and less cost per transplant to the healthcare system. Patient-specific outcomes such as quality of life, relapse-free survival, and overall survival are also important but harder to demonstrate changes with information systems interventions. It is also possible that new evaluation metrics will emerge in the future to assess the impact of information system interventions.
The transplant community has been a leader in various aspects of health care including multidisciplinary team approach, bundled case rate, mandatory national registries, and requirement of accreditation and transparency of outcome data. By 2020 there may be over a half million transplant patients with increasing regulatory and reporting requirements, but without a foreseeable corresponding increase in the transplant workforce. A solution to this problem could be the utilization of information technology to create a safer, more efficient, more cost effective “next-gen” transplant clinic. In this way, the efforts of the transplant team are taken away from documentation, data extraction, and inefficient communication and returned to caring for transplant patients. The transplant programs can lead the way to develop next gen clinics not only at the transplant centers, but weave them into a network of healthcare providers where the long-term transplant patient is the happiest: closer to home.
Footnotes
Conflict of interest: Authors have no conflicts of interest to report
References
- 1.Majhail NS, Tao L, Bredeson C, Davies S, Dehn J, Gajewski JL, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. 2013;19(10):1498–1501. doi: 10.1016/j.bbmt.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahrukh HaL, MR . Late effects after allogeneic hematopoietic stem cell transplantation. In: Savani BN, editor. Blood and Marrow Transplantation Long Term Management: Prevention and Complications. Oxford, UK: John Wiley & Sons Ltd; 2014. pp. 21–31. [Google Scholar]
- 3.Martin PJ, Counts GW, Jr, Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28(6):1011–1016. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savani BN. How can we improve life expectancy and quality of life in long-term survivors after allogeneic stem cell transplantation? Semin Hematol. 2012;49(1):1–3. doi: 10.1053/j.seminhematol.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing Incidence of Chronic Graft-versus-Host Disease in Allogeneic Transplantation - A Report from CIBMTR. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khera N, Storer B, Flowers ME, Carpenter PA, Inamoto Y, Sandmaier BM, et al. Nonmalignant late effects and compromised functional status in survivors of hematopoietic cell transplantation. J Clin Oncol. 2012;30(1):71–77. doi: 10.1200/JCO.2011.38.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Socie G, Rizzo JD. Second solid tumors: screening and management guidelines in long-term survivors after allogeneic stem cell transplantation. Semin Hematol. 2012;49(1):4–9. doi: 10.1053/j.seminhematol.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Majhail NS. Secondary cancers following allogeneic haematopoietic cell transplantation in adults. Br J Haematol. 2011;154(3):301–310. doi: 10.1111/j.1365-2141.2011.08756.x. [DOI] [PubMed] [Google Scholar]
- 9.Hashmi S, Carpenter P, Khera N, Tichelli A, Savani BN. Lost in Transition: The Essential Need for Long-Term Follow-Up Clinic for Blood and Marrow Transplantation Survivors. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Schriber JR, Anasetti C, Heslop HE, Leahigh AK. Preparing for growth: current capacity and challenges in hematopoietic stem cell transplantation programs. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(5):595–597. doi: 10.1016/j.bbmt.2010.02.010. Epub 2010/02/20. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo JD, Wingard JR, Tichelli A, Lee SJ, Van Lint MT, Burns LJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, Center for International Blood and Marrow Transplant Research, and the American Society for Blood and Marrow Transplantation (EBMT/CIBMTR/ASBMT) Bone Marrow Transplant. 2006;37(3):249–261. doi: 10.1038/sj.bmt.1705243. Epub 2006/01/26. [DOI] [PubMed] [Google Scholar]
- 12.Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(3):348–371. doi: 10.1016/j.bbmt.2011.12.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajewski JL, LeMaistre CF, Silver SM, Lill MC, Selby GB, Horowitz MM, et al. Impending challenges in the hematopoietic stem cell transplantation physician workforce. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(12):1493–1501. doi: 10.1016/j.bbmt.2009.08.022. Epub 2009/09/29. [DOI] [PubMed] [Google Scholar]
- 14.Majhail NS, Murphy EA, Omondi NA, Robinett P, Gajewski JL, LeMaistre CF, et al. Allogeneic transplant physician and center capacity in the United States. Biol Blood Marrow Transplant. 2011;17(7):956–961. doi: 10.1016/j.bbmt.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff D, Ayuk F, Elmaagacli A, Bertz H, Lawitschka A, Schleuning M, et al. Current practice in diagnosis and treatment of acute graft-versus-host disease: results from a survey among German-Austrian-Swiss hematopoietic stem cell transplant centers. Biol Blood Marrow Transplant. 2013;19(5):767–776. doi: 10.1016/j.bbmt.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Ruutu T, van Biezen A, Hertenstein B, Henseler A, Garderet L, Passweg J, et al. Prophylaxis and treatment of GVHD after allogeneic haematopoietic SCT: a survey of centre strategies by the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012;47(11):1459–1464. doi: 10.1038/bmt.2012.45. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Vogelsang G, Gilman A, Weisdorf DJ, Pavletic S, Antin JH, et al. A survey of diagnosis, management, and grading of chronic GVHD. Biol Blood Marrow Transplant. 2002;8(1):32–39. doi: 10.1053/bbmt.2002.v8.pm11846354. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Astigarraga CC, Eapen M, Artz AS, Davies SM, Champlin R, et al. Variation in supportive care practices in hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(11):1231–1238. doi: 10.1016/j.bbmt.2008.08.008. Epub 2008/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sichel DE, Oliner SD. Information technology and productivity: where are we now and where are we going? Board of Governors of the Federal Reserve System FEDS. 2002;29 [Google Scholar]
- 20.Buntin MB, Burke MF, Hoaglin MC, Blumenthal D. The benefits of health information technology: a review of the recent literature shows predominantly positive results. Health Aff (Millwood) 2011;30(3):464–471. doi: 10.1377/hlthaff.2011.0178. Epub 2011/03/09. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, McCullough JS, Town RJ. The impact of health information technology on hospital productivity. The RAND Journal of Economics. 2013;44(3):545–568. [Google Scholar]
- 22.Huerta TR, Thompson MA, Ford EW, Ford WF. Electronic health record implementation and hospitals' total factor productivity. Decision Support Systems. 2013;55(2):450–458. [Google Scholar]
- 23.Poissant L, Pereira J, Tamblyn R, Kawasumi Y. The Impact of Electronic Health Records on Time Efficiency of Physicians and Nurses: A Systematic Review. Journal of the American Medical Informatics Association. 2005;12(5):505–516. doi: 10.1197/jamia.M1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halamka J, Aranow M, Ascenzo C, Bates DW, Berry K, Debor G, et al. E-Prescribing collaboration in Massachusetts: early experiences from regional prescribing projects. J Am Med Inform Assoc. 2006;13(3):239–244. doi: 10.1197/jamia.M2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warner J, Hochberg E. Where is the EHR in oncology? J Natl Compr Canc Netw. 2012;10(5):584–588. doi: 10.6004/jnccn.2012.0060. Epub 2012/05/10. [DOI] [PubMed] [Google Scholar]
- 26.Yu P, Artz D, Warner J. Electronic health records (EHRs): supporting ASCO's vision of cancer care. Am Soc Clin Oncol Educ Book. 2014;34:225–231. doi: 10.14694/EdBook_AM.2014.34.225. [DOI] [PubMed] [Google Scholar]
- 27.Yu PP. The evolution of oncology electronic health records. Cancer J. 2011;17(4):197–202. doi: 10.1097/PPO.0b013e3182269629. [DOI] [PubMed] [Google Scholar]
- 28.Ohno-Machado L. Networking the country to promote health and scientific discovery. J Am Med Inform Assoc. 2014;21(4):575. doi: 10.1136/amiajnl-2014-003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dafny L. Hospital industry consolidation--still more to come? The New England journal of medicine. 2014;370(3):198–199. doi: 10.1056/NEJMp1313948. Epub 2013/12/18. [DOI] [PubMed] [Google Scholar]
- 30.Humphreys A. Healthcare & Life Sciences M&A Outlook. Bass: Berry & Sims; 2014. Contract No.: 1. [Google Scholar]
- 31.Koppel R, Lehmann CU. Implications of an emerging EHR monoculture for hospitals and healthcare systems. J Am Med Inform Assoc. 2014 doi: 10.1136/amiajnl-2014-003023. amiajnl-2014-003023. [DOI] [PubMed] [Google Scholar]
- 32.Sittig DF, Wright A. What makes an EHR "open" or interoperable? Journal of the American Medical Informatics Association : JAMIA. 2015 doi: 10.1093/jamia/ocv060. Epub 2015/06/17. [DOI] [PubMed] [Google Scholar]
- 33.Joseph S, Snow M, Furukawa MF, Posnack S, Chaffee MA. HITECH spurs EHR vendor competition and innovation, resulting in increased adoption. The American journal of managed care. 2014;20(9):734–740. Epub 2014/11/05. [PubMed] [Google Scholar]
- 34.Blumenthal D, Tavenner M. The "meaningful use" regulation for electronic health records. N Engl J Med. 2010;363(6):501–504. doi: 10.1056/NEJMp1006114. Epub 2010/07/22. [DOI] [PubMed] [Google Scholar]
- 35.Medicare and Medicaid Programs. Electronic Health Record Incentive Program-Stage 3. Federal register. 2015;80:16731–16804. Epub 2015/03/30. [Google Scholar]
- 36.Williams C, Mostashari F, Mertz K, Hogin E, Atwal P. From the Office of the National Coordinator: the strategy for advancing the exchange of health information. Health Aff (Millwood) 2012;31(3):527–536. doi: 10.1377/hlthaff.2011.1314. [DOI] [PubMed] [Google Scholar]
- 37.Fridsma DBMPFFP, Ceo A. AMIA-Setting the Standard. Journal of the American Medical Informatics Association : JAMIA. 2015;22(3):744–745. doi: 10.1093/jamia/ocv049. Epub 2015/06/07. [DOI] [PubMed] [Google Scholar]
- 38.Aljurf M, Rizzo JD, Mohty M, Hussain F, Madrigal A, Pasquini MC, et al. Challenges and opportunities for HSCT outcome registries: perspective from international HSCT registries experts. Bone Marrow Transplant. 2014;49(8):1016–1021. doi: 10.1038/bmt.2014.78. [DOI] [PubMed] [Google Scholar]
- 39.Ezzone SA. History of Hematopoietic Stem Cell Transplantation. Seminars in oncology nursing. 25(2):95–99. doi: 10.1016/j.soncn.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Kuwatsuka Y, Atsuta Y, Horowitz MM, Inagaki J, Kanda J, Kato K, et al. Graft-versus-host disease and survival after cord blood transplantation for acute leukemia: a comparison of Japanese versus White populations. Biol Blood Marrow Transplant. 2014;20(5):662–667. doi: 10.1016/j.bbmt.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saltz J, Oster S, Hastings S, Langella S, Kurc T, Sanchez W, et al. caGrid: design and implementation of the core architecture of the cancer biomedical informatics grid. Bioinformatics. 2006;22(15):1910–1916. doi: 10.1093/bioinformatics/btl272. [DOI] [PubMed] [Google Scholar]
- 42.Warner JL, Anick P, Drews RE. Physician inter-annotator agreement in the Quality Oncology Practice Initiative manual abstraction task. J Oncol Pract. 2013;9(3):e96–e102. doi: 10.1200/JOP.2013.000931. Epub 2013/08/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abernethy AP, Etheredge LM, Ganz PA, Wallace P, German RR, Neti C, et al. Rapid-learning system for cancer care. J Clin Oncol. 2010;28(27):4268–4274. doi: 10.1200/JCO.2010.28.5478. Epub 2010/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patlak M, Murphy S. A Foundation for Evidence-Driven Practice:: A Rapid Learning System for Cancer Care: Workshop Summary. National Academies Press; 2010. [PubMed] [Google Scholar]
- 45.Friedman CP, Wong AK, Blumenthal D. Achieving a nationwide learning health system. Sci Transl Med. 2010;2(57):57cm29. doi: 10.1126/scitranslmed.3001456. Epub 2010/11/12. [DOI] [PubMed] [Google Scholar]
- 46.Etheredge LM. A rapid-learning health system. Health Aff (Millwood) 2007;26(2):w107–w118. doi: 10.1377/hlthaff.26.2.w107. [DOI] [PubMed] [Google Scholar]
- 47.Institute of Medicine. The Learning Healthcare System: Workshop Summary (IOM Roundtable on Evidence-Based Medicine) Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 48.Middleton B, Bloomrosen M, Dente MA, Hashmat B, Koppel R, Overhage JM, et al. Enhancing patient safety and quality of care by improving the usability of electronic health record systems: recommendations from AMIA. J Am Med Inform Assoc. 2013;20(e1):e2–e8. doi: 10.1136/amiajnl-2012-001458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E. Clinical practice guidelines we can trust. National Academies Press; 2011. [PubMed] [Google Scholar]
- 50.Bright TJ, Wong A, Dhurjati R, Bristow E, Bastian L, Coeytaux RR, et al. Effect of clinical decision-support systems: a systematic review. Annals of internal medicine. 2012;157(1):29–43. doi: 10.7326/0003-4819-157-1-201207030-00450. Epub 2012/07/04. [DOI] [PubMed] [Google Scholar]
- 51.King J, Patel V, Jamoom EW, Furukawa MF. Clinical benefits of electronic health record use: national findings. Health services research. 2014;49(1 Pt 2):392–404. doi: 10.1111/1475-6773.12135. Epub 2013/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grossmann C, Powers B, McGinnis JM, editors. Washington (DC): 2011. Digital Infrastructure for the Learning Health System: The Foundation for Continuous Improvement in Health and Health Care: Workshop Series Summary. [PubMed] [Google Scholar]
- 53.Giuse DA, editor. AMIA annual symposium proceedings. American Medical Informatics Association; 2003. Supporting communication in an integrated patient record system. [PMC free article] [PubMed] [Google Scholar]
- 54.Denny JC, Giuse DA, Jirjis JN. The Vanderbilt Experience with Electronic Health Records. Seminars in Colon and Rectal Surgery. 2005;16(2):59–68. [Google Scholar]
- 55.Giuse NB, Williams AM, Giuse DA. Integrating best evidence into patient care: a process facilitated by a seamless integration with informatics tools. J Med Libr Assoc. 2010;98(3):220–222. doi: 10.3163/1536-5050.98.3.009. Epub 2010/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ragon BK, Clifton C, Chen H, Savani BN, Engelhardt BG, Kassim AA, et al. Geographic distance is not associated with inferior outcome when using long-term transplant clinic strategy. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20(1):53–57. doi: 10.1016/j.bbmt.2013.10.004. Epub 2013/10/15. [DOI] [PubMed] [Google Scholar]
- 57.Duncavage S, Mathe J, Werner J, Malin BA, Ledeczi A, Sztipanovits J, editors. AMIA Annual Symposium Proceedings. American Medical Informatics Association; 2007. A modeling environment for patient portals. [PMC free article] [PubMed] [Google Scholar]
- 58.Collins SA, Vawdrey DK, Kukafka R, Kuperman GJ. Policies for patient access to clinical data via PHRs: current state and recommendations. J Am Med Inform Assoc. 2011;18(Suppl 1):i2–i7. doi: 10.1136/amiajnl-2011-000400. Epub 2011/09/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delbanco T, Walker J, Darer JD, Elmore JG, Feldman HJ, Leveille SG, et al. Open notes: doctors and patients signing on. Ann Intern Med. 2010;153(2):121–125. doi: 10.7326/0003-4819-153-2-201007200-00008. [DOI] [PubMed] [Google Scholar]
- 60.Danciu I, Cowan JD, Basford M, Wang X, Saip A, Osgood S, et al. Secondary use of clinical data: The Vanderbilt approach. J Biomed Inform. 2014;52:28–35. doi: 10.1016/j.jbi.2014.02.003. Epub Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84(3):362–369. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bowton E, Field JR, Wang S, Schildcrout JS, Van Driest SL, Delaney JT, et al. Biobanks and electronic medical records: enabling cost-effective research. Science translational medicine. 2014;6(234):234cm3. doi: 10.1126/scitranslmed.3008604. Epub 2014/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kesselheim AS, Cresswell K, Phansalkar S, Bates DW, Sheikh A. Clinical decision support systems could be modified to reduce 'alert fatigue' while still minimizing the risk of litigation. Health Aff (Millwood) 2011;30(12):2310–2317. doi: 10.1377/hlthaff.2010.1111. Epub 2011/12/08. [DOI] [PubMed] [Google Scholar]
- 64.Lin CP, Payne TH, Nichol WP, Hoey PJ, Anderson CL, Gennari JH. Evaluating clinical decision support systems: monitoring CPOE order check override rates in the Department of Veterans Affairs' Computerized Patient Record System. Journal of the American Medical Informatics Association : JAMIA. 2008;15(5):620–626. doi: 10.1197/jamia.M2453. Epub 2008/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsieh TC, Kuperman GJ, Jaggi T, Hojnowski-Diaz P, Fiskio J, Williams DH, et al. Characteristics and consequences of drug allergy alert overrides in a computerized physician order entry system. Journal of the American Medical Informatics Association : JAMIA. 2004;11(6):482–491. doi: 10.1197/jamia.M1556. Epub 2004/08/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warner JL, Maddux SE, Hughes KS, Krauss JC, Yu PP, Shulman LN, et al. Development, implementation, and initial evaluation of a foundational open interoperability standard for oncology treatment planning and summarization. J Am Med Inform Assoc. 2014 doi: 10.1093/jamia/ocu015. in press. [DOI] [PubMed] [Google Scholar]
- 67.Smith A. Smartphone ownership–2013 update. Washington DC: Pew Research Center; 2013. [Google Scholar]
- 68.Hoverman JR. From the first visit on: information technology and communication. J Oncol Pract. 2013;9(3):152–154. doi: 10.1200/JOP.2013.000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Natl Cancer Inst. 2014;106(9) doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]