Abstract
A number of epidemiological studies have assessed the association of −1304T > G polymorphism in the MKK4 gene and risk of cancer, but the results lack of statistical power due to the limited subjects used in these studies. This study was devised to identify the genetic effects of the −1304T > G polymorphism on cancer risk in a large meta-analysis.
Eligible studies were identified by searching both Chinese and English databases. General as well as subgroup analyses were performed for 8 independent case–control publications with a total of 4623 cases and 5256 cancer-free controls. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to estimate the association.
Overall, this meta-analysis showed that the association between the −1304T > G polymorphism and cancer risk was statistically significant (GG vs TT: OR = 0.63, 95% CI, 0.52–0.75; GG + TG vs TT: OR = 0.85, 95% CI, 0.79–0.91; GG vs TG + TT: OR = 0.67, 95% CI, 0.56–0.80; G vs T: OR = 0.82, 95% CI, 0.77–0.88; TG vs TT: OR = 0.86, 95% CI, 0.79–0.93).
Our meta-analysis reveals that the presence of the −1304T > G polymorphism is likely to decrease risk of cancer. Future larger studies are necessary to validate the current finding.
INTRODUCTION
Environmental carcinogens interacting with inherited factors is the result of cancer.1,2 The interaction is directly or indirectly involved in the activation of the mitogen-activated protein (MAP) kinase pathways that converge on c-Jun N-terminal kinases (JNKs) and p38 MAPKs and function as essential regulators of cellular senescence.3 Mitogen-activated protein kinase 4 (MKK4, also known as MAP2K4, MEK4, JNKK1, and SEK1) is a member of the MAP kinase family, playing a key role in multiple physiologic and pathophysiologic processes such as inflammation and tumor suppression.4MKK4 is highly mutated and has a pro-oncogenic role in cancers of pancreatic, breast, colon, prostate,5 skin,6 and laryngeal squamous cell.7 Meanwhile, MKK4 has also been proved as a suppressor gene in the metastasis of prostate and ovarian cancer cell lines.8,9 Its function in tumorigenesis remains highly controversial.
Mapped to chromosome 17p11.2, the MKK4 gene encoding a 399-amino acid protein in humans spans over 120 kb and consists of 11 exons.4,10 A previous study reporting the association between −1304T > G (rs3826392) polymorphism in the promoter of MKK4 gene and the risk of sporadic colorectal cancer in a southern Chinese population showed a decreased risk correlated with the −1304T > G polymorphism.11 Identical results were also reported in a follow-up investigation focusing on colon caner.12 A number of genetic epidemiological studies looking at other cancers presented substantial evidence that the functional role of the −1304T > G polymorphism in cancer risk differs depending on the type of cancer.13–18 Since the findings are on the ground of relatively small samples restricted to a specific population, thus they may have been underestimated and lack statistical power to elucidate the underlying mechanism of cancer onset associated with this polymorphism.
In an effort to identify the genetic effects of the −1304T > G polymorphism on cancer risk, we performed a meta-analysis composed of the publications evaluating the association between the −1304T > G polymorphism and risk of cancer.
METHODS
Identification and Eligibility of Relevant Studies
We searched both English (PubMed, Embase) and Chinese databases (CNKI) for all publications regarding the association between the −1304T > G polymorphism and cancer risk by using the keywords: MKK4, −1304T > G/rs3826392, polymorphism/polymorphisms/variant/genotype/SNP, and cancer. All references cited in the retrieved articles were also screened to identify the missing data eligible for the meta-analysis. Publications were included in the meta-analysis when satisfying the following criteria: (a) investigating the association between the −1304T > G polymorphism and cancer risk in patients with cancer and control subjects; (b) having genotype data in full detail; (c) published as a full text, rather than a short summary or a comment letter before January 2014. The study was approved by the ethics committee of southwest hospital.
Data Extraction
Based on the inclusion criteria and a consensus reached on all items, 2 investigators independently extracted data including first author, year of publication, country, ethnicity, control source, sex proportion in cases and controls, cancer type, genotyping method, and genotype distributions in cases and controls. In addition, we merged colon cancer into the colorectal cancer when performing meta-analysis.
Statistical Analysis
STATA software, version 12.0 (Stata Corporation, College Station, TX) was used to analyze the data of the −1304T > G polymorphism. The fixed effects model or the random effects model was performed to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for each study. Heterogeneity between studies was measured by Chi-square-based Q test19 and I2 statistic.20 When no obvious inconsistency existed across the studies (P > 0.10 or I2 < 50%), the fixed-effect model (Mantel–Haenszel) was used to pool the ORs; otherwise, the random effects model (DerSimonian and Laird) was selected.21,22 Sensitivity analysis was performed by sequentially excluding each study and rechecking whether the corresponding ORs were altered significantly. Funnel plots and Egger's test23 were used to determine the potential publication bias in the meta-analysis. All tests were 2-sided with P < 0.10 being statistically significant.
RESULTS
Study Characteristics
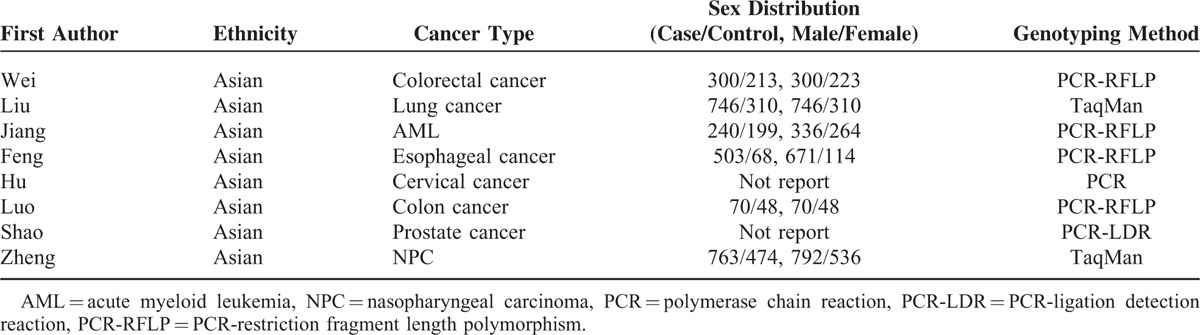
Through the comprehensive search, we yielded a total of 31 papers, whose eligibility was examined by reviewing the key words, titles, abstracts, and the full texts according to the predescribed criteria for inclusion. After excluding the unavailable publications, 8 studies with 4623 cases and 5256 cancer-free controls were included in this meta-analysis11–18 (Figure 1). Of these studies, 2 were published in Chinese12,15 and 6 were in English.11,13,14,16–18 All of the studies were conducted for the Asian populations. Table 1 lists the main characteristics of the eligible studies for this meta-analysis.
FIGURE 1.
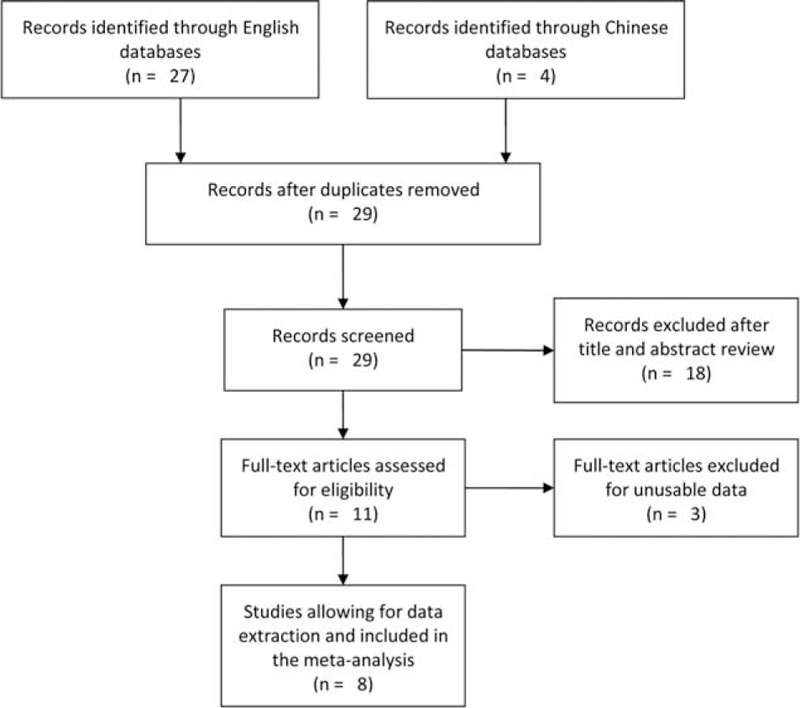
Flow diagram of the study selection process.
TABLE 1.
Distribution of MKK4 −1304T>G Gene in Cancer Cases and Control Subjects
Quantitative Synthesis
As shown in Table 2, pooling all data on the association between the −1304T > G polymorphism and cancer risk into a large dataset revealed a significantly reduced risk of cancer in all genetic models (GG vs TT: OR = 0.63, 95% CI, 0.52–0.75, Figure 2; GG + TG vs TT: OR = 0.85, 95% CI, 0.79–0.91, Figure 3; GG vs TG + TT: OR = 0.67, 95% CI, 0.56–0.80; G vs T: OR = 0.82, 95% CI, 0.77–0.88; TG vs TT: OR = 0.86, 95% CI, 0.79–0.93).
TABLE 2.
Odds Ratio (OR) and Heterogeneity Results for MKK4 −1304T>G Gene in Various Cancers
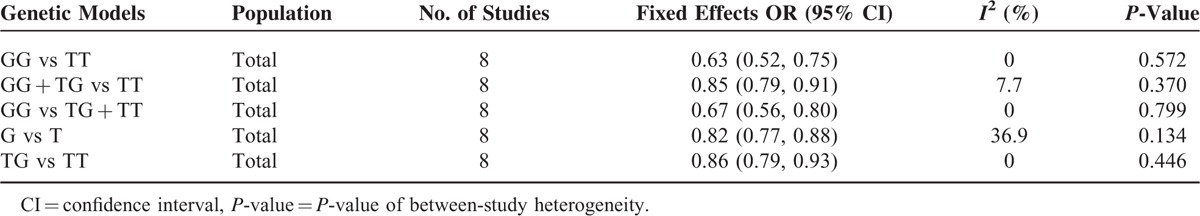
FIGURE 2.
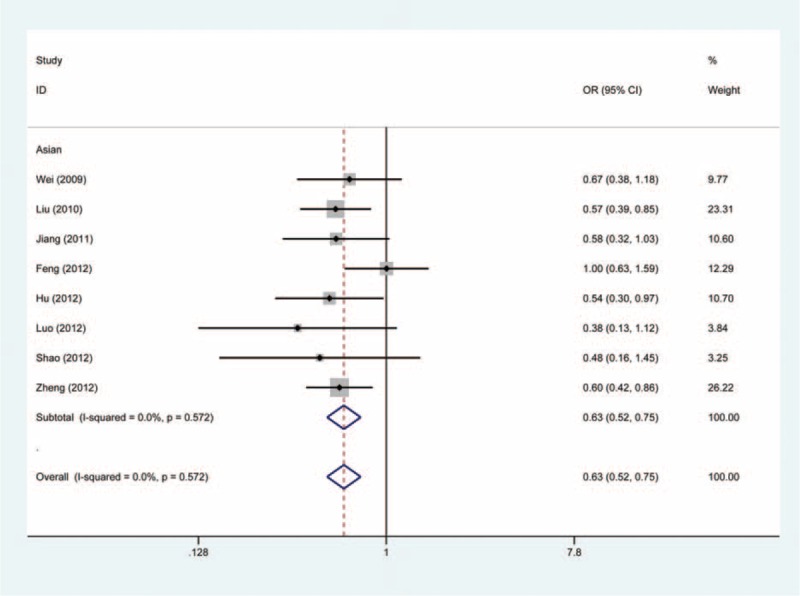
Forest plot of cancer susceptibility associated with MKK4 −1304T>G polymorphism under GG vs TT with fixed-effects model.
FIGURE 3.
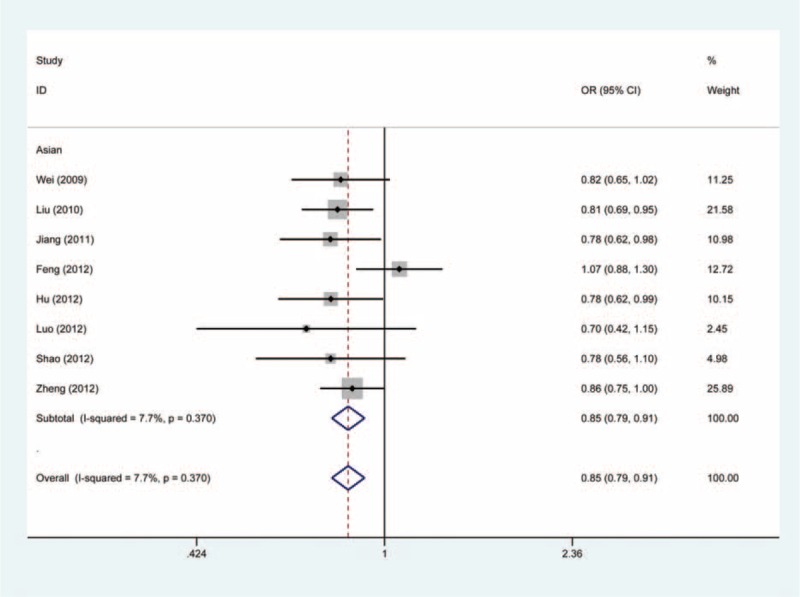
Forest plot of cancer susceptibility associated with MKK4 −1304T>G polymorphism under GG + TG vs TT with fixed-effects model.
No significant between-study heterogeneity was suggested in the meta-analysis (Table 2). Further sensitivity analysis did not reveal any quantitative alternation occurring in the ORs (data not shown). In addition, the funnel plots were symmetrical (P = 0.902) and Egger's test suggested no evidence of obvious publication bias among the studies (GG vs TT: P = 0.894) (Figure 4).
FIGURE 4.
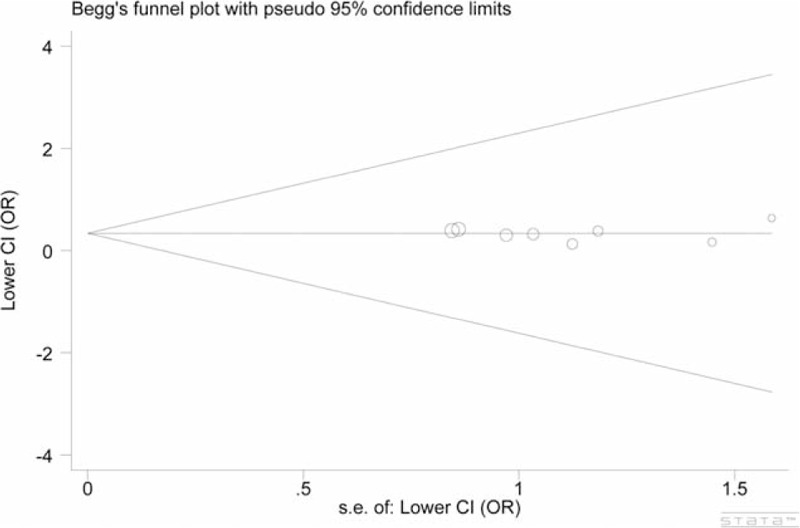
Funnel plots of MKK4 −1304T>G polymorphism cancer risk. Model: GG vs TT, Egger's test: P = 0.894.
DISCUSSION
Based on 8 independent case–control publications with a total of 4623 cases and 5256 cancer-free controls, we performed a meta-analysis to comprehensively assess the relationship between the −1304T > G polymorphism and risk of different types of cancers. From the analysis results, we found statistically significant evidence for a reduction in cancer risk when combining all data together. Since the insufficient data on each cancer did not allow further stratified analysis, such as by type of cancer, thus we failed to estimate the associations of the studied polymorphism and various cancers.
MKK4 belongs to MAPK pathways known to have involvement in the regulation of apoptosis, inflammation, and tumorigenesis.11 An increasing body of evidence has found that the molecular activity of MKK4 is associated with the formation and initiation of cancers.24–26 Frequent mutations of MKK4 have been reported in lung cancer and colorectal cancer.27,28 Also, loss-of-function mutations in the MKK4 gene is shown in a portion of numerous human tissues, accounting for approximately 5%.24,29 Four common polymorphisms in the promotor region of the MKK4 gene have been recorded in Genebank dbSNP database. The genetic variations in the promoter region could affect transcriptional activity and biological function of this gene14 resulting in tumorigeneses.
Since the discovery of a decreased risk associated with the −1304T > G polymorphism in the promotor region of MKK4 was claimed in colorectal cancer,11 a large number of replication studies on various cancers have been successively done in recent years. Most of the studies concluded that the −1304T > G polymorphism have protective effects on the development of cancer.12,13,16 Nevertheless, the susceptibility to esophageal cancer was not found to be associated with this polymorphism.15 Although our meta-analysis revealed an decreased risk in overall cancer, we cannot rule the possibility that the functional −1304T > G polymorphism decreases risk of some cancers by increasing the promoter activity, while it has no biological significance in other cancers, such as esophageal cancer.
Several potential factors must be concerned when interpreting the findings in this meta-analysis. First, the connections of various cancer risks associated with the −1304T > G polymorphism are on the basis of small samples, possibly leading to an underestimate of the true association, which should be further confirmed in a much larger study. Second, we only searched studies conducted among Asian subjects, thus the role of the studied polymorphism in cancer should be widely investigated in more ethnic populations. Third, cancer is known to be a multifactorial disease caused by complex interactions between environmental and genetic factors.30 However, the estimate of such effects was not considered in the present meta-analysis, because the limited data did not allow us to do so.
In summary, this meta-analysis provides evidence that the −1304T > G polymorphism is strongly associated with a decreased risk of cancer. A large well-designed study in diverse ethnic populations is warranted to clarify the true association between the −1304T > G polymorphism and cancer.
Footnotes
Abbreviations: CI = confidence interval, HWE = Hardy–Weinberg equilibrium, JNKs = Jun N-terminal kinases, MAP = mitogen-activated protein, MKK4 = Mitogen-activated protein kinase 4, OR = odds ratio, SNP = single-nucleotide polymorphism.
This work was supported by grant number 81272364 from the National Natural Science Foundation of China (to H.L). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
PG and JO are co-first authors.
The authors have declared that no competing interests exist.
REFERENCES
- 1.Pharoah PD, Dunning AM, Ponder BA, et al. Association studies for finding cancer-susceptibility genetic variants. Nat Rev Cancer 2004; 4:850–860. [DOI] [PubMed] [Google Scholar]
- 2.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature 2001; 411:366–374. [DOI] [PubMed] [Google Scholar]
- 3.Maruyama J, Naguro I, Takeda K, et al. Stress-activated MAP kinase cascades in cellular senescence. Curr Med Chem 2009; 16:1229–1235. [DOI] [PubMed] [Google Scholar]
- 4.Cuenda A. Mitogen-activated protein kinase kinase 4 (MKK4). Int J Biochem Cell Biol 2000; 32:581–587. [DOI] [PubMed] [Google Scholar]
- 5.Teng DH, Perry WL, 3rd, Hogan JK, et al. Human mitogen-activated protein kinase kinase 4 as a candidate tumor suppressor. Cancer Res 1997; 57:4177–4182. [PubMed] [Google Scholar]
- 6.Finegan KG, Tournier C. The mitogen-activated protein kinase kinase 4 has a pro-oncogenic role in skin cancer. Cancer Res 2010; 70:5797–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Huang K, Wang C, et al. Overexpression of mitogen-activated protein kinase kinase 4 and nuclear factor-kappaB in laryngeal squamous cell carcinoma: a potential indicator for poor prognosis. Oncol Rep 2009; 22:89–95. [PubMed] [Google Scholar]
- 8.Robinson VL, Shalhav O, Otto K, et al. Mitogen-activated protein kinase kinase 4/c-Jun NH2-terminal kinase kinase 1 protein expression is subject to translational regulation in prostate cancer cell lines. Mol Cancer Res 2008; 6:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spillman MA, Lacy J, Murphy SK, et al. Regulation of the metastasis suppressor gene MKK4 in ovarian cancer. Gynecol Oncol 2007; 105:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol 1999; 19:2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Y, Wang L, Lan P, et al. The association between −1304T>G polymorphism in the promoter of MKK4 gene and the risk of sporadic colorectal cancer in southern Chinese population. Int J Cancer 2009; 125:1876–1883. [DOI] [PubMed] [Google Scholar]
- 12.Luo HL, Huang J, Lai B. Effects of MKK4 promoter polymorphism on susceptibility of colon cancer. Guangdong Med J 2011; 32:3208–3211. [Google Scholar]
- 13.Liu B, Chen D, Yang L, et al. A functional variant (−1304T>G) in the MKK4 promoter contributes to a decreased risk of lung cancer by increasing the promoter activity. Carcinogenesis 2010; 31:1405–1411. [DOI] [PubMed] [Google Scholar]
- 14.Jiang L, Zhou P, Sun A, et al. Functional variant (−1304T>G) in the MKK4 promoter is associated with decreased risk of acute myeloid leukemia in a southern Chinese population. Cancer Sci 2011; 102:1462–1468. [DOI] [PubMed] [Google Scholar]
- 15.Feng F, Zheng J, Jiang L, et al. No association between the functional polymorphism (−1304T>G) in the MKK4 gene and the risk of esophageal cancer. Chin J Cancer Prev Treat 2012; 19:88–91. [Google Scholar]
- 16.Hu M, Zheng J, Zhang L, et al. The association between −1304T>G polymorphism in the promoter of mitogen-activated protein kinase kinase 4 gene and the risk of cervical cancer in Chinese population. DNA Cell Biol 2012; 31:1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao N, Wang Y, Lu K, et al. Role of the functional MKK4 promoter variant (−1304T>G) in a decreased risk of prostate cancer: case-control study and meta-analysis. J Cancer Res Clin Oncol 2012; 138:1531–1539. [DOI] [PubMed] [Google Scholar]
- 18.Zheng J, Liu B, Zhang L, et al. The protective role of polymorphism MKK4-1304 T>G in nasopharyngeal carcinoma is modulated by Epstein-Barr virus’ infection status. Int J Cancer 2012; 130:1981–1990. [DOI] [PubMed] [Google Scholar]
- 19.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997; 127:820–826. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 21.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22:719–748. [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 23.Egger M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chae KS, Ryu BK, Lee MG, et al. Expression and mutation analyses of MKK4, a candidate tumour suppressor gene encoded by chromosome 17p, in human gastric adenocarcinoma. Eur J Cancer 2002; 38:2048–2057. [DOI] [PubMed] [Google Scholar]
- 25.Ganiatsas S, Kwee L, Fujiwara Y, et al. SEK1 deficiency reveals mitogen-activated protein kinase cascade crossregulation and leads to abnormal hepatogenesis. Proc Natl Acad Sci U S A 1998; 95:6881–6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham SC, et al. MKK4 status predicts survival after resection of gastric adenocarcinoma. Arch Surg 2006; 141:1095–1099.discussion 1100. [DOI] [PubMed] [Google Scholar]
- 27.Davies H, et al. Somatic mutations of the protein kinase gene family in human lung cancer. Cancer Res 2005; 65:7591–7595. [DOI] [PubMed] [Google Scholar]
- 28.Parsons DW, et al. Colorectal cancer: mutations in a signalling pathway. Nature 2005; 436:792. [DOI] [PubMed] [Google Scholar]
- 29.Su GH, Song JJ, Repasky EA, et al. Mutation rate of MAP2K4/MKK4 in breast carcinoma. Hum Mutat 2002; 19:81. [DOI] [PubMed] [Google Scholar]
- 30.Shih CM, Lee YL, Chiou HL, et al. Association of TNF-alpha polymorphism with susceptibility to and severity of non-small cell lung cancer. Lung Cancer 2006; 52:15–20. [DOI] [PubMed] [Google Scholar]


