Abstract
The purpose of this study was to perform a meta-analysis to examine the efficacy and safety of denosumab in postmenopausal women with osteoporosis.
Medline, Cochrane Library, EMBASE, and Google Scholar databases were searched until October 30, 2014 using combinations of the following search terms: osteoporosis, postmenopause, postmenopausal, women, denosumab. The primary outcome was bone mineral density (BMD) change, and secondary outcomes were change in the bone turnover markers β-isomerized carboxy-terminal cross-linking telopeptide of type I collagen (CTX) and serum procollagen type I amino-terminal propeptide (P1NP), and adverse events.
Patients treated with denosumab had significantly increased BMD of the lumbar spine (7.58%), total hip (4.86%), and distal third of the radius (2.92%) than those treated with placebo (all, P < 0.001). Patients treated with denosumab had a significant decrease of CTX (−66.16%) and P1NP (−64.65%) as compared with those treated with placebo (both, P < 0.001). Adverse events were similar between the 2 groups (pooled odds ratio = 1.04, P = 0.625).
Denosumab increases BMD and decreases markers of bone turnover in postmenopausal women with osteoporosis, and is not associated with significant side-effects.
INTRODUCTION
Osteoporosis is common in postmenopausal women, and is defined by a low bone mineral density (BMD).1 It has been estimated that osteoporosis contributes to ≥90% of hip and spine fractures in women 65 to 84 years of age,1 and is thus a major contributor to health care utilization worldwide.2,3 The most commonly used drugs to treat osteoporosis are anti-resorptive medications such as bisphosphonates, and the receptor activator of nuclear factor κB ligand (RANKL) inhibitor denosumab.4 Both bisphosphonates and denosumab inhibit osteoclastic bone resorption. Less commonly used drugs that are typically reserved for patients with more severe osteoporosis are the anabolic parathyroid hormone (PTH) analogs.4
The most commonly prescribed medications used to treat osteoporosis are oral bisphosphonates, for example, alendronate, and they have been shown to reduce the fracture risk in patients with osteoporosis.5 However, study has reported that the majority of postmenopausal women discontinue bisphosphonate therapy within 1 year of initiation, indicating that adherence to long-term bisphosphonate treatment is often inadequate leading to an increased risk of fracture and suboptimal outcomes.5,6
Denosumab is a fully human monoclonal antibody to RANKL that blocks its binding to RANK.5 By blocking RANK binding, denosumab inhibits the development and activity of osteoclasts, decreasing bone resorption and increasing bone density.5 Denosumab is administered by subcutaneous (SC) injection every 6 months, and is thus associated with greater compliance than medications requiring daily administration.5,6 Denosumab has been shown to increase BMD and decrease fracture risk in postmenopausal women with osteopososis.7–13 A recent meta-analysis showed that denosumab was associated with a 42% reduction in the incidence of fractures in postmenopausal women as compared with placebo.14 Bone remodeling, however, is a complex process and RANK is also involved in T-cell function.15
The purpose of this meta-analysis was to examine the effect of denosumab on BMD and bone turnover markers (BTMs) serum β-isomerized carboxy-terminal cross-linking telopeptide of type I collagen (CTX) and serum procollagen type I amino-terminal propeptide (P1NP), and adverse effects, compared with placebo, in postmenopausal women with osteoporosis.
MATERIALS AND METHODS
Literature Search Strategy
This systematic review and meta-analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.16 Medline, Cochrane Library, EMBASE, and Google Scholar databases were searched until October 30, 2014 using combinations of the following search terms: osteoporosis, postmenopause, postmenopausal, women, denosumab. Reference lists of relevant studies were hand-searched.
Study Selection Criteria and Data Extraction
Inclusion criteria were: randomized controlled trials (RCTs); 2-arm prospective studies; participants were postmenopausal women with osteoporosis; the study group was treated with denosumab and the control group with placebo. Subjects were excluded if they had evidence of hyperparathyroidism, vitamin D deficiency, and if they had ever taken parenteral bisphosphonates or teriparatide. Retrospective, cohort study, and crossover study, letters, comments, editorials, case reports, proceedings, and personal communications were excluded. Studies with no quantitative primary outcome were also excluded. Studies were identified by the search strategy by 2 independent reviewers, and a third reviewer was consulted when disagreement arose.
The name of the first author, year of publication, study design, number of participants in each group and age and sex, treatment protocol, BMD and BTM evaluation, length of follow-up, and adverse events were extracted from studies meeting the inclusion criteria. Two independent reviewers performed the data extraction, and a third reviewer was consulted for any uncertainties.
Quality Assessment
The methodological quality of each study was assessed using the risk-of-bias assessment tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0).17
Outcome Measures and Data Analysis
The primary outcome measure was the BMD percent change from baseline between patients who received denosumab and placebo. The secondary outcomes were the percent change in BTMs from baseline, and adverse events. If the median and interquartile range (IQR) was reported in a study, it was assumed that the median of the outcome variable was equal to the mean response and the width of the IQR was approximately 1.35 standard deviations. A χ2-based test of homogeneity was performed using Cochran's Q statistic and I2. I2 reflects the percentage of the total variability in effect estimates among trials that is due to heterogeneity rather than chance. Random-effects models of analysis were used if heterogeneity was detected (I2 > 50%), and fixed-effects models were used if no heterogeneity was noted. Differences in means with 95% confidence intervals (CIs) were calculated for continuous outcomes, whereas odds ratios (ORs) with 95% CIs were calculated for dichotomous outcomes. A 2-sided value of P < 0.05 was considered to indicate statistical significance. Sensitivity analysis was carried out using the leave-one-out approach. If there were ≤5 studies, publication bias was not assessed because >5 studies are required to detect funnel plot asymmetry.18 Comprehensive meta-analysis statistical software, version 2.0 (Biostat, Englewood, NJ) was used to perform all analyses.
RESULTS
A diagram of study selection is presented in Supplemental Figure 1, http://links.lww.com/MD/A466. A total of 89 studies were identified in the literature review after duplicates were removed. After nonrelevant studies were excluded, 34 full-text articles were examined. After further excluding studies that did not meet the inclusion criteria, 4 RCTs were included in the meta-analysis.8,10,19,20 The 4 RCTs included a total of 5806 patients; 4251 received denosumab and 4255 placebo (Table 1). The mean age of patients treated with denosumab ranged from 59.4 to 72.3 years, and for the patients treated with placebo ranged from 58.9 years to 72.3 years.
TABLE 1.
Basic Characteristics of Studies Included in Meta-Analysis
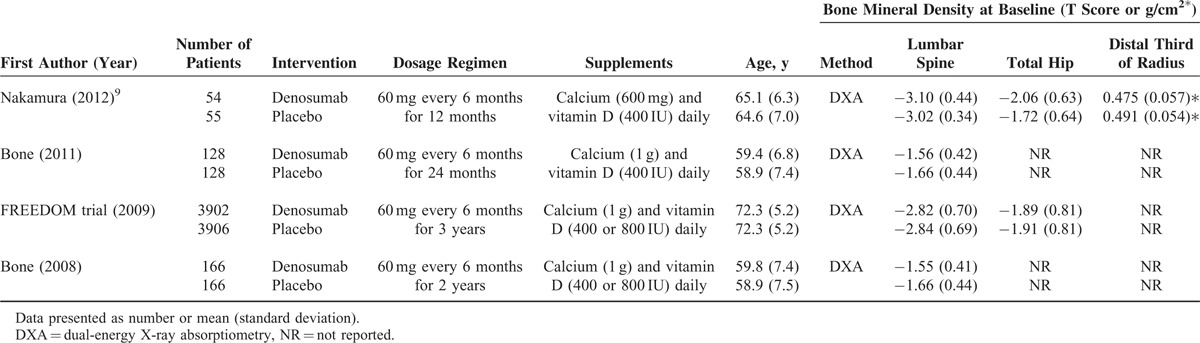
BMD Change
All 4 studies8,10,19,20 provided numerical data regarding the percent change in BMD of the lumbar spine from baseline between patients who received denosumab and placebo, and were included in the meta-analysis. There was evidence of heterogeneity among the 4 studies (Q statistic = 43.47, I2 = 93.10%, P < 0.001); therefore, a random-effects model of analysis was used. The pooled difference in means (7.58%, 95% CI: 6.08%–9.08%, P < 0.001) indicated that patients who received denosumab had significantly increased BMD of the lumbar spine compared with patients who received placebo (Figure 1 A).
FIGURE 1.
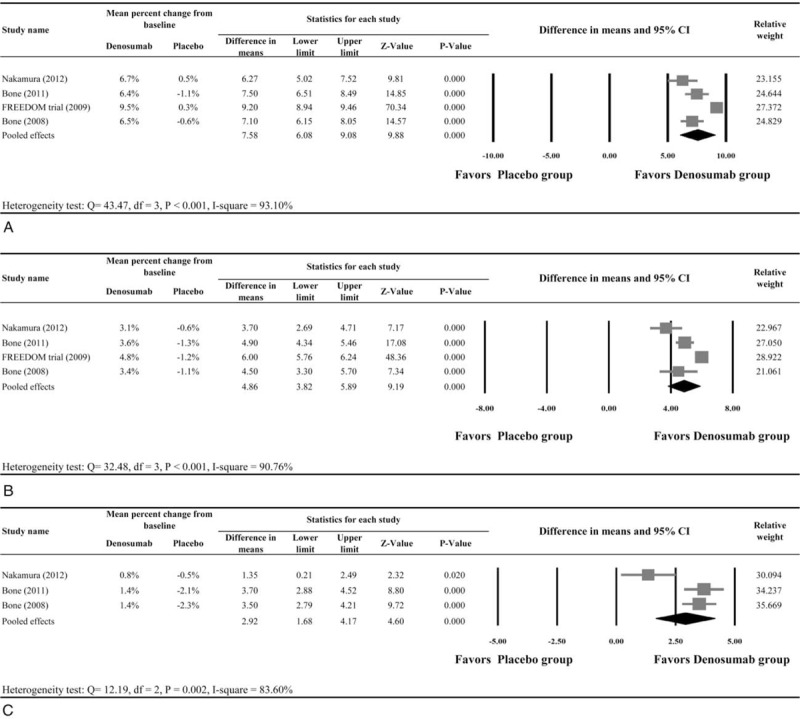
Meta-analysis of the percent change in bone mineral density (BMD) values of the (A) lumbar spine; (B) total hip; and (C) distal third radius.
The 4 studies also provided total hip BMD data, and were included in the analysis. There was evidence of heterogeneity among the 4 studies (Q statistic = 32.48, I2 = 90.76%, P < 0.001); therefore, a random-effects model of analysis was used. The pooled difference in means (4.86%, 95% CI: 3.82%–5.89%, P < 0.001) indicated that patients who received denosumab had significantly increased BMD of the total hip compared with patients who received placebo (Figure 1B).
Three studies9,10,20 provided numerical data for the percent change in BMD of the distal third of the radius from baseline between patients who received denosumab and placebo. A random-effects model of analysis was used because of heterogeneity among the 3 studies (Q statistic = 12.19, I2 = 83.6%, P = 0.002). The pooled difference in means (2.92%, 95% CI: 1.68%–4.17%, P < 0.001) indicated that patients who received denosumab had a significantly increased BMD value of the distal third of the radius compared with those that received placebo (Figure 1C).
Change of BTMs
All 4 studies8,10,19,20 provided numerical data for the percent change in CTX, and were included in the meta-analysis. There was evidence of heterogeneity among the 4 studies (Q statistic = 26.27, I2 = 88.58%, P < 0.001); therefore, a random-effects model of analysis was used. The pooled difference in means of ore, a random-effects model of analysis was used. The po66.16% (95% CI:−77.12% to −55.19%, P < 0.001) indicated that patients who received denosumab had a significant decrease of CTX as compared with those who received placebo (Figure 2A).
FIGURE 2.
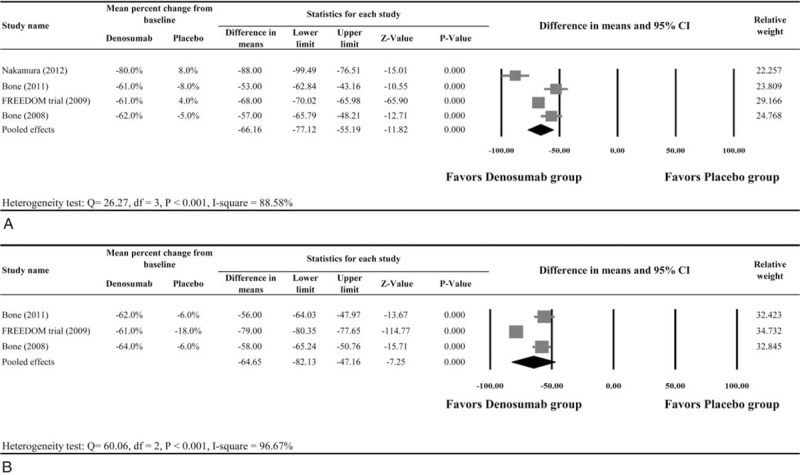
Meta-analysis of bone turnover markers (A) CTX and (B) P1NP.
Three studies10,19,20 provided numerical data for the percent change in P1NP. A random-effects model of analysis was used because of heterogeneity among the 3 studies (Q statistic = 60.06, I2 = 96.67%, P < 0.001). The pooled difference in means of −64.65% (95% CI: −82.13% to −7.16%, P < 0.001) indicated that patients who received denosumab had a significant decrease in P1NP as compared with those who received placebo (Figure 2B).
Adverse Events
All 4 studies were included in the meta-analysis of adverse event rates. A fixed-effects model of analysis was used as there was homogeneity among the 4 studies (Q statistic = 3.885, I2 = 22.78%, P = 0.274). The result indicated that the adverse event rates during the period of treatment were similar between the groups (pooled OR = 1.04, 95% CI: 0.89–1.22, P = 0.625, Figure 3).
FIGURE 3.
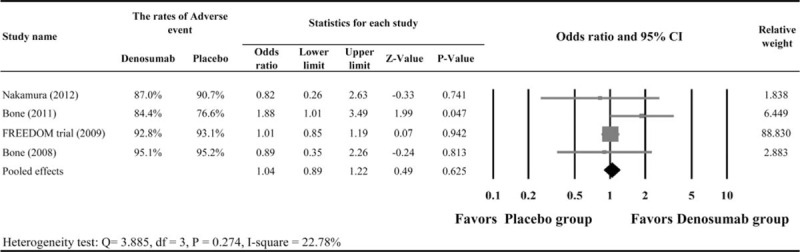
Meta-analysis of adverse events.
Sensitivity Analysis
Sensitivity analysis was performed using the leave-one-out approach (Table 2). The direction and magnitude of combined estimates did not vary markedly with the removal of the studies, indicating that the meta-analysis had good reliability and the data were not overly influenced by any individual study.
TABLE 2.
Sensitivity Analysis
Quality Assessment
Results of the quality assessment are shown in Figure 4. All 4 studies were double-blind, randomized, and placebo-controlled trials. However, the allocation concealment was not clear. All of the studies had incomplete outcome data and selective reporting. Only 1 study19 included an intention-to-treat analysis, and others were unclear. Overall, the results indicated that the studies had a low risk of bias and were of good quality.
FIGURE 4.
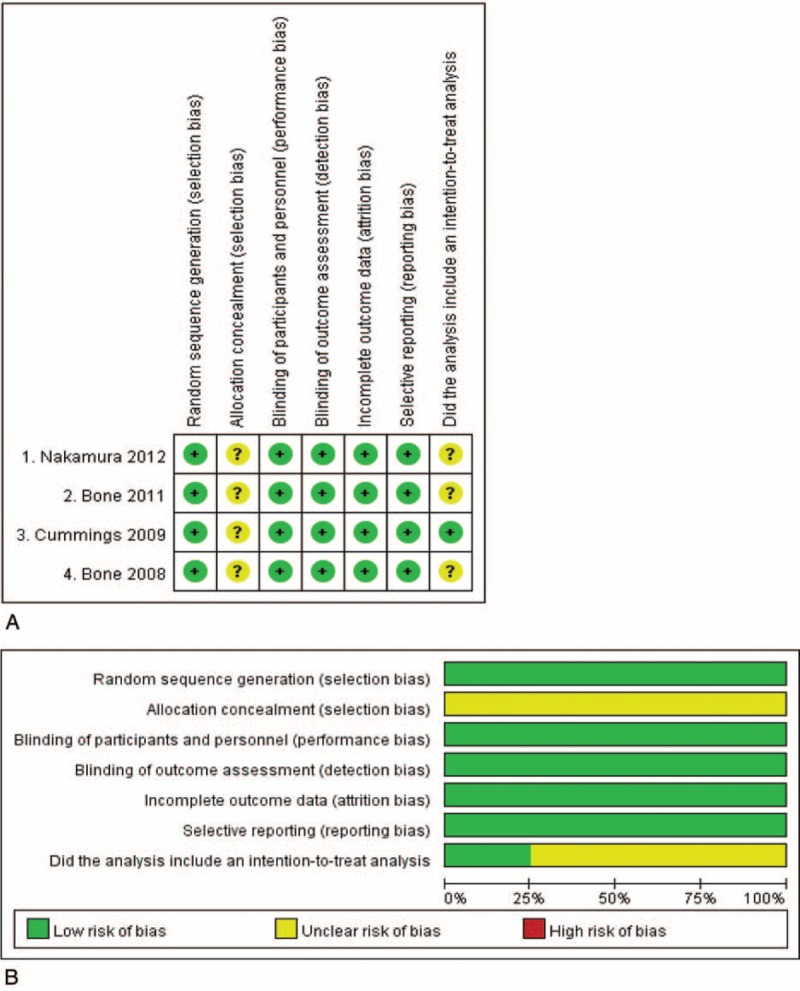
Quality analysis.
DISCUSSION
The results of this meta-analysis including 4 RCTs indicate that treatment of postmenopausal women with denosumab is associated with increased BMD of the distal third of the radius, lumbar spine, and total hip and a significant decrease of the BTMs CTX and P1NP as compared with those that received placebo. Adverse event rates during treatment period were similar between groups. These results indicate that denosumab can effectively prevent the resorption of bone and increase BMD when used in conjunction with calcium and vitamin D.
Osteoporosis has been referred to as the silent disease because bone loss typically occurs without symptoms, and the first indication that the condition is present is the occurrence of a fracture.1 Osteoporotic fractures are associated with chronic pain, disability, and an increased risk of death, thus placing a significant burden on the health care systems of countries worldwide.1 Denosumab was approved by the US Food and Drug administration in 2010 for the treatment of women with osteoporosis at high risk of fracture.15 The drug is administered every 6 months by SC injection, thus improving compliance as compared with daily medications. Its mechanism of action is inhibition of RANKL, and it has been shown to decrease bone resorption, increase BMD, and reduce the risk of fracture in postmenopausal women with osteoporosis.7–13
The largest study to date examining denosumab was the FREEDOM trial.19 The study included 7868 women between 60 and 90 years of age with BMD T scores of <−2.5 but not <−4.0. Women were randomized to receive denosumab 60 mg every 6 months or placebo for 36 months. Denosumab reduced the cumulative incidence of new radiographic vertebral fractures from 7.2% in the placebo group to 2.3% in the denosumab group, a relative decrease of 68%. Denosumab also reduced the risk of hip fractures (hazard ratio [HR] = 0.60; 95% CI: 0.37–0.97; P = 0.04) and nonvertebral fractures (HR = 0.80; 95% CI: 0.67–0.95; P = 0.01). After 36 months, denosumab was associated with a relative increase in BMD of 9.2% (95% CI: 8.2–10.1) of the lumbar spine and 6.0% (95% CI: 5.2–6.7) of the total hip, and decreased CTX and P1NP. The results also showed no increased risk of cancer, infection, cardiovascular disease, delayed fracture healing, or hypocalcemia, and there were no cases of osteonecrosis of the jaw with denosumab. Study of the FREEDOM data also showed that the reduction of new vertebral fracture risk was similar in all subgroups examined (eg, age, body mass index, femoral neck T-score).7 An extension of the FREEDOM trial with denosumab to 6 years indicated gains in BMD, decreased fracture rates, and reduced bone turnover were maintained.21
A 3-dimensional (3D) bone mapping study by Poole et al22 showed that treatment with denosumab increased femoral cortical mass surface density by 5.4% over 3 years, as compared with placebo, with one-third of the increase due to increased cortical density and two thirds from increased cortical thickness. Furthermore, cortical mass surface density and thickness increased by up to 12% at key locations such as the lateral femoral trochanter. Interestingly, Lin et al23 found that at 1 year of treatment, denosumab was more effective at increasing bone mass than alendronate, but the fracture risk reduction was the same with both medications. In a mixed treatment comparison meta-analysis, Migliore et al24 reported that as compared with placebo, zolendronate had the highest probability (52%) of being the most effective treatment to prevent vertebral fractures, followed by denosumab (46% probability), and the ibandronate, alendronate, and risedronate.
Although available evidence indicates that denosumab is safe and effective at increasing BMD and decreasing the risk of fractures in postmenopausal women with osteoporosis, some questions remain unanswered. Further study is needed to examine whether denosumab can decrease the risk of fractures from different mechanisms, for example, compression versus traumatic.7,9,19 Calcium supplementation is generally given with denosumab, and given that a large percentage of patients experience constipation and gastrointestinal upset with the recommended dose of calcium, it remains to be determined whether a lower dose of calcium would still achieve the same result with respect to increase in BMD and decreased risk of fracture. It also remains to be studied whether denosumab is associated with an improved quality of life.
There are a number of limitations of this analysis that should be considered. Although all of the studies included were RCTs of high quality, there were only 4 studies meeting our criteria suggesting that further studies examining the long-term effects of denosumab are necessary. Fracture rates of patients treated with denosumab as compared with control patients were not examined. There were not enough data to examine the effect of denosumab on other important BTMs such as tartrate-resistant acid phosphatase 5b (TRAP-5b), bone alkaline phosphatase (BALP), and osteocalcin. Denosumab and vitamin D levels were not examined.
In summary, denosumab increases BMD and decreases serum markers of bone turnover in postmenopausal women with osteoporosis, and is not associated with significant side effects. Although not examined in the current study, other study has indicated that it decreases the risk of fractures in this population.
Footnotes
Abbreviations: BMD = bone mineral density, BTM = bone turnover markers, CTX = cross-linking telopeptide of type I collagen, P1NP = serum procollagen type I amino terminal propeptide, PTH = parathyroid hormone, RANKL = Receptor activator of nuclear factor kB ligand.
Supplemental Digital Content is available for this article.
This work was financially supported by the construction project of national clinical geriatrics key specialty and Zhejiang Medicine and Health Science and Technology Program (2015119130).
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.NIH. Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. JAMA 2001; 285:785–795. [DOI] [PubMed] [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 2007; 22:465–475. [DOI] [PubMed] [Google Scholar]
- 3.Prevention and management of osteoporosis: WHO technical report series 921. 2003; Geneva: World Health Organization, 1–164. [PubMed] [Google Scholar]
- 4.Bhutani G, Gupta MC. Emerging therapies for the treatment of osteoporosis. J Midlife Health 2013; 4:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fazil M, Baboota S, Sahni JK, et al. Bisphosphonates: therapeutics potential and recent advances in drug delivery. Drug Deliv 2015; 22:1–9. [DOI] [PubMed] [Google Scholar]
- 6.Freemantle N, Satram-Hoang S, Tang ET, et al. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 2012; 23:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClung MR, Boonen S, Törring O, et al. Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Miner Res 2012; 27:211–218. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Matsumoto T, Sugimoto T, et al. Clinical Trials Express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT). J Clin Endocrinol Metab 2014; 99:2599–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura T, Matsumoto T, Sugimoto T, et al. Dose-response study of denosumab on bone mineral density and bone turnover markers in Japanese postmenopausal women with osteoporosis. Osteoporos Int 2012; 23:1131–1140. [DOI] [PubMed] [Google Scholar]
- 10.Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 2011; 96:972–980. [DOI] [PubMed] [Google Scholar]
- 11.Lewiecki EM, Miller PD, McClung MR, et al. Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res 2007; 22:1832–1841. [DOI] [PubMed] [Google Scholar]
- 12.Pitale S, Thomas M, Rathi G, et al. A randomized placebo-controlled trial of the efficacy of denosumab in Indian postmenopausal women with osteoporosis. Indian J Endocrinol Metab 2015; 19:148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 2004; 19:1059–1066. [DOI] [PubMed] [Google Scholar]
- 14.von Keyserlingk C, Hopkins R, Anastasilakis A, et al. Clinical efficacy and safety of denosumab in postmenopausal women with low bone mineral density and osteoporosis: a meta-analysis. Semin Arthritis Rheum 2011; 41:178–186. [DOI] [PubMed] [Google Scholar]
- 15.Chitre M, Shechter D, Grauer A. Denosumab for treatment of postmenopausal osteoporosis. Am J Health Syst Pharm 2011; 68:1409–1418. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. http://www.cochrane-handbook.org. Updated March 2011. [Google Scholar]
- 18.Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000; 320:1574–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361:756–765. [DOI] [PubMed] [Google Scholar]
- 20.Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 2008; 93:2149–2157. [DOI] [PubMed] [Google Scholar]
- 21.Bone HG, Chapurlat R, Brandi ML, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab 2013; 98:4483–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole KE, Treece GM, Gee AH, et al. Denosumab rapidly increases cortical bone in key locations of the femur: a 3D bone mapping study in women with osteoporosis. J Bone Miner Res 2015; 30:46–54. [DOI] [PubMed] [Google Scholar]
- 23.Lin T, Wang C, Cai XZ, et al. Comparison of clinical efficacy and safety between denosumab and alendronate in postmenopausal women with osteoporosis: a meta-analysis. Int J Clin Pract 2012; 66:399–408. [DOI] [PubMed] [Google Scholar]
- 24.Migliore A, Broccoli S, Massafra U, et al. Ranking antireabsorptive agents to prevent vertebral fractures in postmenopausal osteoporosis by mixed treatment comparison meta-analysis. Eur Rev Med Pharmacol Sci 2013; 17:658–667. [PubMed] [Google Scholar]


