Abstract
Previous studies have suggested that procalcitonin is a reliable marker for predicting bacteremia. However, these studies have had relatively small sample sizes or focused on a single clinical entity. The primary endpoint of this study was to investigate the diagnostic accuracy of procalcitonin for predicting or excluding clinically relevant pathogen categories in patients with suspected bloodstream infections. The secondary endpoint was to look for organisms significantly associated with internationally validated procalcitonin intervals. We performed a cross-sectional study that included 35,343 consecutive patients who underwent concomitant procalcitonin assays and blood cultures for suspected bloodstream infections. Biochemical and microbiological data were systematically collected in an electronic database and extracted for purposes of this study. Depending on blood culture results, patients were classified into 1 of the 5 following groups: negative blood culture, Gram-positive bacteremia, Gram-negative bacteremia, fungi, and potential contaminants found in blood cultures (PCBCs). The highest procalcitonin concentration was observed in patients with blood cultures growing Gram-negative bacteria (median 2.2 ng/mL [IQR 0.6–12.2]), and the lowest procalcitonin concentration was observed in patients with negative blood cultures (median 0.3 ng/mL [IQR 0.1–1.1]). With optimal thresholds ranging from ≤0.4 to ≤0.75 ng/mL, procalcitonin had a high diagnostic accuracy for excluding all pathogen categories with the following negative predictive values: Gram-negative bacteria (98.9%) (including enterobacteria [99.2%], nonfermenting Gram-negative bacilli [99.7%], and anaerobic bacteria [99.9%]), Gram-positive bacteria (98.4%), and fungi (99.6%). A procalcitonin concentration ≥10 ng/mL was associated with a high risk of Gram-negative (odds ratio 5.98; 95% CI, 5.20–6.88) or Gram-positive (odds ratio 3.64; 95% CI, 3.11–4.26) bacteremia but dramatically reduced the risk of PCBCs or fungemia. In this large real-life setting experience with more than 35,000 patients, procalcitonin was highly effective at excluding bloodstream infections regardless of pathogen categories. The results from our study are limited by its cross-sectional design and deserve to be validated in prospective longitudinal studies.
INTRODUCTION
Procalcitonin, a prohormone of 116 amino acids, is the precursor for the calcium homeostasis hormone, calcitonin. Procalcitonin circulates at very low concentrations in normal serum and is thought to be produced in physiological conditions by the neuroendocrine cells in the thyroid gland and lungs.1 Procalcitonin is massively synthetized by various types of cells during sepsis, which is defined as a systemic inflammatory response to infection.2,3 In a landmark study, procalcitonin was shown to accurately differentiate between systemic bacterial infections and noninfectious inflammatory states in intensive care unit patients.4 Since then, several studies have evaluated its diagnostic accuracy during sepsis, and 3 meta-analyses demonstrated that procalcitonin was a reliable marker for sepsis in both adult and pediatric populations.5–7 In addition, it has been demonstrated that the use of procalcitonin to guide the initiation and duration of antibiotic therapy in patients with acute respiratory infections was effective in reducing antibiotic exposure without increasing the risks of mortality or treatment failure.8–11
It is now accepted that sepsis and its sequelae are still common causes of acute illness and death in patients with community-acquired or nosocomial infections.6 Although there is no gold-standard test to confirm infection, bacteremia is present in approximately 30% of patients with sepsis.12,13 The average delay in obtaining the result of a blood culture is between 24 and 48 hours. Hence, the availability of a rapid biochemical test to predict the probability of a negative blood culture will target low-risk patients who may not benefit from empirical antibiotic therapy pending blood culture results. Several studies have evaluated the benefit of using procalcitonin to predict blood culture results.14–29 However, these studies have had relatively small sample sizes and short study periods. Furthermore, some of these studies focused only on a single clinical entity such as community-acquired pneumonia29–31 or urosepsis.15,16,18,24
Studies with small sample sizes have evaluated the magnitude of procalcitonin elevations in patients with bacteremia according to Gram stain results and suggested that Gram-negative bacteremia could be associated with higher procalcitonin values compared with Gram-positive bacteremia, regardless of the severity of disease.23,32 However, these results have not been confirmed in large cohorts or through “big data” approaches.
Using a big data approach on standardized data from an electronic database with more than 35,000 consecutive patients over a 7-year study period, we aimed to investigate the diagnostic accuracy of procalcitonin in predicting or excluding pathogen categories in patients with suspected bloodstream infections.
MATERIALS AND METHODS
Study Population
The “Nancy Biochemical Database” is an electronic database that included consecutive patients hospitalized in 67 healthcare departments at the University Hospital of Nancy and underwent concomitant blood procalcitonin assays and blood cultures for suspected bloodstream infections between January 1, 2006 and December 31, 2012 (Supplemental Digital Content: Figure 1, http://links.lww.com/MD/A504). Blood cultures and procalcitonin assays were considered concomitant if the time between the 2 tests was less than 12 hours. All investigations were conducted at the discretion of the physicians of each health care department as part of a standard assessment for bloodstream infection suspicion.
For each patient, biochemical and microbiological data were systematically collected in the electronic database and were retrospectively extracted for the purposes of the study using the GLIMS general laboratory information management system, version 8.11.6 (MIPS France S.a.r.l., Paris, France). The following data were available in the electronic database: patient identification number, patient age at the time of blood collection, date and time of blood sampling for blood culture and procalcitonin assay, patient health care department, blood culture result (positive or negative), microorganism genus and species in case of positive blood culture, blood procalcitonin concentration (ng/mL), and blood C-reactive protein concentration (mg/L). The following data were not available in the electronic database: clinical diagnosis of SIRS, sepsis, severe sepsis, or septic shock; the presence or absence of a central venous catheter, final clinical diagnosis, and infection source in patients with positive blood culture; and antibiotic use. The “Nancy Biochemical Database” was reported to the French National Commission for Data Protection and Liberties (CNIL No 1763197v0), which supervises the protection of individuals with regard to the processing of personal data. The University Hospital of Nancy ethics committee approved the study.
Study Design
We performed a cross-sectional study that included consecutive patients who had concomitant procalcitonin assay and blood culture for suspected bloodstream infection.
Primary and Secondary Endpoints
The primary endpoint of the study was to investigate the diagnostic accuracy of procalcitonin for predicting or excluding pathogen categories in patients with suspected bloodstream infection. The secondary endpoint was to look for organisms significantly associated with internationally validated procalcitonin intervals (0.05–0.1 ng/mL, 0.1–0.25 ng/mL, 0.25–0.5 ng/mL, 0.5–1 ng/mL, 1–2 ng/mL, 2–10 ng/mL, and procalcitonin ≥10 ng/mL).11
Procalcitonin Assay
Plasma procalcitonin concentration was measured using automated immunofluorescent assays of procalcitonin in human plasma (EDTA, heparin) samples (Brahms PCT sensitive KRYPTOR kit for Brahms KRYPTOR, Hennigsdorf, Germany) according to the supplier's protocol. The normal procalcitonin concentration was defined as <0.05 ng/mL according to supplier reference values. The intraassay coefficients of variation for procalcitonin were 6.86% and 5.73% for level 1 and level 2, respectively.
Blood Culture
Routinely, 1 to 3 pairs of blood culture bottles (Bactec Plus Aerobic and Bactec F Lytic Anaerobic, Becton Dickinson, Le Pont de Claix, France) were inoculated with each patient's blood and incubated in the BD Bactec 9240 blood culture system for at least 5 days. If no bacterial growth was detected within the incubation period, the blood culture was considered negative. All bottles flagged positive were removed from the instrument, and aliquots were taken for direct Gram staining and subculture on standard solid media for subsequent analysis. Identification of microorganisms was performed by conventional methods, including biochemical identification (Vitek 2, bioMérieux, Marcy L’Etoile, France), 16S rRNA gene sequencing, and, as of July 2012, mass spectrometry with a Vitek MS (bioMérieux) MALDI-TOF mass spectrometry system. Depending on blood culture results, patients were classified into 1 of the 5 following groups: negative blood culture, Gram-positive bacteremia, Gram-negative bacteremia, fungemia, and potential contaminants found in blood cultures (PCBCs) as defined by Lee et al.33 In the group of patients with potential contaminants (mainly coagulase-negative staphylococci species), the clinical criteria for distinguishing contaminants from true bacteremia as suggested by Lee et al33 were not available due to the design of the study. Patients in the “Gram-negative bacteremia” group were divided into 3 subgroups as follows: enterobacteria (Escherichia, Enterobacter, Klebsiella, and Citrobacter), nonfermenting Gram-negative bacilli (NFGNB) (Pseudomonas and Acinetobacter), and anaerobic bacteria (Bacteroides). The “Gram-positive bacteremia” group included the following organisms: Staphylococcus aureus, Streptococcus (other than viridans-group streptococci), and Enterococcus.
Statistical Analysis
All quantitative variables are described as medians and percentiles (interquartile range [IQR], 25 to 75th percentile). All proportions are expressed as percentages with 95% confidence intervals (95% CIs). The comparison of serum procalcitonin values across groups was performed using the Kruskal-Wallis test. A post-hoc analysis for pairwise group comparisons was performed according to Conover in order to avoid multiple testing issues.
Primary Endpoint
The diagnostic accuracy of procalcitonin was assessed by a receiver operating characteristic (ROC) analysis according to DeLong et al34 using one of the following classification criteria: pathogen group according to blood culture results classification or the specific organism identified in blood cultures. In each ROC analysis, patients who met one of the classification criteria were compared with patients with negative blood cultures and patients with positive blood cultures who did not meet the classification criteria. For each ROC analysis, the diagnostic accuracy output results were as follows: sensitivity, specificity, positive and negative likelihood ratios, positive and negative predictive values (NPVs), and area under the receiver operating characteristic curve with associated P value. For each ROC analysis, the optimal procalcitonin threshold was defined using the Youden index J. Bias-corrected and accelerated (BCa) bootstrap interval after 10,000 iterations for the Youden index and its associated values was performed.35
Secondary Endpoints
To look for organisms significantly associated with internationally validated procalcitonin intervals11 (0.05–0.1 ng/mL, 0.1–0.25 ng/mL, 0.25–0.5 ng/mL, 0.5–1 ng/mL, 1–2 ng/mL, 2–10 ng/mL, and procalcitonin concentrations ≥10 ng/mL), we performed a stepwise multivariate logistic regression analysis. A logistic regression model was constructed for each predefined procalcitonin stratum. In each logistic regression model, the dependent variable was the predefined procalcitonin stratum, and the explanatory variables were either pathogen groups (verbatim) according to the blood culture results classification or pathogen genus identified in blood cultures (verbatim). Results were shown as odds ratios and 95% CIs and the percentage of cases correctly classified. For each logistic regression model, we assessed model discrimination using ROC analysis and model calibration using the Hosmer and Lemeshow goodness-of-fit test. All statistical analyses were conducted with MedCalc for Windows, version 13.3 (MedCalc Software, Ostend, Belgium) on the basis of a 2-sided type I error with an alpha level of 0.05.
RESULTS
Characteristics of the Patients Included in the Study
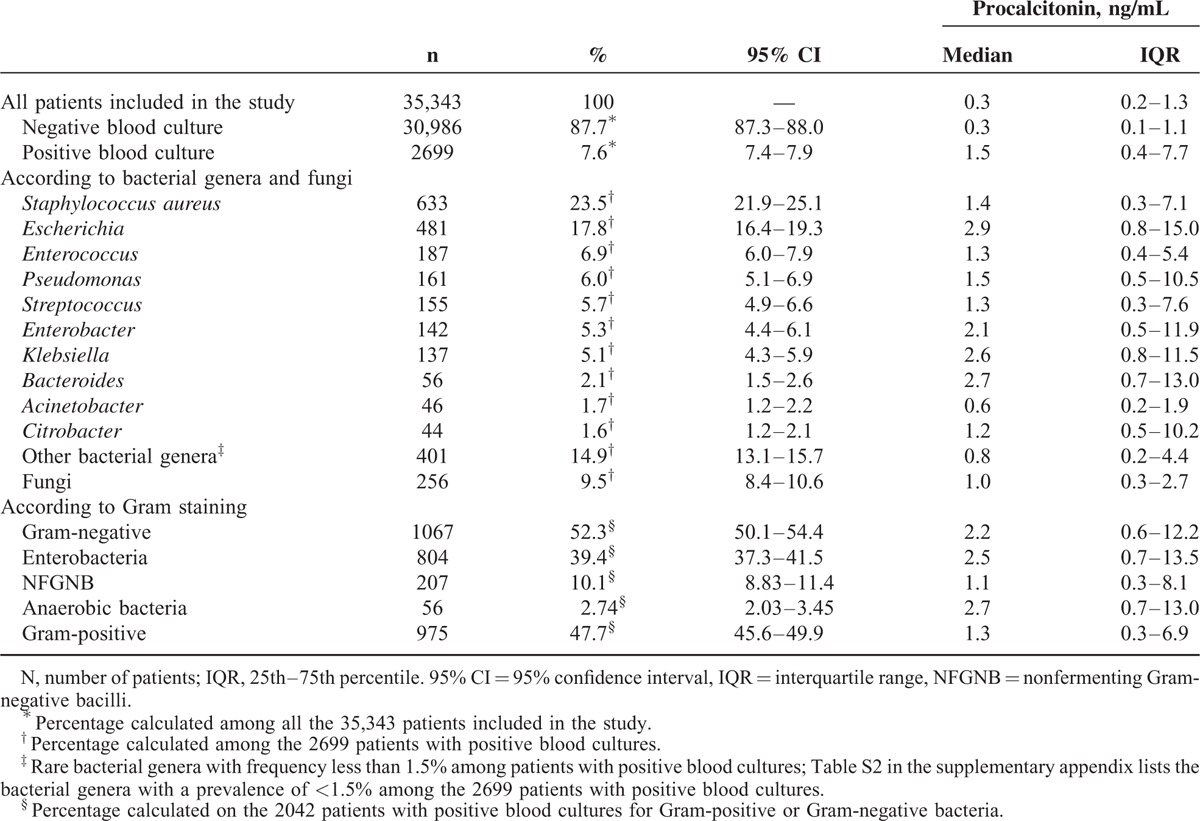
The study included 35,343 consecutive patients. The median age was 49 years (range 0–102; IQR 13–66) and the median procalcitonin value of the entire population was 0.34 ng/mL (IQR 0.15–1.31) (see Supplemental Digital Content: Table 1, http://links.lww.com/MD/A504 for the exhaustive list of departments and corresponding median procalcitonin values according to the health care department). C-reactive protein was measured concomitantly in 6434 of the 35,343 patients included in the study with a median value of 67.1 mg/L (IQR 15.2–150.4). Blood cultures were negative in 87.7% of the 35,343 patients. Among the 2699 (7.6%) patients with positive blood cultures 23.5%, 17.8%, 6.9%, and 6.0% had positive blood cultures for Staphylococcus aureus, Escherichia coli, Enterococcus, and Pseudomonas, respectively. Together, these results represented more than 50% of the positive blood cultures in this study (Table 1 and Supplemental Digital Content: Table 2, http://links.lww.com/MD/A504).
TABLE 1.
Median Procalcitonin Value (ng/mL) in the 35,343 Patients Included in the Study
Influence of the “Blood Culture Results” Group on Procalcitonin Concentration
The median procalcitonin concentration differed significantly across patient groups according to blood culture results (negative blood culture, Gram-positive bacteria, Gram-negative bacteria, and fungi) (P < 0.0001; Kruskal-Wallis test). The highest procalcitonin concentration was observed in patients with blood cultures growing Gram-negative bacteria (median 2.2 ng/mL [IQR 0.6–12.2]), and the lowest procalcitonin concentration was observed in patients with negative blood cultures (median 0.3 ng/mL [IQR 0.1–1.1]) (Table 1 and Supplemental Digital Content: Figure 2, http://links.lww.com/MD/A504). Among the patients with Gram-negative bacteremia, the highest procalcitonin concentrations were noted for anaerobic bacteria (Bacteroides) (median 2.7 ng/mL [IQR 0.7–13.0]) and enterobacteria (Escherichia, Enterobacter, Klebsiella, and Citrobacter) (median 2.5 ng/mL [IQR 0.7–13.5]) in comparison with NFGNB (Pseudomonas, Acinetobacter) (median 1.1 ng/mL [IQR 0.3–8.1]) (P < 0.0001 across subgroups; P < 0.05 for pairwise comparisons: anaerobic bacteria vs NFGNB and enterobacteria vs NFGNB) (Table 1 and Supplemental Digital Content: Figure 2, http://links.lww.com/MD/A504). The patients from the PCBCs group (n = 1658, 4.7%) had blood cultures that were positive for viridans streptococci (n = 86) and coagulase-negative staphylococci (n = 1572) consisting mainly of the 3 following species: epidermidis, hominis, and capitis (Supplemental Digital Content: Table 3, http://links.lww.com/MD/A504).
Primary Endpoint: Diagnostic Accuracy of Procalcitonin for Predicting or Excluding Pathogen Categories in Patients With Suspected Bloodstream Infection
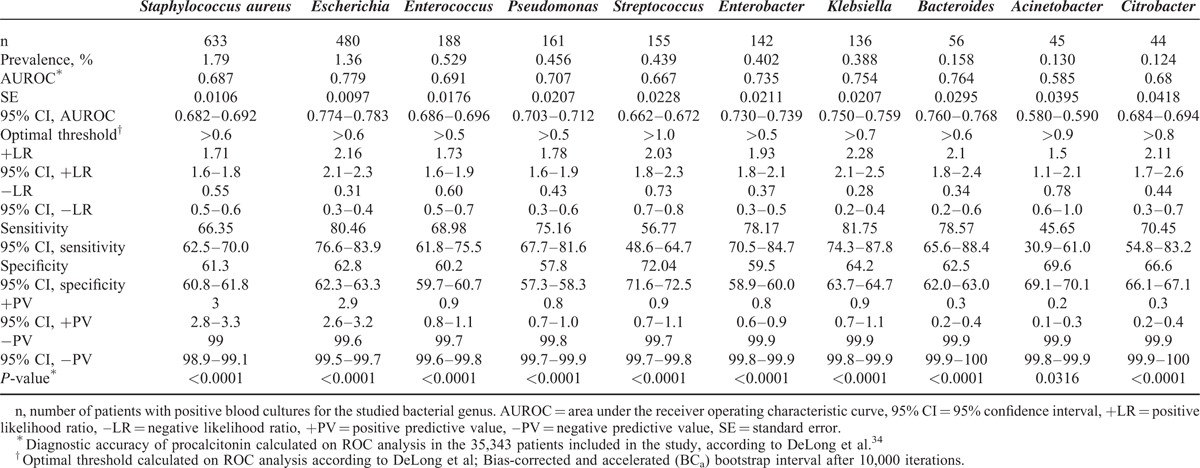
In ROC analysis, optimal procalcitonin thresholds ranging from ≤0.4 to ≤0.75 ng/mL had very high diagnostic accuracies for excluding all pathogen categories with the following NPVs: Gram-negative bacteria (98.9%) (including enterobacteria [99.2%], NFGNB [99.7%], and anaerobic bacteria [99.9%]), Gram-positive bacteria (98.4%), and fungi (99.6%) (Table 2). The diagnostic accuracy of procalcitonin for predicting each specific bacterial genus in patients with bacteremia is reported in Table 3. Consistent with the above-mentioned data, optimal procalcitonin thresholds ranging from ≤0.6 to ≤1.0 ng/mL had very high diagnostic accuracies for excluding all studied bacterial genera with NPVs ranging from 99.6% to 99.9% (Table 3).
TABLE 2.
Diagnostic Accuracy of Procalcitonin for Detecting Positive Blood Cultures and Pathogen Categories in the Study
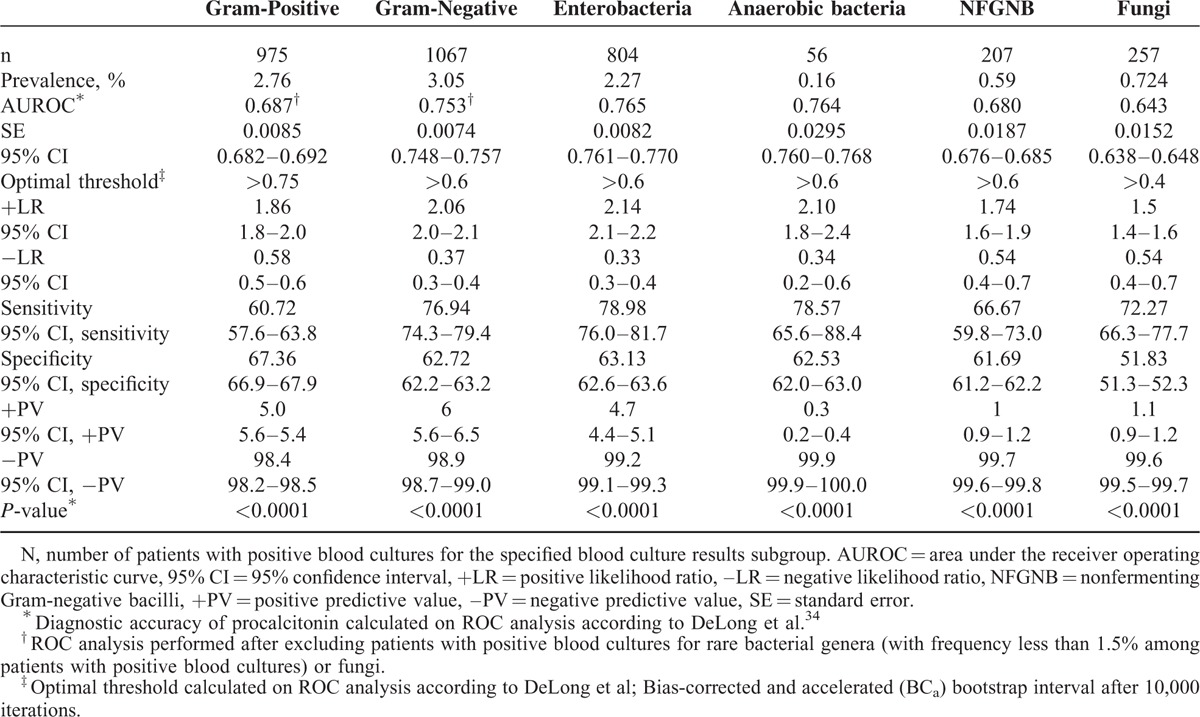
TABLE 3.
Diagnostic Accuracy of Procalcitonin for Detecting Bacterial Genus in the Study
Secondary Endpoint: Association Between Procalcitonin Concentration Strata and Pathogen Categories
Using the whole population of 35,343 patients, we looked for organisms that could be associated with the following internationally validated procalcitonin concentration strata:11 0.05 to 0.1 ng/mL, 0.1 to 0.25 ng/mL, 0.25 to 0.5 ng/mL, 0.5 to 1 ng/mL, 1 to 2 ng/mL, 2 to 10 ng/mL, and procalcitonin concentrations ≥10 ng/mL (Supplemental Digital Content: Table 4, http://links.lww.com/MD/A504). Only patients from the PCBCs group were significantly overrepresented among the patients with procalcitonin concentration between 0.5 and 1 ng/mL (n = 4380), with a small effect size (odds ratio 1.21; 95% CI, 1.05–1.39) (Figure 1 and Supplemental Digital Content: Table 5, http://links.lww.com/MD/A504). In patients with procalcitonin concentration between 1 and 2 ng/mL (n = 3208), there was a significantly increased risk of blood cultures growing Gram-positive bacteria, enterobacteria, and fungi (Figure 1 and Supplemental Digital Content: Table 6, http://links.lww.com/MD/A504). In the next procalcitonin stratum (between 2 and 10 ng/mL, n = 4303), a significantly increased risk was observed for 2 additional Gram-negative pathogen subgroups, namely NFGNB (odds ratio 1.98, 95% CI, 1.41–2.78; P = 0.0001) and anaerobic bacteria (odds ratio 2.59; 95% CI, 1.41–4.74; P = 0.002). Among the patients with procalcitonin concentrations ≥10 ng/mL (n = 2560), there was a significantly increased risk for both “Gram-positive bacteria” (odds ratio 3.64, 95% CI, 3.11–4.26; P < 0.0001) and “Gram-negative bacteria” groups (odds ratio 5.98, 95% CI, 5.20–6.88; P < 0.0001). Interestingly, for the procalcitonin stratum ≥10 ng/mL (n = 2619), there was no significantly increased risk for fungemia or PCBCs (both represented 4.4% of cases in this stratum). The percentage of cases correctly classified in this regression model was 92.7% (Supplemental Digital Content: Tables 5 and 6, http://links.lww.com/MD/A504). All organisms significantly associated with each predefined procalcitonin stratum are shown in Supplemental Digital Content: Table 7, http://links.lww.com/MD/A504 and Figure 3, http://links.lww.com/MD/A504.
FIGURE 1.
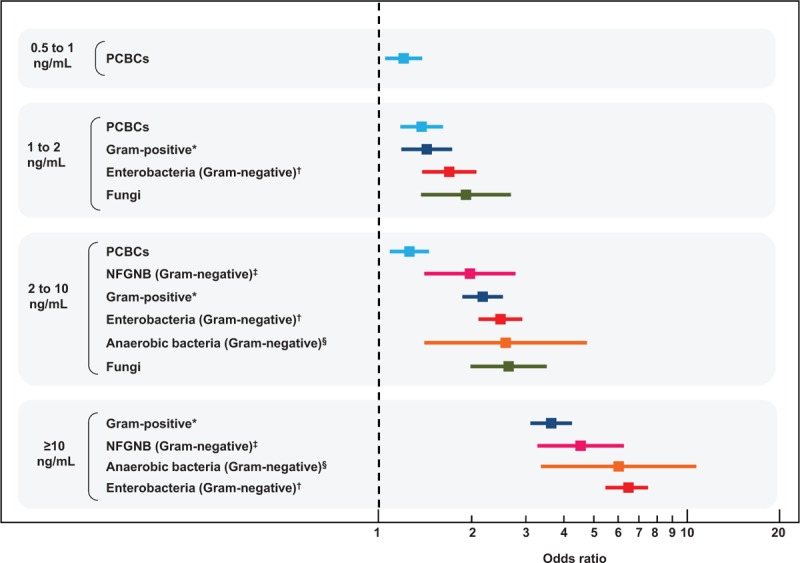
Forest plot showing the odds ratios and confidence intervals of the association between procalcitonin concentration strata and pathogen categories in a stepwise multivariate logistic regression analysis. PCBC = potential contaminants found in blood culture, NFGNB = nonfermenting Gram-negative bacilli, Supplemental Digital Content: Figure 3 illustrates in detail the association between predefined procalcitonin concentration strata and bacterial genera and fungi in stepwise multivariate logistic regression analysis. ∗Staphylococcus aureus, Streptococcus (other than viridans-group streptococci), and Enterococcus. †Enterobacteria: Escherichia, Enterobacter, Klebsiella, and Citrobacter. ‡Nonfermenting Gram-negative bacilli: Pseudomonas and Acinetobacter. §Anaerobic bacteria: Bacteroides.
DISCUSSION
Our study included 35,343 consecutive patients over a 7-year study period and used the most sensitive technique for measuring procalcitonin. In this large, real-life setting study, procalcitonin was highly effective for excluding bloodstream infections regardless of pathogen categories. A recent meta-analysis showed that procalcitonin had a mean sensitivity and specificity of 0.77 and of 0.79, respectively, for the diagnosis of sepsis.6 However, this meta-analysis acknowledged that there was substantial heterogeneity among the studies and considerable variations in procalcitonin cut-offs.6 We found that procalcitonin thresholds ranging from ≤0.4 to ≤0.75 ng/mL had high diagnostic accuracies for excluding all pathogen categories with NPVs ranging from 98.4% for Gram-positive bacteria to 99.9% for anaerobic bacteria.
In healthy subjects, procalcitonin blood concentrations are extremely low but can increase by 1000-fold following bacterial infection.36 It has been shown that following endotoxin treatment of baboons that procalcitonin is produced by several tissues but mainly the liver and kidneys as soon as 6 hours postinjection.36,37 Consistently, massive induction of procalcitonin expression in multiple organs, including the liver, spleen, and lung, was shown in hamsters after Escherichia coli infections.36,38 Both clinical and animal studies have confirmed that a straight lipopolysaccharide (LPS) injection induces production of procalcitonin in the blood stream.39 Furthermore, direct cellular induction of procalcitonin after LPS addition has been demonstrated in cell culture.40 It has been shown that Gram-negative bacteria tend to induce higher levels of blood procalcitonin compared with Gram-positive bacteria.32 This could be explained by the fact that LPS is the major component of the outer membrane of Gram-negative bacteria. On the other hand, Gram-positive bacteria lacking LPS can, to a lesser extent, also provoke procalcitonin stimulation through lipoteichoic acid.1,41 Our results corroborated previously published data and confirmed that Gram-negative bacteria have the greatest ability to stimulate procalcitonin production.
Several randomized trials showed that procalcitonin is an effective tool for reducing antibiotic exposure without harming outcomes in patients with suspected bacterial infections or sepsis.8–11 In an individual patient data meta-analysis, procalcitonin measurements were effective in reducing antibiotic exposure without increasing the risk of mortality or treatment failure in patients with acute respiratory tract infections.8 Consistently, in adult patients with respiratory tract infections and sepsis, a meta-analysis on 14 randomized controlled trials that included 4467 adult patients with respiratory tract infections and sepsis demonstrated that procalcitonin algorithms for antibiotic therapy decisions were effective in reducing antibiotic exposure without worsening patient outcomes or mortality.11 Our results support the use of procalcitonin to guide the initiation of probabilistic antibiotic therapy in patients with suspected bloodstream infection. This would prevent antibiotic exposure in low-risk patients who may not benefit from empirical antibiotic therapy pending blood culture results.
NFGNB – which are intrinsically resistant to many antibiotics and possibly multidrug-resistant – have now emerged as potentially life-threatening healthcare-associated pathogens account for approximately 15% of all bacterial isolates in clinical microbiology laboratories.42,43 We found that procalcitonin concentrations ≤0.6 ng/mL excluded NFGNB bacteremia with an NPV of 99.7%. Furthermore, we observed a significantly increased risk of NFGNB bacteremia only in patients with procalcitonin concentrations of 2 ng/mL or above. These results may help targeting patients at high risk for NFGNB bacteremia among those with suspected bloodstream infection pending blood culture results.
The number of fungal infections is increasing, particularly in patients with cancer.44 This represents a major problem given the relatively poor response rates to and the high cost of empirical antifungal therapy.44 In our study, a procalcitonin concentration of 10 ng/mL or above was associated with a high risk of Gram-positive or Gram-negative bacteremia but dramatically reduced the risk of PCBCs or fungemia. Consequently, this threshold may help identify patients who will not benefit from empirical antifungal therapy pending blood culture results.
Our study had several limitations. First, direct and indirect benefits of the implementation of procalcitonin thresholds in patients with suspected bloodstream infection were not evaluated. These benefits could be assessed through an interventional study that aims to evaluate the benefits of a procalcitonin-based strategy for the reduction of blood culture tests, unnecessary antibiotic prescription rates, and antibiotic resistance. Second, clinical criteria for distinguishing contaminants from true bacteremia were not available and could not be retrieved because of the study design. Consequently, the diagnostic accuracy of procalcitonin in distinguishing contaminants from true bacteremia among the patients in the PCBCs group was not assessed. Nevertheless, the primary endpoint of the study was to investigate the diagnostic accuracy of procalcitonin in predicting or excluding clinically relevant pathogens but not potential contaminants. Interestingly, in our study, the prevalence rates of Gram-positive and Gram-negative bacteremia and fungemia were similar to those reported on a nationally representative sample of acute care hospitals in the United States,45 thereby making our findings applicable to other populations.
CONCLUSIONS
Our study shows that in a large real-life setting experience with more than 35,000 patients, procalcitonin was highly effective at excluding bloodstream infections regardless of pathogen categories. Pending blood culture results, procalcitonin measurements, should be performed in patients with suspected bloodstream infections. Procalcitonin thresholds ranging from ≤0.4 to ≤0.75 ng/mL were highly effective at excluding clinically relevant pathogens. Procalcitonin concentrations ≥10 ng/mL were associated with a high risk of Gram-negative or Gram-positive bacteremia but also dramatically reduced the risk of PCBCs or fungemia. The results from our study are limited by its cross-sectional design and deserve to be validated in prospective longitudinal studies.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, IQR = interquartile range, NFGNB = nonfermenting Gram-negative bacilli, NPV = negative predictive value, ROC = receiver operating characteristic.
Supplemental Digital Content is available for this article.
AO, JF, contributed equally to this work. AL, and JLG shared senior authorship.
AO had full access to all of the data in the study and has taken responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: AO, AL, and LG. Acquisition, analysis, or interpretation of data; Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: AO. Administrative, technical, or material support: MG. Study supervision: AO, AL, and LG.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol 2010; 159:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell JA. Management of sepsis. N Engl J Med 2006; 355:1699–1713. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013; 369:840–851. [DOI] [PubMed] [Google Scholar]
- 4.Assicot M, Gendrel D, Carsin H, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993; 341:515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang BM, Eslick GD, Craig JC, et al. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis 2007; 7:210–217. [DOI] [PubMed] [Google Scholar]
- 6.Wacker C, Prkno A, Brunkhorst FM, et al. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13:426–435. [DOI] [PubMed] [Google Scholar]
- 7.Vouloumanou EK, Plessa E, Karageorgopoulos DE, et al. Serum procalcitonin as a diagnostic marker for neonatal sepsis: a systematic review and meta-analysis. Intensive Care Med 2011; 37:747–762. [DOI] [PubMed] [Google Scholar]
- 8.Schuetz P, Briel M, Christ-Crain M, et al. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin Infect Dis 2012; 55:651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med 2008; 177:498–505. [DOI] [PubMed] [Google Scholar]
- 10.Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375:463–474. [DOI] [PubMed] [Google Scholar]
- 11.Schuetz P, Chiappa V, Briel M, et al. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med 2011; 171:1322–1331. [DOI] [PubMed] [Google Scholar]
- 12.Bates DW, Cook EF, Goldman L, et al. Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med 1990; 113:495–500. [DOI] [PubMed] [Google Scholar]
- 13.Bates DW, Sands K, Miller E, et al. Predicting bacteremia in patients with sepsis syndrome. Academic Medical Center Consortium Sepsis Project Working Group. J Infect Dis 1997; 176:1538–1551. [DOI] [PubMed] [Google Scholar]
- 14.Chirouze C, Schuhmacher H, Rabaud C, et al. Low serum procalcitonin level accurately predicts the absence of bacteremia in adult patients with acute fever. Clin Infect Dis 2002; 35:156–161. [DOI] [PubMed] [Google Scholar]
- 15.Tokuda Y, Miyasato H, Stein GH. A simple prediction algorithm for bacteraemia in patients with acute febrile illness. QJM 2005; 98:813–820. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro NI, Wolfe RE, Wright SB, et al. Who needs a blood culture? A prospectively derived and validated prediction rule. J Emerg Med 2008; 35:255–264. [DOI] [PubMed] [Google Scholar]
- 17.Muller F, Christ-Crain M, Bregenzer T, et al. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial. Chest 2010; 138:121–129. [DOI] [PubMed] [Google Scholar]
- 18.van Nieuwkoop C, Bonten TN, van’t Wout JW, et al. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: a prospective observational study. Crit Care 2010; 14:R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albrich WC, Mueller B. Predicting bacteremia by procalcitonin levels in patients evaluated for sepsis in the emergency department. Expert Rev Anti Infect Ther 2011; 9:653–656. [DOI] [PubMed] [Google Scholar]
- 20.Riedel S, Melendez JH, An AT, et al. Procalcitonin as a marker for the detection of bacteremia and sepsis in the emergency department. Am J Clin Pathol 2011; 135:182–189. [DOI] [PubMed] [Google Scholar]
- 21.Mencacci A, Leli C, Cardaccia A, et al. Procalcitonin predicts real-time PCR results in blood samples from patients with suspected sepsis. PLoS One 2012; 7:e53279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong S, Park Y, Cho Y, et al. Diagnostic utilities of procalcitonin and C-reactive protein for the prediction of bacteremia determined by blood culture. Clin Chim Acta 2012; 413:1731–1736. [DOI] [PubMed] [Google Scholar]
- 23.Brodska H, Malickova K, Adamkova V, et al. Significantly higher procalcitonin levels could differentiate Gram-negative sepsis from Gram-positive and fungal sepsis. Clin Exp Med 2013; 13:165–170. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto K, Adomi S, Koike H, et al. Procalcitonin as an indicator of urosepsis. Res Rep Urol 2013; 5:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortegiani A, Russotto V, Montalto F, et al. Procalcitonin as a marker of Candida species detection by blood culture and polymerase chain reaction in septic patients. BMC Anesthesiol 2014; 14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naffaa M, Makhoul BF, Tobia A, et al. Procalcitonin and interleukin 6 for predicting blood culture positivity in sepsis. Am J Emerg Med 2014; 32:448–451. [DOI] [PubMed] [Google Scholar]
- 27.Hattori T, Nishiyama H, Kato H, et al. Clinical value of procalcitonin for patients with suspected bloodstream infection. Am J Clin Pathol 2014; 141:43–51. [DOI] [PubMed] [Google Scholar]
- 28.Arai T, Kumasaka K, Nagata K, et al. Prediction of blood culture results by measuring procalcitonin levels and other inflammatory biomarkers. Am J Emerg Med 2014; 32:330–333. [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Hwang SS, Kim K, et al. Bacteremia prediction model using a common clinical test in patients with community-acquired pneumonia. Am J Emerg Med 2014; 32:700–704. [DOI] [PubMed] [Google Scholar]
- 30.Kruger S, Ewig S, Papassotiriou J, et al. Inflammatory parameters predict etiologic patterns but do not allow for individual prediction of etiology in patients with CAP: results from the German competence network CAPNETZ. Respir Res 2009; 10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller F, Christ-Crain M, Bregenzer T, et al. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial. Chest 2010; 138:121–129. [DOI] [PubMed] [Google Scholar]
- 32.Charles PE, Ladoire S, Aho S, et al. Serum procalcitonin elevation in critically ill patients at the onset of bacteremia caused by either Gram negative or Gram positive bacteria. BMC Infect Dis 2008; 8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CC, Lin WJ, Shih HI, et al. Clinical significance of potential contaminants in blood cultures among patients in a medical center. J Microbiol Immunol Infect 2007; 40:438–444. [PubMed] [Google Scholar]
- 34.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44:837–845. [PubMed] [Google Scholar]
- 35.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, Florida, USA: Chapman & Hall/CRC; 1994. [Google Scholar]
- 36.Matwiyoff GN, Prahl JD, Miller RJ, et al. Immune regulation of procalcitonin: a biomarker and mediator of infection. Inflamm Res 2012; 61:401–409. [DOI] [PubMed] [Google Scholar]
- 37.Morgenthaler NG, Struck J, Chancerelle Y, et al. Production of procalcitonin (PCT) in non-thyroidal tissue after LPS injection. Horm Metab Res 2003; 35:290–295. [DOI] [PubMed] [Google Scholar]
- 38.Muller B, White JC, Nylen ES, et al. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab 2001; 86:396–404. [DOI] [PubMed] [Google Scholar]
- 39.Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab 1994; 79:1605–1608. [DOI] [PubMed] [Google Scholar]
- 40.Oberhoffer M, Stonans I, Russwurm S, et al. Procalcitonin expression in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis-related cytokines in vitro. J Lab Clin Med 1999; 134:49–55. [DOI] [PubMed] [Google Scholar]
- 41.Ryu YH, Baik JE, Yang JS, et al. Differential immunostimulatory effects of Gram-positive bacteria due to their lipoteichoic acids. Int Immunopharmacol 2009; 9:127–133. [DOI] [PubMed] [Google Scholar]
- 42.Troillet N, Samore MH, Carmeli Y. Imipenem-resistant Pseudomonas aeruginosa: risk factors and antibiotic susceptibility patterns. Clin Infect Dis 1997; 25:1094–1098. [DOI] [PubMed] [Google Scholar]
- 43.Gales AC, Jones RN, Forward KR, et al. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997–1999). Clin Infect Dis 2001; 32 Suppl 2:S104–S113. [DOI] [PubMed] [Google Scholar]
- 44.Klastersky J. Empirical antifungal therapy. Int J Antimicrob Agents 2004; 23:105–112. [DOI] [PubMed] [Google Scholar]
- 45.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348:1546–1554. [DOI] [PubMed] [Google Scholar]


