Abstract
This study aims to investigate the impact of psammoma body (PB) on papillary thyroid carcinoma (PTC), and evaluate the association among PB, Hashimoto thyroiditis (HT), and other clinicopathologic characteristics in PTC patients.
We conducted a retrospective case-control study involving 1052 PTC patients who underwent total thyroidectomy or lobectomy with lymph node dissection.
Psammoma body was observed in 324 out of 1052 PTC (30.8%) patients. Ultrasonographic (US) calcification (P < 0.001), multifocality of the tumor (P = 0.047), lymph node metastasis (LNM) (P < 0.001), HT (P < 0.001), and Primary tumor (T), Regional lymph nodes (N), Distant metastasis (M) staging (P = 0.001) were significantly related to the presence of PB. The presence of PB was significantly associated with US microcalcification (P < 0.001). In the subgroup with HT, compared with the patients without PB, the patients with PB exhibited a higher frequency of central LNM (54.7% vs 32.1%; P < 0.001) and US microcalcification (94.7% vs 38.8%; P < 0.001), as well as smaller tumors (0.9 ± 0.6 vs 1.3 ± 0.9 cm; P < 0.001). In the subgroup without HT, the patients with PB displayed a higher incidence of lateral LNM (25.8% vs 14.6%; P < 0.001), US microcalcification (87.3% vs 52.5%; P < 0.001), and extrathyroidal extension (47.2% vs 34.8%; P = 0.001), as well as larger tumors (1.3 ± 0.9 vs 1.0 ± 0.8 cm; P < 0.001) than without PB. Moreover, in the subgroup with PB, the PTC patients with HT showed a higher LNM (77.9% vs 57.2%; P < 0.001) and a lower frequency of extrathyroidal extension (20.0% vs 47.2%; P < 0.001) than without HT.
Psammoma body is a useful predictor of aggressive tumor behavior in PTC patients. HT with PB shows more aggressive behaviors than non-HT with PB in PTC patients.
INTRODUCTION
Thyroid carcinoma is the most common type of head and neck malignant tumor. Recently, the number of thyroid carcinoma cases had annually increased by 4% globally1 and has become the fastest growing type of cancer worldwide.2,3 Papillary thyroid carcinoma (PTC) accounts for 80% to 85% of all types of thyroid carcinomas.4
A psammoma body (PB) is a concentric lamellated calcified structure about 50 to 70 μm in size in tumor tissues.5 PBs are most commonly observed in PTC, meningioma, and papillary serous cystadenocarcinoma of the ovary6; thus, PB is of great significance in the diagnosis of these tumors. The presence of PB, which often indicates PTC, makes it one of the important bases for the pathological diagnosis of PTC.5 Several studies have reported that PB is found in about 50% of PTC cases6,7; thus, PB is a reliable diagnostic marker for PTC.
Calcification characteristics are the most important ultrasonographic (US) criterion in evaluating thyroid nodules. Histologically, calcification is classified as macrocalcification (diameter ≥2 mm) and microcalcification (diameter <2 mm). Although US calcification can be detected in both benign and malignant thyroid nodules, microcalcification often represents a potential risk for malignancy.8,9 Clinically, microcalcification exists in more than 50% of malignant thyroid nodules, especially in PTC, whereas microcalcification occurs in only about 3% to 5% of benign thyroid nodules.10 Shi et al11 found that the incidence of malignancy was significantly higher in patients with microcalcification (96.5%) than in those with macrocalcification (41.1%)—a result suggesting that the occurrence of microcalcification may be a reliable characteristic of thyroid carcinoma. Despite the similar effect of calcification and PB in PTC, however, microcalcification does not equate to PB.5,12
To date, few studies have investigated the correlation between PB and the clinicopathologic characteristics of PTC.7,13 In addition, the relationship between US microcalcification and PB remains unclear. Therefore, this study aims to investigate the influence of PB on PTC, and to evaluate the association among HT, PB, US microcalcification, and other clinicopathologic characteristics of PTC.
MATERIALS AND METHODS
Study Protocol and Data Collection
We conducted a retrospective case-control study involving 1052 patients who underwent total thyroidectomy or lobectomy with prophylactic central lymph node and/or therapeutic lateral lymph node dissection at the First Affiliated Hospital of Wenzhou Medical University, from January 2009 to December 2012. All patients had no history of thyroid or neck surgery for nonthyroidial cancer, as well as of neck irradiation. The patients were divided into the case group (324 patients) and the control group (728 patients), according to the presence of PB.
The surgical procedure conducted in this study was in accordance with the National comprehensive cancer network guidelines for thyroid carcinoma. The patients’ demographic, preoperative laboratory, and pathologic data were obtained from their electronic medical records. The research protocol used in this study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University, and informed consent was obtained from each patient.
Clinicopathological Parameters
The clinical and pathologic data were age, gender, tumor size, US calcification, preoperative level of thyrotropin (TSH) and thyroglobulin (Tg), multifocality of the tumor, central lymph node metastasis (CLNM), lateral lymph node metastasis (LLNM), extrothyroid extension (ETE), lymph node metastasis (LNM), Hashimoto thyroiditis (HT), TNM staging, and PB. The presence of PB was ascertained through hematoxylin and eosin staining (Fig. 1A). Based on location, PBs were categorized as intratumoral PB, extratumoral PB, and lymph node PB. The primary tumor size determined through ultrasonography was measured on the basis of the standard change in echo edge. When multiple PTCs were found in the specimen, the largest tumor or the most suspicious dominant nodule was analyzed. US calcification was defined as the hyperechoic spots with or without acoustic shadows or as the simple fine acoustic shadows in ultrasound14 (Fig. 1B). The multifocality of a tumor was confirmed when more than one mass was found according to the final pathological examination of the specimens. ETE was defined as the tumor perimeter in contact with >25% of the thyroid capsule in a malignant lesion, or the loss of the capsule line.15 LNM was obtained from the final pathologic reports. HT was confirmed in the final pathological examination when the specimens exhibited diffused lymphoplasmacytic infiltration with germinal centers, parenchymal atrophy with oncocytic changes, and variable amounts of stromal fibrosis throughout the thyroid gland16 (Fig. 1C). TNM staging was based on the 7th Edition of the American Joint Cancer Committee TNM classifications.17
FIGURE 1.
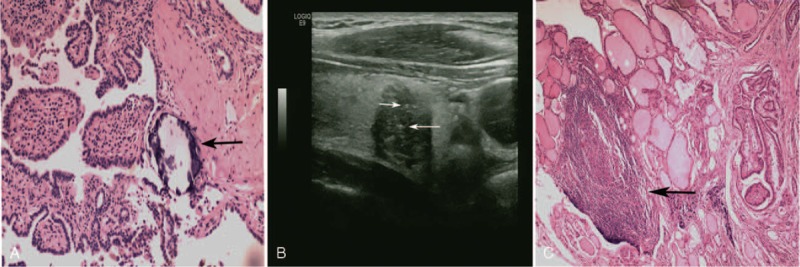
A, A 30-year-old man with psammoma body and diagnosed as having papillary thyroid carcinoma. This is an intratumoral PB (Black arrow) with concentric lamellated calcified structure (H&E, 100×). B, A 30-year-old man with several microcalcifications and diagnosed as having papillary thyroid carcinoma. The lesion of PTC shows some fine sand-like hyperechoic spots in the ultrasonoscopy (white arrow). C, A 58-year-old woman with HT and diagnosed as having papillary thyroid carcinoma. The lesion of HT (black arrow) shows diffused lymphoplasmacytic infiltration and parenchymal atrophy in the pathology (H&E, 40×). HT = Hashimoto thyroiditis, PB = psammoma body, PTC = papillary thyroid carcinoma.
Ultrasonography was performed with Acuson Sequoia and 128XP sonographic scanners (Siemens Medical Solutions, Mountain View, CA) equipped with commercially available 8 to 13 MHz linear probes. The ultrasonic calcification was classified as macrocalcification (diameter ≥2 mm) and microcalcification (diameter <2 mm). Two experienced pathologists performed all the pathological assessments.
Statistical Analysis
Data on normal distribution were expressed as mean ± standard deviation (SD) and were compared with t test. Categorical variables were expressed as percentage and were compared with chi-square test or Fisher exact test, as appropriate. Logistic regression analysis was also performed to estimate the odds ratios (ORs) of certain parameters. Variables with P < 0.05 in the univariate analysis were progressed to a multivariate analysis using forward stepwise selection. All P values were 2-sided, and a P value of < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS software version 19.0 (SPSS, Chicago, IL).
RESULTS
Baseline Characteristics
Our study enrolled 1052 patients, including 827 women and 225 men. Table 1 shows the baseline clinicopathological characteristics of all patients. PB was observed in 324 out of 1052 PTC (30.8%) patients. Intratumoral PB, extratumoral PB, and lymph node PB were observed in 314 (96.9%), 126 (38.9%), and 93 (28.7%) patients, respectively.
TABLE 1.
Baseline Characteristics of Papillary Thyroid Carcinoma Patients Stratified by Psammoma Body (n = 1052)

Association Between PB and Clinicopathological Parameters
The patients were divided into 2 groups on the basis of PB status. Table 1 shows the differences in clinicopathological and US features of the 2 groups. Among the 11 factors, 5 factors were significantly related to the presence of PB, namely, US calcification (P < 0.001), multifocality of the tumor (P = 0.047), LNM (P < 0.001), HT (P < 0.001), and TNM staging (P = 0.001). Age, gender, tumor size, preoperative level of TSH and Tg, and ETE were not significantly correlated with the presence of PB (P > 0.05). Multivariate analyses showed that LNM (OR 1.421, 95% confidence interval [CI] 1.169–1.727, P < 0.001) and HT (OR 1.704, 95% CI 1.245–2.334, P = 0.001) were independent predictors of PB.
Relationship Between PB and US Calcification
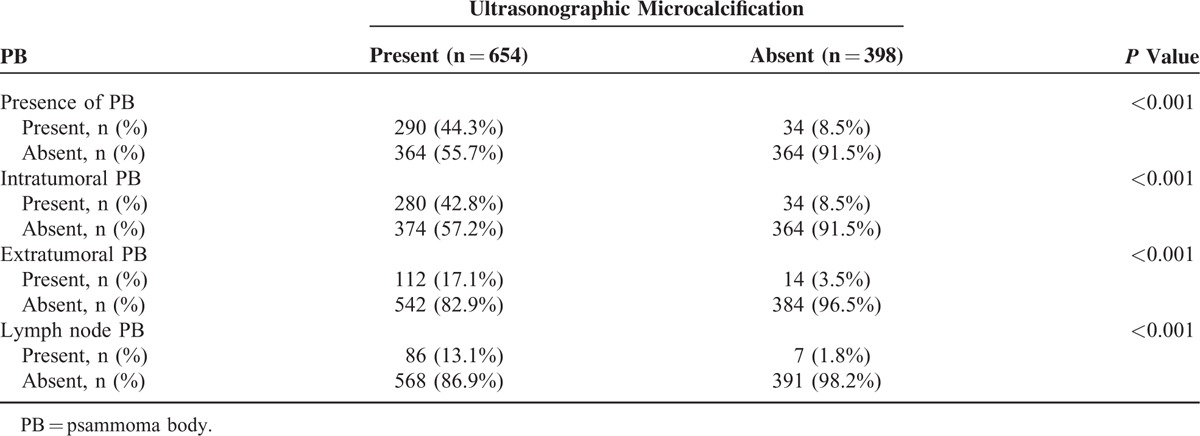
Few studies have reported on the correlation between US calcification and the presence of PB in PTC. Two types of US calcifications exist, namely, microcalcification and macrocalcification. Our univariate analysis showed that US microcalcification is a predictor of risk factor for PB. US calcifications were detected in 654 (62.2%) out of 1052 PTC patients. Using the final pathological findings, we classified PB based on location, namely, intratumoral PB, extratumoral PB, and lymph node PB. Table 2 shows that a strong association between US microcalcification and PB exists. In patients with US microcalcification, as much as 42.8%, 17.1%, and 13.1% exhibit intratumoral PB, extratumoral PB, and lymph node PB (all P < 0.001), respectively. Thus, US calcification may be a reliable predictor of PB in PTC.
TABLE 2.
The Correlation Between the Ultrasonographic Microcalcification and the Presence of Psammoma Body in Papillary Thyroid Carcinoma
Influence of Aggressive Factors on the Different Statuses of PB and HT in PTCs
To further investigate the influence of HT in patients with and without PB, we divided the PTC patients into 4 subgroups on the basis of the presence or absence of PB and HT; these groups were HT (+) PB (+), HT (+) PB (−), HT (−) PB (+), and HT (−) PB (−) (Table 3).
TABLE 3.
The Correlation Between the Hashimoto Thyroiditis and Psammoma Body in Papillary Thyroid Carcinoma

HT (+) PB (+) Versus HT (+) PB (−)
Compared with the HT (+) PB (−)group, the HT (+) PB (+) group exhibited a higher frequency of CLNM (54.7% vs 32.1%; P < 0.001), LLNM (23.2% vs 20.9%; P < 0.001), and US microcalcification (94.7% vs 38.8%; P < 0.001). In addition, the latter exhibited smaller tumors (0.9 ± 0.6 vs 1.3 ± 0.9 cm; P < 0.001). These results showed that the presence of PB was an aggressive factor of PTC in patients with HT.
HT (+) PB (+) Versus HT (−) PB (+)
The HT (+) PB (+) group exhibited a higher LNM (77.9% vs 57.2%; P < 0.001) and a lower frequency of ETE (20.0% vs 47.2%; P < 0.001) than the HT (−) PB (+) group, a result indicating that under the influence of PB, HT exhibited an aggressive effect on PTC.
HT (+) PB (−) Versus HT (−) PB (−)
The HT (+) PB (−) and HT (−) PB (−) groups were not significantly different in terms of LNM and ETE (P = 0.152, P = 0.313). However, larger tumors (1.3 ± 0.9 vs 1.0 ± 0.8 cm; P = 0.004) and a higher incidence of multifocality (30.6% vs 20.2%; P = 0.011) were observed in the HT (+) PB (−) group compared with the HT (−) PB (−) group. In brief, in the absence of PB, the HT (+) group showed the larger tumor size and more frequent multifocality than the HT (−) group; however, there was no significant difference of lymph node metastasis between these 2 groups. These results suggested that without the presence of PB, HT was not an aggressive factor for PTC.
HT (−) PB (+) Versus HT (−) PB (−)
The HT (−) PB (+) group showed a higher incidence of LLNM (25.8% vs 14.6%; P < 0.001), US microcalcification (87.3% vs 52.5%; P < 0.001), ETE (47.2% vs 34.8%; P = 0.001), and larger tumors (1.3 ± 0.9 vs 1.0 ± 0.8 cm; P < 0.001) than the HT (−) PB (−) group. Although the HT (+) PB (+) group had a lower incidence of ETE than the HT (−) PB (+) patients, the PB (+) HT (+) group had higher incidence of lymph node metastasis. These results showed that PB, even in the absence of HT, was an aggressive factor for PTC.
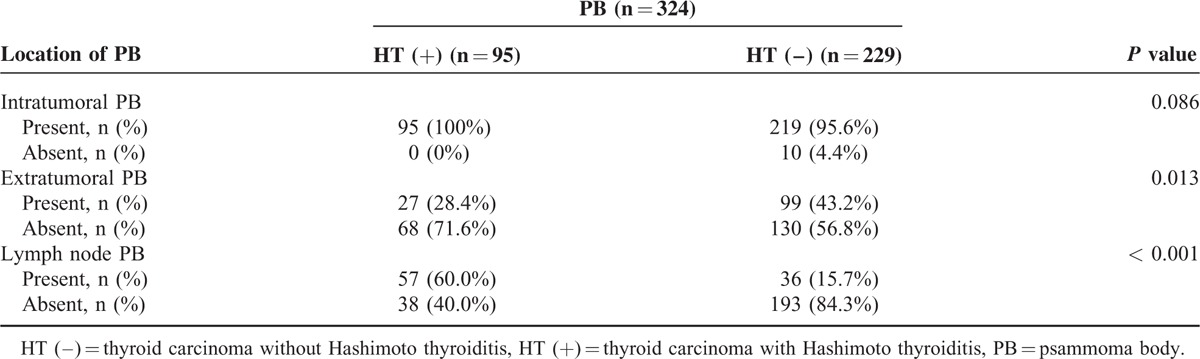
To confirm the effect of HT in patients with PB, we analyzed the relationship between HT and the location of PB (Table 4). HT is highly associated with the location of PB (Table 4). The HT (+) group exhibited a higher incidence of lymph node PB than the HT (−) group (60.0% vs 15.7%; P < 0.001). By contrast, the HT (−) group showed a higher incidence of extratumoral PB than the HT(+) group (42.3% vs 28.4%; P = 0.013).
TABLE 4.
The Correlation Between the Hashimoto Thyroiditis and the Location of Psammoma Body in Papillary Thyroid Carcinoma
DISCUSSION AND CONCLUSIONS
Although PBs are found in about 50% of the PTC cases,6,7 the importance of PB in PTC diagnosis and prognosis has yet to be fully understood. Therefore, we performed this retrospective study involving 1052 PTC patients to investigate the correlation between the presence of PB and clinicopathological characteristics, as well as the relationship between US microcalcification and PB.
Our univariate analysis showed that PB is significantly associated with LNM, TNM staging, and tumor multifocality. Our multivariate analyses also proved that LNM and HT are independent predictors of PB. The presence of PB was previously reported to be associated with LNM.13 Similarly, a study involving 258 patients demonstrated that the presence of PB is significantly correlated with tumor multifocality and LNM.7 Recent studies showed that nodal involvement is commonly associated with local recurrence and cancer-specific mortality.18,19 Therefore, PB is possibly a useful predictor of aggressive tumor behavior in PTC patients—a finding that is consistent with that of previous research.
Ultrasonographic calcification was significantly associated with the presence of PB—a result indicating that US calcification may indicate the presence of PB. Previous studies reported that PB originates from the calcification of intravascular or intralymphatic tumor thrombi and from the intracellular calcifications of viable cells in lesions.13 PB is also a predictive factor for the poor prognosis of PTC patients,13 and the presence of PB often strongly suggests malignancy in PTC.5 Although PB plays an important role in PTC diagnosis and prognosis, its clinical application is limited because PB can be only detected after surgery or fine needle biopsy, in which PB is often neglected. The literature indicates that US microcalcification instead of cytological and histological examinations may be used to a certain extent in predicting the presence of PB.5,7 Consistent with those of the previous studies, our results showed that US microcalcification is significantly associated with PB. Moreover, this relationship holds true regardless of the type of PB involved, be it an intratumoral PB, extratumoral PB, or lymph node PB. Our findings thus indicate that US microcalcification is a potential indicator of the presence of PB. The sensitivity, specificity, positive predictive value, and negative predictive value of US microcalcification reach up to 89.5%, 50.0%, 44.3%, and 91.5%, respectively. The absence of US microcalcification, which is represented by the negative predictive value (91.5%), indicates that the possibility of the presence of PB is very low.
Many studies have reported that PTC accompanied by HT is associated with improved prognosis of PTC, such as in reduced incidence of LNM.20,21 By contrast, PB has been reported as a predictive factor for the poor prognosis of PTC patients, especially the increased incidence of LNM.13 The present study found that the presence of PB indicates a higher incidence of LNM regardless of whether PB is accompanied by HT or not. In the HT group, the patients with PB exhibited a higher frequency of LNM than those patients without PB (77.9% vs 53.0%; P < 0.001). A similar pattern was observed in the non-HT group (57.2% vs 46.0%; P < 0.001). Therefore, PB promoted the development of PTC, and this result is consistent with that of the previous studies. Moreover, the frequency of LNM is much higher in the HT (+) PB (+) group than in the HT (−) PB (+) group (77.9% vs 57.2%; P < 0.001) —a result indicating that HT may indicate poor prognosis rather than good prognosis in PTC patients with PB. This finding is contrary to that of most reports, in which HT is associated with an improved prognosis of PTC. There are some possible explains for these inverted results: long-term impact of HT leads to high levels of serum thyroid-stimulating hormone, which acts as a risk factor for developing PTC22,23; at the molecular level, the proto-oncogene RET, which is located on chromosome 10q11.2 and encodes a transmembrane receptor-tyrosine kinase,24 may play an important role between HT and PTC development by RET/PTC rearrangement. Some studies also demonstrated that the mitogen-activated protein kinase signaling pathway, which is activated by the RET/PTC rearrangement, is crucial in the association between HT and PTC.25
We also found an interesting phenomenon about LNM. In the HT group, CLNM occurred in 54.7% (52/95) of the PTC patients with PB—a value accounting for more than two-thirds (70.3%, 52/74) of all patients exhibiting LNM. As shown in Table 4, about 60% of lymph node PB cases were found in the HT (+) group. This result suggested that PTC that is accompanied by PB and HT easily leads to CLNM via the lymphatic drainage. By contrast, in the non-HT group, LLNM occurred in 25.8% (59/229) of the PTC patients with PB—a value accounting for nearly half (45%, 59/131) of all patients with LNM. About 43.2% were extrathyroidal PB in the non-HT group. Moreover, previous findings showed that ETE is higher in the PB (+) HT (−) group—a result indicating that PTC patients with PB, but without HT, exhibit a prior tumor local invasion leading to LLNM. Some studies have shown that LNM often occurs first in the central lymph node of the PTC patients.26–28 Other studies have demonstrated that PTC accompanied by ETE is more likely to directly result in LLNM than when ETE is absent.29,30 In the HT (+) PB (+) and the HT (+) PB (−) groups, the presence of PB was not significantly correlated with ETE. However, in the non-HT group, ETE was significantly associated with the presence of PB. In addition, the frequency of ETE was much higher in the HT (−) PB (+) group than in the HT (+) PB (+) group (47.2% vs 20.0%; P < 0.001) —a result showing that HT restrained the effect of PB on ETE in PTC.
Furthermore, we found that the localization of PB is not similar between the HT and the non-HT groups (Table 4). In the HT group, most patients (60.0%) exhibited lymph node PB. By contrast, about 43.2% of PB cases were extratumoral in the non-HT group. On the basis of these results, we may conclude that the mechanism of LNM varies between the 2 groups. In the PB (+) HT (+) group, LNM occurred mainly through the lymphatic vessels, and this led to CLNM, whereas in the HT (−) PB (+) group, LNM occurred mainly through ETE, and this resulted in LLNM. Therefore, HT may play a crucial role in this process. When PB is accompanied by HT in PTC, the effect of HT in promoting LNM was stronger than that of PB on ETE. By contrast, when PB is not accompanied by HT in PTC, the incidence of LNM was low despite the high amount of ETE, and LLNM mainly occurred. This result indicated that under the influence of PB, HT may act as an aggressive factor of PTC.
The microcalcification is reliable characteristic for papillary thyroid carcinoma.8–10 The specificity of microcalcifications for a diagnosis of malignant thyroid carcinoma was 96.5%.11 Some studies indicated that there was no association between HT and tumor size.31–33 Similar to previous researches, the present study did not show the statistical relationship between HT and tumor size (1.10 ± 0.82 vs 1.10 ± 0.78 cm; P = 0.936, data not show). However, the PB (+) HT (+) group had smaller tumor size than the PB (−) HT (+) group (0.9 ± 0.6 vs 1.3 ± 0.9 cm; P < 0.001), and the HT (+) PB (−) group had bigger tumor size than the HT (−) PB (−) group (1.3 ± 0.9 vs 1.0 ± 0.8 cm; P = 0.004). One possible explanation is that in the HT (+) subgroup, the PB (+) group had a higher incidence of microcalcification than in the PB (−) group (94.7% vs 38.8%; P < 0.001) and thyroid nodules with microcalcification would be more likely earlier diagnosed as PTC than without microcalcification. In the PB (−) subgroup, the patients with HT had higher incidence of microcalcification than HT (−) (52.5% vs 38.8%; P < 0.001). In addition, the patients with HT would have more frequent and longer-term follow-ups than those without HT before diagnosis of PTC." should be "In addition, the patients with HT would have more frequent and longer-term follow-ups than those without HT before diagnosis of PTC, so the PB in thyroid nodules could be better monitored and the PTC in patients with HT would be possibly diagnosed earlier.
We conducted the study on the clinical characteristics of PB in PTC. However, the current study has several limitations. First, the source of the above results was among populations in coastal regions, where there is a high incidence of PTC.34,35 Additionally, the tumor size of 91.1% (958/1052) patients is ≤20 mm (T1 staging) in our study. Therefore, a selection bias possibly exists in this retrospective cross-sectional study. Second, single-center verification is not accurate enough. Third, our study did not analyze data from the long-term follow-up period, such as disease recurrence and disease-free survival; thus, we cannot directly conclude on the relationship between PB and prognosis of PTC. Further investigation with a longer duration is needed.
In conclusion, our study indicated that the presence of PB may indicate aggressive tumor behaviors, such as tumor multifocality, LNM, ETE, and TNM staging, in PTC patients. US microcalcification is a useful predictor for the presence of PB. Under the influence of PB, HT acted as an aggressive factor for PTC. Further studies are needed to elucidate the biochemical mechanisms of PB in PTC patients.
Footnotes
Abbreviations: CLNM = central lymph node metastasis, ETE = extrathyroidal extension, HT = Hashimoto thyroiditis, LLNM = lateral lymph node metastasis, LNM = lymph node metastasis, PB = psammoma body, PTC = papillary thyroid carcinoma, US = ultrasonographic.
Y-FC and Q-XW have contributed equally to the writing of this article.
X-HZ, JY, Q-XW, and Y-FC designed the study. C-JN, LW, H-YD, and QL collected data. JY and G-LG did the statistical analyses. Y-FC, Q-XW, JY, X-HZ, and O-CW reviewed the results, interpreted data, and wrote the manuscript. All authors saw and approved the final version of the article.
This work was supported by grants from the Wenzhou City Science and Technology Bureau (Y20140124) and the National High Technology Research and Development Program of 863 project of China (NO. 2012AA02A210).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 2009; 115:3801–3807. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA 2014; 64:9–29. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 4.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nature Rev Cancer 2013; 13:184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triggiani V, Guastamacchia E, Licchelli B, et al. Microcalcifications and psammoma bodies in thyroid tumors. Thyroid 2008; 18:1017–1018. [DOI] [PubMed] [Google Scholar]
- 6.Das DK. Psammoma body: a product of dystrophic calcification or of a biologically active process that aims at limiting the growth and spread of tumor? Diagn Cytopathol 2009; 37:534–541. [DOI] [PubMed] [Google Scholar]
- 7.Pyo JS, Kang G, Kim DH, et al. The prognostic relevance of psammoma bodies and ultrasonographic intratumoral calcifications in papillary thyroid carcinoma. World J Surg 2013; 37:2330–2335. [DOI] [PubMed] [Google Scholar]
- 8.Chen G, Zhu XQ, Zou X, et al. Retrospective analysis of thyroid nodules by clinical and pathological characteristics, and ultrasonographically detected calcification correlated to thyroid carcinoma in South China. European surgical research. Europaische chirurgische Forschung. Recherches chirurgicales europeennes 2009; 42:137–142. [DOI] [PubMed] [Google Scholar]
- 9.Moon WJ, Jung SL, Lee JH, et al. Benign and malignant thyroid nodules: US differentiation: multicenter retrospective study. Radiology 2008; 247:762–770. [DOI] [PubMed] [Google Scholar]
- 10.Kim BK, Choi YS, Kwon HJ, et al. Relationship between patterns of calcification in thyroid nodules and histopathologic findings. Endocrine J 2013; 60:155–160. [DOI] [PubMed] [Google Scholar]
- 11.Shi C, Li S, Shi T, et al. Correlation between thyroid nodule calcification morphology on ultrasound and thyroid carcinoma. J Int Med Res 2012; 40:350–357. [DOI] [PubMed] [Google Scholar]
- 12.Katoh R, Kawaoi A, Muramatsu A, et al. Birefringent (calcium oxalate) crystals in thyroid diseases. A clinicopathological study with possible implications for differential diagnosis. Am J Surg Pathol 1993; 17:698–705. [DOI] [PubMed] [Google Scholar]
- 13.Bai Y, Zhou G, Nakamura M, et al. Survival impact of psammoma body, stromal calcification, and bone formation in papillary thyroid carcinoma. Modern Pathol 2009; 22:887–894. [DOI] [PubMed] [Google Scholar]
- 14.Solbiati L, Cioffi V, Ballarati E. Ultrasonography of the neck. Radiol Clin N Am 1992; 30:941–954. [PubMed] [Google Scholar]
- 15.Moon HJ, Kim EK, Yoon JH, et al. Differences in the diagnostic performances of staging US for thyroid malignancy according to experience. Ultrasound Med Biol 2012; 38:568–573. [DOI] [PubMed] [Google Scholar]
- 16.Zeng RC, Jin LP, Chen ED, et al. The potential relationship between hashimoto's thyroiditis and BRAF Mutation status in papillary thyroid cancer. Head Neck 2015. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 18.Cho SY, Lee TH, Ku YH, et al. Central lymph node metastasis in papillary thyroid microcarcinoma can be stratified according to the number, the size of metastatic foci, and the presence of desmoplasia. Surgery 2015; 157:111–118. [DOI] [PubMed] [Google Scholar]
- 19.Ito Y, Tomoda C, Uruno T, et al. Ultrasonographically and anatomopathologically detectable node metastases in the lateral compartment as indicators of worse relapse-free survival in patients with papillary thyroid carcinoma. World J Surg 2005; 29:917–920. [DOI] [PubMed] [Google Scholar]
- 20.Jara SM, Carson KA, Pai SI, et al. The relationship between chronic lymphocytic thyroiditis and central neck lymph node metastasis in North American patients with papillary thyroid carcinoma. Surgery 2013; 154:1272–1280.[discussion 1280–1272]. [DOI] [PubMed] [Google Scholar]
- 21.Loh KC, Greenspan FS, Dong F, et al. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab 1999; 84:458–463. [DOI] [PubMed] [Google Scholar]
- 22.Boelaert K, Horacek J, Holder RL, et al. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab 2006; 91:4295–4301. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Li H, Ji QH, et al. The clinical features of papillary thyroid cancer in Hashimoto's thyroiditis patients from an area with a high prevalence of Hashimoto's disease. BMC Cancer 2012; 12:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi M, Buma Y, Iwamoto T, et al. Cloning and expression of the ret proto-oncogene encoding a tyrosine kinase with two potential transmembrane domains. Oncogene 1988; 3:571–578. [PubMed] [Google Scholar]
- 25.Unger P, Ewart M, Wang BY, et al. Expression of p63 in papillary thyroid carcinoma and in Hashimoto's thyroiditis: a pathobiologic link? Human Pathol 2003; 34:764–769. [DOI] [PubMed] [Google Scholar]
- 26.Xiao GZ, Gao L. Central lymph node metastasis: is it a reliable indicator of lateral node involvement in papillary thyroid carcinoma? World J Surg 2010; 34:237–241. [DOI] [PubMed] [Google Scholar]
- 27.Machens A, Hauptmann S, Dralle H. Lymph node dissection in the lateral neck for completion in central node-positive papillary thyroid cancer. Surgery 2009; 145:176–181. [DOI] [PubMed] [Google Scholar]
- 28.Roh JL, Park CI. Sentinel lymph node biopsy as guidance for central neck dissection in patients with papillary thyroid carcinoma. Cancer 2008; 113:1527–1531. [DOI] [PubMed] [Google Scholar]
- 29.Zeng RC, Zhang W, Gao EL, et al. Number of central lymph node metastasis for predicting lateral lymph node metastasis in papillary thyroid microcarcinoma. Head Neck 2014; 36:101–106. [DOI] [PubMed] [Google Scholar]
- 30.Kim SS, Lee BJ, Lee JC, et al. Preoperative serum thyroid stimulating hormone levels in well-differentiated thyroid carcinoma is a predictive factor for lateral lymph node metastasis as well as extrathyroidal extension in Korean patients: a single-center experience. Endocrine 2011; 39:259–265. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Ma XP, Deng FS, et al. The effect of chronic lymphocytic thyroiditis on patients with thyroid cancer. World J Surg Oncol 2014; 12:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwak HY, Chae BJ, Eom YH, et al. Does papillary thyroid carcinoma have a better prognosis with or without Hashimoto thyroiditis? Int J Clin Oncol 2015; 20:463–473. [DOI] [PubMed] [Google Scholar]
- 33.Ye ZQ, Gu DN, Hu HY, et al. Hashimoto's thyroiditis, microcalcification and raised thyrotropin levels within normal range are associated with thyroid cancer. World J Surg Oncol 2013; 11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakazawa T, Kondo T, Kobayashi Y, et al. RET gene rearrangements (RET/PTC1 and RET/PTC3) in papillary thyroid carcinomas from an iodine-rich country (Japan). Cancer 2005; 104:943–951. [DOI] [PubMed] [Google Scholar]
- 35.Ezaki H, Ebihara S, Fujimoto Y, et al. Analysis of thyroid carcinoma based on material registered in Japan during 1977–1986 with special reference to predominance of papillary type. Cancer 1992; 70:808–814. [DOI] [PubMed] [Google Scholar]


