Abstract
T helper (Th) 17 cells were reported to have the property of proinflammation and profibrosis. We first investigate the levels of Th17 cells in primary biliary cirrhosis (PBC) patients, and then explore their distribution and fibrotic role in the disease.
We compared the circulating Th17 and hepatic interleukin (IL)-17-positive cells between patients and healthy controls (HCs) at different disease stages by flow cytometry and immunohistochemistry, respectively. The levels of chemokine (c-c motif) ligand (CCL) 20 were then measured. For exploration of the reason why Th17 cells increased, CD4+CD161+ populations were sorted and cultured with IL-23 and IL-1β to analyze their proliferation and IL-17 secretions. The serum IL-23 and IL-1β were tested by enzyme-linked immunosorbent assay. The proliferation and expressions of α-smooth muscle actin and IL-8 of hepatic stellate cells (HSCs) were identified after stimulated by different concentrations of IL-17.
Circulating and hepatic Th17 cells were elevated in PBC patients compared with HCs. Early PBC patients presented with more Th17 cells in periphery blood and less in the liver than advanced PBC patients. Accordingly, the levels of both serum and hepatic CCL20 for Th17 cells were higher, especially in those with advanced disease. The progenitor of Th17, CD4+CD161+ cell was increased in PBC. Moreover, the percentage of Th17 cells was positively related with CD4+CD161+ cell. After stimulation with IL-23 and IL-1β which were improved in PBC patients, CD4+CD161+ cells from PBC patients expressed more IL-17, although their proliferation were not different between 2 groups. IL-17 can promote the proliferation of HSCs at a dose-dependent method, and also increase the IL-8 expression in a dose/time-dependent way. Anti-IL-17 can neutralize the above reactions.
CD4+CD161+ cells are a source of increased Th17 in PBC patients. With disease progression, Th17 population decreased in the circulation, accompanied by greater accumulation in the liver, which is regulated by CCL20 in advanced patients. IL-17 may be involved in the process of PBC fibrosis.
INTRODUCTION
Primary biliary cirrhosis (PBC) is a typical organ-specific autoimmune liver disease characterized by the presence of serum anti-mitochondrial antibodies (AMAs) and the destruction of small- and medium-sized intrahepatic bile ducts.1 In addition to genetic susceptibility2 and environmental factors,3,4 the immunological or inflammatory component is one of the most crucial players in PBC pathogenesis.5 It is commonly accepted that immune dysfunction, unbalanced T helper (Th) cell response, and corresponding cytokines/chemokines play a significant role in PBC.5
Recently, Th17 cells have been proposed to represent a novel cell lineage due to their unique cytokine production and transcription factor profile. Th17 cells are of particular importance for host mucosal defense against extracellular infections 6 and development of autoimmune diseases such as experimental autoimmune encephalitis,7,8 rheumatoid arthritis,9,10 and inflammatory bowel disease.11 Interleukin (IL)-17, the signature cytokine produced by Th17 cells, participates in tissue destruction and induces proinflammatory mediators.12 It also contributes to organ fibrosis.13–15 Not surprisingly, clinical trials testing the potential of targeting the Th17 cell pathway as a treatment for autoimmune diseases are currently underway.16,17
The ratio of Th17 to Treg cells as well as the level of serum Th17-correlated cytokines was found to be significantly elevated in peripheral blood mononuclear cells (PBMCs) of patients with PBC compared with those of healthy individuals, leading researchers to hypothesize a pathogenic role of Th17 in PBC.18 Researchers found that biliary epithelial cells hold the ability to produce Th17-inducible cytokines (IL-6, IL-1β, and IL-23) when stimulated with pathogen-associated molecular patterns.19 In addition, IL-17+ cells were shown to accumulate around the damaged bile ducts.19,20 Furthermore, IL-12p35−/− dominant-negative transforming growth factor-β receptor II mice demonstrated a distinct cytokine profile characterized by a shift from Th1 to a Th17 response associated with occurrence of liver fibrosis, implying the involvement of a Th17 response in the development of biliary fibrosis.21 However, no studies have identified the source of elevated Th17 cells and their distribution during different disease phases in PBC. The probable mechanism for liver fibrosis of Th17 cells is also unclear.
In this study, we first investigated whether circulating Th17 cells and levels of IL-17 in liver were increased in PBC. The percentage of Th17 cells at different disease stages was also analyzed. The level of chemokine (c-c motif) ligand (CCL) 20, a chemokine for Th17 cells, was measured to explain the reason for distribution of Th17 cells at diverse phases of the disease. Then, we explored the source of elevated Th17 cells in PBC patients. Finally, we identified the effect of IL-17 on hepatic stellate cells (HSCs) to reveal the probable mechanisms of Th17 population for disease progression in PBC.
MATERIALS AND METHODS
Patients and Samples
Thirty-five patients with PBC and 15 patients with chronic hepatitis B, as well as 35 age- and sex-matched healthy controls (HCs) were enrolled in the study. The study was approved by the Ethics Committee of Peking Union Medical College Hospital in Beijing. Written informed consent was obtained from each study participant. PBMCs were isolated within 4 hours after blood collection. Serums were stored at −80°C until measurement. Liver biopsy specimens from 16 patients with PBC were obtained for immunohistological analysis. Owing to the ethical issues, liver specimens were from 4 patients with large hepatic hemangioma who underwent hemangioma resection when the tumor was growing or the abdominal manifestation was obvious as control group.
PBC was diagnosed by the following criteria: elevated alkaline phosphatase and γ-glutamyltransferase levels for >6 months, and the presence of AMAs in serum or representative histological manifestations of bile duct injury.
Inclusion criteria of chronic hepatitis B in our study were as follows: patients with positive results for serum hepatitis B surface antigen or hepatitis B virus DNA > 10,000 copies/mL for >6 months, and persistently elevated alanine aminotransferase or aspartate transaminase level for 3 months before treatment.
Disease Stage
Patients with liver biopsies were grouped into early (E-PBC group, stages I and II) and advanced PBC (A-PBC group, stages III and IV) in accordance with the Ludwig classification.22 A-PBC group also included patients who lacked liver biopsies, but presented with hepatic decompensation indications such as portal hypertension, hypoproteinemia, and coagulant function abnormalities.23
Flow Cytometry
PBMCs were isolated by Ficoll-Hypaque density centrifugation. The cells were stained with FITC-conjugated anti-CD4 mAb (eBioscience: USA) and PE-Cy5-conjugated anti-CD161 mAb (BD Pharmingen: USA) following published protocols. Isotype controls were used to ensure antibody specificity. For intracellular staining, 1 × 106 cells/well were cultured in 24-well plates in a total volume of 1 mL. The culture medium consisted of RPMI 1640, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, and 10% inactivated fetal bovine serum (Hyclone: USA) in the presence of 20 ng/mL phorbol-12-myristate-13-acetate (Sigma Aldrich: USA) and 1000 ng/mL ionomycin (Sigma Aldrich: USA) at 37°C in humidified air with 5% CO2 for 5 hours. After 1 hour of culture, 1 μL GolgiPlug (BD Pharmingen: USA) was added. PE-conjugated anti-IL-17 mAb (eBioscience: USA) and appropriate isotype controls were used for identifying the expression of IL-17. Analysis was carried out with a BD FACS Aria II instrument using FCS Express V3 software.
Isolation and Culture of CD161+CD4+ Cells
Because of the needs for plenty of cells, CD4+CD161+ cells were sorted by flow cytometry from another 6 PBC patients and 6 age- and sex-matched HCs.
PBMCs were stained with PE-Cy5-conjugated anti-CD161 mAb (BD Pharmingen: USA) and PE-Cy7-conjugated anti-CD4 mAb (eBioscience: USA), and CD4+CD161+ cells were sorted with a FACS Aria II flow cytometer (Becton Dickinson: BD, USA). With anti-CD3/CD28-conjugated T cell expander, purified CD4+CD161+ cells were cultured in the presence of IL-23 (50 ng/mL) (Invitrogen: USA) and IL-1β (50 ng/mL) (Invitrogen: USA) for 5 days as described in a previous study.24 After culture, IL-17 production was determined by enzyme-linked immunosorbent assay (ELISA) (eBioscience: USA) in cultural supernatants.
Culture of HSCs and Proliferation Assay
Cell counting kit-8 assay was used to identify the ability of cell proliferation based on the manufacturers’ instructions. 5 × 103 human HSC cells (Shang Hai, China)/well were cultured in 96-well plates containing DMEM (Gibico: Australia) and 10% inactivated fetal bovine serum in a total volume of 100 μL in the presence of 0, 1, 5, and 10 ng/mL IL-17 (Peprotech: USA) or 1 μg/mL anti-IL-17 (Peprotech: USA) at 37°C in humidified air with 5% CO2 for 24, 72, and 144 hours, respectively. Then, cell counting kit-8 solution (Dojindo: Japan) was added to the cell culture medium to a final concentration of 10 μL/100 μL and incubated for another 1 hour. Absorbance was measured at 490 and 630 nm to determine cell proliferation using a microplate reader. All experiments were performed in quadruplicate. 5 × 104 HSCs/well were cultured in 24-well plates with 1 mL medium as described before. The culture supernatant were collected and stored for IL-8 measurement by ELISA.
ELISA
The concentrations of CCL20 (R&D Systems: USA), IL-23 (R&D Systems: USA), and IL-1β (R&D Systems: USA) in serums of patients with PBC and HCs were measured with ELISA kits according to the manufacturers’ instructions. IL-17 (eBioscience: USA) and IL-8 (eBioscience: USA) in culture supernatant were also tested with corresponding ELISA kits after cell culture.
Immunohistochemistry
All fresh liver specimens were fixed in 10% buffered formalin overnight at 4°C, embedded in paraffin, and cut into 4 μm sections. Hematoxylin-eosin staining was performed to evaluate the disease stage by histology.
Before immunohistochemical staining, the tissue sections were deparaffinized and rehydrated to water. Endogenous peroxidase was blocked with 3% H2O2 for 20 minutes at room temperature. The slides were treated with a 0.1 mol/L concentration of citrate buffer in an 800-W microwave oven for 15 minutes for antigen retrieval, and then rinsed in distilled water and in phosphate buffered saline for 5 minutes, respectively.
For immunohistochemical staining of IL-17 and CCL20, respectively, 5% normal horse serum was used to suppress nonspecific protein binding, and 5 μL/mL goat anti-human-IL-17 antibody (R&D Systems: USA) or anti-human-CCL20 antibody (R&D Systems: USA) was applied to the tissue sections overnight at 4°C. The slides were washed and incubated with horseradish peroxidase-conjugated mouse anti-goat IgG for 1 hour at room temperature, and then incubated with avidin-biotin complex for 45 minutes. Color was developed with diaminobenzidine tetrahydrochloride.
Statistical Analysis
SPSS17.0 software package (SPSS Inc, Chicago, IL) was used for statistical analysis. The data were presented as the mean ± standard deviation in intergroup comparisons. The Student t test and the nonparametric Mann–Whitney U test were used to determine differences between 2 groups, whereas one-way ANOVA was used to compare differences among the multiple groups. Repeated measures analysis of variance was used to evaluate the continuous data. Statistical significance was defined as a P value <0.05. The statistical power is 0.8, and the sample size was estimated accordingly except for functional examination (CD4+CD161+ cells culture) in the paper.
RESULTS
Circulating Th17 Cells Are Elevated in PBC Patients, Especially in Those With Early-Stage Disease
The basic characteristics of the research subjects were shown in Table 1. According to criteria mentioned in “Methods” section, 22 E-PBC and 13 A-PBC patients were included, respectively.
TABLE 1.
The Basic Characteristics of the Study Patients
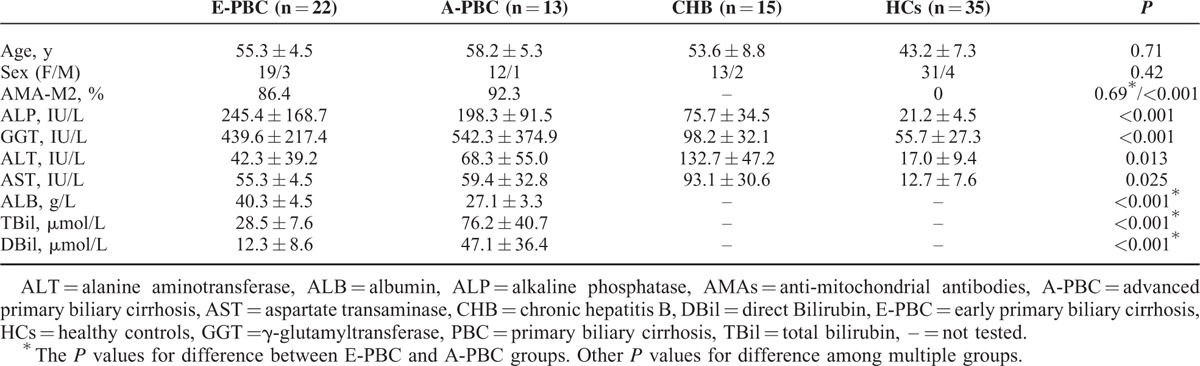
We first examined the IL-17 expression in CD4+ T cells in PBC patients (n = 35), chronic hepatitis B patients (n = 15), and HCs (n = 35) by flow cytometry. Circulating Th17 cells was elevated in PBC patients compared with HCs (1.03% ± 0.22% and 0.55% ± 0.20%, respectively; P < 0.001), and higher than those in chronic hepatic B (1.03% ± 0.22% and 0.67% ± 0.20%, respectively; P < 0.001; Figure 1A). In addition, E-PBC patients had higher levels of Th17 cells than A-PBC patients (1.09% ± 0.23% and 0.93% ± 0.16%, respectively; P = 0.035; Figure 1B). Representative flow cytometry results for Th17 cells from PBC patients, chronic hepatitis B, and HCs were shown in Figure 1C.
FIGURE 1.
Expression of IL-17 in CD4+ T cells in patients with PBC (n = 35), chronic hepatitis B (n = 15), and HCs (n = 35). (A) Frequency of Th17 cells in PBC patients, chronic hepatitis B, and HCs, respectively. (B) Frequency of Th17 cells in E-PBC and A-PBC patient groups, respectively. (C) Representative flow cytometry results for Th17 cells from PBC patients, CHB, and HCs. A-PBC = advanced primary biliary cirrhosis, CHB = chronic hepatitis B, E-PBC = early primary biliary cirrhosis, HCs = healthy controls, IL = interleukin, PBC = primary biliary cirrhosis, Th = T helper.
IL-17+ Expression Cells Infiltrated in Liver Are Evident in PBC Patients, Especially in Those With Advanced-Stage Disease
In our study, 11 liver biopsy specimens of E-PBC patients were acquired among 16 samples. In this E-PBC group, 9 (81.8%) patients were AMA-M2 positive. Among 5 A-PBC hepatic specimens, 1 (20%) was from AMA-M2 negative PBC patients.
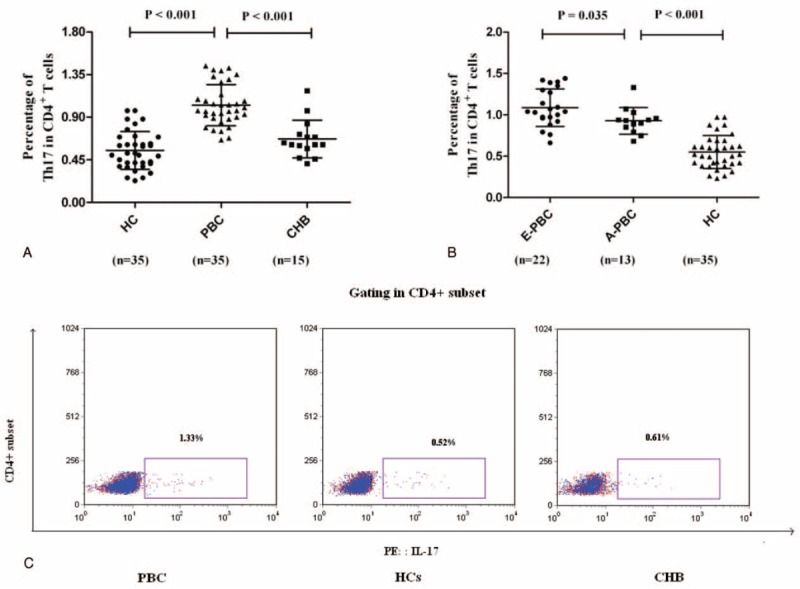
In PBC, cells in the specific organ are more vital than those in the circulation.25 This led us to explore IL-17 levels in the liver by immunohistochemistry. As expected, the IL-17+ cells were higher in the liver portal area in PBC patients, particularly of those in the advanced phase (Figure 2).
FIGURE 2.
Detection of IL-17+ cells in the liver in patients with PBC (n = 16) and HCs (n = 4). (×100) (Blank arrows indicate the IL-17+ cells, which were stained brown). HCs = healthy controls, IL = interleukin, PBC = primary biliary cirrhosis.
A-PBC Patients Have Higher Serum CCL20 That Might Lead to Liver Infiltration of IL-17+ Cells
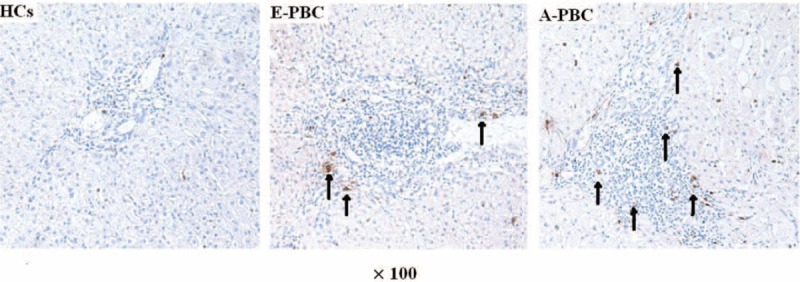
Serum CCL20, a chemokine for the Th17 population,26,27 was also enhanced in PBC patients (n = 35) compared with HCs (n = 35) (57.34 ± 36.21 pg/mL and 22.17 ± 18.50 pg/mL, respectively; P < 0.001; Figure 3A), and was much higher in the A-PBC group (n = 13) than in the E-PBC group (n = 22) by ELISA (89.37 ± 30.16 pg/mL and 29.02 ± 12.38 pg/mL, respectively; P < 0.001; Figure 3A). We also found hepatic cells secrete CCL20 by immunohistochemistry. Furthermore, the level of CCL20 in the liver was more obvious in PBC patients (Figure 3B). However, the percentage of CCR6+ cells was not significantly increased among CD4+ T cells in PBC patients no matter of disease stages (data were shown in the Supplementary 1).
FIGURE 3.
The level of CCL20 in serum and liver in PBC patients and HCs. (A) Levels of CCL20 in PBC patients (n = 35) and HCs (n = 35), respectively. (B) Hepatic CCL20 in PBC patients (n = 6) and HCs (n = 4), respectively. CCL20 = chemokine (c-c motif) ligand 20, HCs = healthy controls, PBC = primary biliary cirrhosis.
Increased CD4+CD161+ Cells Produce More IL-17 After Stimulation With IL-1β and IL-23 in PBC Patients, Contributing to the Elevated Th17 Population of PBC Patients
It is generally accepted that CD161 is a typical surface marker of the Th17 lineage and CD4+CD161+ cells are progenitors of Th17 cells.28–30 We found that the CD4+CD161+ population was larger among the CD4+ T cells in patients (n = 20) than HCs (n = 20) (22.22% ± 5.09% and 13.50% ± 3.78%, respectively; P < 0.001; Figure 4A). The percentage of CD4+CD161+ cells was significantly positively related to the Th17 cells (r2 = 0.25, P = 0.001; Figure 4B).
FIGURE 4.
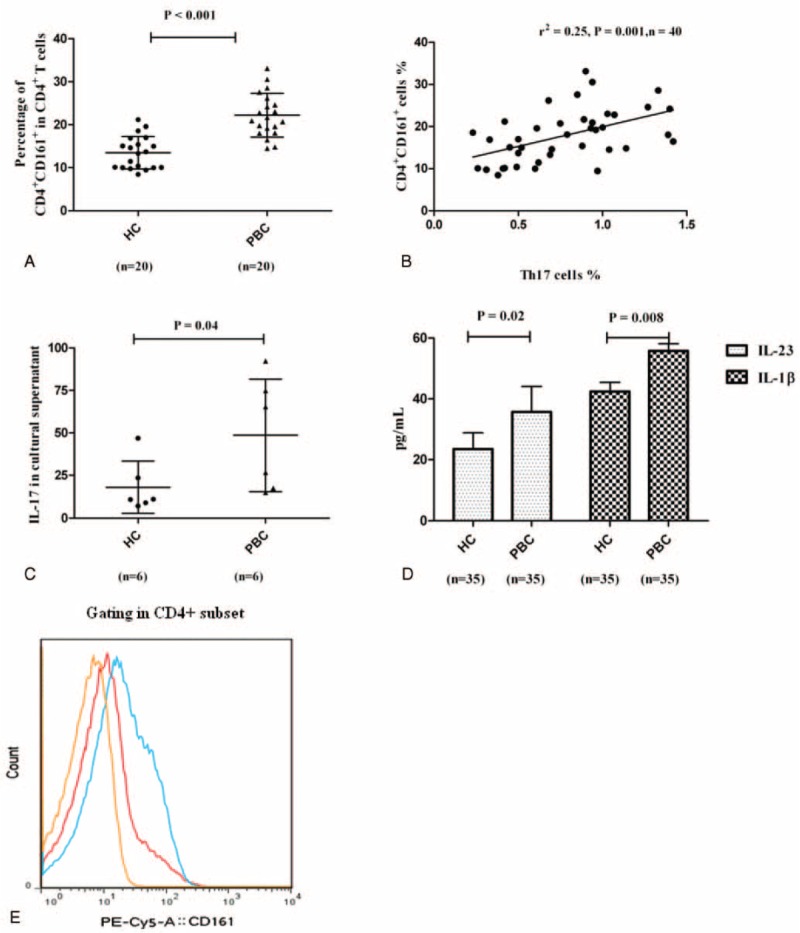
The source for elevated Th17 cells in PBC patients. (A) Frequency of CD161+CD4+ cells in the CD4+ T compartment in PBC patients (n = 20) and HCs (n = 20), respectively. (B) Relationship between Th17 cells and CD161+CD4+ cells (n = 40). (C) IL-17 expression of CD161+CD4+ cells after stimulation in vitro in PBC patients (n = 6) and HCs (n = 6), respectively. (D) Levels of serum IL-23 and IL-1β in PBC patients (n = 35) and HCs (n = 35), respectively. (E) Representative flow cytometry results for CD161+CD4+ cells in the CD4+ T compartment from PBC patients and HCs. HCs = healthy controls, IL = interleukin, PBC = primary biliary cirrhosis, Th = T helper.
CD4+CD161+ cells were then sorted by flow cytometry and cultured in 96-well plates. After stimulation with IL-23 and IL-1β for 5 days, IL-17 in cultural supernatant was higher in PBC patients (48.63 ± 32.99 pg/mL and 18.11 ± 15.23 pg/mL, respectively; P = 0.04; Figure 4C). Accordingly, the serum IL-1β (59.10 ± 9.38 pg/mL and 45.20 ± 8.45 pg/mL, respectively; P = 0.008) and IL-23 levels (35.8 ± 8.34 pg/mL and 23.5 ± 5.35 pg/mL, respectively; P = 0.02) were higher in patients compared with HCs (Figure 4D). However, the proliferation of CD4+CD161+ cells was not statistically different between 2 groups (data were shown in the Supplementary 2). Representative flow cytometry results for CD161+CD4+ cells in the CD4+ T compartment from PBC patients and HCs were shown in Figure 4E.
IL-17 Promotes the Proliferation and the IL-8 Expression of HSCs
The HSCs play a critical role in hepatic fibrogenesis. HSCs can be activated by profibrotic cytokines such as IL-8 and transforming growth factor-β in an autocrine manner, sequentially producing collagen type I. We used IL-17 to intervene human HSCs at different concentration of 0, 1, 5, and 10 ng/mL for diverse periods (Table 2). In preliminary experiment, we found proliferation of HSCs reached a maximum after stimulated with IL-17 at the concentration of 10 ng/mL. So, we added anti-IL-17 to detect its inhibitory effect on HSCs proliferation in the presence of 10 ng/mL IL-17. We found that IL-17 could promote HSCs proliferation dose-dependently (P < 0.001; Figure 5A), but there were no statistically difference among diverse time groups (P = 0.48; Figure 5A). This effect can be neutralized by IL-17 antagonist (P = 0.04; Figure 5A); however, the efficacy of anti-IL-17 attenuated with time extending.
TABLE 2.
The Proliferation of HSC After Stimulation With IL-17 at Different Concentrations
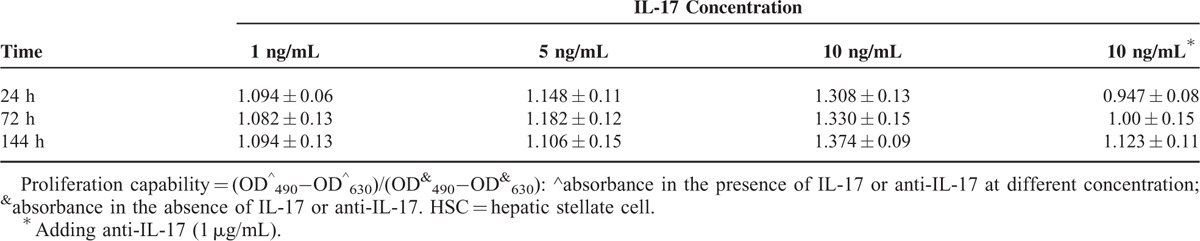
FIGURE 5.
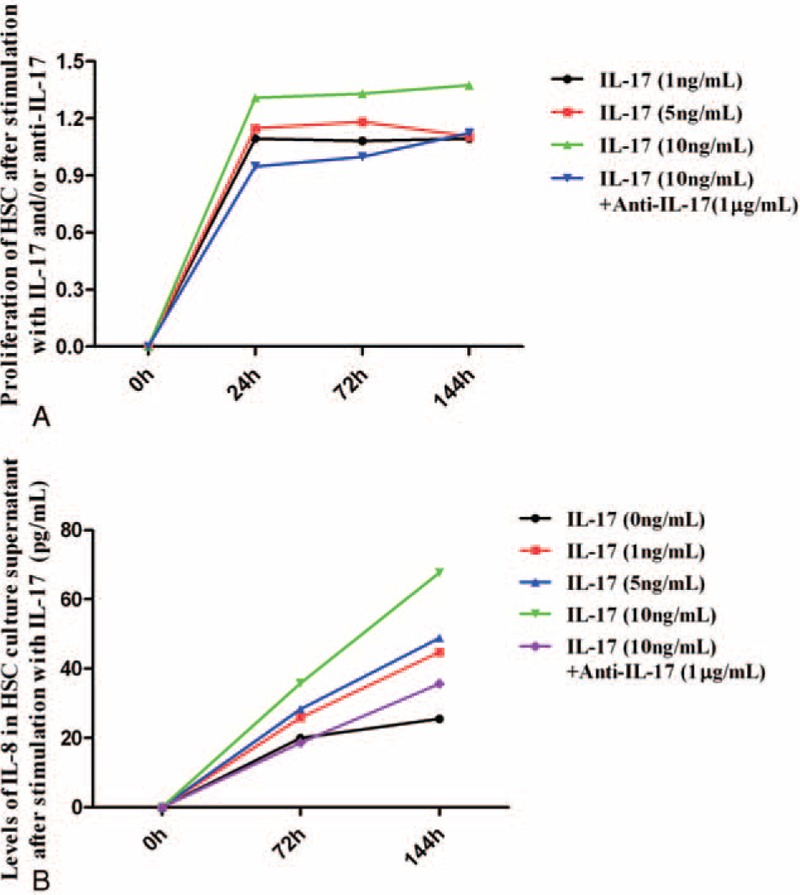
The influence of IL-17 on HSCs. (A) Proliferation of HSCs after stimulation with IL-17 at different concentrations. (B) Levels of IL-8 in HSCs culture supernatant after stimulation with IL-17 at different concentrations. HSC = hepatic stellate cell, IL = interleukin.
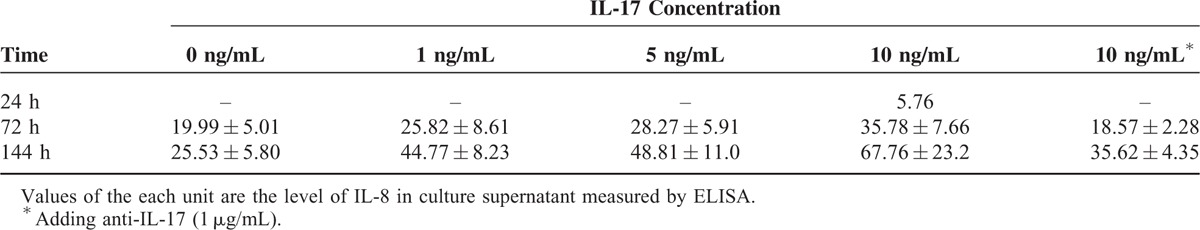
In our study, almost no IL-8 was tested in supernatant after culture of 24 hours. IL-8 secretion of HSC was elevated with IL-17 concentration or cultural duration increased, and deceased due to anti-IL-17 added (P < 0.001; Figure 5B, Table 3). However, the production of α-SMA may not be influenced by IL-17 tested by real-time reverse transcription polymerase chain reaction (data were shown in the Supplementary 3).
TABLE 3.
The Levels of IL-8 in Culture Supernatant After Stimulation With IL-17 at Different Concentrations
DISCUSSION
In our present study, we not only investigated the frequency of IL-17-expressing cells in the circulation and liver portal area in patients with PBC, but also analyzed the levels of Th17 cells during different disease stages. Our study is the first to report the source of increased IL-17 secretion from CD4+ T cells in these patients. The actions of effector IL-17 on HSCs for liver fibrosis were also investigated. Taken together, our results revealed a pathogenic role of Th17 cells in PBC.
CD4+ T cells have been classified into distinct subsets, namely Th1, Th2, Th17, Th9, and Tregs, depending on the major cytokines that they express. To date, numerous studies have demonstrated that Th17 cells are also involved in autoimmune diseases.31–33 In our study, we found Th17 cells in peripheral blood was significantly elevated as reported previously,18 especially in E-PBC patients, suggestive of their proinflammtory role in disease.
In PBC, T cells that have infiltrated the liver may be more important than circulating cells,25 as they can lead to the destruction of the biliary ducts.34,35 Therefore, we further examined IL-17+ cells in the liver, and particularly in the portal area. We found that IL-17+ cells were increased in the portal area of patients as previously described14,20; however, we did not find that they localized around the damaged bile ducts. Unlike in PBMCs, the higher frequency of IL-17+ cells occurred in the liver in later stage of the disease, which paralleled the altered levels of CCL20. One possible mechanism is that enhanced CCL20 expression in advanced stage of the disease causes Th17 cells to migrate to the liver, resulting in a decrease in Th17 cell numbers among the PBMCs. Meanwhile, these consequences suggest that IL-17 may participate in fibrosis in PBC. Unfortunately, we had no enough specimens to evaluate the hepatic CCL20 at different disease phases in our study.
In 2008, Cosmi et al29 reported CD161 was a novel surface indicator for human Th17 cells and that these cells originated from the CD161+CD4+ T cell progenitor. Moreover, CD161+CD4+ T cells can mediate destructive tissue inflammation in inflammatory bowel disease.36 To further confirm the mechanism for Th17 cell elevation in PBC, we assessed the levels of CD161+CD4+ T cells in the CD4+ T cell population. As expected, the CD161+ fraction of CD4+ T cells was increased in PBC patients compared with HCs. We found CD161+CD4+ T cells from PBC patients were prone to secrete more IL-17 than HCs after activation with proinflammatory cytokines IL-23 and IL-1β. Therefore, based on these data, we can conclude that the increased IL-17 expression in CD4+ T cells originates from the CD4+CD161+ cells.
The profibrotic characteristic of IL-17 has already been demonstrated in many tissues. For example, in bleomycin-induced idiopathic pulmonary fibrosis, early IL-17 production was essential during late fibrosis.14,37 In addition, IL-17 can lead to myocardial fibrosis in an isoproterenol-induced heart failure model.38 A study showed that IL-17A contributed to skin fibrosis in systemic sclerosis mouse.39 Moreover, immunization of the 2-octynoic acid coupled to bovine serum albumin-PBC mouse model with α-galactosylceramide, which is an invariant natural killer T cell activator, can exacerbate liver fibrosis and increase NKT and CD4+ T cells,40 which are the major IL-17 producers in the liver.41 The HSCs play a critical role in fibrogenesis in cirrhosis. After activation by different triggers, HSCs express collagen type I, secrete profibrogenic cytokines, and produce inhibitors of matrix-degrading enzymes (such as tissue inhibitor of matrix metalloproteinase-1),42 which cause the production of extracellular matrix deposition over degradation.43 Interestingly, we found IL-17 can promote HSCs proliferation dose-dependently, and anti-IL-17 can antagonize this action especially at earlier time. The cytokine IL-8 has been reported to be related to poorer survival in alcoholic liver disease 44 and chronic hepatitis C.45 In our study, we demonstrated that IL-17 could activate HSCs to express IL-8 in a dose- and time-dependent manner. The higher levels of IL-8 have been previously observed in late PBC.46 Therefore, our results, combined with these studies, suggest that Th17 cells in the liver of A-PBC patients have a profibrotic effect, which leads to disease progression.
There are also some limitations of the current study. First, only a small number of PBC patients had available liver biopsy specimens, and A-PBC patients with liver histology was an even smaller group in clinical practice, so we had no enough hepatic samples to analyze the levels of CCL20 according to disease stage at the last of experiment. Second, because of the relative low quantity of Th17 cells in CD4+ T cells, we did not sort them to co-culture with HSCs to confirm their specific fibrotic role. Lastly, A-PBC group in our study was heterogeneous, including both patients with histological evidence of fibrosis and patients without biopsies but clinical signs of decompensated cirrhosis; in this case, it can impact results to some extent.
CONCLUSION
Our study reveals that the increased number of CD4+CD161+ cell is the predominant source of the elevated Th17 levels in PBC patients. As the disease progresses, enhanced CCL20 levels lead to IL-17-expressing cells to home to the liver. Therefore, the Th17 population was more abundant in peripheral blood and lower in the liver of E-PBC patients. Th17 cells may play a profibrotic role in PBC.
Footnotes
Abbreviations: AMAs = anti-mitochondrial antibodies, CCL = chemokine (c-c motif) ligand, ELISA = enzyme-linked immunosorbent assay, HCs = healthy controls, HSCs = hepatic stellate cells, IL = interleukin, PBC = primary biliary cirrhosis, PBMCs = peripheral blood mononuclear cells, Th = T helper.
This work was supported by grants from the National Science Technology Pillar Program in the 11th Five-Year Plan (2008BAI59B03), the National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2012ZX09303006-002), and the Research Special Fund for Public Welfare Industry of Health (201202004).
TYS and TZ contributed equally to this work.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med 2005; 353:1261–1273. [DOI] [PubMed] [Google Scholar]
- 2.Hirschfield GM, Xie G, Lu E, et al. Association of primary biliary cirrhosis with variants in the CLEC16A, SOCS1, SPIB and SIAE immunomodulatory genes. Genes Immun 2012; 13:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dronamraju D, Odin J, Bach N. Primary biliary cirrhosis: environmental risk factors. Dis Markers 2010; 29:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNally RJ, James PW, Ducker S, et al. Seasonal variation in the patient diagnosis of primary biliary cirrhosis: further evidence for an environmental component to etiology. Hepatology 2011; 54:2099–2103. [DOI] [PubMed] [Google Scholar]
- 5.Shi TY, Zhang FC. Role of autoimmunity in primary biliary cirrhosis. World J Gastroenterol 2012; 18:7141–7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aujla SJ, Chan YR, Zheng M, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 2008; 14:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noubade R, Krementsov DN, Del Rio R, et al. Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood 2011; 118:3290–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jadidi-Niaragh F, Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol 2011; 74:1–13. [DOI] [PubMed] [Google Scholar]
- 9.van Hamburg JP, Asmawidjaja PS, Davelaar N, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum 2011; 63:73–83. [DOI] [PubMed] [Google Scholar]
- 10.Hot A, Miossec P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann Rheum Dis 2011; 70:727–732. [DOI] [PubMed] [Google Scholar]
- 11.Brand S. Crohn's disease: Th1 Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut 2009; 58:1152–1167. [DOI] [PubMed] [Google Scholar]
- 12.Romagnani S, Maggi E, Liotta F, et al. Properties and origin of human Th17 cells. Mol Immunol 2009; 47:3–7. [DOI] [PubMed] [Google Scholar]
- 13.Cortez DM, Feldman MD, Mummidi S, et al. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am J Physiol Heart Circ Physiol 2007; 293:H3356–3365. [DOI] [PubMed] [Google Scholar]
- 14.Wilson MS, Madala SK, Ramalingam TR, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med 2010; 207:535–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonian PL, Roark CL, Wehrmann F, et al. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol 2009; 182:657–665. [PMC free article] [PubMed] [Google Scholar]
- 16.Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med 2010; 2:52ra72. [DOI] [PubMed] [Google Scholar]
- 17.Genovese MC, Van den Bosch F, Roberson SA, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum 2010; 62:929–939. [DOI] [PubMed] [Google Scholar]
- 18.Rong G, Zhou Y, Xiong Y, et al. Imbalance between T helper type 17 and T regulatory cells in patients with primary biliary cirrhosis: the serum cytokine profile and peripheral cell population. Clin Exp Immunol 2009; 156:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harada K, Shimoda S, Sato Y, et al. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol 2009; 157:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan RY, Salunga TL, Tsuneyama K, et al. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J Autoimmun 2009; 32:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuda M, Zhang W, Yang GX, et al. Deletion of interleukin (IL)-12p35 induces liver fibrosis in dominant-negative TGFbeta receptor type II mice. Hepatology 2013; 57:806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol 1978; 379:103–112. [DOI] [PubMed] [Google Scholar]
- 23.Shi TY, Zhang LN, Chen H, et al. Risk factors for hepatic decompensation in patients with primary biliary cirrhosis. World J Gastroenterol 2013; 19:1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samson M, Audia S, Fraszczak J, et al. Th1 and Th17 lymphocytes expressing CD161 are implicated in giant cell arteritis and polymyalgia rheumatica pathogenesis. Arthritis Rheum 2012; 64:3788–3798. [DOI] [PubMed] [Google Scholar]
- 25.Kita H, Naidenko OV, Kronenberg M, et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology 2002; 123:1031–1043. [DOI] [PubMed] [Google Scholar]
- 26.Hirata T, Osuga Y, Takamura M, et al. Recruitment of CCR6-expressing Th17 cells by CCL 20 secreted from IL-1 beta-, TNF-alpha-, and IL-17A-stimulated endometriotic stromal cells. Endocrinology 2010; 151:5468–5476. [DOI] [PubMed] [Google Scholar]
- 27.Annunziato F, Cosmi L, Liotta F, et al. Type 17 T helper cells-origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol 2009; 5:325–331. [DOI] [PubMed] [Google Scholar]
- 28.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 2007; 8:639–646. [DOI] [PubMed] [Google Scholar]
- 29.Cosmi L, De Palma R, Santarlasci V, et al. Human interleukin 17-producing cells originate from a CD161 + CD4 + T cell precursor. J Exp Med 2008; 205:1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maggi L, Santarlasci V, Capone M, et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol 2010; 40:2174–2181. [DOI] [PubMed] [Google Scholar]
- 31.Carbajal KS, Mironova Y, Ulrich-Lewis JT, et al. Th cell diversity in experimental autoimmune encephalomyelitis and multiple sclerosis. J Immunol 2015; 195:2552–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun 2015; pii s0896-8411 (15) 30011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkatesha SH, Dudics S, Weingartner E, et al. Altered Th17/Treg balance and dysregulated IL-1beta response influence susceptibility/resistance to experimental autoimmune arthritis. Int J Immunopathol Pharmacol 2015; 28:318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang GX, Wu Y, Tsukamoto H, et al. CD8 T cells mediate direct biliary ductule damage in nonobese diabetic autoimmune biliary disease. J Immunol 2011; 186:1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuda M, Ambrosini YM, Zhang W, et al. Fine phenotypic and functional characterization of effector cluster of differentiation 8 positive T cells in human patients with primary biliary cirrhosis. Hepatology 2011; 54:1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleinschek MA, Boniface K, Sadekova S, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med 2009; 206:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasse P, Riteau N, Vacher R, et al. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS One 2011; 6:e23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng W, Li W, Liu W, et al. IL-17 induces myocardial fibrosis and enhances RANKL/OPG and MMP/TIMP signaling in isoproterenol-induced heart failure. Exp Mol Pathol 2009; 87:212–218. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto Y, Hasegawa M, Matsushita T, et al. Potential roles of interleukin-17A in the development of skin fibrosis in mice. Arthritis Rheum 2012; 64:3726–3735. [DOI] [PubMed] [Google Scholar]
- 40.Wu SJ, Yang YH, Tsuneyama K, et al. Innate immunity and primary biliary cirrhosis: activated invariant natural killer T cells exacerbate murine autoimmune cholangitis and fibrosis. Hepatology 2011; 53:915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan S, Wang L, Liu N, et al. Critical role of interleukin-17/interleukin-17 receptor axis in mediating Con A-induced hepatitis. Immunol Cell Biol 2011; 90:421–428. [DOI] [PubMed] [Google Scholar]
- 42.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 2015; 44-46:247–254. [DOI] [PubMed] [Google Scholar]
- 43.Su TH, Kao JH, Liu CJ. Molecular mechanism and treatment of viral hepatitis-related liver fibrosis. Int J Mol Sci 2014; 15:10578–10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang YS, Chan CY, Wu JC, et al. Serum levels of interleukin-8 in alcoholic liver disease: relationship with disease stage, biochemical parameters and survival. J Hepatol 1996; 24:377–384. [DOI] [PubMed] [Google Scholar]
- 45.Polyak SJ, Khabar KS, Rezeiq M, et al. Elevated levels of interleukin-8 in serum are associated with hepatitis C virus infection and resistance to interferon therapy. J Virol 2001; 75:6209–6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmermann HW, Seidler S, Gassler N, et al. Interleukin-8 is activated in patients with chronic liver diseases and associated with hepatic macrophage accumulation in human liver fibrosis. PLoS One 2011; 6:e21381. [DOI] [PMC free article] [PubMed] [Google Scholar]


