Abstract
Impact of atorvastatin on carotid intima-media thickness (CIMT) in patients with type 2 diabetes is still debating.
The aim of our study is to investigate atorvastatin as adjuvant treatment on CIMT in Chinese patients with type 2 diabetes by conducting a meta-analysis based on the randomized controlled trials (RCTs).
A systematic search of electronic database of the Pubmed, EMBASE, Cochrane Library, VIP database, China National Knowledge Infrastructure, and Wangfang up to January 2015 was conducted. Randomized controlled trials (RCTs) comparing atorvastatin adjuvant treatment to the hypoglycemic therapies or high-dose atorvastatin versus low-dose atorvastatin therapies for patients with type 2 diabetes were selected.
A total of 14 RCTs involving 1345 patients were included. Adjuvant treatment with atorvastatin was associated with a significant reduction in CIMT (weighted mean difference [WMD] = −0.17 mm; 95% confidence interval [CI] −0.22 to −0.12). Compared with the low-dose atorvastatin, high-dose atorvastatin treatment was associated with a significant reduction in CIMT (WMD = −0.17 mm; 95% CI: −0.32 to −0.02). Adjuvant treatment with atorvastatin reduced serum total cholesterol, triglyceride, low-density lipoproteins, and high sensitivity C-reactive protein levels. However, atorvastatin had no significant impact on blood glucose levels.
This meta-analysis demonstrated that treatment with atorvastatin significantly reduced CIMT in Chinese patients with type 2 diabetes. Moreover, high-dose atorvastatin appeared to have additional benefits in reducing CIMT than the low-dose atorvastatin.
INTRODUCTION
Diabetes mellitus is a global health problem. It is estimated that type 2 diabetes affects at least 285 million people worldwide and the total number of people will rise to 438 million in 2030.1 In China, an epidemic study indicated that there were 92.4 million diabetes adults in 2010.2 Diabetic patients have at least a 2-fold greater risk of developing cardiovascular disease (CVD) than the general population.3 The high mortality and morbidity of patients with type 2 diabetes is mainly because of its vascular complications. Atherosclerosis is the main pathological feature of type 2 diabetic macrovascular complications. Therefore, early management of subclinical atherosclerosis is necessary to prevent serious diabetic complications.
Carotid intima-media thickness (CIMT) is a well-known surrogate marker of subclinical atherosclerosis4 and CVD.5 Carotid intima-media thickness scanning is a safe, noninvasive, and relatively inexpensive method of assessing subclinical atherosclerosis.6 Detection and intervention of CIMT in diabetic patients allows timely treatment and prevention of diabetic vascular complications. Statins therapy has been demonstrated to decrease in the CIMT value, but the drug-specific effects of statins on CIMT are conflicting.7 Atorvastatin, is a well-accepted 3-hydroxy-3-methyl-glutaryl coenzyme A reductase drug for management of dyslipidemia in patients with CVD. The role of atorvastatin in CIMT progression has been established in a previously published meta-analysis.8However, the impact of atorvastatin on CIMT in patients with type 2 diabetes is still debating.9 Therefore, we conducted this meta-analysis based on the available randomized controlled trial (RCT) to provide a comprehensive summary of atorvastatin on CIMT progression in Chinese patients with type 2 diabetes.
METHODS
Search Strategies
We systematically conducted a search through PubMed, EMBASE, Cochrane Library, China National Knowledge Infrastructure, Wanfang, and VIP database up to January 2015. The following medical subject headings [Mesh] were used to identify studies: carotid artery intima-media thickness ([Mesh] OR CIMT [Mesh] OR carotid atherosclerosis [Mesh]) AND atorvastatin [Mesh] AND diabetes [Mesh] AND (random [Free Item] OR randomized controlled trials [Free Item] OR RCTs [Free Item]). We also hand searched reference lists of the retrieved papers to identify the additional eligible studies.
Study Selection
Inclusion criteria were as follows: (1) RCTs investigating atorvastatin treatment or comparison of high-dose versus low-dose atorvastatin in Chinese patients; (2) use of the ultrasound method to measure CIMT at baseline and at end of treatment; and (3) participants were diagnosed of type 2 diabetes based on the diagnostic criteria. High-dose atorvastatin treatment is defined at least 2 times bigger than the low-dose. Carotid intima-media thickness is defined as the measured distance between the luminal-intimal interface and the media-adventitial interface of the common carotid artery.10 Trials were excluded if: (1) trial did not evaluate CIMT change as an endpoint; (2) different therapeutic approaches had been used apart from atorvastatin intervention between 2 groups; and (3) nonrandomized controlled trials, case-control study, or cohort study. Patients who had already received atorvastatin or other statins therapy within 2 weeks were excluded.
Data Extraction and Quality Assessment
Two authors (NF and WH) extracted the following data from eligible articles independently: name of the first author, year of publication, sample size, age, gender, atorvastatin dose/control group dose, duration of treatment, CIMT at baseline, and at end of treatment. The quality of the individual study was evaluated according to the methodological quality of included RCTs using Cochrane risk of bias tool. This tool is based on the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome data, incomplete outcome data, and selective reporting and others.
Data Synthesis and Analysis
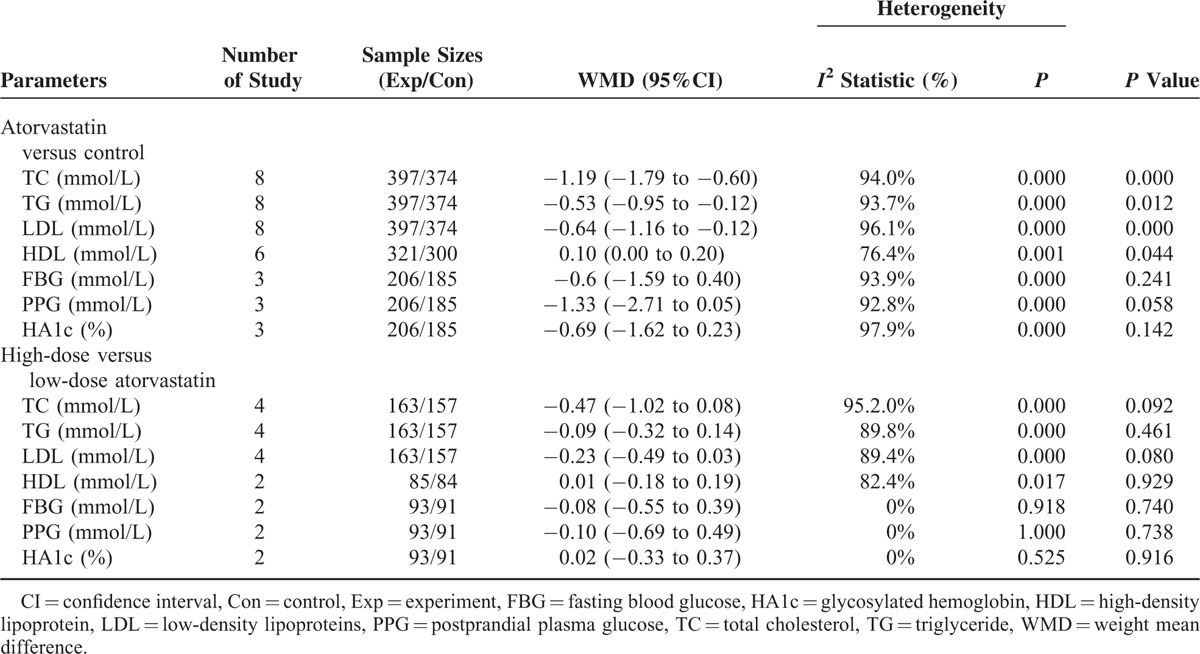
Carotid intima-media thickness was calculated as the weighted mean difference (WMD) or standardized mean difference (SMD) with 95% confidence interval (CI). Analyses were stratified by the atorvastatin versus control or high-dose versus low-dose atorvastatin. Statistical homogeneity was tested using Cochrane's Q test and I2 statistics. I2 statistic > 50% or P < 0.10 in Cochrane's Q test was deemed to have significant heterogeneity.11 Pooled effect sizes were calculated with a random effects model in the presence of significant heterogeneity; otherwise, a fixed-effect model was selected. Publication bias was determined by using the funnel plot, Begg’ s rank correlation test,12 and Egger's regression test.13 All the statistical analyses were performed by using STATA statistical software version 11.0 (StataCorp, College Station, TX).
RESULTS
Study Characteristics
The primary search yielded 238 records. After applying the predefined inclusion criteria, 14 RCTs14–27 were ultimately included in this meta-analysis. The process of selection of the trials is shown in Figure 1. The baseline characteristics of the eligible trials are presented in Table 1 . Of 14 RCTs, 10 trials14–23 compared the add-on effect of atorvastatin, and 4 trials24–27 provided the comparison of high-dose versus low-dose atorvastatin. A total of 1345 diabetic patients were identified. There was no significant difference in baseline CIMT between 2 groups. The duration of follow-up ranged from 24 weeks to 12 months. Sample size varied from 50 to 155 in the individual trials. The dose of atorvastatin was 10 mg, 20 mg, and 40 mg per day. Carotid intima-media thickness was measured by high-resolution B-mode carotid ultrasonography. In general, the included trials were in moderate quality and the detail quality assessments are shown in Figure 2.
FIGURE 1.
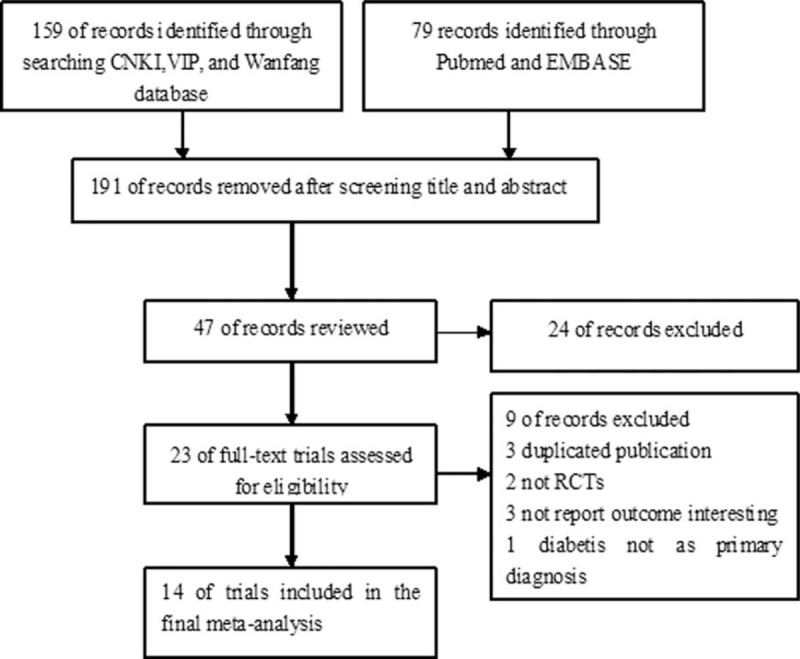
Flow diagram of study selection process.
TABLE 1.
Summary of the Characteristics of the Included Studies
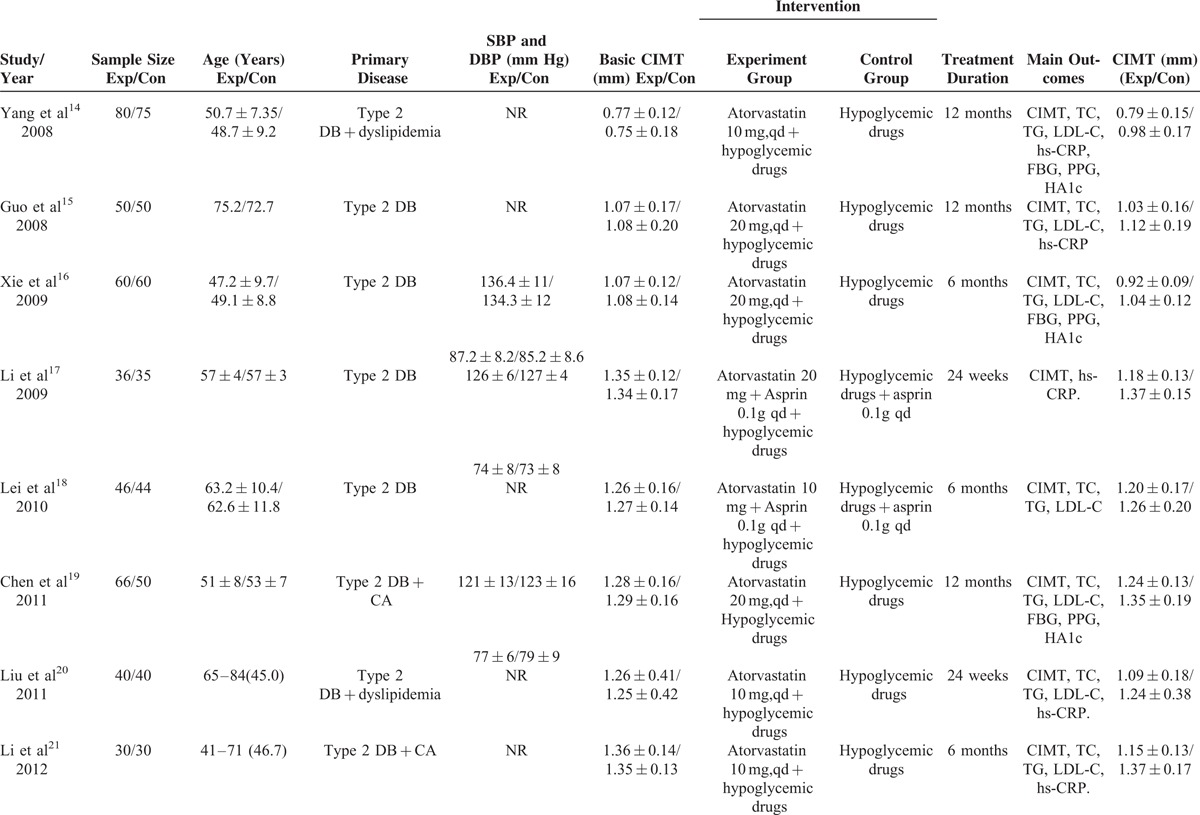
FIGURE 2.
Quality assessment of the included studies. Risk of bias graph (A); risk of bias summary (B).
Changes of CIMT
Ten trials14–23 provided the add-on effect of atorvastatin treatment on CIMT changes. Of 1025 patients, 524 were allocated to the atorvastatin group, whereas 501 were allocated to the control group. As shown in Figure 3(1), evidence of significant heterogeneity was found (I2 = 81.0%, P < 0.001), so we chose the random effects model. Adjuvant treatment with atorvastatin was associated with a significant reduction in CIMT (WMD = −0.17, 95% CI: −0.22 to−0.12). No evidences of publication bias were observed according to Begg's rank correlation test (P = 0.283), Egger's linear regression test (P = 0.233), and funnel plots (Fig. 4.)
FIGURE 3.
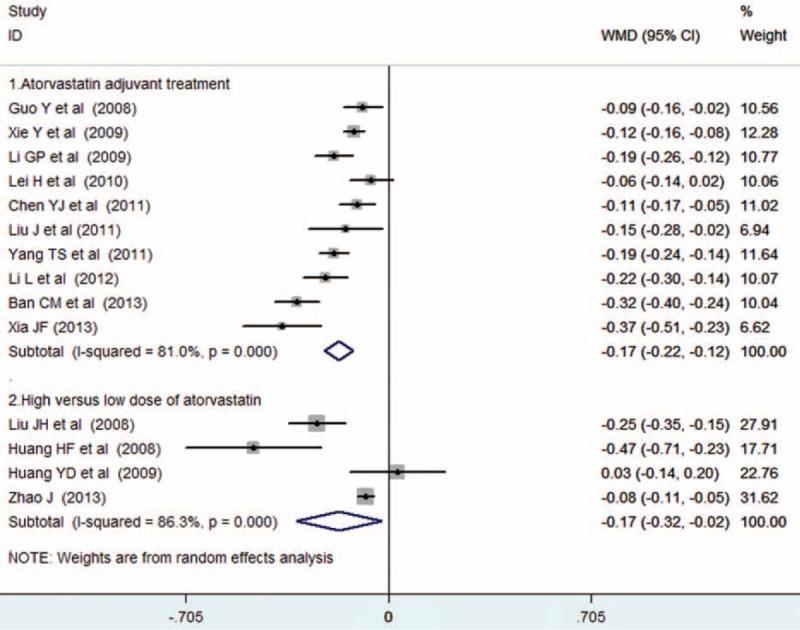
Forest plots showing weighted mean differences with 95% confidence intervals for reduction in carotid intima–media thickness in a random effects model.
FIGURE 4.

Funnel plots based on the changes of carotid intima–media thickness.
Four trials24–27 provided the comparison of high-dose versus low-dose atorvastatin on CIMT changes. Of 320 patients, 163 were allocated to the high-dose atorvastatin group, whereas 157 were allocated to the low-dose group. As shown in Figure 3(2), strong evidence of heterogeneity was also observed (I2 = 86.3%, P < 0.001), so we chose the random effects model. High-dose atorvastatin treatment was associated with a significant decrease in CIMT (WMD = −0.17, 95% CI: −0.32 to −0.02). Publication bias was not observed by Begg's rank correlation test (P = 0.350) and Egger's linear regression test (P = 0.203).
Changes of Metabolic Parameters
Table 2 lists the changes of total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoproteins (LDL), fasting blood glucose (FBG), glycosylated hemoglobin (HA1c), and postprandial plasma glucose (PPG) between the 2 groups. Overall, adjuvant treatment with atorvastatin was associated with a significant increase in TC (WMD = −1.19 mmol/L, 5% CI −1.79 to −0.60), TG (WMD = 0.53 mmol/L, 95% CI: −0.95 to −0.12), and LDL (WMD = −0.64 mmol/L, 95% CI: −1.16 to −0.12) and increase in HDL-C (WMD = 0.10 mmol/L, 95% CI: 0.00 to 0.20). There were no significant differences in the changes of FPG, PPG, and HA1c between the 2 groups. Compared with the low-dose atorvastatin, high-dose atorvastatin showed some trends in improvement in TC, TG, HDL, LDL, FBG, PPG, and HA1c; however, there were no significant differences in the changes of metabolic parameters between the 2 groups.
TABLE 1 (Continued).
Summary of the Characteristics of the Included Studies
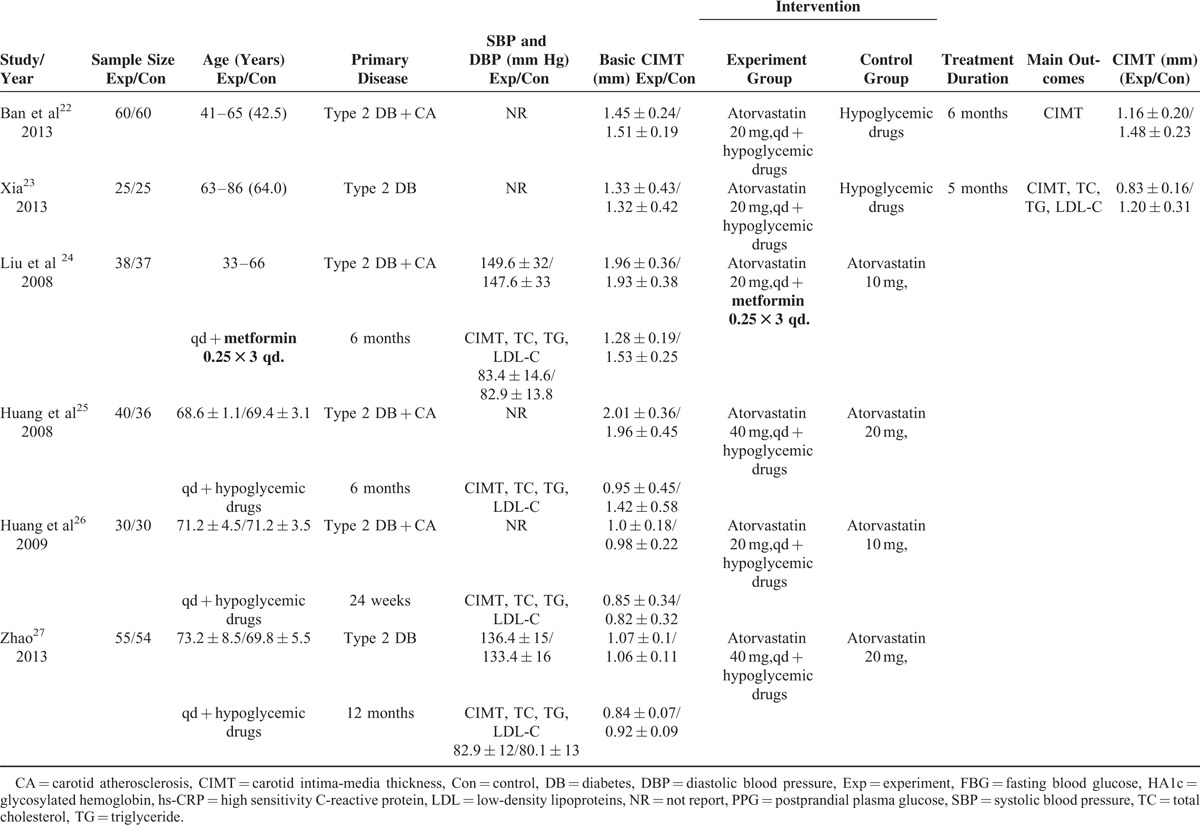
TABLE 2.
Comparison of the Change of Metabolic Parameters
Change of High Sensitivity C-reactive protein (hs-CRP)
Changes of serum hs-CRP levels were reported in 5 trials.14,15,17,20,21 Of 466 patients, 236 were allocated to the high-dose atorvastatin group, whereas 230 were allocated to the low-dose group. As shown in Figure 5, adjuvant treatment with atorvastatin was associated with a significant decrease in hs-CRP (SMD −3.26 mg/L, 95% CI: −4.78 to −1.74) in a random effects model. Evidence of significant heterogeneity was found (I2 = 97%, P < 0.001).
FIGURE 5.
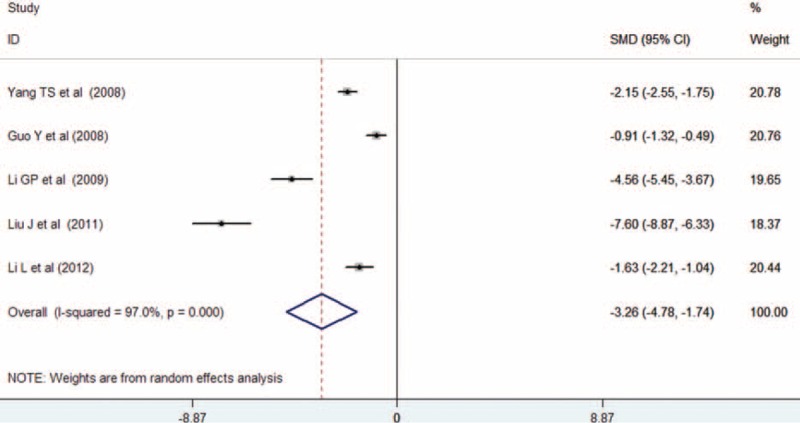
Forest plots showing standardized mean difference with 95% confidence intervals for improvement in serum high sensitivity C-reactive protein levels comparing atorvastatin to the control in a random effects model.
Subgroup Analyses and Sensitivity Analyses
Subgroup analyses were conducted based on the changes of CIMT by the presence of complication (carotid atherosclerosis or dyslipidemia). As shown in Figure 6, adjuvant treatment with atorvastatin reduced WMD of CIMT to −0.21 mm (95% CI: −0.22 to −0.12) among patients complicated with carotid atherosclerosis, −0.18 mm (95% CI: −0.23 to −0.14) among patients complicated with dyslipidemia, and −0.15 mm (95% CI: −0.22 to −0.08) among type 2 diabetic patients. Sensitivity analyses were performed by omitting 1 study at each turn to investigate the change of the overall WMD and 95%CI of CIMT. The results showed that there was just a slight change in the WMD or 95% CI, and no change in the direction of WMD when anyone study was omitted (Data not shown).
FIGURE 6.
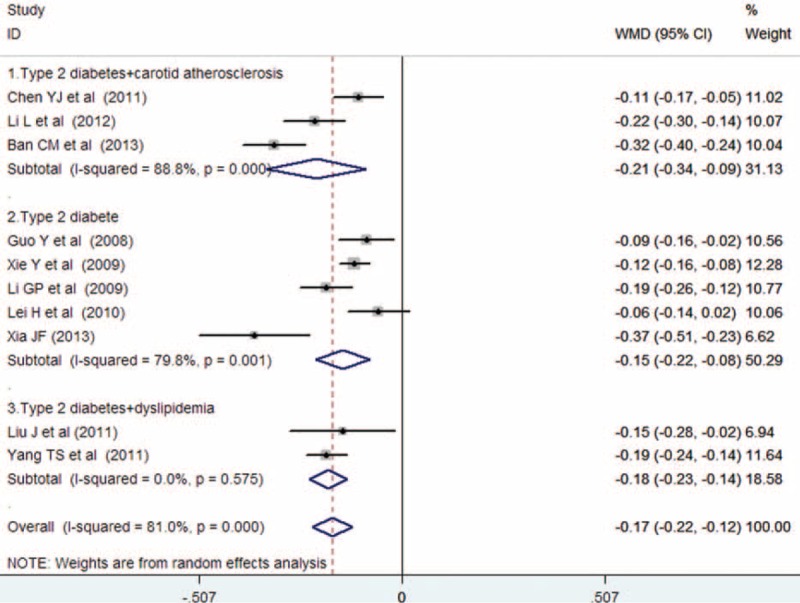
Subgroup analyses of carotid intima-media thickness changes based on the presence of carotid atherosclerosis or dyslipidemia.
DISCUSSION
The main finding of the study is that treatment with atorvastatin significantly reduces CIMT in Chinese patients with type 2 diabetes. High-dose atorvastatin appears to have an additional benefit on the progression of CIMT than the low-dose one. In addition, atorvastatin treatment is associated with reduction in serum hs-CRP levels as well as improvement in serum lipid levels. The beneficial effects of atorvastatin on CIMT regression or slowed progression might be attributable in part to the improvement in lipid profile as well as anti-inflammatory properties. However, atorvastatin treatment has no impact on serum FBG, HA1c, and PPG levels.
The efficacy of atorvastatin on the progression CIMT has been well established.8 Although high-dose atorvastatin produced favorable effects on lipid profiles in type 2 diabetes,28,29 the impact of atorvastatin on CIMT in patients with type 2 diabetes remains controversial. A study conducted in Greece showed that treatment 10 to 80 mg atorvastatin for 12 months significantly improved the lipid profile but without change of CIMT in type 2 diabetic patients.9 A recent report in China found that atorvastatin effectively reduced CIMT in new-onset type 2 diabetes patients.30 The relatively short duration of atorvastatin use might be a possible explanation for the conflicting results on CIMT progression.
To our best knowledge, this is the first meta-analysis to investigate atorvastatin on CIMT in type 2 diabetic patients. A systematic review revealed that type 2 diabetes was associated with a 0.13 mm increase in CIMT than the controls.31 CIMT, as measured by B-mode ultrasound, is an index of atherosclerotic progression. In the present study, adjuvant treatment with atorvastatin significantly decreased CIMT (WMD = −0.17 mm). Additionally, high-dose atorvastatin treatment appeared to produce greater reduction in CIMT (WMD = −0.17 mm). This result is in agreement with patients treated with high-dose of atorvastatin considerably suppressed CIMT after 12 months than the low-dose atorvastatin 9. Subgroup analysis suggested that adjuvant treatment with atorvastatin resulted in a greater reduction in CIMT (WMD = −0.21 mm) among patients complicated with carotid atherosclerosis, suggesting that atorvastatin had beneficial effect on CIMT regression. Moreover, atorvastatin reduced 0.18 mm CIMT in patients complicated with dyslipidemia and 0.15 mm in type 2 diabetic patients. These findings indicated that atorvastatin also had a beneficial effect on slowed CIMT progression. However, all the patients were Chinese people, and generalization of these findings to diverse populations should be cautioned.
Patients with type 2 diabetes typically present with a dyslipidemic profile. Dyslipidaemia in diabetes is mainly characterized by raised triglycerides and reduced HDL cholesterol levels.32 Atorvastatin is an effective and safe treatment for hyperlipidemia in Taiwanese diabetic patients.33 In the present study, we found that adjuvant treatment with atorvastatin was associated with a significant decrease in serum levels of TC, TG, and LDL levels as well as serum hs-CRP levels. Hs-CRP was a cardiovascular risk predictor in type 2 diabetics with normal lipid profile.34These findings suggested that the beneficial effects of atorvastatin on CIMT regression or slowed progression might be attributed to improve the dyslipidemic profile and serum hs-CRP levels.
Increased new-onset insulin resistance and type 2 diabetes risk have raised concerns regarding the use of statin.35 Most studies which reported an increased risk of diabetes were conducted in older patients in whom statins were started very late in life.36,37 However, drug-specific effects of statins on diabetes risk remain inconclusive. A well-designed meta-analysis suggested that it was important to balance the risks and benefits when administering specific statins.38 Animal study showed atorvastatin could prevent the development of type 2 diabetes in the rat model.39 Therefore, the risk of new-onset type 2 diabetes in relationship with atorvastatin treatment needs to be long-term follow-up.
Some limitations of this meta-analysis should be noted. First, the relatively low quality of the individual trials reduced the evidence. Second, high heterogeneity (I2 from 81% and 86.3%) was observed in the analysis of CIMT changes. The most likely sources of heterogeneity might be correlated with the duration of diabetes, dose of atorvastatin treatment, variation in the ultrasound method, and different duration of atorvastatin regimen. Third, blood pressure itself and antihypertensive agents may affect diversely on the results of the CIMT40,41; however, the information on blood pressure or antihypertensive agents uses was unavailable in most of the included trials. Finally, despite no evidence of the publication bias on CTIM changes was observed according to Begg's rank correlation test, Egger's linear regression test, and funnel plot, potential publication bias cannot be excluded due to all the included studies were conducted in China and published in Chinese.
CONCLUSIONS
This meta-analysis suggests that treatment with atorvastatin significantly reduce CIMT in Chinese patients with type 2 diabetes, and particularly contributes to the regression or slowed progression in those complicated with carotid atherosclerosis or dyslipidemia patients. Moreover, high-dose atorvastatin appears to have additional benefits in regression of CIMT than the low-dose atorvastatin. However, due to the methodological drawbacks, more well-designed RCTs are warranted to confirm our findings. In addition, long-term follow-up studies are needed to investigate potential adverse effects following atorvastatin treatment.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, CIMT = carotid intima-media thickness, CVD = cardiovascular disease, FBG = fasting blood glucose, HA1c = glycosylated hemoglobin, HDL = high-density lipoprotein, hs-CRP = high sensitivity C-reactive protein, LDL = low-density lipoproteins, PPG = postprandial plasma glucose, RCTs = randomized clinical trials, SMD = standardized mean difference, TC = total cholesterol, TG = triglyceride, WMD = weighted mean difference.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Whiting DR, Guariguata L, Weil C, et al. Idf diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011; 94:311–321. [DOI] [PubMed] [Google Scholar]
- 2.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in china. N Engl J Med 2010; 362:1090–1101. [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA 2004; 292:2495–2499. [DOI] [PubMed] [Google Scholar]
- 4.Jung CH, Baek AR, Kim KJ, et al. Association between cardiac autonomic neuropathy, diabetic retinopathy and carotid atherosclerosis in patients with type 2 diabetes. Endocrinol Metab (Seoul) 2013; 28:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sibal L, Agarwal SC, Home PD. Carotid intima–media thickness as a surrogate marker of cardiovascular disease in diabetes. Diabetes Metab Syndr Obes 2011; 4:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liviakis L, Pogue B, Paramsothy P, et al. Carotid intima–media thickness for the practicing lipidologist. J Clin Lipidol 2010; 4:24–35. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Li W, Dong L, et al. Effect of statin therapy on the progression of common carotid artery intima–media thickness: an updated systematic review and meta-analysis of randomized controlled trials. J Atheroscler Thromb 2013; 20:108–121. [DOI] [PubMed] [Google Scholar]
- 8.Takagi H, Yamamoto H, Iwata K, et al. Effects of atorvastatin on carotid intima media thickness: a meta-analysis of randomized controlled trials. Int J Cardiol 2012; 159:69–72. [DOI] [PubMed] [Google Scholar]
- 9.Kadoglou NP, Sailer N, Kapelouzou A, et al. Effects of atorvastatin on apelin, visfatin (nampt), ghrelin and early carotid atherosclerosis in patients with type 2 diabetes. Acta Diabetol 2012; 49:269–276. [DOI] [PubMed] [Google Scholar]
- 10.Pignoli P, Tremoli E, Poli A, et al. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986; 74:1399–1406. [DOI] [PubMed] [Google Scholar]
- 11.The Cochrane Collaboration; 2011; Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated march 2011]. Available from www.Cochrane-handbook.Org. [Google Scholar]
- 12.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 13.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang ST, Shao HG, Ouy J. Modulation effect on dislipidemia and atherosclerosis by atorvastatin in newly diagnosed type 2 diabetes patients. J Hunan Nomal UIliv (Med Sci) 2008; 5:38–41. [Google Scholar]
- 15.Guo Y, Sun LX, Li W, et al. Effect of atorvastatin on carotid atherosclerosis in elderly patients with type 2 diabetes mellitus. Chin J Geriatr Heart Brain Vessel Dis 2008; 10:757–759. [Google Scholar]
- 16.Xie Y, Duan SH, Hu T. The effect of atorvastatin on macroangiopathy in patients with type 2 diabetes mellitus and possible mechanism. Chin J Arterioscler 2009; 17:761–764. [Google Scholar]
- 17.Li GP, Li YB, Pen N, et al. The effect of atorvastatin on the level of serum adiponectin and carotid intima media thickness in type 2 diabetes mellitus. Chin J Diffic Compl Cases 2009; 8:335–337. [Google Scholar]
- 18.Lei H, Chen GC, Lu P, et al. Observation of therapeutic effect of atorvastatin in treatment of patients with type 2 diabetes with carotid atherosclerosis. Mod Med J 2010; 38:387–389. [Google Scholar]
- 19.Chen YJ, Liu ZM, Zhang YP. The therapeutic effect of atorvastatin intervention on the carotid intima medial thickness in newly diagnosed type 2 diabetic patients complicated with carotid atherosclerosis. Chin J Diffic Complic Cases 2011; 10:744–747. [Google Scholar]
- 20.Liu J, Mei QC, Huang T. Effects of atorvastatin on carotid artery plaque in elderly patients in type 2 diabetis with dyslipidemia. Chin J Microcirc 2011; 21:57–59. [Google Scholar]
- 21.Li L, Qiu P, Chen YH. The effect of atorvastatin detection and measurement of serum crp levels in patients with type 2 diabetes with carotid atherosclerosis and effect of atorvastatin intervention. Guide China Med 2012; 10:461–463. [Google Scholar]
- 22.Ban CM. Intervention study of atorvastatin for carotid artery intima–media thickness of type 2 diabetes. Mod Diagn Treat 2013; 24:2886–2888. [Google Scholar]
- 23.Xia JF. Effect of atorvastatin on carotid atherosclerosis plaque in patients with type 2 diabetes mellitus. Anhui Med J 2013; 10:1529–1531. [Google Scholar]
- 24.Liu JH, Hu CH, Zhu SW, et al. Clinical observation of different doses of atorvastatin in treatment of type 2 diabetes with carotid atherosclerosis. Prev Treat Cardio-Cerebral-Vascular Dis 2008; 8:423–424. [Google Scholar]
- 25.Huang HF, Yu HF, Zhao BB, et al. Effects of different doses of atorvastatin on carotid atherosclerosis in patients with type 2 diabetes mellitus. Prev Treat Cardio-Cerebral-Vascular Dis 2008; 8:110–111. [Google Scholar]
- 26.Huang YD, Zhang L, Zhao XP, et al. Clinical observation of different doses of atorvastatin in treatment of 60 cases of senile diabetes with carotid artery atherosclerotic plaque. Hainan Med J 2009; 20:47–48. [Google Scholar]
- 27.Zhao RH. Effect of atorvastatin on intima–media thickness of carotid and endothelial function in elderly patients with type 2 diabetes mellitus. Chin J Postgr Med 2013; 36:16–21. [Google Scholar]
- 28.Lawrence JM, Reid J, Taylor GJ, et al. The effect of high dose atorvastatin therapy on lipids and lipoprotein subfractions in overweight patients with type 2 diabetes. Atherosclerosis 2004; 174:141–149. [DOI] [PubMed] [Google Scholar]
- 29.Akalin A, Temiz G, Akcar N, et al. Short term effects of atorvastatin on endothelial functions and oxidized ldl levels in patients with type 2 diabetes. Endocr J 2008; 55:861–866. [DOI] [PubMed] [Google Scholar]
- 30.Yu D, Wang Y, Chi J, et al. Impacts of atorvastatin on blood lipids and arterial media thickness in new-onset type 2 diabetes patients. Zhonghua Liu Xing Bing Xue Za Zhi 2014; 35:733–736. [PubMed] [Google Scholar]
- 31.Brohall G, Oden A, Fagerberg B. Carotid artery intima–media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23:609–616. [DOI] [PubMed] [Google Scholar]
- 32.Jaiswal M, Schinske A, Pop-Busui R. Lipids and lipid management in diabetes. Best Pract Res Clin Endocrinol Metab 2014; 28:325–338. [DOI] [PubMed] [Google Scholar]
- 33.Sheu SJ, Liu NC, Ger LP, et al. High hba1c level was the most important factor associated with prevalence of diabetic retinopathy in taiwanese type II diabetic patients with a fixed duration. Graefes Arch Clin Exp Ophthalmol 2013; 251:2087–2092. [DOI] [PubMed] [Google Scholar]
- 34.Asegaonkar SB, Marathe A, Tekade ML, et al. High-sensitivity c-reactive protein: a novel cardiovascular risk predictor in type 2 diabetics with normal lipid profile. J Diabetes Complications 2011; 25:368–370. [DOI] [PubMed] [Google Scholar]
- 35.Preiss D, Sattar N. Pharmacotherapy: statins and new-onset diabetes—the important questions. Nat Rev Cardiol 2012; 9:190–192. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (prosper): a randomised controlled trial. Lancet 2002; 360:1623–1630. [DOI] [PubMed] [Google Scholar]
- 37.Waters DD, Ho JE, DeMicco DA, et al. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol 2011; 57:1535–1545. [DOI] [PubMed] [Google Scholar]
- 38.Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol 2013; 111:1123–1130. [DOI] [PubMed] [Google Scholar]
- 39.Madhu SV, Aslam M, Galav V, et al. Atorvastatin prevents type 2 diabetes mellitus—an experimental study. Eur J Pharmacol 2014; 728:135–140. [DOI] [PubMed] [Google Scholar]
- 40.Ohta Y, Kawano Y, Iwashima Y, et al. Control of home blood pressure with an amlodipine- or losartan-based regimen and progression of carotid artery intima–media thickness in hypertensive patients: the HOSP substudy. Clin Exp Hypertens 2013; 35:279–284. [DOI] [PubMed] [Google Scholar]
- 41.Baguet JP, Asmar R, Valensi P, et al. Effects of candesartan cilexetil on carotid remodeling in hypertensive diabetic patients: the MITEC study. Vasc Health Risk Manag 2009; 5:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]


