Abstract
Recent studies have demonstrated that exon 19 deletion (19 Del) and exon 21 L858R mutation (L858R) are 2 different types of sensitive epidermal growth factor receptor (EGFR) mutations in nonsmall cell lung cancer (NSCLC). However, whether there are some differences between those 2 groups in baseline clinical characteristics is still unclear.
We enrolled consecutive 1271 NSCLC patients detected with either 19 Del or L858R and collected their baseline clinical characteristics including age, sex, comorbidity, smoking and drinking status, body mass index (BMI), TNM stage, histologic type, differentiation, tumor maximum diameter (TMD), and CEA level. χ2 test and multivariate logistic regression analysis were used to compare the difference.
We found a higher percentage of 19 Del in younger patients group (< = 50 yr) than L858R (P < 0.001) through χ2 test. Besides, patients with 19 Del have higher risk of lymph node metastasis (P < 0.001). However, there were no significant differences in other items of clinical characteristics between 19 Del and L858R. Multivariate analysis showed similar significant results. Subgroup analysis in different age groups (10 yr as an interval) and N stages (stratified by N0, N1, N2, and N3) also indicated above-mentioned trends.
NSCLC patients with 19 Del are more likely to be young and have lymphatic metastasis than those with L858R. Age and N stage might be considered in predicting EGFR mutation type in NSCLC.
INTRODUCTION
Nonsmall-cell lung cancer (NSCLC) is the predominant form of lung cancer, the leading cause of cancer-related mortality worldwide, and patients are usually diagnosed in the advanced stages of disease.1–4 NSCLC could be caused by the accumulation of genetic alterations. The most common one is kirsten rat sarcoma viral oncogene (KRAS) mutations (22%), followed by epidermal growth factor receptor (EGFR) mutations (17%), and anaplastic lymphoma kinase (ALK) rearrangement (7%).5 EGFR mutations can be divided into common EGFR mutations (19 Del/ L858R) and rare EGFR mutations. While common EGFR mutations considering EGFR tyrosine kinase inhibitors (EGFR-TKIs) as first-line treatment,6 platinum-based chemotherapy should be a first-line treatment for rare EGFR mutation.7–9
However, 19 Del and L858R are 2 different types of sensitive EGFR mutations in NSCLC. Recent studies have reported that 19 Del and L858R have different responses to EGFR-TKIs. EGFR-TKIs treatment is more effective than chemotherapy in 19 Del patients. But for the patients with L858R, EGFR-TKIs treatment and chemotherapy have similar effect and chemotherapy might even be better.10,11 This breaks the previous idea that EGFR mutation patients should use TKI as possible. It also indicated that the 2 population, 19 Del patients and L858R ones, are different. However, whether there were any differences between those 2 groups in clinical characteristics is still unclear.
Therefore, we sought to conduct a retrospective study to assess the difference of clinical characteristics between patients with 19 Del and those with L858R in nonsmall cell lung cancer in southern Chinese.
MATERIALS AND METHODS
Study Population
We identified 1323 records of patients who had EGFR gene mutation positive in 5248 patients from October 2008 to April 2014 at Sun Yat-Sen University Cancer Center, Guangzhou, China. The study was approved by the Institutional Review Board of Sun Yat-Sen University Cancer Center. All the patients had provided written informed consent before samples were collected. In these records, 5 are neither 19 Del nor L858R. Two records are missing too much information while another 5 are repeat records. Therefore, we included 1311 records of patients. Figure 1 summarizes the flow chart. Among these patients, 4 are mixed mutation of 19 Del and L858R. Two are small cell lung cancer patients and 25 of them are not lung cancer patients. In addition, 3 patients’ lung cancer is metastatic. Another 4 patients are cancer in situ of the lung with T-stage marking Tis. The above patients were excluded and finally 1271 patients were included in the study. The clinicopathological features of the patients included age, sex, and comorbidity, smoking history, drinking history, body mass index (BMI), TNM stage, histologic type, differentiation, tumor maximum diameter (TMD), and carcino-embryonic antigen (CEA) level.
FIGURE 1.
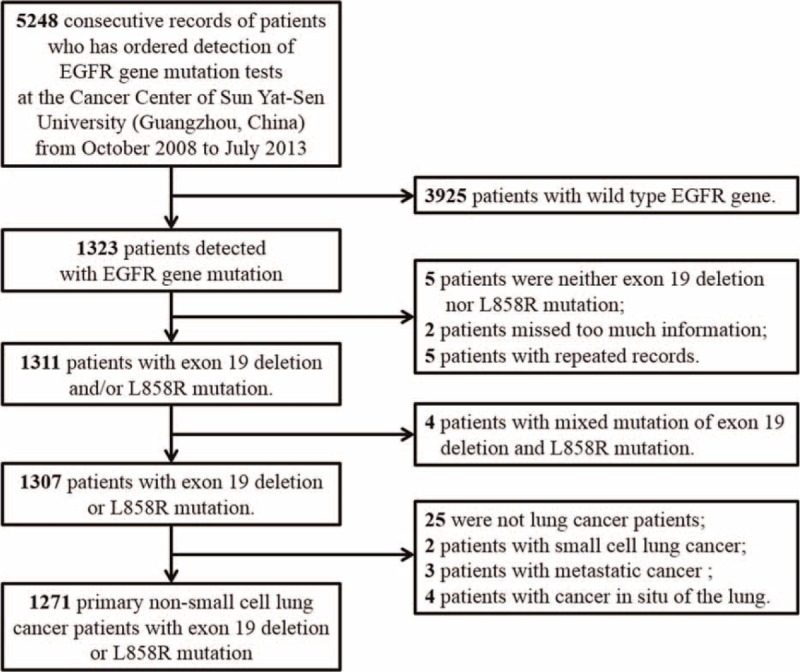
Flow chart of the enrollment.
Categories of each characteristic were divided as follows: for age, patients more than 50 years old were considered the older group. Smoking, alcohol history, and comorbidities were noted as yes or no. BMI equal to or larger than 24 kg/m2 was considered overweight group and the rest as normal group. Histologic type is divided into adenocarcinoma and non-adenocarcinoma. Differentiation is considered high, moderate, and low. High CEA level was defined, if CEA level in serum was >5 ng/mL. Similarly, large tumor was defined when the TMD was >3 cm. For N stage, we considered N0 as “without lymphatic metastasis” group and N1, N2, N3 as “with lymphatic metastasis” group. Similarly, M0 was defined as nonmetastasis group and M1 as metastasis group.
EGFR Mutation Detection
EGFR mutations were detected using PCR-based direct sequencing of exons 18–21. The method is briefly introduced as follows.
First, genomic DNA was extracted from tumors embedded in paraffin blocks or fresh frozen tumors. Then, use Hot Star Taq DNA polymerase (Qiagen Inc, Valencia, CA) to complete PCR amplification with a forward primer (5′-GGATCGGCCTCTTCATGC-3′) and a reverse primer (5′-TAAAATTGATTCCAATGCCATCC-3′). Sequencing was performed by ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA) using Applied Biosystems PRISM dye terminator cycle sequencing method (Perkin-Elmer Corp., Foster City, CA) directly on PCR products. Any in-frame deletions in exon 19 or point mutations in exon 21 (L858R substitutions), which confer sensitivity to EGFR-TKIs therapy, were considered EGFR mutant.
Statistical Analyses
SPSS 16.0 software was used for the statistical analysis. Continuous variables were divided into different categories as mentioned above. All the cut-off values were obtained by X-tile software (Version 3.6.1, Yale University, New Haven, CT), taking clinical expertise into consideration. Further investigations of multivariable analyses were performed by Cox regression for factors which were significantly associated in univariate survival analyses. Results were reported with odd ratio (OR), corresponding 95% confidence intervals (CI). A P value <0.05 was considered statistically significant.
RESULTS
Clinicopathological Characteristics of the Patients
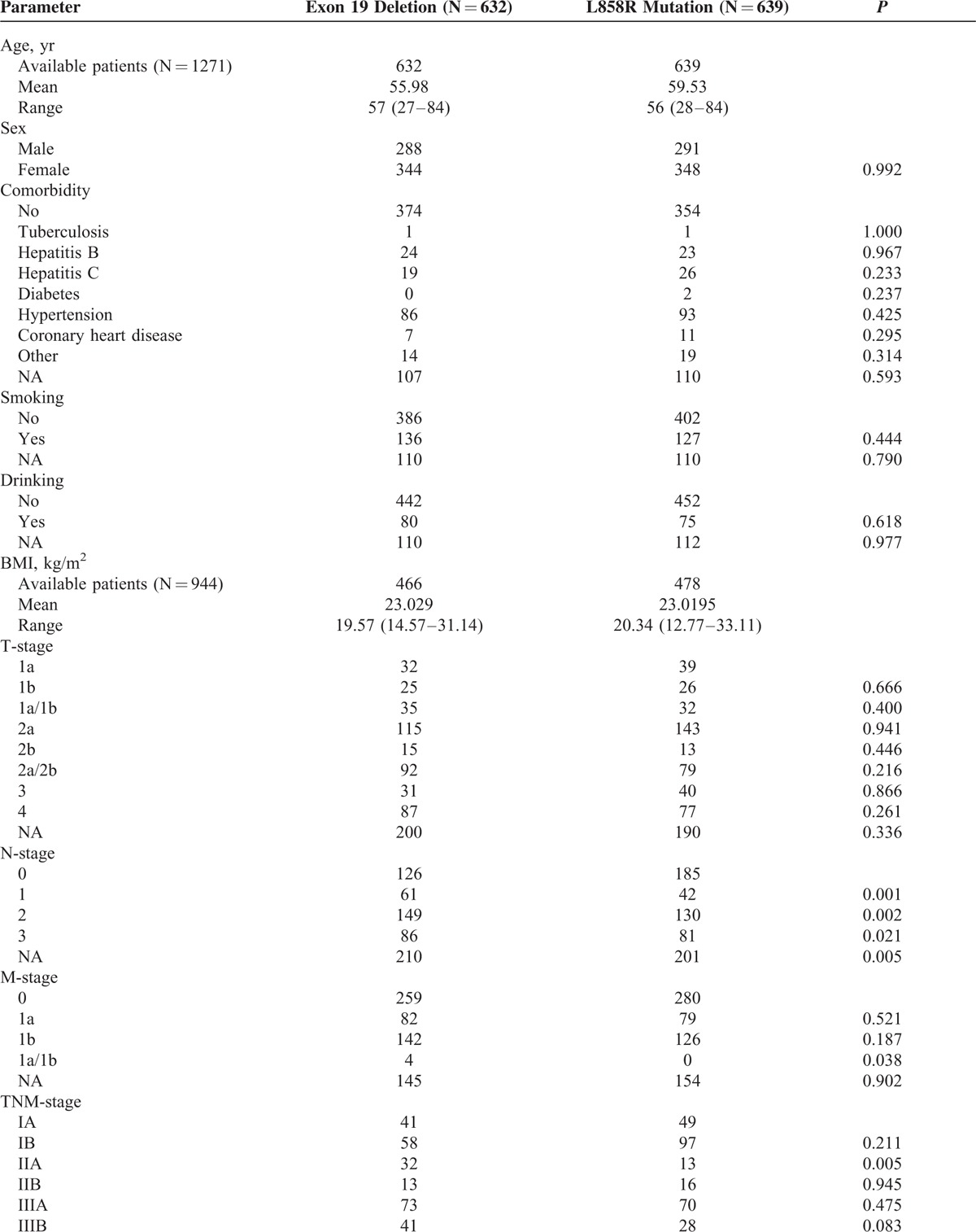
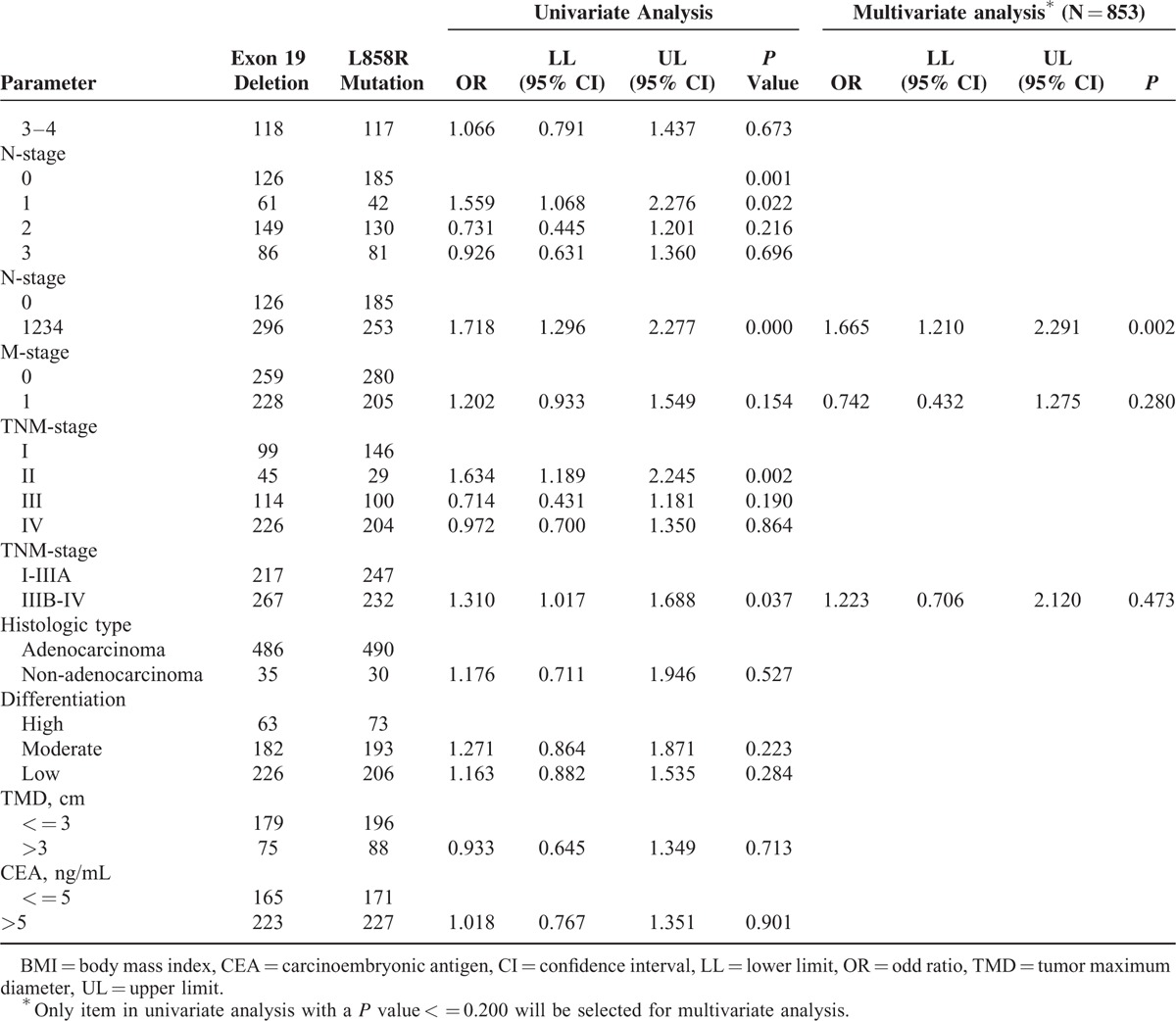
A total of 1271 NSCLC patients were enrolled in this study. Nineteen Del and L858R accounted for 49.7% (632/1271) and 50.3% (639/1271), respectively. The gender distribution was 579 males and 692 females. There was no difference in the distribution of other basic characteristics (all P value ≥0.05), except age and N stage. Clinicopathological features of the patients are presented in Table 1 .
TABLE 1.
Baseline Characteristics of Enrolled 1271 Nonsmall-Cell Lung Cancer Patients With Either Exon 19 Deletions or Exon 21 L858R Mutations
Higher Percentage of 19 Del in Younger Patients Group (< = 50 yr) Than L858R
We sought to find out the age difference between 19 Del and L858R patients. The percentages of older group (>50 yr) and younger group (< = 50 yr) were 68.4% (432/632) and 31.6% (200/632) in 19 Del and 79.8% (510/639) and 20.2% (129/639) in L858R, respectively. Univariate analyses indicated that the higher percentage of 19 Del in younger patients group (< = 50 yr) than L858R was significant (P < 0.001). Multivariate analysis also showed similar significant result (P = 0.011) (Table 2 )
TABLE 1 (Continued).
Baseline Characteristics of Enrolled 1271 Nonsmall-Cell Lung Cancer Patients With Either Exon 19 Deletions or Exon 21 L858R Mutations
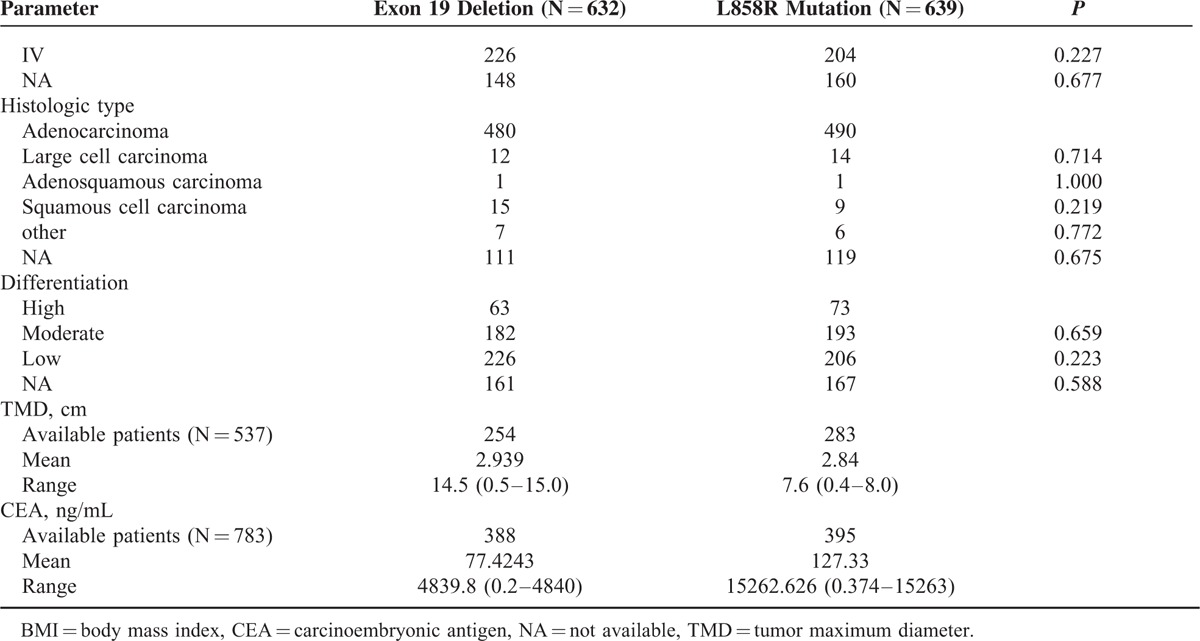
TABLE 2.
The Difference of Baseline Characteristics Between NonSmall-Cell Lung Cancer Patients With Exon 19 Deletions and Those With Exon 21 L858R Mutations in Univariate and Multivariate Logistic Regression Analysis
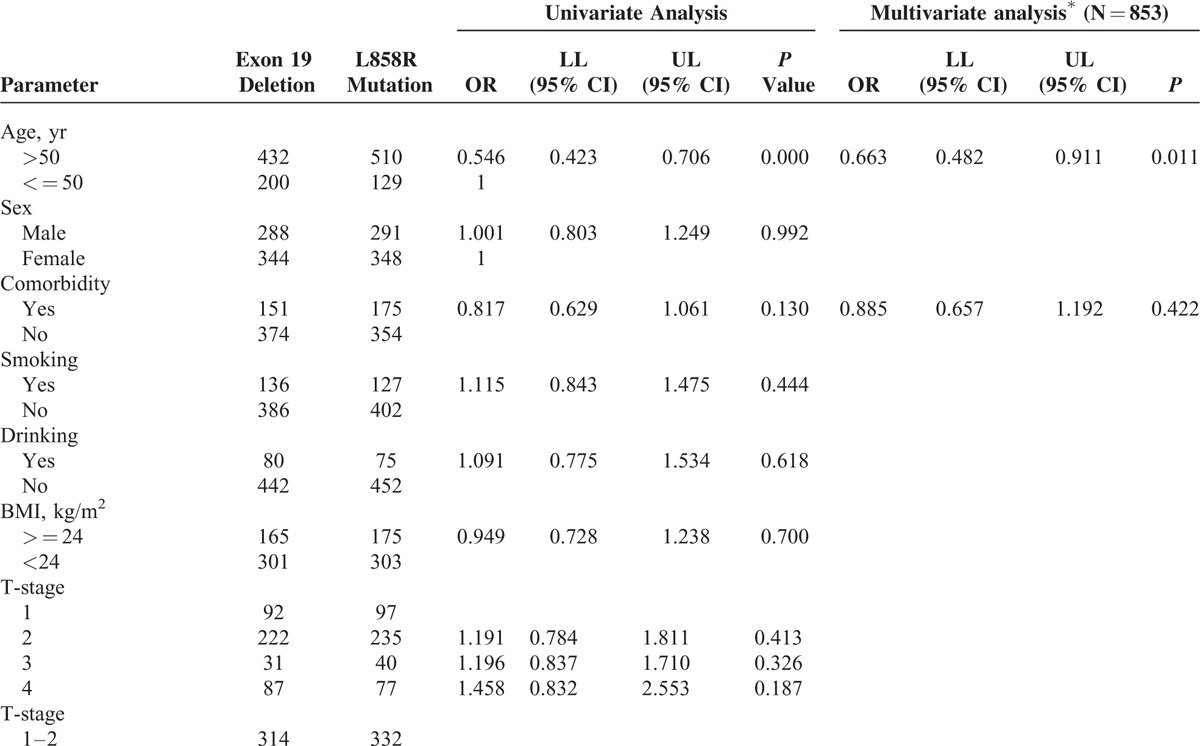
TABLE 2 (Continued).
The Difference of Baseline Characteristics Between NonSmall-Cell Lung Cancer Patients With Exon 19 Deletions and Those With Exon 21 L858R Mutations in Univariate and Multivariate Logistic Regression Analysis
We also conducted a subgroup analysis in different age groups. Age group was classified as 21–30, 31–40, 41–50, 51–60, 61–70, 71–80, and 81–90 yr (10 yr as an interval). Nineteen Del patients showed higher percentage than L858R patients in all age groups below 61 yr, including 21–30, 31–40, 41–50, 51–60 yr, while lower percentage in all age groups above 60 yr, including 61–70, 71–80, and 81–90 yr (Fig. 2A). This indicated same trends we found in univariate and multivariate analyses.
FIGURE 2.
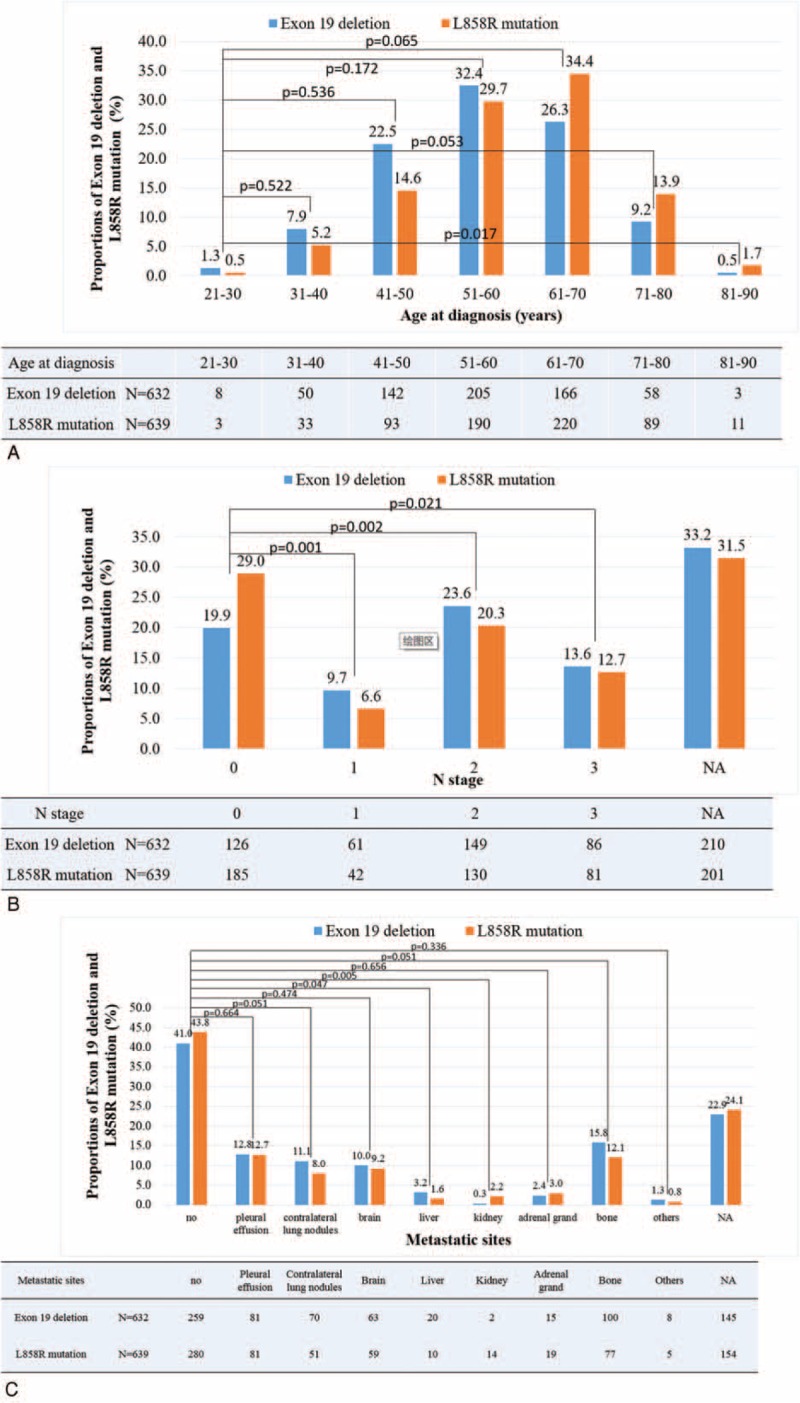
The incidence of exon 19 deletion and exon 21 L858R mutation in nonsmall-cell lung cancer patients according to different age groups (A), different N-stage groups (B), and different M-stage groups (C).
Patients With 19 Del Have Higher Risk of Lymph Node Metastasis Than L858R
We assessed the lymph node metastasis rate of 19 Del and L858R patients. Lymphatic metastasis rates of 19 Del and L858R were 46.8% (296/632) and 39.6% (253/639), respectively. In univariate analysis, significant difference was observed in distribution of “without lymphatic metastasis” group (N0) and “with lymphatic metastasis” group (N1, N2, and N3) of each mutation. Patients with 19 Del had higher risk of lymph node metastasis than L858R ones (P < 0.001). Multivariate analysis showed the same result (P = 0.002) (Table 2 ).
We also conducted subgroup analysis in N stages (stratified by N0, N1, N2, and N3). The result showed that the percentages of N1, N2, and N3 in 19 Del were 9.7%, 23.6%, and 13.6%, respectively. And in L858R those numbers were 6.6%, 20.3%, and 12.7%, respectively. In each N stage, 19 Del had a higher percentage than L858R, except N0 where the percentage of 19 Del was 19.9% and L858R 29.0% (Fig. 2B).
No Significant Differences in Other Items of Clinical Characteristics Between 19 Del and L858R
There was no statistical difference in T or M or TNM stages between 19 Del and L858R patients in univariate analyses. Subgroup analysis in metastatic sites was performed and no statistical difference was found (Fig. 2C). Similarly, no statistical significance was found in histologic types or differentiation as well as in tumor size and CEA level (Table 2 ).
DISCUSSION
NSCLC Patients With 19 Del Are More Likely to Be Young and Have Lymphatic Metastasis Than Those With L858R
NSCLC, as one of the leading causes of cancer-related mortality around the globe, could be caused by the accumulation of genetic alterations. EGFR mutations, usually 19 Del or L858R, count for the second common type of genetic alteration in NSCLC. EGFR tyrosine kinase inhibitors (EGFR-TKIs) were considered to be the first-line therapy for advanced NSCLC patients harboring EGFR 19 Del and L858R.12–15 Although 19 Del and L858R are both common-type EGFR mutations and under the same first-line therapy recommendation, recent studies have shown that 19 Del patients benefit more from EGFR-TKI therapy than L858R patients.10,11,16 However, few studies reported the difference of clinical characteristics between 19 Del and L858R patients.
Based on these investigations, we hypothesized that there are some differences between the clinical characteristics of 19 Del and L858R patients. In our study, NSCLC patients with 19 Del did show differences with those L858R patients. NSCLC patients with 19 Del were more likely to be young. Besides, they are more likely to have lymphatic metastasis than L858R patients. We confirmed our findings by multivariate analysis. Subgroup analysis was also performed. Multivariate analysis showed the same trends above, as well as further subgroup analysis. However, there was no statistically significant difference among other aspects we observed in this study.
The difference between 19 Del and L858R may help explain, in some aspects, why 19 Del patients benefit more from EGFR-TKI therapy than L858R patients. The reasons, to some degree, might lay to the young age of 19 Del patients and the inhibition of lymphatic metastasis of EGFR-TKI. Nineteen Del patients are more likely to be younger than L858R patients. Therefore, their basic conditions are better than L858R patients which could help in better prognosis. In addition, EFGR-TKI might inhibit the lymphatic metastasis of NSCLC patients and the inhibition effect would be greater in 19 Del patients because they are more likely to have lymphatic metastasis. The reason why 19 Del patients tend to be younger and have lymphatic metastasis is worthy of further research.
Therefore, we concluded that NSCLC patients with 19 Del are more likely to be young and have lymphatic metastasis than those with L858R.
Age and N Stage Might Be Considered in Predicting EGFR Mutation Type in NSCLC
Although testing of the mutations in EGFR, KRAS, and ALK is the today's standard of care,15,17 a recent study has reported that the detection rate of epidermal growth factor receptor (EGFR) mutation in NSCLC patients in China was only 9.6% because of the limited prevalence of testing technology and that EGFR-TKIs were used more frequently as salvage rather than upfront therapy.18 This indicated the importance of predicting EGFR mutation status and type in NSCLC patients. Previous studies had revealed that Asian nonsmoking women with adenocarcinoma is the population with higher EGFR mutation possibility but not specific to the type of EGFR mutation.19–22 This population also showed greater benefit in EGFR-TKI therapy.23–25 However, some recent studies have shown that 19 Del and L858R have different responses to EGFR-TKIs and chemotherapy. Patients with 19 Del benefited more from EGFR-TKIs treatment than chemotherapy, while for the patients with L858R, EGFR-TKIs treatment and chemotherapy have similar effect and chemotherapy might be even better.10,11 Therefore, prediction specific to the type of EFGR mutation is essential for treatment selection and additional factors for EGFR mutation type prediction are needed.
In our study, we observed that 19 Del patients presented higher percentage of in younger group (< = 50 yr) than L858R, as well as higher lymphatic metastasis risk. It suggested that NSCLC patients with 19 Del are more likely to be young and have lymphatic metastasis than those with L858R. Subgroup analysis performed for age and N-stages also indicates the same conclusion. The above data suggested that EGFR mutation type might be predicted by the patient's age and lymphatic metastasis condition. Young patients with lymph node metastasis at diagnosis would probably be 19 Del rather than L858R. However, the efficiency of this prediction needs further investigation.
Summary
In conclusion, our investigation suggested that NSCLC patients with 19 Del are more likely to be young and have lymphatic metastasis than those with L858R. It is worthy of further investigation on the underlying mechanism. Besides, age and N stage might be considered in predicting EGFR mutation type in NSCLC, which might help in choosing the initial therapy for EGFR mutation NSCLC.
Footnotes
Abbreviations: 19 Del = exon 19 deletion, ALK = anaplastic lymphoma kinase, BMI = body mass index, CEA = carcino-embryonic antigen, EGFR = epidermal growth factor receptor, EGFR-TKI = EGFR tyrosine kinase inhibitor, KRAS = kirsten rat sarcoma, L858R = exon 21 L858R mutation, NSCLC = nonsmall-cell lung cancer, OR = odd ratio, TMD = tumor maximum diameter.
YZ, DH, and WF contributed equally to this work.
All the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
This study was supported by the following funds: (1) National High Technology Research and Development Program of China (Grant No. 2012AA02A502); (2) Innovative drug R&D center based on real-time high-throughput cell-based screening platform and large capacity compound library (Grant No. 2013ZX09401003-002); (3) National Natural Science Funds of China (Grant No. 81372502); (4) Natural Science Foundation of Guangdong (Grant No. S2013010016564); (5) Specialized Research Fund for the Doctoral Program of Higher Education (20120171120116); (6) Young Teacher Training Program of Sun Yat-Sen University (14ykpy38); (7) Outstanding Young Talent Cultivation Project of Sun Yat-Sen University Cancer Center (04140701); (8) Wu Jieping Medical Foundation Project (Grant No. 320.6750.131).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2010; 8:740–801. [DOI] [PubMed] [Google Scholar]
- 3.Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008; 83:584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakelee H, Belani CP. Optimizing first-line treatment options for patients with advanced NSCLC. Oncologist 2005; 10 Suppl 3:1–10. [DOI] [PubMed] [Google Scholar]
- 5.Kris MG, Johnson BE, Kwiatkowski DJ, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: the NCI's Lung Cancer Mutation Consortium (LCMC). J Clin Oncol 2011; 29S:A7506. [Google Scholar]
- 6.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31:3327–3334. [DOI] [PubMed] [Google Scholar]
- 7.Arrieta O, Cardona AF, Corrales L, et al. The impact of common and rare EGFR mutations in response to EGFR tyrosine kinase inhibitors and platinum-based chemotherapy in patients with non-small cell lung cancer. Lung Cancer 2015; 87:169–175. [DOI] [PubMed] [Google Scholar]
- 8.Ellis PM, Coakley N, Feld R, et al. Use of the epidermal growth factor receptor inhibitors gefitinib, erlotinib, afatinib, dacomitinib, and icotinib in the treatment of non-small-cell lung cancer: a systematic review. Curr Oncol 2015; 22:e183–e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi L, Tang J, Tong L, et al. Risk of interstitial lung disease with gefitinib and erlotinib in advanced non-small cell lung cancer: a systematic review and meta-analysis of clinical trials. Lung Cancer 2014; 83:231–239. [DOI] [PubMed] [Google Scholar]
- 10.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16:141–151. [DOI] [PubMed] [Google Scholar]
- 11.Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res 2006; 12:3908–3914. [DOI] [PubMed] [Google Scholar]
- 12.Rossi A, Pasquale R, Esposito C, et al. Should epidermal growth factor receptor tyrosine kinase inhibitors be considered ideal drugs for the treatment of selected advanced non-small cell lung cancer patients? Cancer Treat Rev 2013; 39:489–497. [DOI] [PubMed] [Google Scholar]
- 13.Caicun Zhou YWGC. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011; 12:735–742. [DOI] [PubMed] [Google Scholar]
- 14.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004; 350:2129–2139. [DOI] [PubMed] [Google Scholar]
- 15.Keedy VL, Temin S, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol 2011; 29:2121–2127. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Li RQ, Ai YQ, et al. Exon 19 deletion was associated with better survival outcomes in advanced lung adenocarcinoma with mutant EGFR treated with EGFR-TKIs as second-line therapy after first-line chemotherapy: a retrospective analysis of 128 patients. Clin Transl Oncol 2015; 17:727–736. [DOI] [PubMed] [Google Scholar]
- 17.Stella GM, Scabini R, Inghilleri S, et al. EGFR and KRAS mutational profiling in fresh non-small cell lung cancer (NSCLC) cells. J Cancer Res Clin Oncol 2013; 139:1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue C, Hu Z, Jiang W, et al. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer 2012; 77:371–375. [DOI] [PubMed] [Google Scholar]
- 19.Lee DH, Srimuninnimit V, Cheng R, et al. Epidermal growth factor receptor mutation status in the treatment of non-small cell lung cancer: lessons learned. Cancer Res Treat 2015; 47:549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. New Engl J Med 2009; 361:938–958. [DOI] [PubMed] [Google Scholar]
- 21.Pham D, Kris MG, Riely GJ, et al. Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J Clin Oncol 2006; 24:1700–1704. [DOI] [PubMed] [Google Scholar]
- 22.Yano T, Haro A, Shikada Y, et al. Non-small cell lung cancer in never smokers as a representative ‘non-smoking-associated lung cancer’: epidemiology and clinical features. Int J Clin Oncol 2011; 16:287–293. [DOI] [PubMed] [Google Scholar]
- 23.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005; 366:1527–1537. [DOI] [PubMed] [Google Scholar]
- 24.Peled N, Yoshida K, Wynes MW, et al. Predictive and prognostic markers for epidermal growth factor receptor inhibitor therapy in non-small cell lung cancer. Ther Adv Med Oncol 2009; 1:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cioffi P, Marotta V, Fanizza C, et al. Effectiveness and response predictive factors of erlotinib in a non-small cell lung cancer unselected European population previously treated: a retrospective, observational, multicentric study. J Oncol Pharm Pract 2013; 19:246–253. [DOI] [PubMed] [Google Scholar]


