Abstract
Acute kidney injury (AKI) is a common complication after cardiac surgery. Recent studies have revealed emerging associations between the magnitude of acute glycemic fluctuations and intensive care unit (ICU) mortality rates. However, the effect of acute glycemic fluctuations on the development of postoperative AKI remains unclear. Thus, we aim to investigate the effect of the magnitude of acute perioperative glycemic fluctuations on the incidence of postoperative AKI.
We conducted a prospective cohort study by prospectively obtaining data from all patients who underwent elective coronary artery bypass grafting in a tertiary heart institution from 2009 to 2011. The magnitude of the difference between the highest and lowest perioperative glucose levels within 48 hr was calculated as a measure of perioperative glycemic fluctuation. Patients were divided into 4 groups for analysis based on the magnitude of perioperative glycemic fluctuation-A: 0 to 2 mmol/L; B: >2 to 4 mmol/L; C: >4 to 6 mmol/L; and D: >6 mmol/L. We analyzed the incidence of postoperative AKI, ICU mortality and ICU length of stay as primary and secondary outcomes, respectively. Both univariate and multivariate analyses were used.
We analyzed data from 1386 patients. The overall incidence of AKI was 29.9% and increased with wider glycemic fluctuation. The incidence of AKI was statistically highest in Group D (38.3%), followed by Groups C (28.6%), B (21.7%), and A (17.4%), respectively (P�=�0.001). A similar trend was observed among both diabetics and nondiabetics (P�=�0.001 and P�=�0.002, respectively). Multivariate logistic regression showed the magnitude of perioperative glycemic fluctuations to be an independent risk factor in the development of AKI (P < 0.001, odds ratio 1.180, 95% confidence interval 1.116-1.247). ICU length of stay was statistically highest in Group D (58.3�hr) compared with Groups C (44.5�hr), B (37.3�hr), and A (32.8�hr, P�=�0.003). ICU mortality rate was comparable among all 4 groups (P�=�0.172).
Wide acute perioperative glycemic fluctuations should be avoided as they are associated with a significantly increased risk of AKI and ICU length of stay in both the diabetics and the nondiabetics.
INTRODUCTION
Acute kidney injury (AKI) is a common complication following cardiac surgery, occurring in up to 30% of patients postoperatively.1 Besides being an independent risk factor for mortality, AKI also poses higher risks of developing long-term complications including chronic kidney disease and end-stage renal failure.2–5
Although hyperglycemia is a well-known risk factor for AKI and poor intensive care unit (ICU) outcomes, recent studies have revealed emerging associations between variations in glucose levels and ICU mortality rates in medical and surgical ICUs.6–8 Further study involving septic patients also showed glycemic variability to be an independent risk factor for mortality.9 Two studies looking at cardiac surgical patients demonstrated that perioperative glycemic variation was a predictor of adverse ICU composite outcomes and events such as hospital mortality, renal morbidity, myocardial infarction, neurological impairment, and wound infection among other complications.10,11 Higher glycemic variability has also been associated with adverse renal outcomes in diabetic patients suffering from urinary tract infection.12
Therefore, we aimed to investigate the effect of the magnitude of acute perioperative glycemic fluctuations on the development of postoperative AKI in a multiethnic Southeast Asian population undergoing coronary artery bypass grafting (CABG) in a tertiary heart centre.
METHODS
Following institutional review board approval from the Singhealth Centralised Institutional Review Board, we prospectively obtained data from all patients receiving elective isolated CABG from January 1, 2009 to December 31, 2011, at a tertiary heart centre in Singapore. Written informed consent was obtained from all patients. Patients with pre-existing renal impairment were excluded from the study. The minimum sample size required was predetermined to be 1082 based on a 30% prevalence rate of AKI, minimum number of positive incidences of AKI of 300, and a 95% probability.
Perioperative clinical practices and surgical management followed strict international standards. Anesthesia was induced with intravenous induction agents (Propofol or Etomidate) and maintained with a balanced anesthesia regime using low-dose Fentanyl (10–20 μg kg−1) in addition to volatile agents (primarily Sevoflourane). Conventional cardiopulmonary bypass circuits with heat exchangers, roller pumps, membrane oxygenators, arterial blood filters, venous reservoirs, and cardiotomy suction were utilized. Typically 1300 to 1400 mL of prime was used in the CPB circuits. Perfusion targets include a hematocrit of >22%, mild-to-moderate hypothermia (32–35°C), nonpulsatile flow rate of 2.2 to 2.4 L min−1 m−2, mean arterial pressure of 50 to 70 mm Hg, glucose levels of below 10 mmol/L, and activated clotting times of more than 400 sec. Cold blood cardioplegia was used to achieve myocardial protection. Aprotinin was not utilized in any patients.
All patients were kept to a tight baseline blood glucose control of within a range of 4 to 10 mmol/L, in accordance with hospital protocol and the Society of Thoracic Surgeons Practice Guideline series: Blood Glucose Management During Adult Cardiac Surgery.13 In line with hospital protocol, glycemic targets were strictly adhered to via insulin infusion and monitoring every 2 to 4 hr or more frequently if required, up to the first 48 hr.
Serum creatinine values were obtained from the preoperative period till discharge from the Cardiothoracic Surgical Intensive Care Unit. The peak percentage and absolute change in postoperative creatinine in the first 48 hr was calculated. Serum creatinine level was determined via a dry-slide enzymatic reflectance technique.
Patient demographic data and characteristics were collected. Preoperative, intraoperative and postoperative glucose levels were collected. The magnitude of perioperative glycemic fluctuation was defined as the difference between the highest and lowest perioperative glucose levels documented within 48 hr. This was employed as a surrogate measure of acute perioperative glycemic variation. Patients were then subdivided into 4 groups for analysis based on the magnitude of glycemic fluctuation—Group A: 0 to 2 mmol/L; Group B: >2 to 4 mmol/L; Group C: >4 to 6 mmol/L; and Group D: >6 mmol/L.
The primary outcome was the incidence of AKI as characterized using the Acute Kidney Injury Network (AKIN) criteria. A positive incidence of AKI was defined by a minimum increase of 150% or at least a 0.3 mg/dL rise in serum creatinine from the preoperative baseline level. Patients with a new need for renal replacement therapy were also determined to have AKIN Stage 3 AKI. ICU length of stay and mortality rate were analyzed as secondary outcomes.
Univariate analysis of population demographics, medical history, preoperative risk factors, intra-operative variables, and postoperative data was carried out. The Chi-squared test was used in the analysis of categorical variables. A 2-tailed independent samples t test was used in the analysis of continuous variables. Variables with P < 0.05 on univariate analysis were selected for inclusion in the multivariate logistic regression model.
For additional comparison of outcomes among the 4 subgroups of glycemic fluctuation, a 1-way ANOVA with post hoc tests was performed for continuous variables.
All analyses were carried out using SPSS version 20.
RESULTS
A total of 1481 patients underwent elective CABG with open vein harvesting at the Singapore General Hospital from 2009 to 2011. Complete data were obtained for 1443 patients, while 57 patients were excluded as they had pre-existing renal impairment. Therefore, a total of 1386 patients were analyzed. The overall incidence of AKI was 29.9%.
On univariate analysis (refer to Table 1), patients who developed AKI were more likely to be older, diabetic, and hypertensive. They were also more likely to have a, higher euroSCORE logistic, higher preoperative creatinine levels and lower hemoglobin and hematocrit levels. Perioperatively, patients who required intra-aortic balloon pumps, red blood cell transfusion and with higher bypass times, and aortic cross clamping times were more likely to develop AKI postoperatively.
TABLE 1.
Clinical Characteristics and Acute Kidney Injury

A multivariate logistic regression of all significant factors on univariate analysis was performed. Factors which significantly affect the incidence of postoperative AKI are shown in Table 2. The magnitude of perioperative glycemic fluctuation was revealed to be an independent risk factor in the development of AKI (P < 0.001, odds ratio 1.180, 95% confidence interval 1.116–1.247). Other independent predictors of postoperative AKI are depicted fully in Table 2.
TABLE 2.
Significant Risk Factors of AKI on Multivariate Logistic Regression
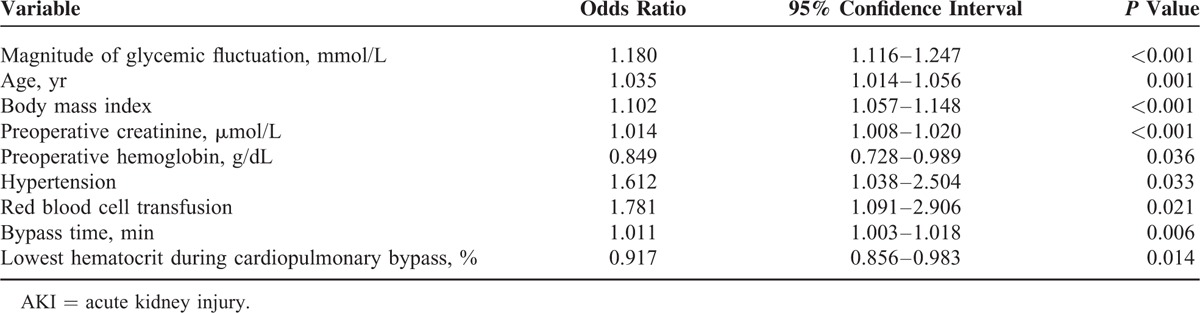
The incidence of AKI increased as the magnitude of glycemic fluctuation increased (Figure 1). Upon comparison of the 4 subgroups of glycemic fluctuation, the incidence of AKI was statistically highest (P = 0.001) in Group D (38.3%), followed by Groups C (28.6%), B (21.7%), and A (17.4%), respectively (refer to Table 3). A similar trend was observed among both the diabetics and the nondiabetics (P = 0.001 and P = 0.002, respectively).
FIGURE 1.
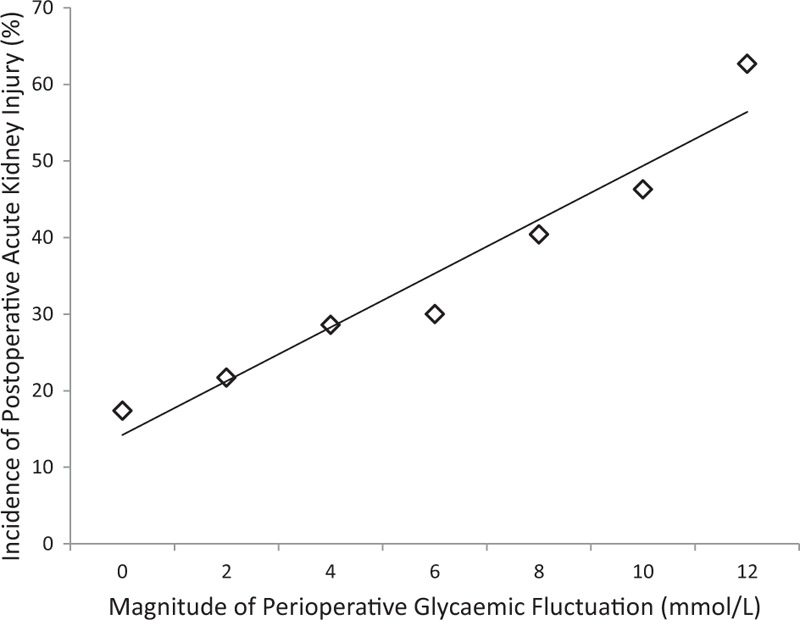
Graph depicting the relationship between the magnitude of perioperative glycemic fluctuation and the incidence of postoperative acute kidney injury (%). The incidence of postoperative acute kidney injury increases as the magnitude of perioperative glycemic fluctuation increases.
TABLE 3.
Incidence of AKI Among 4 Glycemic Groups

Secondary outcomes of ICU mean length of stay and mortality rate are presented in Table 4. The magnitude of glycemic fluctuation was associated with significantly increased ICU length of stay, but not ICU mortality rate. ICU mortality rate was not significantly different among Groups A, B, C, and D (P = 0.172). However, ICU length of stay was statistically highest (P = 0.003) in Group D (58.3 hr) as compared with Groups C (44.5 hr), B (37.3 hr), and A (32.8 hr), respectively.
TABLE 4.
Secondary Outcomes Among 4 Glycemic Groups

DISCUSSION
AKI is common but contributes significantly to postcardiac surgery mortality and morbidity.14 Our incidence of AKI was 29.9%, which is consistent with previously reported incidences of up to 30%.15
Our study showed that the magnitude of glycemic fluctuation is independently associated with the development of postoperative AKI in both the diabetics and the nondiabetics. This corroborates with the aforementioned studies involving general medical patients, surgical patients, ICU populations, outpatient diabetics, and septic patients.8–11,16,17
The surgical stress associated with CABG predisposes patients to stress-induced hyperglycemia and associated complications including increased mortality.18,19 Hyperglycemia is known to induce neutrophil, monocyte, and endothelial dysfunction, as well as proinflammatory cytokine expression.20 Similarly, acute hyperglycemia has also been postulated to cause AKI via oxidative damage, iNOS activation, and hypoxic reperfusion injury in addition to the aforementioned mechanisms.21
However, it is increasingly recognized that acute glucose fluctuations are a more specific trigger of oxidative stress, when compared with chronic sustained hyperglycemic states.17 This is consistent with our findings, and postulated mechanisms to explain this include the augmentation of the proapoptotic effects of high glucose via the production of mitochondrial superoxide, inflammatory cytokines, and nitrotyrosine production (a marker of oxidative stress).22,23 Thus, these aforementioned changes associated with glycemic fluctuation increase the risk of endothelial damage, oxidative stress, inflammation, and end organ dysfunction associated with CABG.24 Although these mechanisms are generalized, we hypothesize that these changes are enhanced in the renal vasculature of our patients who already suffer from a CABG-induced systemic proinflammatory state.25 It is therefore unsurprising that our findings revealed an increased incidence of AKI with increasing glycemic fluctuation.
Subgroup analysis of diabetic versus nondiabetic patients revealed that both cohorts were sensitive to acute glycemic fluctuations. Our findings contrast with a previous study by Krinsley, who observed higher mortality rates associated with increasing glycemic variability in only the nondiabetics.26 Although there is currently insufficient evidence to ascertain the mechanism of this phenomenon, it has been proposed that nondiabetics were less tolerant to acute glucose fluctuations due to their lack of chronic exposure to these fluctuations. However, this was not observed in our study. As the prevalence rate of diabetes mellitus in the local Southeast-Asian population is markedly high, we postulate that there may be patients in the nondiabetic group who could have had prediabetes or impaired glucose tolerance. In addition, there is a high incidence of metabolic syndrome and insulin insensitivity among the Southeast-Asians as compared with Western populations, and it has been shown that these patients may also have increased artherosclerotic changes and plaque instability in the coronary vasculature. These changes may also exist in the renal vasculature, thus inherently predisposing these patients to AKI.27–33
Our study also concurred with Krinsley's work in demonstrating the association between higher glycemic variation and ICU length of stay.34 The difference in ICU length of stay between the highest and lowest glycemic variation group was almost 24 hr. In the local context, this is equivalent to approximately an additional US$852 incurred per patient due to an extra day's stay in the ICU. In our study, mortality rates did not correlate with the magnitude of fluctuation, this contrasts with Krinsley's work. This could be perhaps due to the overall low mortality rate in our study of 1%.
Compared with previous studies utilizing the mean, standard deviation, mean amplitude of glycemic excursions, and glycemic variability index in measuring glycemic fluctuation, our use of the maximum perioperative glycemic fluctuation to assess changes in glucose control over time has greater use as a tool and is more intuitive to clinicians. This offers greater clinical utility and may be applied on a daily basis. Moreover, in our subgroup analysis, we have based our ranges of glucose fluctuation based on 2 landmark glycemic control protocols developed in the last decade—the Society of Thoracic Surgeons Practice Guideline series and Van den Berghe's study which allowed for a maximum glucose fluctuation of 6 and 2 mmol/L, respectively.13,35
In addition, this study has several other strengths. It is a prospective study of patients undergoing a uniform, quantifiable, and predictable stressor of elective CABG, in a controlled perioperative environment with intensive patient monitoring. It is also the first investigating the relationship between glycemic fluctuation and AKI in the Southeast-Asian population. We have also utilized an extensively validated AKI scoring criteria (AKIN) in the definition of our primary outcome.
However, our study may be limited due to the lack of data for continuous glucose monitoring which might shed greater insight into effect of minute glycemic fluctuations on postoperative AKI. This has been countered by the strict adherence to hospital protocol in keeping the glucose control targets within a range of 4 to 10 mmol/L. However, we recognize that a continuous glucose monitoring system would be ideal, as this would track minute glycemic variations, being akin to a physiological feedback loop system.
CONCLUSIONS
We conclude that greater perioperative glycemic fluctuations are an independent risk factor of the development of AKI postoperatively in both diabetic and nondiabetic patients undergoing CABG.
Despite the strict adherence to baseline glucose control targets, large glycemic fluctuations may still occur, which increases the risk of developing postoperative AKI. Therefore, our findings also emphasize the need for more stringent glucose control and monitoring in addition to the strict baseline glucose control targets already in place.
The need for stringent glucose and monitoring with continuous devices is highly anticipated. With advances in technology, it is hopeful that this may help to avoid minute perioperative fluctuations in glucose levels, and thereby contribute to improved patient outcomes.
Footnotes
Abbreviations: AKI = acute kidney injury, BMI = body mass index, CABG = coronary artery bypass graft, ICU = intensive care unit.
This manuscript was presented in part in the form of a poster presentation at the ERA-EDTA 52nd Congress in May 2015.
This study was supported by the Khoo Research Grant from the Duke-NUS Graduate Medical School (Singapore).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. Clin J Am Soc Nephrol 2004; 15:1597–1605. [DOI] [PubMed] [Google Scholar]
- 2.Nisula S, Kaukonen K, Vaara S, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med 2013; 39:420–428. [DOI] [PubMed] [Google Scholar]
- 3.Ng R, Chew S, Liu W, et al. Persistent kidney injury at hospital discharge after cardiac surgery with cardiopulmonary bypass in patients with normal preoperative serum creatinine and normal estimated glomerular filtration rate. J Cardiothorac Vasc Anesth 2014; 28:1453–1458. [DOI] [PubMed] [Google Scholar]
- 4.Pannu N, James M, Hemmelgarn B, et al. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 2013; 8:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coca S, Singanamala S, Parikh C. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2011; 81:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriyama N, Ishihara M, Noguchi T, et al. Admission hyperglycaemia is an independent predictor of acute kidney injury in patients with acute myocardial infarction. Circ J 2014; 78:1475–1480. [DOI] [PubMed] [Google Scholar]
- 7.Meynaar I, Eslami S, Abu-Hanna A, et al. Blood glucose amplitude variability as predictor for mortality in surgical and medical intensive care unit patients: a multicenter cohort study. J Crit Care 2012; 27:119–124. [DOI] [PubMed] [Google Scholar]
- 8.Egi M, Bellomo R, Stachowski E, et al. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 2006; 105:244–252. [DOI] [PubMed] [Google Scholar]
- 9.Ali N, OʼBrien J, Dungan K, et al. Glucose variability and mortality in patients with sepsis. Crit Care Med 2008; 36:2316–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan A, Abd-Elsayed A, Maheshwari A, et al. Role of intraoperative and postoperative blood glucose concentrations in predicting outcomes after cardiac surgery. Anesthesiology 2010; 112:860–871. [DOI] [PubMed] [Google Scholar]
- 11.Subramaniam B, Lerner A, Novack V, et al. Increased glycaemic variability in patients with elevated preoperative HbA1C predicts adverse outcomes following coronary artery bypass grafting surgery. Anesth Analg 2014; 118:277–287. [DOI] [PubMed] [Google Scholar]
- 12.Chiu P, Wu C, Huang C, et al. Lower blood glucose and variability are associated with earlier recovery from renal injury caused by episodic urinary tract infection in advanced type 2 diabetic chronic kidney disease. PLoS ONE 2014; 9:e108531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazar HL, McDonnell M, Chipkin SR, et al. The society of thoracic surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009; 87:663–669. [DOI] [PubMed] [Google Scholar]
- 14.Chertow G, Levy E, Hammermeister K, et al. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 1998; 104:343–348. [DOI] [PubMed] [Google Scholar]
- 15.Rosner M, Okusa M. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2005; 1:19–32. [DOI] [PubMed] [Google Scholar]
- 16.Jung H. Clinical implications of glucose variability: chronic complications of diabetes. Endocrinol Metab 2015; 30:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295:1681–1687. [DOI] [PubMed] [Google Scholar]
- 18.McCowen K, Malhotra A, Bistrian B. Stress-induced hyperglycaemia. Crit Care Clin 2001; 17:107–124. [DOI] [PubMed] [Google Scholar]
- 19.Krinsley J. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clinic Proc 2003; 78:1471–1478. [DOI] [PubMed] [Google Scholar]
- 20.Doenst T, Wijeysundera D, Karkouti K, et al. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 2005; 130:1144.e1–1144 e8. [DOI] [PubMed] [Google Scholar]
- 21.Mehta RL. Glycemic control and critical illness: is the kidney involved? J Am Soc Nephrol 2007; 18:2623–2627. [DOI] [PubMed] [Google Scholar]
- 22.Piconi L, Quagliaro L, Assaloni R, et al. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab Res Rev 2006; 22:198–203. [DOI] [PubMed] [Google Scholar]
- 23.Rizzo M, Barbieri M, Marfella R, et al. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: role of dipeptidyl peptidase-IV inhibition. Diabetes Care 2012; 35:2076–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerritsen W, Van Boven W, Driessen A, et al. Off-pump versus on-pump coronary artery bypass grafting: oxidative stress and renal function. Eur J Cardiothorac Surg 2001; 20:923–929. [DOI] [PubMed] [Google Scholar]
- 25.Fransen E, Maessen J, Dentener M, et al. Systemic inflammation present in patients undergoing CABG without extracorporeal circulation. Chest 1998; 113:1290–1295. [DOI] [PubMed] [Google Scholar]
- 26.Krinsley J. Glycaemic variability and mortality in critically ill patients: the impact of diabetes. J Diabetes Sci Technol 2009; 3:1292–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raji A, Gerhard-Herman MD, Warren M, et al. Insulin resistance and vascular dysfunction in non-diabetic Asian Indians. J Clin Endocrinol Metab 2004; 89:3965–3972. [DOI] [PubMed] [Google Scholar]
- 28.Palaniappan LP, Kwan AC, Abbasi F, et al. Lipoprotein abnormalities are associated with insulin resistance in South Asian Indian women. Metabolism 2007; 56:899–904. [DOI] [PubMed] [Google Scholar]
- 29.Hodge AM, Dowse GK, Collins VR, et al. Abdominal fat distribution and insulin levels only partially explain adverse cardiovascular risk profile in Asian Indians. J Cardiovasc Risk 1996; 3:263–270. [PubMed] [Google Scholar]
- 30.Vimaleswaran KS, Radha V, Anjana M, et al. Effect of polymorphisms in the PPARGC1A gene on body fat in Asian Indians. Int J Obes 2006; 30:884–891. [DOI] [PubMed] [Google Scholar]
- 31.Bhopal R, Unwin N, White M, et al. Heterogeneity of coronary heart disease risk factors in Indian, Pakistani, Bangladeshi, and European origin populations: cross sectional study. Br Med J 1999; 319:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vimaleswaran KS, Radha V, Ghosh S, et al. Peroxisome proliferator-activated receptor-gamma co-activator-1alpha (PGC-1alpha) gene polymorphisms and their relationship to type 2 diabetes in Asian Indians. Diabetic Med 2005; 22:1516–1521. [DOI] [PubMed] [Google Scholar]
- 33.Chew S, Mar W, Ti L. Association of ethnicity and acute kidney injury after cardiac, surgery in a South East Asian population. Brit J Anaesth 2013; 110:397–401. [DOI] [PubMed] [Google Scholar]
- 34.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med 2008; 36:3008–3013. [DOI] [PubMed] [Google Scholar]
- 35.Van Den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345:1359–1367. [DOI] [PubMed] [Google Scholar]


