Abstract
Objective
To investigate the impact of postoperative acute kidney injury (AKI) on early health outcome and on long-term survival in patients undergoing redo coronary artery bypass grafting (CABG).
Methods
We performed a Cox analysis with 398 consecutive patients undergoing redo CABG over a median follow-up of 7 years (interquartile range, 4-12.2 years). Renal function was assessed using baseline and peak postoperative levels of serum creatinine. AKI was defined according to the risk, injury, failure, loss, and end-stage (RIFLE) criteria. Health outcome measures included the rate of in-hospital AKI and all-cause 30-day and long-term mortality, using data from the United Kingdom's Office of National Statistics. Propensity score matching, as well as logistic regression analyses, were used. The impact of postoperative AKI at different time points was related to survival.
Results
In patients with redo CABG, the occurrence of postoperative AKI was associated with in-hospital mortality (odds ratio [OR], 3.74; 95% confidence interval [CI], −1.3 to 10.5; P < .01], high Euroscore (OR, 1.27; 95% CI, 1.07-1.52; P < .01), use of IABP (OR, 6.9; 95% CI, 2.24-20.3; P < .01), and reduced long-term survival (hazard ratio [HR], 2.42; 95% CI, 1.63-3.6; P = .01). Overall survival at 5 and 10 years was lower in AKI patients with AKI compared with those without AKI (64% vs 85% at 5 years; 51% vs 68% at 10 years). On 1:1 propensity score matching analysis, postoperative AKI was independently associated with reduced long term survival (HR, 2.8; 95% CI, 1.15-6.7).
Conclusions
In patients undergoing redo CABG, the occurrence of postoperative AKI is associated with increased 30-day mortality and major complications and with reduced long-term survival.
Key Words: coronary surgery, redo surgery, renal failure, long-term survival
Abbreviations and Acronyms: AKI, acute kidney injury; ARF, acute renal failure; CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society; CI, confidence interval; CPB, cardiopulmonary bypass; CT, computed tomography; eGFR, estimated glomerular filtration rate; HR, hazard ratio; IABP, intra-arterial balloon pump; LVEF, left ventricular ejection fraction; OR, odds ratio; RIFLE, risk, injury, failure, loss, and end-stage; SCr, serum creatinine

CONSORT diagram of patients undergoing redo CABG between 1998 and 2014, showing exclusion criteria, rate of AKI, and 1:1 propensity score matching.
Central Message.
The occurrence of postoperative AKI following redo CABG surgery is associated with increased early and long-term mortality. This finding might help clinical decision making and guide patient consent.
Perspective.
Our findings improve the understanding of the impact of postoperative AKI on short-term health outcome and on long-term survival in patients undergoing redo CABG. This is of potential benefit to surgeons and intensivists managing redo CABG cases during their hospital stay, as well as to patients who might benefit from a more informative consent process.
See Editorial Commentary page 243.
In developed countries, the number of patients undergoing any redo cardiac surgery procedures across all surgical categories is on the rise.1 This increase can be attributed to various factors, including prolonged overall longevity, improved survival following cardiac surgery, progression of native coronary disease and/or limited long-term patency rate of saphenous vein grafts, an increasing number of patients requiring palliation for complex congenital malformations, and the limited long-term durability of bioprosthetic valves.2, 3, 4, 5 Conversely, the incidence of patients requiring redo coronary artery bypass grafting (CABG) has been declining gradually over time6, 7; however, evidence suggests that despite refinements in surgical, perfusion, and anesthetic techniques, redo CABG is still associated with increased in-hospital mortality.6, 7
Acute kidney injury (AKI) is recognized as an independent risk factor for adverse outcomes in patients undergoing cardiac surgery.8, 9, 10, 11 Introduction of the risk, injury, failure, loss, and end-stage (RIFLE) criteria has standardized the definition of AKI, allowing more objective comparisons among different studies.12 The combination of redo CABG and occurrence of postoperative AKI is potentially lethal, yet no data are available on the rate of postoperative AKI defined according to the RIFLE criteria in patients undergoing redo CABG, or on the impact of postoperative AKI on early health outcomes and long-term survival in these patients.7, 13, 14
The aim of this study was to evaluate the impact of postoperative AKI on early health outcomes as well as long-term survival in a consecutive series of patients undergoing redo CABG.
Materials and Methods
We conducted a retrospective analysis of prospectively collected data for all patients undergoing redo CABG from our institutional database registry. These data are prospectively collected, validated, and stored by a dedicated independent team as part of the United Kingdom's National Registry. The study protocol was in compliance with the local Institutional Clinical Audit Review Board and received full approval; the need for patient consent was waived.
Patient Selection
A Consolidated Standards of Reporting Trials flow diagram for patient selection is shown in Figure 1. Data from 15,436 consecutive patients undergoing isolated CABG between 1998 and 2014 were evaluated, of whom 398 patients were identified as having undergone redo CABG. Of these, 18 were excluded from final analysis due to either the use of preoperative dialysis or the incompleteness of postoperative AKI data. The remaining 380 patients who underwent redo CABG were included in the analysis and were divided into 2 groups based on the occurrence of postoperative AKI.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram of patients undergoing redo CABG between 1998 and 2014, showing exclusion criteria, rate of AKI, and 1:1 propensity score matching. CABG, Coronary artery bypass grafting; AKI, acute kidney injury.
Definition of AKI
All patients had baseline serum creatinine (SCr) and estimated glomerular filtration rate (eGFR) values derived from our institutional database registry. AKI was defined according to the RIFLE criteria by the change in SCr from baseline to postoperative values. Based on a previous report,13 we opted to use the highest SCr value (ie, poorest measurement) recorded during postoperative recovery to compare with the baseline value. According to the RIFLE criteria, SCr must be increased by at least 50% from baseline value for a classification as AKI.12
Data Collection, Definitions, and Clinical Management
Baseline, intraoperative, and postoperative data collection and recording of health outcomes were done prospectively out at our institution according to the standards of the mandatory National Cardiac Surgery Audit. Baseline data collection included a detailed risk profile, symptom status, past medical history, and baseline renal function, including SCr and eGFR values as calculated from the Modification of Diet in Renal Disease equation. Postoperative peak SCr values were obtained manually from medical records, and eGFR values were calculated accordingly. Intraoperative and postoperative clinical management was maintained according to strict predefined protocols. Detailed evaluation of baseline computed tomography (CT) angiography results, along with evaluation of previous CABG surgery, distribution of native coronary disease, as well as patency and/or occlusion of bypass grafts, to inform the surgical approach. Surgical, anesthesia, perfusion, and organ protection techniques were applied as reported previously by our group.15, 16
To minimize the risk of injury at reentry, preoperative CT angiography scanning was used at the surgeon's discretion to ascertain the anatomic relationship of any patent grafts with the planned entry route. Other factors considered included a “no touch” technique for blocked vein grafts, along with isolation of any patent left internal mammary artery on a vessel loop, followed by soft clamping of previous cardioplegia. Occasionally, when the baseline CT angiography scan indicated a close anatomic relationship between the heart and/or any patent grafts with the sternum, femoro-femoral cardiopulmonary bypass (CPB) before chest reentry was established, again based on surgeon preference. Similarly, the use of off-pump surgery was based on surgeon preference.
Intraoperative data collection included use of CPB, cross-clamp time, perfusion time, use of off-pump coronary surgery, preoperative use of an intra-aortic balloon pump (IABP), and degree of procedural urgency, defined as elective (admitted from home) or urgent (admitted as an in-hospital transfer after CT angiography owing to clinical factors or the severity of coronary disease). Early postoperative data collection included 30-day mortality, occurrence of postoperative AKI, all postoperative complications, and length of hospital stay.
Patient management after surgery was provided according to our established protocols. Patients with no pending clinical issues after surgery were discharged directly to home with a visit to our outpatient clinic scheduled for 6 weeks after discharge. If clinically necessary, this visit was repeated at 3 months. At this stage, patients exhibiting complete surgical recovery were discharged back to the care of the referring cardiologist and general practitioner. Patients developing postoperative wound healing issues were referred to our wound clinic, with repeated long-term visits until complete recovery before discharge to the general practitioner. Patients developing noncardiac single-organ renal, respiratory, and/or neurologic dysfunction/failure were treated at our center until complete recovery and referred to a specialist center for further management beyond the 30-day cutoff. Late survival data after discharge were obtained from the National Health Service's tracking service, with the latest data obtained on February 21, 2015.
Statistical Analysis
Data are presented as mean ± SD for continuous variables and as number and percentage for dichotomous variables. Continuous variables were tested for normality using the Shapiro-Wilk test and then compared between groups with the unpaired Student t test if normally distributed or the Mann-Whitney U test if non-normally distributed. In cases of dichotomous variables, the Pearson χ2 or Fisher exact test was used as appropriate.
Univariate analyses for in-hospital and long-term mortality were carried out, and the predictors thus obtained were subsequently tested in a multivariable logistic regression model. The model was built using the following variables: AKI after surgery, emergency procedure, use of on-pump surgery, preoperative use of nitrates, evidence of preoperative cardiogenic shock, use of an IABP, evidence of previous myocardial infarction, Euroscore, and reduced left ventricular ejection fraction (LVEF). A stepwise approach was used and was confirmed by backward and forward methods. The significance within the final models was evaluated with the Wald test, whereas the strength of the association of each variable with postoperative death was estimated by calculating the odds ratio (OR) and 95% confidence interval (CI). The model was calibrated using the Hosmer-Lemeshow goodness-of-fit test. Model discrimination was evaluated using the area under the receiver operating characteristic curve.
The event-free survival rate was estimated for the patients who developed postoperative AKI and those who did not using the Kaplan-Meier method and then compared with the log-rank test. A Cox proportional hazard model (with Breslow likelihood approximation) was developed to address the predictors for long-term survival.
To further adjust for patient selection and preoperative characteristics, a propensity score-matched analysis was conducted. The group of patients who did not develop AKI was 1:1 matched to the group of patients who did develop AKI using the following variables: age (years), sex, basal SCr, emergency surgery, LVEF category, New York Heart Association class, Canadian Cardiovascular Society (CCS) class, and Euroscore. This resulted in 2 paired groups with 60 patients each, designated AKI and non-AKI. After matching, the 2 groups were compared using the paired t test or Wilcoxon test for continuous variables and the McNemar or Fisher exact test for categorical variables. A conditional logistic regression model was also developed to identify predictors of in-hospital mortality, including the same variables used for the nonmatched unconditional logistic regression. Event-free survival rates were estimated by the Kaplan-Meier method and subsequently compared with the log-rank test even in this subset of matched patients. A Cox proportional hazard model was developed, stratifying for matching index. Missing values were addressed via multiple imputation. All tests were 2-sided, with an α level set at 0.05 for statistical significance. Clinical data were recorded and subsequently tabulated with Excel (Microsoft, Redmond, Wash). All statistical analyses were conducted using R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Overall In-Hospital and 30-Day Clinical Outcomes
The distributions of baseline patient characteristics for the overall population and for the propensity score–matched population are presented in Table 1. The total study cohort of 380 patients comprised 323 males (85%) and 57 females (15%), with an overall mean age of 66.9 ± 9 years. Of the 380 patients recruited, 237 (62.3%) underwent redo CABG using CPB and cardioplegic arrest, whereas the remaining 143 (37.6%) underwent beating heart off-pump redo CABG.
Table 1.
Preoperative characteristics of the study cohort
| Characteristic | Overall (n = 380) |
Overall population |
Propensity score–matched population |
||||
|---|---|---|---|---|---|---|---|
| AKI (n = 60) |
No AKI (n = 320) |
P value | AKI (n = 60) |
No AKI (n = 60) |
P value | ||
| Age, yr, mean ± SD | 66.9 ± 9 | 68.7 ± 8.9 | 66.6 ± 8.9 | .08 | 68.7 ± 8.9 | 66.9 ± 9.4 | .31 |
| Female sex, n (%) | 57 (15) | 8 (13.3) | 49 (15.3) | .89 | 8 (13.3) | 6 (10) | .77 |
| BMI, kg/m2, mean ± SD | 27.2 ± 4.4 | 26.1 ± 4.6 | 27.4 ± 4.3 | .07 | 26.2 ± 4.7 | 27.4 ± 4.7 | .15 |
| LVEF, n (%) | .04 | .65 | |||||
| Good | 247 (65.1) | 34 (56.7) | 213 (66) | 34 (56.7) | 35 (58.3) | ||
| Moderate | 109 (28.7) | 18 (30) | 91 (28.4) | 18 (30) | 18 (30) | ||
| Poor | 24 (6.31) | 8 (13.3) | 16 (8) | 8 (13.3) | 7 (11.7) | ||
| Diabetes, n (%) | .54 | .90 | |||||
| No | 294 (77.4) | 46 (76.7) | 248 (77.5) | 46 (76.7) | 47 (78.3) | ||
| Diet | 16 (4.2) | 3 (5) | 13 (4.1) | 3 (5) | 2 (3.3) | ||
| Oral treatment | 45 (11.8) | 5 (8.3) | 40 (12.5) | 5 (8.3) | 7 (11.7) | ||
| Insulin treatment | 25 (6.5) | 6 (10) | 19 (5.9) | 6 (10) | 4 (6.7) | ||
| Hypertension, n (%) | 271 (71.3) | 51 (85) | 220 (68.8) | .06 | 41 (68.3) | 38 (63.3) | .23 |
| Previous CVA, n (%) | 49 (12.8) | 10 (16.7) | 39 (12.2) | .60 | 10 (16.7) | 8 (13.3) | .12 |
| PVD, n (%) | 78 (20.5) | 16 (26.7) | 62 (19.4) | .19 | 16 (26.7) | 13 (21.7) | .64 |
| COPD, n (%) | 57 (15) | 12 (20) | 45 (14.1) | .23 | 12 (20) | 8 (13.3) | .45 |
| Smoking, n (%) | .63 | .87 | |||||
| Never smoked | 101 (26.6) | 16 (26.7) | 85 (26.5) | 16 (26.7) | 16 (26.7) | ||
| Ex-smoker | 246 (64.7) | 39 (65) | 207 (64.7) | 39 (65) | 36 (60) | ||
| Current smoker | 33 (8.6) | 5 (8.3) | 28 (8.8) | 5 (8.3) | 8 (13.3) | ||
| NYHA class, n (%) | .06 | .37 | |||||
| Class I | 63 (16.6) | 7 (11.7) | 56 (17.5) | 7 (11.7) | 5 (8.3) | ||
| Class II | 149 (39.2) | 17 (28.3) | 132 (41.2) | 17 (28.3) | 20 (33.3) | ||
| Class III | 148 (38.9) | 32 (53.3) | 116 (36.2) | 32 (53.3) | 32 (53.3) | ||
| Class IV | 20 (5.2) | 4 (6.7) | 16 (5) | 4 (6.7) | 3 (5) | ||
| CCS class, n (%) | <.01 | .78 | |||||
| Class 0 | 41 (10.8) | 10 (16.7) | 31 (9.7) | 10 (16.7) | 10 (16.9) | ||
| Class I | 25 (6.6) | 10 (16.7) | 15 (4.7) | 10 (16.7) | 4 (6.8) | ||
| Class II | 82 (21.6) | 11 (18.3) | 71 (22.2) | 11 (18.3) | 10 (16.9) | ||
| Class III | 132 (34.7) | 16 (26.7) | 117 (36.6) | 16 (26.7) | 15 (25.4) | ||
| Class IV | 99 (26) | 13 (21.7) | 86 (26.9) | 13 (21.7) | 20 (33.9) | ||
| Euroscore II, mean ± SD | 5.11 ± 4.03 | 6.12 ± 3.6 | 4.91 ± 3.8 | <.01 | 6.12 ± 3.6 | 5.91 ± 3.4 | .12 |
| Left main disease, n (%) | 89 (23.4) | 16 (26.7) | 73 (22.8) | .51 | 16 (26.7) | 14 (23.3) | .82 |
| Atrial fibrillation, n (%) | 29 (7.6) | 7 (11.7) | 22 (6.9) | .19 | 7 (11.7) | 9 (15) | .79 |
| Reduced eGFR, n (%) | 112 (29.5) | 30 (50) | 82 (25.6) | <.01 | 30 (50) | 30 (50) | 1 |
| Baseline SCr, μmol/L, mean ± SD | 102.8 ± 30.8 | 117 ± 35.9 | 100 ± 29 | <.01 | 117 ± 35.9 | 114 ± 41.8 | .26 |
| Peak SCr, μmol/L, mean ± SD | 133.3 ± 78.8 | 248 ± 121 | 111 ± 39.8 | <.01 | 248 ± 121 | 120 ± 52.7 | <.01 |
| Urgency, n (%) | .34 | .91 | |||||
| Elective | 235 (61.8) | 33 (55) | 202 (63.1) | 33 (55) | 34 (56.7) | ||
| Urgent | 134 (35.2) | 24 (40) | 110 (34.4) | 24 (40) | 22 (36.7) | ||
| Emergency | 10 (2.6) | 3 (5) | 7 (2.2) | 3 (5) | 4 (6.7) | ||
| Salvage | 1 (0.26) | 0 | 1 (0.3) | 0 | 0 | ||
| Off-pump, n (%) | 143 (37.6) | 18 (30) | 125 (39.1) | .23 | 18 (30) | 24 (40) | .46 |
| Number of grafts, mean ± SD | 2.22 ± 0.8 | 2.21 ± 0.88 | 2.33 ± 0.9 | .26 | 2.2 ± 0.9 | 2.2 ± 20.9 | .30 |
AKI, Acute kidney injury; SD, standard deviation; BMI, body mass index; LVEF, left ventricular ejection fraction; CVA, cerebrovascular accident; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association; CCS, Canadian Cardiovascular Society; eGFR, estimated glomerular filtration rate; SCr, serum creatinine.
Overall postoperative outcomes are presented in Table 2. The 30-day mortality was 4.7%, with 18 deaths, including 11 due to multiorgan failure/sepsis, 5 due to cardiogenic shock, and 2 due to permanent stroke. For cause-and-effect considerations, the 18 patients experiencing early death also sustained the following postoperative complications: 7 cases of acute renal failure (ARF) requiring dialysis, 7 cases of cardiogenic shock, 2 cases of permanent stroke, 4 cases of respiratory failure requiring tracheostomy, and 1 case of deep mediastinitis. In addition, 5 patients (1.4%) sustained postoperative stroke, 8 patients (3.7%) needed surgical reexploration for bleeding, and 27 patients (7.1%) required an IABP. The overall average postoperative in-hospital length of stay was 9.17 ± 8.0 days.
Table 2.
Postoperative outcome
| Characteristic | Overall (n = 380) |
Overall population |
Propensity score–matched population |
||||
|---|---|---|---|---|---|---|---|
| AKI (n = 60) |
No AKI (n = 320) |
P value | AKI (n = 60) |
No AKI (n = 60) |
P value | ||
| Periprocedural mortality, n (%) | 18 (4.7) | 8 (13.3) | 10 (3.1) | <.01 | 8 (13.3) | 3 (5) | .22 |
| CVA, n (%) | 5 (1.4) | 2 (3.3) | 3 (0.9) | .17 | 2 (3.3) | 2 (3.3) | 1 |
| Reoperation for bleeding, n (%) | 8 (3.7) | 4 (6.7) | 4 (1.2) | .02 | 4 (6.6) | 2 (3.3) | .37 |
| IABP use, n (%) | 27 (7.1) | 9 (15) | 18 (33.3) | .02 | 9 (15) | 11 (18.3) | .8 |
| Length of stay, d, mean ± SD | 9.17 ± 8.0 | 13.6 ± 12.8 | 8.3 ± 6.4 | <.01 | 13.6 ± 12.8 | 10.7 ± 10.5 | .06 |
AKI, Acute kidney injury; CVA, cerebrovascular accident; IABP, intra-aortic balloon pump; SD, standard deviation.
Occurrence of In-Hospital AKI and Impact on 30-Day Health Outcomes
Sixty of the 380 patients (15.8%) developed postoperative AKI according to the following RIFLE criteria classes: R, n = 39 (65%); I, n = 15 (25%); F, n = 6 (10%). For the overall population, the AKI group had a higher average incidence of moderate to poor LVEF, higher angina class, reduced eGFR (50% vs 25.6%; P < .01), higher SCr level (117 ± 35.9 μmol/L vs 100 ± 29 μmol/L; P < .01) and higher Euroscore (Tables 1 and 2). There were no baseline differences between the 2 groups following 1:1 propensity score matching, however.
In the overall cohort, the occurrence of in-hospital AKI was associated with increased 30-day mortality (13.3% vs 3.1% for no AKI; P < .01), reoperation for bleeding (6.7% vs 1.3%; P = .03), and prolonged hospital stay (13.6 ± 12.8 days vs 8.35 ± 6.4 days; P < .01). After propensity score matching, a between-group difference in mortality of approximately 8% remained, although this did not reach statistical significance. Similarly, the AKI group had an average of 3-day longer hospital, although again this difference did not reach statistical significance. With regard to postoperative renal function, the occurrence of AKI was associated with a higher postoperative peak SCr value in the AKI group in both the overall and the matched study cohorts. In addition, 11 of 60 patients in the AKI group (18.3%) developed complete ARF requiring renal replacement therapy, whereas there was no occurrence of ARF in the non-AKI group. Of note, the 11 cases of ARF occurred in 6 of the 6 patients with class F AKI and in 5 of the 15 patients with class I AKI.
Univariate analyses identified AKI, preoperative nitrate use, Euroscore, emergency operation, and preoperative IABP use as associated with increased 30-day mortality. However, our multivariate logistic regression analysis suggested that AKI (OR, 3.74; 95% CI, −1.3 to 10.5; P < .01), Euroscore (OR, 1.27; 95% CI, 1.07-1.52; P < .01), and preoperative IABP (OR, 6.9; 95% CI, 2.24-20.3; P < .01) were independent predictors of 30-day mortality in patients undergoing redo CABG (Table 3). After propensity score matching, only the use of IABP remained as an independent predictor of 30-day mortality (OR, 7.52; 95% CI, 2.08-27.14; P < .01).
Table 3.
Independent predictors of 30-day mortality before and after propensity matching
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Overall population | |||
| AKI | 3.74 | 1.3-10.5 | .01 |
| Euroscore | 1.27 | 1.07-1.52 | <.01 |
| Use of IABP | 6.9 | 2.24-20.29 | <.01 |
| Propensity score–matched population | |||
| Use of IABP | 7.52 | 2.08-27.14 | <.01 |
OR, Odds ratio; CI, confidence interval; AKI, acute kidney injury; IABP, intra-aortic balloon pump.
Occurrence of In-Hospital AKI and Impact on Long-Term Survival
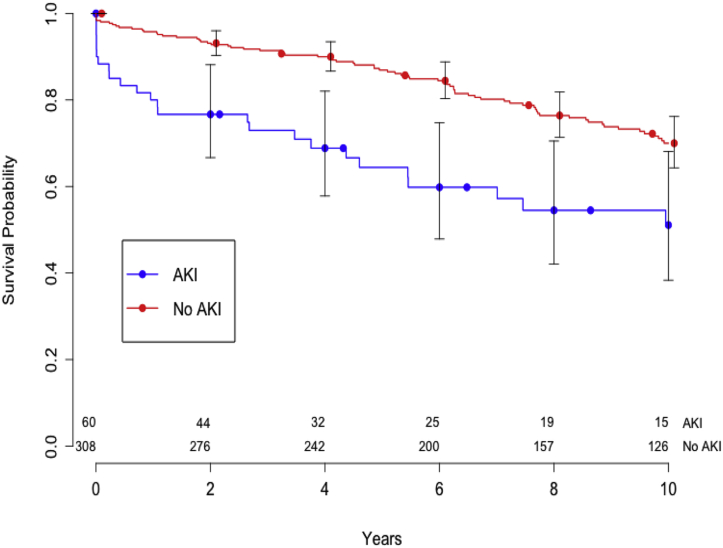
The median duration of follow-up was 7 years (interquartile range, 4-12.2 years). Late survival in the AKI and non-AKI groups is shown in Figure 2. The 1-year follow-up found an actuarial overall survival of 90.3% after redo CABG (80% for AKI vs 93% for non-AKI). Overall 5-year survival was 79% (64% for AKI vs 85% for non-AKI), and 10-year survival was 64% (51% for AKI vs 68% for non-AKI). Table 4 presents results from our Cox proportional hazard model identifying postoperative AKI, age, IABP use, and previous myocardial infarction as independent predictors of long-term mortality. After propensity score matching, only postoperative AKI and IABP use remained as independent predictors of long-term mortality, and the survival rate remained different between the 2 groups (P < .01, log-rank test).
Figure 2.
Impact of AKI on 10-year survival. The log-rank test shows a significant difference between the 2 groups. AKI, Acute kidney injury.
Table 4.
Cox proportional hazard model for mortality
| Variable (reference level) | Overall population HR (95% CI) |
P value | Propensity score–matched population HR (95% CI) |
P value |
|---|---|---|---|---|
| AKI∗ | 2.42 (1.63-3.6) | <.01 | 2.8 (1.15-6.7) | .02 |
| Age | 1.03 (1.01-1.05) | <.01 | ||
| Use of IABP | 1.66 (0.98-2.8) | .059 | 5.7 (1.04-31.1) | .04 |
| Previous MI | 1.4 (0.99-1.97) | .055 |
HR, Hazard ratio; CI, confidence interval; AKI, acute kidney injury; IABP, intra-aortic balloon pump; MI, myocardial infarction.
Defined as any RIFLE criterion.
Discussion
Cardiac reoperation remains an integral part of daily cardiac surgery practice, accounting for 5% to 8% of the entire surgical workload.6, 17 These procedures are technically challenging because of the recognized difficulty in gaining access to the heart owing to adhesions, scarring, fibrosis, or calcifications at the operative site, leading to prolonged operating times and high rates of early mortality and postoperative complications.
Redo CABG procedures still account for 2.5% to 4% of the surgical coronary practice in developed countries, with associated mortality as high as 4% to 5%6 despite advances in surgical techniques. Sabik and colleagues7 evaluated the outcomes of 21,568 CABG procedures, of which 4518 were reoperations and reported in-hospital mortality of 4.3% for a first redo, 5.1% for a second redo, and 6.4% for third or subsequent redos. These figures are in agreement with the findings published by Ghanta and colleagues,6 who recently reported the outcomes of 1.5 million isolated CABG operations (including 72,322 redo CABGs) from the Society of Thoracic Surgeons' adult cardiac surgery database. Nonetheless, very little data are available in the literature on the impact of patient characteristics and postoperative complications on early health outcomes and long-term mortality in patients undergoing redo CABG taking into account the RIFLE criteria.
Our main finding in the present study is the association between postoperative AKI and increased 30-day mortality and other major postoperative complications, as well as long-term mortality. Previous studies have confirmed that in redo CABG, a high baseline Euroscore and IABP use are also associated with 30-day mortality, and that advanced patient age and CCS class ≥3 are independent predictors of all-cause long-term mortality.2, 6, 7
For the purpose of this study, we defined AKI based on the RIFLE criteria, which have been widely validated and accepted as a standard methodology for qualifying and quantifying AKI after cardiac surgery. Strong associations have been reported between AKI as defined by the RIFLE criteria and outcomes in patients undergoing cardiac surgery.11, 13, 15, 16, 18, 19, 20 Some studies have suggested an association between AKI as defined by the RIFLE criteria and late health outcome as well, although in patients undergoing first-time cardiac surgery.14, 20 In a wide analysis of 2973 patients undergoing any cardiothoracic surgery, Hobson and colleagues21 reported that the risk of death associated with AKI defined by the RIFLE criteria after cardiothoracic surgery remains high for 10 years regardless of the presence of other risk factors. The hazard ratio (HR) for the subgroup of patients undergoing first-time CABG was 1.28 (95% CI, 1.05-1.54; P = .01). Unfortunately, however, those authors did not report on redo CABG. Another recent study evaluating the impact of AKI by the RIFLE criteria in patients undergoing first-time CABG compared AKI and non-AKI groups using a propensity score matched analysis and found higher mortality for the AKI group.11 The HR for AKI was 1.8 (95% CI, 1.5-2.16; P < .001) before matching and 1.52 (95% CI, 1.19-1.93; P < .01) after matching. That study also did not report on patients undergoing redo CABG, however.
In the present study, the incidence of postoperative AKI by the RIFLE criteria was 15.8%. Bove and colleagues,14 in an observational study of 5068 cardiac surgeries (including 498 redos), reported postoperative AKI incidence of 21.6% following redo CABG. That study also identified redo CABG as an independent predictor of postoperative AKI, although it did not address the impact of AKI on survival. It is worth noting that Bove and colleagues reported a higher incidence of AKI compared with our results despite the fact that their definition of AKI was stricter and based on a 100% increase in SCr, compared with the 50% increase defined by the RIFLE criteria.
There is little in the literature on the long-term outcome of redo CAB surgery and its association with postoperative AKI. The impact of postoperative AKI on long-term survival in patients undergoing redo CABG was another key funding of our present study. In a small study of 82 patients (with 11 reoperations) evaluating long-term prognosis in patients requiring renal replacement therapy postsurgery, Srivastava and colleagues22 reported survival rates of 54% at 5 years and 38% at 7 years. Another study of 739 elderly patients undergoing redo CABG found an in-hospital mortality rate of 7.6% and actuarial survival rates of 75% at 5 years and 49% at 10 years.23 The authors also reported that increased in-hospital mortality was associated with preoperative SCr value >1.6 mg/dL, but that postoperative AKI did not appear to be an independent risk factor for mortality.23 One possible explanation for their findings is that the study was undertaken before the advent of the RIFLE criteria, and thus was based on a less-sensitive definition of postoperative AKI.
Additional findings of the present study are the associations between high baseline Euroscore and IABP use and the observed 30-day mortality, as well as the associations between baseline advanced age and CCS class ≥3 and all-cause long-term mortality. These findings are confirmatory, in keeping with the outcomes reported by previous studies.10, 14
Identifying sensitive predictors of health outcomes is of value. In the present study, the occurrence of postoperative AKI, bedsides predicting early mortality, also predicted postoperative ARF requiring dialysis. Indeed, 6 of 6 patients with class F AKI and 5 of 15 patients with class I AKI subsequently developed ARF requiring dialysis, although none of the patients with class R AKI developed ARF requiring dialysis. In addition, our findings suggest that the occurrence of AKI by the RIFLE criteria also may be predictive of long-term health outcomes, although we believe that more evidence is needed to support this suggestion.
Predicting health outcomes with AKI by the RIFLE criteria in patients undergoing redo CABG might lead to changes in clinical approaches. One possible application might be before surgery during the patient consent process, allowing a more accurate description of the risk-to-benefit ratio associated with the intended procedure. In addition, the knowledge of a possible association between the occurrence of postoperative AKI and early/long-term survival might help stimulate the development/implementation of new clinical approaches aimed at preventing AKI. In our series, approximately 37% of the patients were treated with off-pump coronary surgery as an alternative surgical technique. This approach was based on surgeon preference in a generic attempt to reduce postoperative complications. Whether this approach might have been beneficial or detrimental, we cannot say, because our series did not contain enough patients undergoing redo CABG to ascertain the impact of this alternative technique.
This study has several limitations. First, its retrospective design might be suggestive of residual bias and unconsidered confounding factors. In addition, the study represents only a relatively small cohort of patients from a single institution. A second limitation is our lack of data on the timing of CT angiography in relation to the date of surgery. This might be an important factor, given that CT angiography performed close to the time of surgery might have acted as confounding factor in the incidence of AKI. This lack of data was only partially addressed by our inclusion of degree of urgency in our analysis, which can be considered a surrogate measure of timing of CT angiography related to time of surgery, given that urgent patients generally undergo surgery soon after CT angiography as part of the in-hospital urgent transfer process. A third limitation is that our database does not include postoperative SCr values, and thus although it was possible to derive these values manually from the medical records for our 380 patients in our study cohort, this was not possible for >15,000 patients in the database undergoing first-time CABG. This prevents us from comparing the relationship between redo CABG surgery and the occurrence of postoperative AKI in patients undergoing first-time CABG and those undergoing redo CABG. A fourth limitation is a lack of prospective follow-up, which might have prevented us from identifying additional clinical factors occurring late after discharge that possibly contributed to our long-term survival data. For similar reasons, we do not have data on causes of late deaths, and thus could not explore a long-term cause-and-effect relationship. Finally, our patient cohort was treated over a long period, thus possibly introducing confounding factors owing to changes in clinical practice over time.
Conflict of Interest Statement
Authors have nothing to disclose with regard to commercial support.
Footnotes
This study was supported by the National Institute for Health Research Biomedical Research Unit in Cardiovascular Disease at the University Hospitals Bristol National Health Service Foundation Trust. R.A. is supported by grants from the Medical Research Council, the British Heart Foundation, and the National Institute for Health Research.
References
- 1.Lloyd-Jones D., Adams R., Carnethon M., De Simone G., Ferguson T.B., Flegel K. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Awad W.I., De Souza A.C., Magee P.G., Walesby R.K., Wright J.E., Uppal R. Re-do cardiac surgery in patients over 70 years old. Eur J Cardiothorac Surg. 1997;12:40–46. doi: 10.1016/s1010-7940(97)00147-4. [DOI] [PubMed] [Google Scholar]
- 3.Elahi M., Dhannapuneni R., Firmin R., Hickey M. Direct complications of repeat median sternotomy in adults. Asian Cardiovasc Thorac Ann. 2005;13:135–138. doi: 10.1177/021849230501300208. [DOI] [PubMed] [Google Scholar]
- 4.Warnes C.A. The adult with congenital heart disease: born to be bad? J Am Coll Cardiol. 2005;46:1–8. doi: 10.1016/j.jacc.2005.02.083. [DOI] [PubMed] [Google Scholar]
- 5.Ruel M., Kulik A., Rubens F.D., Bédard P., Masters R.G., Pipe A.L. Late incidence and determinants of reoperation in patients with prosthetic heart valves. Eur J Cardiothorac Surg. 2004;25:364–370. doi: 10.1016/j.ejcts.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Ghanta R.K., Kaneko T., Gammie J.S., Sheng S., Aranki S.F. Evolving trends of reoperative coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons Adult Cardiac Surgery Database. J Thorac Cardiovasc Surg. 2013;145:364–372. doi: 10.1016/j.jtcvs.2012.10.051. [DOI] [PubMed] [Google Scholar]
- 7.Sabik J.F., III, Blackstone E.H., Houghtaling P.L., Walts P.A., Lytle B.W. Is reoperation still a risk factor in coronary artery bypass surgery? Ann Thorac Surg. 2005;80:1719–1727. doi: 10.1016/j.athoracsur.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Anderson R.J., O'brien M., MaWhinney S., VillaNueva C.B., Moritz T.E., Sethi G.K. Renal failure predisposes patients to adverse outcome after coronary artery bypass surgery. VA Cooperative Study 5. Kidney Int. 1999;55:1057–1062. doi: 10.1046/j.1523-1755.1999.0550031057.x. [DOI] [PubMed] [Google Scholar]
- 9.Litmathe J., Kurt M., Feindt P., Gams E., Boeken U. The impact of pre- and postoperative renal dysfunction on outcome of patients undergoing coronary artery bypass grafting (CABG) Thorac Cardiovasc Surg. 2009;57:460–463. doi: 10.1055/s-0029-1185877. [DOI] [PubMed] [Google Scholar]
- 10.Mangano C.M., Diamondstone L.S., Ramsay J.G., Aggarwal A., Herskowitz A., Mangano D.T. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194–203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher S., Jones D.A., Lovell M.J., Hassan S., Wragg A., Kapur A. The impact of acute kidney injury on midterm outcomes after coronary artery bypass graft surgery: a matched propensity score analysis. J Thorac Cardiovasc Surg. 2014;147:989–995. doi: 10.1016/j.jtcvs.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Bellomo R., Ronco C., Kellum J.A., Mehta R.L., Palevsky P. Acute renal failure: definition, outcome measures, animal models, fluid therapy and information technology needs. The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dardashti A., Ederoth P., Algotsson L., Brondén B., Bjursten H. Incidence, dynamics, and prognostic value of acute kidney injury for death after cardiac surgery. J Thorac Cardiovasc Surg. 2014;147:800–807. doi: 10.1016/j.jtcvs.2013.07.073. [DOI] [PubMed] [Google Scholar]
- 14.Bove T., Calabrò M.G., Landoni G., Aletti G., Marino G., Crescenzi G. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:442–445. doi: 10.1053/j.jvca.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Ascione R., Nason G., Al-Ruzzeh S., Ko C., Ciulli F., Angelini G.D. Coronary revascularization with or without cardiopulmonary bypass in patients with preoperative non–dialysis-dependent renal insufficiency. Ann Thorac Surg. 2001;72:2020–2025. doi: 10.1016/s0003-4975(01)03250-7. [DOI] [PubMed] [Google Scholar]
- 16.Angelini G.D., Taylor F.C., Reeves B.C., Ascione R. Early and midterm outcome after off-pump and on-pump surgery in Beating Heart Against Cardioplegic Arrest Studies (BHACAS 1 and 2): a pooled analysis of two randomised controlled trials. Lancet. 2002;359:1194–1199. doi: 10.1016/S0140-6736(02)08216-8. [DOI] [PubMed] [Google Scholar]
- 17.Onorati F., Biancari F., De Feo M., Mariscalco G., Messina A., Santarpino G. Mid-term results of aortic valve surgery in redo scenarios in the current practice: results from the multicentre European RECORD (REdo Cardiac Operation Research Database) initiative. Eur J Cardiothorac Surg. 2015;47:269–280. doi: 10.1093/ejcts/ezu116. [DOI] [PubMed] [Google Scholar]
- 18.Arnaoutakis G.J., Bihorac A., Martin T.D., Hess P.J., Jr., Klodell C.T., Ejaz A.A. RIFLE criteria for acute kidney injury in aortic arch surgery. J Thorac Cardiovasc Surg. 2007;134:1554–1560. doi: 10.1016/j.jtcvs.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 19.Nina V.J., Matias M.M., Brito D.J., Figueiredo Neto J.A., Coutinho L.B., Rodrigues R.F. Acute kidney injury after coronary artery bypass grafting: assessment using RIFLE and AKIN criteria. Rev Bras Cir Cardiovasc. 2013;28:231–237. doi: 10.5935/1678-9741.20130033. [DOI] [PubMed] [Google Scholar]
- 20.Bastin A.J., Ostermann M., Slack A.J., Diller G.P., Finney S.J., Evans T.W. Acute kidney injury after cardiac surgery according to Risk/Injury/Failure/Loss/End-Stage, Acute Kidney Injury Network, and Kidney Disease: Improving Global Outcomes classifications. J Crit Care. 2013;28:389–396. doi: 10.1016/j.jcrc.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Hobson C.E., Yavas S., Segal M.S., Schold J.D., Tribble C.G., Layon A.J. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava V., D'Silva C., Tang A., Sogliani F., Ngaage D.L. The impact of major perioperative renal insult on long-term renal function and survival after cardiac surgery. Interact Cardiovasc Thorac Surg. 2012;15:14–17. doi: 10.1093/icvts/ivs106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamuro M., Lytle B.W., Sapp S.K., Cosgrove D.M., III, Loop F.D., McCarthy P.M. Risk factors and outcomes after coronary reoperation in 739 elderly patients. Ann Thorac Surg. 2000;69:464–474. doi: 10.1016/s0003-4975(99)01076-0. [DOI] [PubMed] [Google Scholar]