Abstract
Rationale
Increased activity of prefrontal D1 dopamine receptors (D1R) is involved in reward-related behavior found in bipolar disorder and drug addiction. While the effects of elevated D1R are known, depressive-like behaviors also occur in these disorders after reward-seeking ends.
Objectives
The goal is to characterize how termination of D1R over-expression influences depressive-like behaviors.
Methods
An inducible (Tet.On), lentiviral vector was used to manipulate the expression of the DRD1 gene in glutamate neurons within the prefrontal cortex in male, adult rats. Sexual activity and sucrose preference were studied in both D1R elevated ‘ON’ and relatively reduced ‘OFF’ states. Following termination of the D1R ‘ON’ state, depressive-like behavior was determined in the ‘OFF’ state. Expression of the transcriptional regulator, cyclic AMP-responsive element binding protein (CREB), was used as an indication of down-stream effects in the NA.
Results
‘ON’ D1R expression increased sexual activity that returned to baseline in the ‘OFF’ state. Sucrose preferences increased ∼ 6% in ‘ON’ state but fell 11% below control levels when ‘OFF’. Consistent with a depressive-like phenotype, D1R ‘OFF’ decreased activity by 40%, impaired the ability to control (43%) and motivation to escape shock (27% more impaired) relative to dsRed ‘OFF’. CREB increased 29% in the NA in the D1R ‘OFF’ state relative to the ‘ON’ state.
Conclusions
This novel approach demonstrates that elevated D1R expression increased hedonic behavior, whereas the termination of D1R over-expression often resulted in depressive-like behavior. These observations support a role for D1R expression cycling in bipolar-associated behaviors and addiction.
Keywords: addiction, bipolar disorder, hedonia, lentivirus, prefrontal cortex, rat
Introduction
Dopamine dysfunction has been observed in a number of disorders, but the course of these disorders often wax and wane. Elevations in prefrontal (PFC) dopamine D1 receptors (D1R) are observed in schizophrenia (Paspalas et al. 2013), bipolar disorder (Suhara et al. 1992; Gonul et al. 2009; Yao et al. 2013), and addiction (Kalivas et al. 2005). Too much or too little PFC D1R is also associated with working memory deficits (Goldman-Rakic 1999; Floresco and Phillips 2001; Paspalas et al. 2013). Increased PFC D1Rs on pyramidal neurons, some of which project to the nucleus accumbens (NA), are evident in reinstatement processes associated with cocaine addiction (Kalivas and McFarland 2003; Sonntag et al. 2014), impulsivity (Loos et al. 2010; Sonntag et al. 2014), sucrose preferences (Sonntag et al. 2014), and rewarding social interactions (Tanda et al. 2009). While the role of increased D1R in a number of behaviors is known, we raise the question of what happens to behavior when D1R over-expression on glutamate neurons is terminated.
Bidirectional changes in dopamine function have been studied with sensitizing doses of amphetamine or cocaine followed by their withdrawal (Barr et al. 1999; Stoker and Markou 2011). While studies show that reward-related behaviors are associated with increased D1R (see above), it is possible that relative reduction of D1R may result in decreased reward or anhedonia. Studies have primarily assessed changes in either hedonia or anhedonia with sucrose preference tests (Powell et al. 2011; Overstreet 2012), and these results are often in good agreement with shifts in the threshold in the intracranial self-stimulation paradigm (Der-Avakian and Markou 2012; Donahue et al. 2014). However, these behavioral tests of anhedonia do not fully capture the broader spectrum of behavioral symptoms observed in the human condition of depression (reviewed by Andersen 2015). Other behavioral changes associated with depression include decreased sexual activity (Kennedy and Rizvi 2009), activity, and reduced motivation (avolition). These are rarely studied in animals as a constellation of behaviors, but typically studied independently.
Dopamine-related neural mechanisms and structures associated with depressive-like states are also involved in reward processing, motivation, attention, and decision-making. These brain systems include ventral tegmental projections to the NA, amygdala, anterior cingulate cortex, PFC, and the hypothalamus (Koob and Kreek 2007; Der-Avakian and Markou 2012). Drug withdrawal-precipitated anhedonia reduces general dopamine activity within the PFC (Goldstein and Volkow 2002; Bolla et al. 2004; Kroener and Lavin 2010) and the NA (Salamone et al. 1994; Correa et al. 2002; Der-Avakian and Markou 2012). In contrast, elevated D1R in the PFC reduced NA dopamine D2 receptor expression (Sonntag et al. 2014), further demonstrating a functional link between these two regions.
Currently, we have limited mechanistic insight into the underlying neurobiological processes involved in anhedonia (Kato et al. 2007; Nestler and Hyman 2010). Increases in the transcription-regulating factor CREB (cAMP response element-binding protein) in the NA play a role in depressive- and anxiety-like states (Carlezon et al. 2005) in general and following drug-withdrawal (Muschamp and Carlezon 2013). For example, increased accumbens CREB levels are associated with increased immobility in the forced swim test (Pliakas et al. 2001) and following cocaine withdrawal (Chandra et al., 2015). However, reduced levels of CREB in the NA shell impair the initiation of sexual behavior, with no effect on ejaculation (Barrot et al. 2005; Wallace et al. 2009).
Viral-mediated gene expression selectively targets specific neuronal populations within a single region. Here, the use of a lentiviral vector allowed us to study the role of D1R in motivated behaviors related to hedonia. Through this approach, we previously found that D1R over-expression on glutamate neurons in the medial PFC (mPFC; although more specifically the prelimbic region) increased impulsivity, sucrose preferences, and cocaine intake (Sonntag et al. 2014). Since D1R is sufficient to elevate reward-related salience that increases hedonic activities, we hypothesized that the absence of D1R elevation is associated with the loss of salience and hedonic activity.
In the present study, increased prefrontal dopaminergic signaling was produced by an inducible, cell-specific viral over-expression of D1R in the mPFC. Changes in behavior following changes in D1R expression were examined with an inducible virus system that allowed for over-expression of D1R (‘ON’ state) and its termination (‘OFF’ state); animals were tested in both states for sexual behavior, sucrose preferences, and locomotor activity, which were not contaminated by the previous state. Depressive-like behavior was determined in the ‘OFF’ state following ‘ON’. Depressive-like behaviors were studied with active avoidance, which is sensitive to the initial stressor environment (Banasr and Duman 2008), and the triadic model of learned helplessness and is not context-dependent. Both tests involve the mPFC (Amat et al. 2005; Moscarello and LeDoux 2013).
2.0 Experimental procedures
2.1 Animals
Adult, male Sprague Dawley rats (350-375g) were obtained from Charles River Laboratories (Boston, MA). Rats were housed with food and water available ad libitum in constant temperature and humidity conditions (22 ± 2°C and 55 ± 25 %) on a 12-hr light/dark cycle (light period 07:00–19:00). Different sets of rats were used for each experiment (including Western blot analysis), except the animals that were evaluated for sexual behavior who were also assessed for locomotor activity. Only males were used in this study to avoid an interference of estrous cycle-dependent hormonal changes in females on our induced high/low D1R state cycle. The experiments were conducted in accordance with the principles of animal care as set forth in the Guide for the Care and Use of Laboratory Animals (NIH), and were approved by the Institutional Animal Care and Use Committee at McLean Hospital.
2.2 Lentiviral vector and associated procedures
2.2.1 Production
A third generation Tetracycline-On inducible lentiviral vector system (Tet.On) was used. The system expressed the rat D1R (or the control, red fluorescent protein dsRed) driven by a CamKIIα promoter in the presence of the tetracycline derivative, DOX. Virus production, concentration by ultracentrifugation, qRT-PCR-based titer determination was performed at the Massachusetts General Hospital Viral Core according to published protocols (Sonntag et al. 2014). Viral expression is shown in cultured cells (Figure 1a), as in vivo ‘OFF’ expression is not possible to detect. Briefly, cells were stained for D1R with rat anti-D1 DAR IgG (1:250; Sigma; secondary: anti-rat TRITC coupled IgG [1:200; Molecular Probes]), or CamKIIα in mouse CamKIIα IgG (1:250; Chemicon; secondary: anti-mouse Alexa 488-coupled IgG [1:200; Molecular Probes]) in the presence or absence of doxycylcline to demonstrate ‘ON’ or ‘OFF’.
Fig. 1.
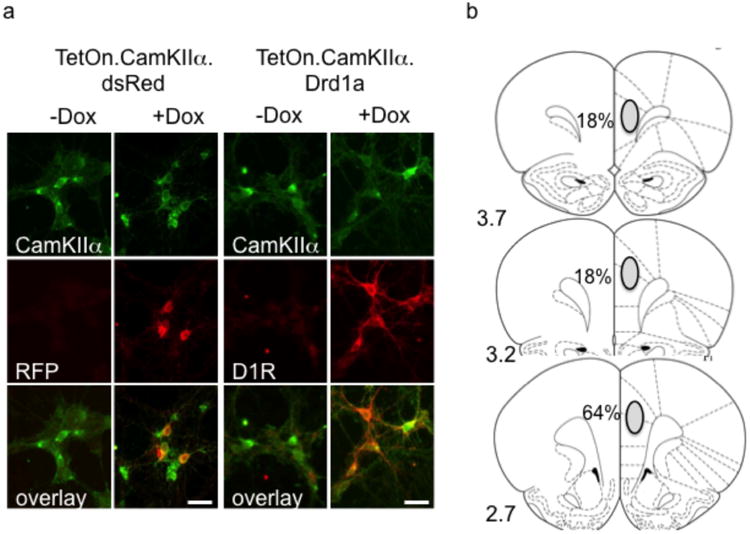
The inducible CamKIIα dependent lentiviral system in vitro and histological confirmation in vivo. (a) Virus-transduced rat E18 cortical primary cultures showing that D1R and dsRed (red fluorescent protein; RFP) are expressed in CamKIIα positive cells depending on the presence of doxycycline (here in a concentration of 200 ng/ml). The size bar represents 20 μm. (b) Location of D1R virus injections in the mPFC by histological confirmation and the percentage of subjects where the main portion of the bolus was found. Site location referenced to bregma coordinates
2.2.2 Surgery
Rats were anesthetized with a ketamine/xylazine mixture (80/12 mg/kg) and received 1 μl of virus (2 × 107 transducing units per μl) bilaterally into the mPFC at stereotaxic coordinates (AP +2.7, ML: 0.4; DV: -2.8) of (Paxinos et al. 1980).
2.2.3 Virus placement and expression
Virus placement was confirmed histologically after the behavioral testing by inducing expression with DOX (Sigma-Aldrich, St. Louis, MO; see below). Subjects were perfused with 4% paraformaldehyde, the brains cryoprotected and cut into 40 μm sections. After blocking in 10% donkey serum, sections were exposed to 1:250 antibody to the rat D1R IgG (Sigma-Aldrich), amplified with anti-rat IgG secondary, and visualized using standard diaminobenzidine or fluorochrome procedures (Brenhouse et al. 2008). Figure 1b shows where viral placement occurred. Subjects where the majority of placement was not within the mPFC were excluded from all analyses (n=3). D1R expression in the D1R ‘ON’ condition was increased ∼ 183% of the dsRed ‘OFF’ group — about half of what we previously reported with the constitutive virus (Sonntag et al., 2014), but within physiological levels.
2.3.4 Doxycycline (DOX) treatment
Virus expression was controlled by 0.5 g/l DOX hyclate (Sigma-Aldrich) in the drinking water to produce an ‘ON’ state. This dose was based on preliminary studies conducted in the elevated plus maze, as we have shown constitutive D1R over-expression increases time spent in the open arms (Sonntag et al. 2014; data not shown). Full viral expression occurred within seven days after surgery/DOX exposure was initiated (‘ON’) and over-expression was reduced after three days of DOX removal (‘OFF’ state). D1R expression was confirmed for each subject in the ‘ON’ state prior to sacrifice.
2.4 Behavioral Assessments
Behaviors were selected to show a contrast between hedonic and anhedonic behavior. However, sexual behavior and sucrose preferences demonstrated state changes (e.g., ‘ON’ and ‘OFF’ states of D1R modulation) that did not appear contaminated by prior D1R expression states. Depressive-like behavior was demonstrated with active avoidance and the triadic model, where controllability in the escapable group is linked to the PFC (Amat et al. 2005) and avolition has been associated with lack of escape in the no shock group (Pryce et al., 2011). The depressive behaviors were not amenable to repeated testing due to carryover effects of the prior shock exposure.
2.4.1 Sexual behavior
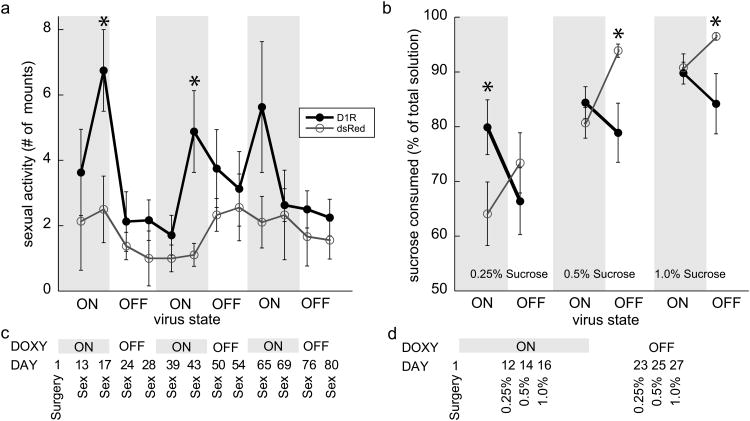
Sexual behavior was tested for two days in the ‘ON’ state and ‘OFF’ state across throughout three cycles (timeline in Figure 2c). Testing occurred during the dark cycle. Each animal was put in a 26.5 × 50.8 × 32.4 cm glass observation chamber and allowed to habituate for 5 min. An ovariectomized female was treated with estradiol benzoate (20 μg, subcutaneous 48h before testing) and progesterone (500 μg, subcutaneous 4h before testing) to induce sexual receptivity and was placed in the chamber with the male. Females were randomized between subjects and testing days. Mounts, intromissions and ejaculations were recorded for 25 min (Ågmo 1997; Barr et al. 1999). These same animals were then removed from DOX water for seven days and then re-tested in the ‘OFF’ state. This ‘ON’/’OFF’ cycle was repeated 3 times (timeline in Figure 2b).
Fig. 2.
Cycling of behaviors. (a) Number of sexual mounts following over-expression of D1R (black circles) following doxycycline exposure (ON' [grey shaded area]) compared to dsRed controls (white circles). Over-expression is terminated (‘OFF’) when doxycycline is removed. n=8 D1R and n=9 dsRed subjects (b) Sucrose preferences in the ON' (grey shaded area) and ‘OFF’ states. (c) + (d) Timelines of the testing schedule for sexual behavior and sucrose preferences. Means ± SEM are presented for n=10 D1R and n=9 dsRed subjects * p<0.05, Bonferrroni correction indicating significant differences within each state and/or concentration
2.4.2 Sucrose preference test
A two-bottle preference test (Willner et al. 1987; John et al. 2012) with three different sucrose concentrations was conducted. Rats were singly housed, and two water bottles in their home cage were weighed daily. Both bottles were filled with 0.5g/l DOX in the water and baseline drinking was assessed; no pre-existing side preferences were detected (44.1 ± 3% consumed from one bottle). After four days of baseline, sucrose concentration was increased every two days from 0.25% to 0.5% to 1%. The amount of sucrose consumed was expressed as total liquid consumed each day. Animals were then placed in the ‘OFF’ condition for three days and the procedure was repeated (Figure 2d).
2.4.3 Locomotor activity
As we have previously shown that D1R over-expression does not produce changes in activity (Sonntag et al. 2014), subjects were initially tested in the ‘OFF’ stage after seven days in the ‘ON’ state and then tested again in the ‘ON’ state after seven days of DOX. Rats were placed in a 24 × 18 × 33 cm dimly lit box (MedAssociates Inc, St Albans, VT) and the number of beam breaks was recorded for 1 hour during the light cycle.
2.4.4 Active avoidance
Active avoidance measures depressive-like behavior within the same context as the initial shock exposure. Subjects were tested in the ‘OFF’ state, three days after the termination of seven days in the ‘ON’ state. On Day 1, subjects received 60 0.65mA shocks (inter-trial interval of 40 ± 10 sec for both days) that were signaled by a tone and light and not allowed to escape from one side of a two-sided shuttlebox (Med Associates Inc, St Albans, VT). On Day 2, the tone and light signaled a 0.85mA shock, but subjects could avoid the shock by escaping to a second chamber. Latency to escape shock was measured.
2.4.5 Learned helplessness
The triadic model of learned helplessness allowed us to parse different aspects of depressive-like behavior (Amat et al. 2005; Pryce et al. 2011; Freund et al. 2013). Three conditions were tested. 1) escapable shock (ES), where the subject controls shock termination by turning a wheel on Day 1; this condition reflects activity within the PFC (Amat et al. 2005). 2) Inescapable shock (IS), where subjects were yoked to ES animals and likely reflects hippocampal activity. 3) No shock (NS), where subjects were restrained in a wheel-turn box and received no shock until Day 2; NS reflects motivation (Pryce et al., 2011). This helplessness paradigm tests in a second context than training, unlike active avoidance. Animals were tested after three days in the ‘OFF’ state, which followed the ‘ON’ state. On Day 1, subjects underwent 100 trials of a tail shock delivered on a variable interval (45 s) schedule that had escalated shock intensity to prevent habituation (1.0 mA for trials 1-30, 1.3 mA for trials 31-60, and 1.6 mA for trials 61-100). On Day 2, subjects were placed into a shuttlebox (Med Associates Inc, St Albans, VT) and could terminate a 1 mA foot shock (signaled by a 3s warning tone) by shuttling to the other side for trials 1-5 (FR1), or by shuttling to the other side and back again for trials 6-30 (FR2). The shock was terminated after 30 s if the animal failed to shuttle as required. Depressive-like behavior was measured as the mean latency to escape in the last 25 trials.
2.5 Western immunoblot for CREB expression
Subjects were either sacrificed in the ‘ON’ state (9 days of DOX exposure) or sacrificed in the ‘OFF’ state (seven days on DOX followed by three days of DOX termination to assure that the contrast between ‘ON’ and ‘OFF’ would manifest). The mPFC was dissected for confirmation of D1R expression in all groups, which was used as a covariate in the analyses. The NA was also dissected, although subregions were not assessed, to determine CREB levels and stored at -80 °C until processing. Tissue was then homogenized in 1% sodium dodecylsulfate solution (SDS) coxntaining a protease inhibitor cocktail (Pierce, Rockford, IL). Protein concentration was determined by the Bradford method (BioRad Laboratories, Hercules, CA; Bradford, 1976) and 40 μg of protein was mixed in 6 × SDS, centrifuged, and boiled for 3 min prior to separation by 15% SDS-PAGE. Following electrophoresis, proteins were transferred to a nitrocellulose membrane (BioRad Laboratories). The membranes were blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) in phosphate-buffered saline for 60 minutes at RT and incubated with primary polyclonal antibodies to CREB (43kDa; 1:400; rabbit; Cell Signaling Technologies, Danvers, MA), and D1 receptors (52kDa; 1:400; rat; Sigma, St. Louis, MO) in Odyssey blocking buffer (LI-COR Biosciences) in PBS containing 0.1-0.2% Tween overnight 4 °C. The membranes were rinsed four times for five minutes at RT in PBS-T. After the rinsing procedure, the membranes were incubated for one hour at room temperature in IRDye 800-conjugated anti- rabbit (LI-COR Biosciences) or anti-rat (LI-COR Biosciences) 1:20,000 in Odyssey blocking buffer in 0.1% PBS-T. Protein loading was assessed by β-tubulin (55 kDa; 1:10,000, Covance Laboratories, Dedham, MA; secondary: anti-mouse β-tubulin for IRDye 700-conjugated anti-IgG [H&L; LI-COR Biosciences]) in PBS-T. An Odyssey infrared imaging system (LI-COR Biosciences) with excitation/emission filters at 780 nm/820 nm range was used to detect fluorescence. Protein values were normalized to β -tubulin expression.
2.6 Statistical analysis
Data were analyzed by 3-way ANOVA with Virus (D1R or dsRed), State (‘ON’ or ‘OFF’), and Session (1-3; for sexual behavioral data) or Concentration (0.25%, 0.5%, 1%: for sucrose preference data) as variables using SPSS v.20 (IBM Corp). Virus was a between-subject variable and State, Session, or Concentration were within-subjects variables. A one-way ANOVA was used to analyze the active avoidance paradigm. Data for the learned helplessness paradigm were analyzed with a 2-way ANOVA with Virus and Group (ES, IS or NS) as independent variables. Bonferroni correction was used for any post-hoc comparisons between individual Group conditions to determine the effects of Virus.
CREB data were corrected for tubulin amounts, and analyzed with a Virus × State (in this case it was a between-subjects variable) ANCOVA and covaried for levels of mPFC D1R from each subject (included both endogenous and virally-tranduced expression levels). Bonferroni correction was used for post-hoc comparison in the D1R condition alone, consistent with our a priori hypothesis that change would only occur in the D1R group. Significance was set at p<0.05.
3.0 Results
3.1 Sexual behavior
Animals transduced with either the D1R or dsRed were tested for sexual behavior across three ‘ON’/’OFF’ cycles. An ‘ON’/’OFF’ X Virus interaction for the frequency of sexual mounts was found (F1,14 = 4.658; P=0.049). When in the ‘ON’ state, D1R animals showed more sexual mounts compared to dsRed animals, but in the ‘OFF’ state, D1R animals returned to control levels. Overall, D1R subjects had more mounts than dsRed subjects (Virus main effect: F1,14 = 10.847; P=0.005; Figure 2a). Post-hoc comparisons shows that increased mounting behavior occurred during the first two ‘ON’ cycles (Bonferroni correction, P<0.05). The same pattern was found for successful intromissions (i.e., percentage of intromissions that resulted in ejaculation; not shown) with D1R ‘ON’ animals having more ejaculations per intromission. When D1R was turned ‘OFF’, D1R subjects did not differ from dsRed animals (‘ON’/’OFF’ X Virus interaction: F1,15 = 10.727; P=0.005). Taken together, sexual activity increased following the over-expression of D1R and returned to baseline when the over-expression was terminated. This cycling of sexual activity was observed throughout multiple ‘ON’/’OFF’ cycles.
3.2 Sucrose preference test
This second test of hedonic activity showed cycling between hedonia and anhedonia. In the two-bottle test of sucrose preference where animals could chose between water or 0.25, 0.5, or 1.0% sucrose, both D1R and dsRed subjects demonstrated concentration-dependent preferences for sucrose (Concentration main effect: F1,20 = 156.74; P<0.0001). A Virus × ‘ON’/’OFF’ interaction (F2,20 = 3.43; P=0.05; Figure 2b) shows that the sensitivity to sucrose concentrations depended on ‘ON’ or ‘OFF’ states indicating a 95% chance of correctly rejecting the null hypothesis. Sucrose preference was noticeably increased at the 0.25% sucrose solution in the D1R ‘ON’ animals compared to dsRed animals (post-hoc comparison with Bonferroni correction, P<0.05). In the D1R ‘OFF’ state, however, sucrose preferences not only returned to baseline, but decreased compared to controls (for the high concentrations; Bonferroni correction, P<0.05).
3.3 Locomotor activity
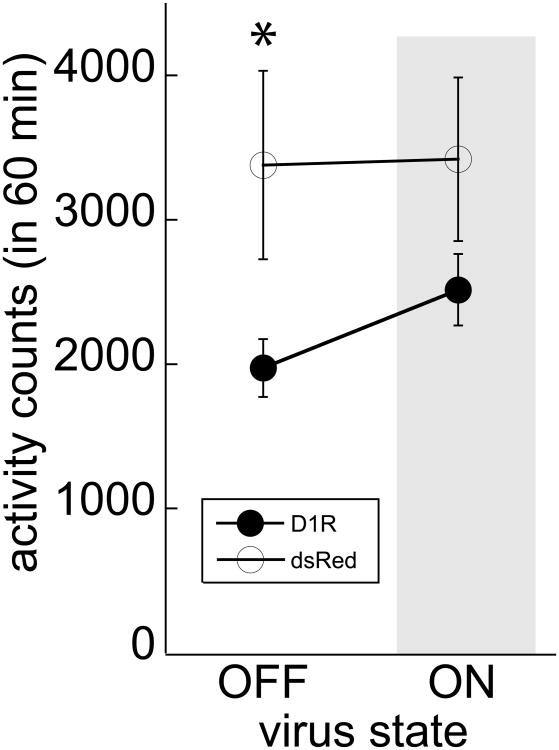
A 60 min open field test was used to determine the relationship of D1R to general locomotor activity (Figure 3). As this behavior is subject to novelty-induced activity when initially assessed, transduced subjects were tested in the ‘OFF’ state first (after experiencing seven days in the ‘ON’ state followed by three days ‘OFF’), and then re-tested in the ‘ON’ state after seven days on DOX. D1R animals show a trend to be less active compared to dsRed animals (Virus main effect: F1,15 = 3.571, P=0.079; Figure 3) that is driven by changes within the ‘OFF’ state (Bonferroni correction, P<0.05).
Fig. 3.
Locomotor activity in the ‘OFF’ and ‘ON’ state. D1R animals (black circles) in the ‘OFF’ state were less active but did not show activity differences in the ‘ON’ (grey shaded area) state compared to dsRed animals (white circles). Means ± SEM are presented for n=8 D1R and n=9 dsRed; * p<0.05
3.4 Active avoidance
Depressive-like behavior was assessed in the ‘OFF’ phase following prior ‘ON’ state exposure. The results from this paradigm indicated no difference between Virus groups (Figure 4a; One-way ANOVA: F1,18 = 0.01, P=0.9).
Fig. 4.
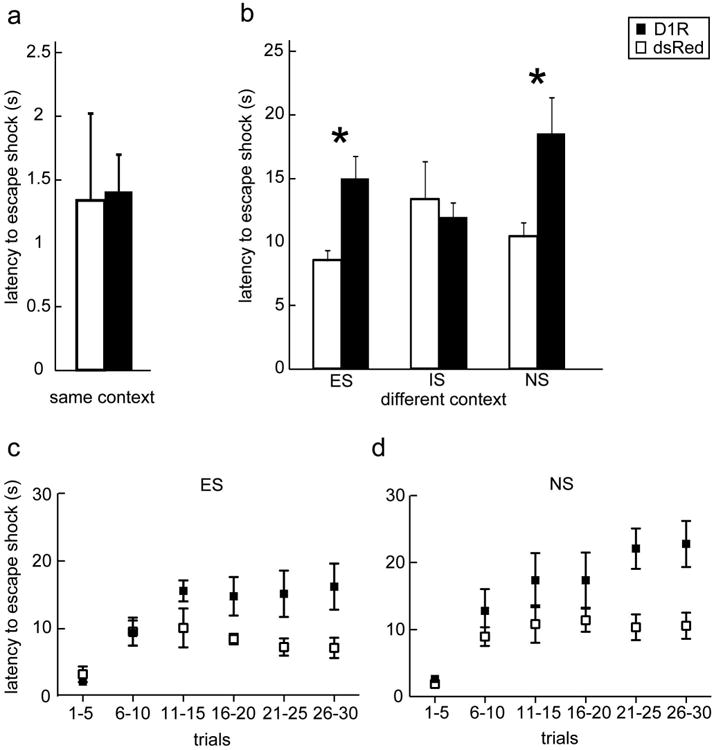
Depressive-like behavior in the ‘OFF’ state. (a) In an active avoidance paradigm where training and testing occur in the same context; D1R (black bars; n= 11) and dsRed (white bars; n=8) do not differ. (b) In the triadic learned helplessness paradigm, behavior was trained and tested in different contexts; during testing, shock was escapable (ES group), inescapable (IS group), or no shock (NS group). n = 5-7 per virus condition and group. (c) and (d) illustrate how escape behavior changes across trials; D1R (black squares) and dsRed (white squares) do not differ in the first block of trials, suggesting no impairment in learning to escape initially. Bars represent means ± SEM; * p<0.05
3.5 Learned helplessness
D1R ‘OFF’ animals showed increased helplessness relative to dsRed controls that depended on the helplessness condition (latency to escape the shock; Virus X Group interaction: F2,35= 3.3, P=0.05, indicating a 95% chance of correctly rejecting the null hypothesis). To determine which helplessness condition was different between virus group, post-hoc directed comparisons revealed that D1R ‘OFF’ subjects in the ES (P=0.006) and NS (P=0.03) group demonstrated significantly longer latencies to escape relative to dsRed controls; no differences were found between D1R ‘OFF’ subjects and controls in the IS group (Figure 4b).
3.6 CREB Western immunoblot in the NA
A Virus × ‘ON’/’OFF’ ANCOVA was conducted, where ‘ON’/’OFF’ was a between-subjects variable and data co-varied for individual mPFC D1R expression (both endogenous- and virally-mediated expression), which was assessed in separate blots. While the overall interaction was not significant (F1,19=1.49, P=0.2), the a priori prediction was that only a difference in CREB expression would be evident in the D1 animals. As such, an additional t-test with Bonferroni correction between D1R ‘ON’ and D1R ‘OFF’ was significant (t10 = 2.58, P=0.03).
4.0 Discussion
We show for the first time that manipulation of D1R within a single animal induces different states of behavior. Similar mechanisms may be found in bipolar disorder (Suhara et al. 1992; Yao et al. 2013), addiction (Ponce et al. 2015), and schizophrenia (Paspalas et al. 2013). Over-expression of D1R increases sexual activity and sucrose preferences, which fell below control values when D1R over-expression is reduced. Importantly, the data show that a reduction in D1R is sufficient to increase learned helplessness that is mediated by changes in the mPFC in the ES controllable shock condition (Amat et al. 2005) or PFC modulation of the NA in the case of the NS condition (Pryce et al. 2011). The use of the CK.D1 virus provides strong evidence that mPFC D1R on glutamatergic outputs mediates these behaviors.
The present data maybe most appropriately applied to the cycling between mania and depression (DelBello et al. 2008). Moreover, these data focus the broad dopamine hypothesis of bipolar disorder (Berk et al. 2007) on D1R in the mPFC by demonstrating its role in many of these behavioral states. Previous studies have linked mania to increased dopaminergic signaling (Murphy et al. 1971; Young et al. 2011) and depression with decreased dopaminergic signaling (Xing et al. 2006; Tye et al. 2013). Postmortem studies indicate a role for D1R in bipolar disorder (Pantazopoulos et al. 2004; Yao et al. 2013;Suhara et al. 1992). Here, D1R ‘ON’ increases sexual activity, which is often found patients with mania (Basco and Celis-de Hoyos 2012; Dvorak et al. 2013), and induces hedonia (indicated by increased sucrose preference). Furthermore, we have previously demonstrated that elevated D1R on glutamatergic neurons in the mPFC increases impulsivity, novelty seeking, drug- seeking and taking while it decreases anxiety (Sonntag et al. 2014). All these behaviors are evident in the D1R ‘ON’ animals and consistent with a mania-like state.
The neural circuitry that underlies the effects of D1R ‘OFF’ on depressive-like behavior may further help define the role of the mPFC in the different symptoms of depression (Andersen 2015). The PFC modulates multiple regions in the brain that have been implicated in depression, including the NA, the dorsal raphe nucleus, the hippocampus, and the amygdala – the first three of which are affected by D1R changes.
Behaviors associated with hedonia/anhedonia are typically associated with changes in the NA. Relative to dsRed subjects, the increased sexual activity (e.g., the number of intromissions) in the D1R ‘ON’ subjects returns to a low baseline when D1R is ‘OFF.’ Reduced locomotion, also associated with depression (Pryce et al., 2011), is also evident in the ‘OFF’ state. While reduced activity could impair sexual behavior, the same pattern is observed for intromissions that result in an ejaculation, which requires little locomotion. A relative increase in CREB expression in the D1R ‘OFF’ condition was observed in the NA. Increased levels of accumbens CREB are associated with depressive-like states in rats with the forced swim test (Pliakas et al., 2001) or following drug withdrawal (Muschamp et al., 2011; Chandra et al., 2015). We observed an increase in CREB in the ‘OFF’ D1R condition, but not during the ‘ON’ state when sexual behavior was most effected; no change in CREB was observed in the dsRed subjects in either state. Our findings of a return to baseline of sexual activity in the D1 ‘OFF’ seem to be in contrast to a report of reduced CREB expression (by a dominant negative mutant CREB virus) in the NA shell that is associated with increased latency to mount a receptive female and time to intromission during the first experience; mutant CREB was not associated with the number of mounts needed for ejaculation (Barrot et al. 2005). These findings were observed during the first encounter (D1R ‘ON’ state). Our CREB changes could possibly reflect downstream effects of D1R overexpression on different populations of neurons than those targeted by the mutant CREB virus. To this end, both core and shell of the NA were included in our sample.
Depressive behavior that is relevant to PFC D1R expression likely involves neural circuits that include the dorsal raphe nucleus and the NA. Under circumstances where helplessness is induced and context is associated with a predictive cue that signals shock (e.g., the tone in the active avoidance paradigm), the D1R ‘OFF’ animals show no apparent deficits. This finding is in line with Kram et al. (2002) who showed no correlation between mPFC D1R and helplessness when the context between training and testing did not change. However, the context change between training and testing in the triadic model reveals escape deficits in ES and NS animals. ES behavior involves mPFC modulation of the dorsal raphe nucleus (Amat et al. 2005). Consistent with reduced mPFC output during depressive states (Robbins 2005), D1R ‘OFF’ animals are impaired in the ES condition as they fail to transfer the ability control shock to a new environment. This behavioral observation is similar to previous observations in rats that underwent maternal separation and have reduced D1R on glutamate output neurons in the mPFC (Leussis et al. 2012; Brenhouse et al. 2013).
Avolition and hedonia/anhedonia are associated with the NA (Muschamp et al. 2011; Berridge and Kringelbach 2013) and the mPFC ‘OFF’ state may have reduced modulation of the NA. The observed increased latency to escape in the NS group is probably related to motivational changes associated with the NA (Pryce et al. 2011). D1R expression in the mPFC projections to the accumbens plays a role in motivated behaviors (Richard and Berridge 2013l; McFarland et al. 2003; Sonntag et al. 2014). While it is possible that the animals may have difficulty learning the contingency to escape or that reduced activity contributed to a “depressive-like” phenotype, the data in Figures 4 c and d argue against this hypothesis. D1R animals show no differences from dsRed animals for trials 1-5 (FR1 to escape) or trials 6-10 (FR2). Rather, a progressive impairment in escape latencies is observed across sessions. The constellation of reduced sexual behavior, stressor controllability (the ES group), motivation (the NS group), and sucrose-drinking in the D1R ‘OFF’ state are all suggestive of an anhedonic/depressive-like state. These behaviors may be due to either the contrast of the D1R ‘ON’ experience or a compensatory mechanism; the title “When the party is over…” describes the contrast that appears during drug withdrawal or post-mania.
This hypothesis of reduced dopamine signaling leading to depressive-like behavior is further supported by clinical data showing that dopamine agonists are helpful in bipolar depression (Dell’Osso et al. 2013). Extensive PFC D1R stimulation can result in a functional disconnection (Hains and Arnsten 2008). We hypothesize that the D1R over-expression induces autoregulatory mechanisms leading to a hypo-expression in the ‘OFF’ state. The result is decreased dopaminergic output signaling of the mPFC that leads to anhedonia and depressive-like behavior. Parkinson's patients who are treated with dopamine agonists can exhibit mania (Weintraub 2008), while tapering off of a dopamine agonist increases risk to develop depressions (Nirenberg 2013). These observations may be mediated by the D1R.
Here, we focused on accumbens CREB as a downstream target of reduced PFC modulation. In line with our findings, others have shown that CREB over-expression in accumbens reduces sucrose preference (Barrot et al. 2002), induces depressive-like behavior (Pliakas et al., 2001), and is associated with aversive states of drug withdrawal (Muschamp and Carlezon, 2013). Our earlier study on mPFC D1R overexpression demonstrated a reduction in D2 receptors in the NA (Sonntag et al., 2014). By isolating D1- and D2-expressing medium spiny neurons (MSNs) in the NA, a recent study demonstrated that repeated cocaine followed by withdrawal increased and decreased CREB in D1- or D2-expressing MSNs, respectively (Chandra et al., 2015). The data by Chandra and colleagues offer insight into the complexity of CREB signaling. Medial PFC D1R overexpression may affect the D2 MSNs more directly, whereas the compensatory actions of either D1R ‘OFF’ or cocaine withdrawal might be reflected in the D1 MSNs. Bi-functionality of CREB activities within the NA can facilitate reward-associated behaviors while simultaneously modulating dysphoria (Dineri et al., 2009). CREB regulation of reward behaviors is complex and is region- (even sub-region) and form- (phosphorylated or not) dependent (Zhong et al., 2014; Olson et al., 2005). The main focus of the current study is the induction of cycling behavior by manipulating a single receptor in the mPFC. Inclusion of the CREB Western blots was to simply show downstream pathways are also affected by our manipulation.
Future studies will examine other regions downstream of the mPFC that are involved in depressive behaviors. Decreased mPFC modulation of the raphe in the ‘OFF’ state may underlie increased escape latency of the ES group in the triadic paradigm (Amat et al., 2005). Reduced activity in the ventral tegmental area (Eisch et al. 2003; Minton et al. 2009) could also induce depressive-like behaviors. Taken together, elevated mPFC D1R increased hedonic-like behaviors (sex, sucrose preferences), but more importantly, termination of D1R over-expression induced anhedonic- and depressive-like behaviors (decreased sucrose preferences, locomotor retardation, helplessness). The D1R expression pattern of ‘ON’/‘OFF’ may therefore play an important role in the switch from mania/hedonia to depression/anhedonia that is clearly evident in bipolar patients, but also in individuals with schizophrenia or addiction. An inducible virus provides a new tool to study specific circuits underlying multiple behaviors.
Fig. 5.
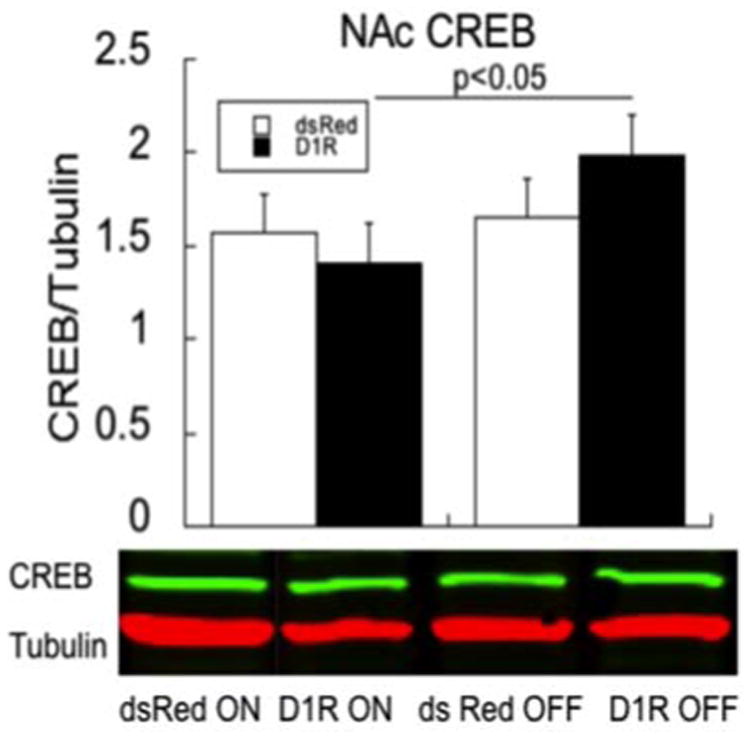
CREB changes in the ‘ON’ and ‘OFF’ states in the NA in dsRed (white bars) and D1R (black bars) subjects; n=6 for each of the four groups. Data were co-varied for plPFC D1R expression. Bars represent means ± SEM; * p<0.05. Shown below are representative bands of CREB and the control protein, tubulin. Bars represent means ± SEM
Acknowledgments
We want to thank the Leopoldina Fellowship Program LPDS-40, the NARSAD Young Investigator Grant 2012, the van Ameringen Foundation (to NF), DA-026485 and DA-015403 and the Simches Family (to SLA) for support, Dr. David Sibley (NIH) for providing the D1 receptor plasmid, Dr. Karl Deisseroth (Stanford University) for the CamKIIα promotor, Heather Brenhouse for her help with conducting the experiments and Nellie Mary for helpful discussions.
Footnotes
Financial disclosure: The authors declare that they have no competing financial interests.
References
- Ågmo A. Male rat sexual behavior. Brain Res Protoc. 1997;1:203–209. doi: 10.1016/S1385-299X(96)00036-0. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Exposure to early adversity: Points of cross-species translation that can lead to improved understanding of depression. Dev Psychopathol. 2015;27:477–491. doi: 10.1017/S0954579415000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Fiorino DF, Phillips AG. Effects of withdrawal from an escalating dose schedule of d-amphetamine on sexual behavior in the male rat. Pharmacol Biochem Behav. 1999;64:597–604. doi: 10.1016/s0091-3057(99)00156-2. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JDA, Perrotti LI, et al. CREB activity in the NA shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Wallace DL, Bolaños CA, et al. Regulation of anxiety and initiation of sexual behavior by CREB in the NA. Proc Natl Acad Sci U S A. 2005;102:8357–8362. doi: 10.1073/pnas.0500587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basco MR, Celis-de Hoyos CE. Biopsychosocial model of hypersexuality in adolescent girls with bipolar disorder: strategies for intervention. J Child Adolesc Psychiatr Nurs Off Publ Assoc Child Adolesc Psychiatr Nurses Inc. 2012;25:42–50. doi: 10.1111/j.1744-6171.2011.00312.x. [DOI] [PubMed] [Google Scholar]
- Berk M, Dodd S, Kauer-Sant'anna M, et al. Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand Suppl. 2007:41–9. doi: 10.1111/j.1600-0447.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol. 2013;23:294–303. doi: 10.1016/j.conb.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, et al. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci Off J Soc Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H, Lukkes J, Andersen S. Early life adversity alters the developmental profiles of addiction-related prefrontal cortex circuitry. Brain Sci. 2013;3:143–158. doi: 10.3390/brainsci3010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chandra R, Francis TC, Konkalmatt P, Amgalan A, Gancarz AM, Dietz DM, Lobo MK. Opposing role for Egr3 in NA cell subtypes in cocaine action. J Neurosci. 2015;35:7927–37. doi: 10.1523/JNEUROSCI.0548-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa M, Carlson BB, Wisniecki A, Salamone JD. NA dopamine and work requirements on interval schedules. Behav Brain Res. 2002;137:179–187. doi: 10.1016/s0166-4328(02)00292-9. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Adler CM, Cerullo MA, et al. In: Bipolar Disorder. Squire LR, editor. Elsevier; Amsterdam: 2008. [Google Scholar]
- Dell'Osso B, Ketter TA, Cremaschi L, et al. Assessing the roles of stimulants/stimulant-like drugs and dpamine-agonists in the treatment of bipolar depression. Curr Psychiatry Rep. 2013;15:1–12. doi: 10.1007/s11920-013-0378-z. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinieri JA, Nemeth CL, Parsegian A, Carle T, Gurevich VV, Gurevich E, Neve RL, Nestler EJ, Carlezon WA., Jr Altered sensitivity to rewarding and aversive drugs in mice with inducible disruption of cAMP response element-binding protein function within the NA. J Neurosci. 2009;29:1855–9. doi: 10.1523/JNEUROSCI.5104-08.2009s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SJ, et al. Effects of striatal ΔFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak RD, Wray TB, Kuvaas NJ, Kilwein TM. Mania and sexual risk: associations with behavioral self-regulation. J Affect Disord. 2013;150:1076–1081. doi: 10.1016/j.jad.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, et al. Brain-derived neurotrophic factor in the ventral midbrain-NA pathway: a role in depression. Biol Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;115:934–939. [PubMed] [Google Scholar]
- Freund N, Thompson BS, Denormandie J, et al. Windows of vulnerability: Maternal separation, age, and fluoxetine on adolescent depressive-like behavior in rats. Neuroscience. 2013;249:88–97. doi: 10.1016/j.neuroscience.2013.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The “psychic” neuron of the cerebral cortex. Ann N Y Acad Sci. 1999;868:13–26. doi: 10.1111/j.1749-6632.1999.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonul AS, Coburn K, Kula M. Cerebral blood flow, metabolic, receptor, and transporter changes in bipolar disorder: the role of PET and SPECT studies. Int Rev Psychiatry Abingdon Engl. 2009;21:323–335. doi: 10.1080/09540260902962131. [DOI] [PubMed] [Google Scholar]
- Hains AB, Arnsten AFT. Molecular mechanisms of stress-induced prefrontal cortical impairment: Implications for mental illness. Learn Mem. 2008;15:551–564. doi: 10.1101/lm.921708. [DOI] [PubMed] [Google Scholar]
- John CS, Smith KL, Van't Veer A, et al. Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology. 2012;37:2467–75. doi: 10.1038/npp.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacol Berl. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kato T, Kubota M, Kasahara T. Animal models of bipolar disorder. Neurosci Biobehav Rev. 2007;31:832–42. doi: 10.1016/j.neubiorev.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Rizvi S. Sexual dysfunction, depression, and the impact of antidepressants. J Clin Psychopharmacol. 2009;29:157–164. doi: 10.1097/JCP.0b013e31819c76e9. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kram ML, Kramer GL, Ronan PJ, et al. Dopamine receptors and learned helplessness in the rat: an autoradiographic study. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:639–645. doi: 10.1016/s0278-5846(01)00222-6. [DOI] [PubMed] [Google Scholar]
- Kroener S, Lavin A. Altered dopamine modulation of inhibition in the prefrontal cortex of cocaine-sensitized rats. Neuropsychopharmacology. 2010;35:2292–2304. doi: 10.1038/npp.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis MP, Freund N, Brenhouse HC, et al. Depressive-like behavior in adolescents after maternal separation: sex differences, controllability, and GABA. Dev Neurosci. 2012;34:210–7. doi: 10.1159/000339162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M, Pattij T, Janssen MCW, et al. Dopamine receptor D1/D5 gene expression in the medial prefrontal cortex predicts impulsive choice in rats. Cereb Cortex N Y N 1991. 2010;20:1064–1070. doi: 10.1093/cercor/bhp167. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the NA mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci Off J Soc Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton GO, Young AH, McQuade R, et al. Profound changes in dopaminergic neurotransmission in the prefrontal cortex in response to flattening of the diurnal glucocorticoid rhythm: implications for bipolar disorder. Neuropsychopharmacol. 2009;34:2265–2274. doi: 10.1038/npp.2009.53. [DOI] [PubMed] [Google Scholar]
- Moscarello JM, LeDoux JE. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J Neurosci. 2013;33:3815–23. doi: 10.1523/JNEUROSCI.2596-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Brodie HKH, Goodwin FK, Bunney WE. Regular induction of hypomania by L-Dopa in “bipolar” manic-depressive patients. Nature. 1971;229:135–136. doi: 10.1038/229135a0. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Carlezon WA. Roles of NA CREB and dynorphin in dysregulation of motivation. Cold Spring Harb Perspect Med. 2013;3:a012005. doi: 10.1101/cshperspect.a012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Veer AV, Parsegian A, et al. Activation of CREB in the NA shell produces anhedonia and resistance to extinction of fear in rats. J Neurosci. 2011;31:3095–3103. doi: 10.1523/JNEUROSCI.5973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ. Dopamine agonist withdrawal syndrome: Implications for patient care. Drugs Aging. 2013;30:587–592. doi: 10.1007/s40266-013-0090-z. [DOI] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci. 2005;25:5553–62. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH. Modeling depression in animal models. Methods Mol Biol Clifton NJ. 2012;829:125–144. doi: 10.1007/978-1-61779-458-2_7. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Stone D, Walsh J, Benes FM. Differences in the cellular distribution of D1 receptor mRNA in the hippocampus of bipolars and schizophrenics. Synapse. 2004;54:147–155. doi: 10.1002/syn.20076. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Wang M, Arnsten AFT. Constellation of HCN channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex: potential substrate for working memory deficits in schizophrenia. Cereb Cortex N Y N 1991. 2013;23:1643–1654. doi: 10.1093/cercor/bhs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980;3:129–149. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, et al. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in NA. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce G, Quiñones-Lombraña A, Martín-Palanco NG, et al. The addiction-related gene Ankk1 is oppositely regulated by D1R- and D2R-like dopamine receptors. Neurotox Res. 2015 doi: 10.1007/s12640-015-9545-9. [DOI] [PubMed] [Google Scholar]
- Powell TR, Fernandes C, Schalkwyk LC. Depression-related behavioral tests. John Wiley & Sons, Inc.; 2011. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Azzinnari D, Spinelli S, et al. Helplessness: A systematic translational review of theory and evidence for its relevance to understanding and treating depression. Pharmacol Ther. 2011;132:242–267. doi: 10.1016/j.pharmthera.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Prefrontal cortex modulates desire and dread generated by NA glutamate disruption. Biol Psychiatry. 2013;73:360–370. doi: 10.1016/j.biopsych.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Controlling stress: how the brain protects itself from depression. Nat Neurosci. 2005;8:261–262. doi: 10.1038/nn0305-261. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and NA dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Sonntag KC, Brenhouse HC, Freund N, et al. Viral over-expression of D1 dopamine receptors in the prefrontal cortex increase high-risk behaviors in adults: Comparison with adolescents. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-013-3399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Markou A. Withdrawal from chronic cocaine administration induces deficits in brain reward function in C57BL/6J mice. Behav Brain Res. 2011;223:176–81. doi: 10.1016/j.bbr.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhara T, Nakayama K, Inoue O, et al. D1 dopamine receptor binding in mood disorders measured by positron emission tomography. Psychopharmacology (Berl) 1992;106:14–18. doi: 10.1007/BF02253582. [DOI] [PubMed] [Google Scholar]
- Tanda K, Nishi A, Matsuo N, et al. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain. 2009;2:19. doi: 10.1186/1756-6606-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, et al. CREB regulation of NA excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D. Dopamine and Impulse Control Disorders in Parkinson's Disease. Ann Neurol. 2008;64:S93–100. doi: 10.1002/ana.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, et al. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Xing G, Zhang L, Russell S, Post R. Reduction of dopamine-related transcription factors Nurr1 and NGFI-B in the prefrontal cortex in schizophrenia and bipolar disorders. Schizophr Res. 2006;84:36–56. doi: 10.1016/j.schres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Yao J, Pan Y, Ding M, et al. Meta-analysis shows dopamine receptor D1 gene polymorphism is associated with bipolar disorder but not with schizophrenia. Psychiatry Res. 2013;210:1324–1325. doi: 10.1016/j.psychres.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Young JW, van Enkhuizen J, Winstanley CA, Geyer MA. Increased risk-taking behavior in dopamine transporter knockdown mice: further support for a mouse model of mania. J Psychopharmacol. 2011;25:934–43. doi: 10.1177/0269881111400646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Liu X, Zhang Z, Hu Y, Liu SJ, Lezama-Ruiz M, Joksimovic M, Liu QS. Cyclin-dependent kinase 5 in the ventral tegmental area regulates depression-related behaviors. J Neurosci. 2014;34:6352–66. doi: 10.1523/JNEUROSCI.3673-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]