Abstract
Background
Several factors may mitigate the efficacy of repetitive transcranial magnetic stimulation (rTMS) over sham rTMS in patients with treatment-resistant depression (TRD). These factors include unilateral stimulation (i.e., treatment of only the left dorsolateral prefrontal cortex [DLPFC]), suboptimal methods of targeting the DLPFC and insufficient stimulation intensity (based on coil-to-cortex distance).
Methods
We recruited patients with TRD between the ages of 18 and 85 years from a university hospital, and participants were randomized to receive sequential bilateral rTMS (600 pulses at 1 Hz followed by 1500 pulses at 10 Hz), unilateral high-frequency left (HFL)-rTMS (2100 pulses at 10 Hz) or sham rTMS for 3 or 6 weeks depending on treatment response. Stimulation was targeted with MRI localization over the junction of the middle and anterior thirds of the middle frontal gyrus, using 120% of the coil-to-cortex adjusted motor threshold. Our primary outcome of interest was the remission rate.
Results
A total of 121 patients participated in this study. The remission rate was significantly higher in the bilateral group than the sham group. The remission rate in the HFL-rTMS group was intermediate and did not differ statistically from the rate in the 2 other groups. There were no significant differences in reduction of depression scores among the 3 groups.
Limitations
The number of pulses used per session in the unilateral group was somewhat lower in our trial than in more recent trials, and the sham condition did not involve active stimulation.
Conclusion
Our findings suggest that sequential bilateral rTMS is superior to sham rTMS; however, adjusting for coil-to-cortex distance did not yield enhanced efficacy rates.
Introduction
Major depressive disorder (MDD) is a highly prevalent mental illness with an estimated lifetime prevalence of 10.8%.1 It is associated with decreased quality of life, increased mortality and substantial social and economic costs.2,3 Less than one-third of patients achieve remission within 12 weeks of starting treatment with a first-line antidepressant.4 Despite advances in psychopharmacological augmentation and combination treatments, up to one-third of patients remain treatment-resistant to these interventions.5 Thus, alternative treatment strategies are required to improve outcomes.
Repetitive transcranial magnetic stimulation (rTMS) has emerged as another treatment for MDD with established efficacy in patients with treatment-resistant depression (TRD). Several rTMS treatment protocols have demonstrated efficacy of active rTMS compared with sham rTMS (placebo) in patients with TRD. Most of these protocols have used high-frequency (> 5 Hz) rTMS applied to the left dorsolateral prefrontal cortex (DLPFC) (HFL-rTMS).6,7 Low-frequency (1 Hz) rTMS applied to the right DLPFC (LFR-rTMS)8,9 has also been shown to be more efficacious than sham rTMS, though fewer studies have used this protocol compared with the HFL protocol in patients with TRD. While HFL-rTMS has demonstrated superior efficacy to sham rTMS, the differential effect has typically been modest.10–12 In a meta-analysis of 29 sham-controlled randomized controlled trials (RCTs), the overall response rate was 29.3% with HFL-rTMS and 10.4% with sham stimulation.12 Studies have also proven LFR-rTMS to be superior to sham.8,9,13 In a meta-analysis of 8 RCTs, the response rate was 38.2% with LFR-rTMS and 15.1% with sham stimulation.13 Studies comparing HFL-rTMS and LFR-rTMS have found equivalent response rates with the 2 treatments. 14–16 Thus, combining these 2 treatments may result in better outcomes than HFL- or LFR-rTMS alone. Sequential bilateral rTMS has been shown to be more effective than sham.17,18 However, it has not been proven superior to unilateral rTMS. In 2 trials, Fitzgerald and colleagues14,19 found no difference in response or remission rates between bilateral and unilateral rTMS. Neither study was sham-controlled. In a previous study, we used the 5 cm method of targeting the DLPFC and fixed intensities of 100% and 120% motor threshold (MT) to compare bilateral rTMS to HFL-rTMS and sham rTMS and reported a 34.6% remission rate with bilateral rTMS, 4.5% with HFL-rTMS and 5% with sham rTMS.20 Other sham-controlled RCTs have failed to demonstrate a superior efficacy of bilateral rTMS.21,22 In a meta-analysis, bilateral rTMS and unilateral rTMS (LFR or HFL) were found to have comparable efficacy.23 Thus, the question remains as to whether sequential bilateral rTMS is a superior treatment approach for depressed patients.
The studies mentioned above have several limitations that may explain their divergent findings. First, most of them used the 5 cm anterior method of localizing the DLPFC, which does not account for individual variations in the distance between motor areas and the DLPFC.24 More recent studies have suggested a potential increased efficacy of rTMS treatment when the DLPFC is targeted using neuronavigation methods; however, no difference in remission rates were found.25 In one of the larger rTMS studies, the approximation method would have resulted in stimulation of the premotor cortex rather than the DLPFC in 33% of patients.10 Second, stimulation intensity may have been insufficient to induce an antidepressant response, as most studies used an unadjusted motor threshold.26 Adjusting the MT for coil-to-cortex distance increases the stimulation intensity and may improve treatment outcomes.26,27 Third, several studies had a relatively short treatment duration (i.e., 4 wk).
We conducted a study to address these limitations and to more definitively compare the efficacy of sequential bilateral rTMS and unilateral rTMS using a sham control in patients with TRD. We targeted the DLPFC using MRI cortical coregistration, treatment intensity was adjusted based on coil-to-cortex distance, and treatment duration was up to 6 weeks. We hypothesized that with these optimized methods sequential bilateral rTMS would have higher remission rates than both HFL-rTMS and sham rTMS. We also hypothesized that HFL-rTMS would be superior to sham rTMS.
Methods
Participants
We recruited participants from the adult and geriatric mood and anxiety services at the Centre for Addiction and Mental Health (CAMH) between 2008 and 2012. The CAMH Research Ethics Board approved the study, and written informed consent was obtained from all participants before completing any study procedures. Patients were included if they were between the ages of 18 and 85 years, had a DSM-IV diagnosis of MDD based on the Structured Clinical Interview for DSM-IV (SCID), were experiencing a current major depressive episode (MDE) with a score of 20 or higher on the 17-item Hamilton Rating Scale for Depression (HAMD-17), had failed to achieve a clinical response to or did not tolerate at least 2 separate antidepressants from different classes at sufficient doses for at least 6 weeks according to Stage II criteria outlined by Thase and colleagues,28 and were receiving stable doses of psychotropic medications for at least 4 weeks before randomization. Patients were excluded if they had a history of DSM-IV substance dependence (excluding nicotine) in the 6 months preceding the study or DSM-IV substance abuse in the month preceding the study; met DSM-IV criteria for borderline personality disorder or antisocial personality disorder based on the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II); had an unstable medical or neurologic illness or a history of seizures; were acutely suicidal; were pregnant; had metal implants in the skull; had a cardiac pacemaker, an implanted defibrillator, or a medication pump; had a diagnosis of dementia or a current Mini Mental State Examination (MMSE) score less than 24; or were taking lorazepam (> 2 mg/d) or equivalent medication during the 4 weeks preceding the study.
Treatment protocol
After enrolment and collection of basic demographic and clinical data, participants were randomized using a computer-generated list with a permuted, random block design, to receive sequential bilateral rTMS, HFL-rTMS, or sham stimulation. By necessity, the treatment operator was aware of the treatment allocation. However, clinical evaluators and participants were all blinded to the treatment condition. We followed strict instructions throughout the trial to ensure that operators did not communicate with raters.
In the first phase, all participants received treatment 5 times per week over a period of 3 weeks for a total of 15 treatments. Based on blinded clinical ratings at the end of this 3-week period, participants were classified as either remitters (HAMD-17 score < 8) or nonremitters (HAMD-17 score ≥ 8).29 Those who achieved remission completed the study at this point. Those classified as nonremitters entered a second phase during which they received an additional 3 weeks of the same treatment under double-blind conditions. Participants were allowed to make up missed sessions; however, participants could not miss 2 consecutive scheduled sessions and no more than 1 session per week. If a session was missed it was added on to the end of the course until all of the sessions were completed (15 or 30). If a session was cancelled owing to a statutory holiday it was not considered a scheduled session. Treatments occurred on weekdays only, with 1 treatment occurring per day.
rTMS treatment and neuronavigation
We administered rTMS using a Magventure RX-100 repetitive magnetic stimulator (Tonika/Magventure) and a cool B-65 figure-8 coil. Intensity was derived using the resting motor threshold (RMT) obtained before treatment according to previously published methods.30,31 Unadjusted stimulation may lead to both understimulation and overstimulation depending on the coil-to-cortex distance.32 Greater coil-to-cortex distance has been associated with reduced efficacy of therapeutic TMS.33 Thus, to adjust for coil-to-cortex distance, stimulation was delivered at 120% of the distance-adjusted RMT (AdjRMT) according to the equation AdjRMT = RMT + 2.8 × (DDLPFC–DM1), where AdjRMT is the adjusted RMT in % stimulator output, RMT is the unadjusted RMT in % stimulator output, DM1 is the distance between the scalp and the motor cortex (M1), and DDLPFC is the distance between the scalp and DLPFC.32 In order to localize the DLPFC stimulation site, a structural MRI (parameters: high-resolution, T1-weighted, 128 slices, repetition time [TR] 9.08 ms, echo time [TE] 2.70 ms, matrix size 256 × 256, slice thickness 1.40 mm, 1.5 T Signa General Electric scanner) was coregistered to participants’ heads using a magnetic tracking device (miniBIRD, Ascension Technology Group) for coil-to-cortex coregistration. The targeted stimulation site for the DLPFC was an area between the centre of Brodmann area (BA) 9 and the border of BA 9 and 46, based on the conservative definition of these areas in previous research.34 Stimulation was targeted at the Talairach coordinates x, y, z = −45 or 45 (depending on hemisphere), 45, 35. Details of the neuronavigation procedure can be found in the supplementary material.
Treatment parameters adhered with safety guidelines and are detailed in Table 1.35,36 It should be noted that the study was designed before publication of the results of the large multicentre RCTs10,11 that have led to 3000 pulses per session becoming the standard of care in clinical practice. The sham stimulation was administered in randomized fashion either as sham HFL-rTMS or sham bilateral rTMS with the coil angled 90° away from the skull in a single-wing tilt position, producing some scalp sensation and similar sound intensity to that of active stimulation. At the time of study design a feasible active placebo coil was not available to use instead of the angled coil approach. Participants were unable to see the coil, which reduced the likelihood that they could detect the treatment condition. This method has been shown to produce minimal intracortical activity, but causes an auditory and tactile experience that is similar to active treatment.37 After the final treatment session, we asked participants whether they thought they had received active or sham stimulation.
Table 1.
Treatment parameters
| Unilateral | Bilateral | |
|---|---|---|
| Frequency: 10Hz | Frequency: 1 Hz | Frequency: 10 Hz |
| Intensity: 120% | Intensity: 120% | Intensity: 120% |
| AdjRMT | AdjRMT | AdjRMT |
| Pulses per train: 30 | Pulses per train: 100 | Pulses Per Train: 30 |
| Trains: 70 | Trains: 6 | Trains: 50 |
| Intertrain interval: 30 s | Intertrain interval: 30 s | Intertrain interval: 30 s |
| Total pulses: 2100 | Total pulses: 600 | Total pulses: 1500 |
AdjRMT = resting motor threshold adjusted for distance.
Assessments and outcome measures
We confirmed the participants’ diagnosis and symptom severity before study entry using the SCID and HAMD-17. To assess exclusion criteria, we asked participants to complete the antisocial and borderline personality disorders modules of the SCID-II and the MMSE before study entry.
Clinical measures were assessed at randomization and at week 3 and week 6 by a trained rater (M.M.) blinded to the randomized treatment condition. The same rater assessed the outcome at baseline, week 3 and week 6. The primary outcome measure was the remission rate, with remission defined as a score of 7 or less on the HAMD-17 at the week 3 or week 6 assessment. Secondary outcome measures included the response rate (with response defined as > 50% reduction in HAMD-17 score), absolute change in HAMD-17 scores,38 remission and response rates based on the Beck Depression Inventory (BDI-II)39 and adverse events based on an interview focused on the subjective experiences of TMS. We used the Antidepressant Treatment History Form (ATHF) to quantify the adequacy of prior treatment for depression.40
Statistical analysis
All statistical analyses were conducted using SPSS software for Mac version 22.0 (IBM Inc.). Power analysis, using an α level of 0.05 and a β level of 0.90, called for an overall sample size of 38 participants per group based on the between-group difference in remission rates in our previous trial.20 We compared baseline differences in demographic and clinical variables among the treatment groups. Continuous variables were analyzed with 1-way analysis of variance (ANOVA), and categorical variables were analyzed with χ2 analyses or a Fisher exact test when less than 80% of the cells had a frequency of less than 5. All procedures were 2-tailed, and we used a significance level set at α = 0.05 for the primary outcome. Intention to treat and completer analyses were conducted for all outcome variables. We used a linear mixed-model regression analysis to assess changes in HAMD-17 scores over time.
Results
Participant flow, follow-up, and sample characteristics
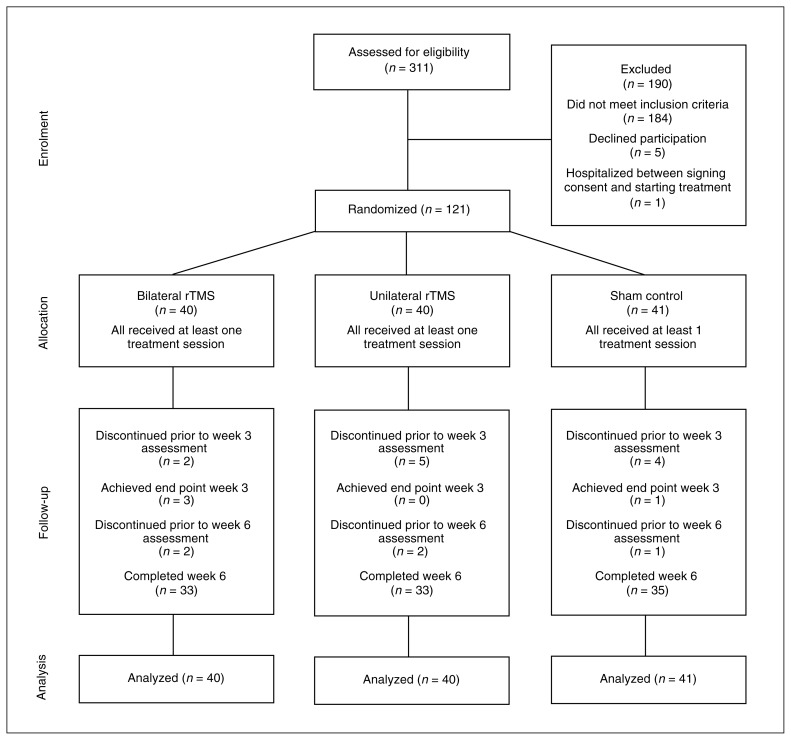
The flow of patients through the study is presented in Fig. 1. Of the 311 patients screened, 183 did not meet our eligibility criteria, 6 declined to participate, and 1 was admitted to hospital between giving consent and randomization. Thus, 121 patients were randomized, participated in at least 1 treatment session and were included in the analyses.
Fig. 1.
Flow of patients through the study. rTMS = repetitive transcranial magnetic stimulation.
The participants’ baseline clinical and demographic characteristics are summarized in Table 2. The bilateral group had a significantly higher ATHF score than the other 2 groups. There were no other clinically important differences among the 3 groups. With respect to the intensity adjustment based on scalp to cortex distance, most participants had an intensity adjustment within 3% of their unadjusted RMT.
Table 2.
Demographic and clinical characteristics of study participants
| Group; mean ± SD or no. (%)* | |||
|---|---|---|---|
|
|
|||
| Characteristic | Bilateral (n = 40) | Unilateral (n = 40) | Sham (n = 41) |
| Age, yr | 46.4 ± 12.5 | 46.5 ± 14.1 | 48.1 ± 12.0 |
| Sex, male:female | 17:23 | 10:30 | 17:24 |
| Education, yr | 16.1 ± 3.4 | 15.6 ± 3.9 | 16.2 ± 3.2 |
| Age at illness onset, yr | 22.6 ± 12.9 | 21.5 ± 12.3 | 25.8 ± 12.9 |
| Recurrent episodes | 37 (92.5) | 37 (92.5) | 37 (90.2) |
| Duration of current episode, mo† | 51.5 ± 70.5 | 30.9 ± 64.1 | 46.9 ± 110.3 |
| No. of episodes‡ | 4.7 ± 4.1 | 4.1 ± 2.1 | 5.7 ± 6.7 |
| Current episode severity | |||
| Severe | 6 (15) | 10 (25) | 13 (31.7) |
| Moderate | 34 (85) | 30 (75) | 28 (68.3) |
| Comorbid anxiety disorder | 3 (7.5) | 5 (12.5) | 6 (14.6) |
| Medication hstory | |||
| ECT | 3 (7.5) | 3 (7.5) | 2 (4.9) |
| SSRI | 25 (62.5) | 18 (45) | 23 (56.1) |
| SNRI | 19 (47.5) | 19 (47.5) | 23 (56.1) |
| TCA | 7 (17.5) | 8 (20) | 4 (9.8) |
| Mirtazapine | 7 (17.5) | 3 (7.5) | 4 (9.8) |
| MAOI | 3 (7.5) | 3 (7.5) | 3 (7.3) |
| Buproprion | 15 (37.5) | 14 (37.5) | 14 (34.1) |
| Lithium | 3 (7.5) | 1 (2.5) | 1 (2.4) |
| Active medication during study | |||
| Benzodiazepine | 20 (50) | 16 (40) | 16 (39.0) |
| Atypical antipsychotic | 12 (30) | 10 (25) | 13 (31.7) |
| Antidepressant–antipsychotic combo | 12 (30) | 9 (22.5) | 12 (29.3) |
| Antidepressant and lithium | 3 (7.5) | 0 (0) | 1 (2.4) |
| Two antidepressants | 20 (50) | 19 (47.5) | 18 (43.9) |
| No antidepressant | 0 (0) | 4 (10) | 2 (4.9) |
| Past alcohol dependence | 2 (5) | 3 (7.5) | 3 (7.3) |
| ATHF for current episode§ | 8.9 ± 8.2) | 5.4 ± 2.6 | 7.8 ± 5.2 |
| Baseline HAMD | 24.1 ± 3.2 | 26 ± 3.4 | 25.5 ± 3.6 |
| Baseline BDI-II | 35.0 ± 10.4 | 35.8 ± 9.8 | 36.0 ± 10.1 |
| Left intensity % adjustment | −1.2 ± 4.0 | 0.7 ± 3.0 | −2.0 ± 7.1 |
| Right intensity % adjustment | −1.1 ± 4.1 | 1.0 ± 3.5 | −0.9 ± 6.1 |
| No. of treatments | 27.1 ± 7.3 | 26.2 ± 8.8 | 26.7 ± 8.4 |
ATHF = Antidepressant History Treatment Form; BDI-II = Beck Depression Inventory; ECT = electroconvulsive therapy; HAMD = Hamilton Rating Scale for Depression; TCA = tricyclic antidepressant; MAOI = monoamine oxidase inhibitor; SD = standard deviation; SNRI = serotonin norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor.
Unless indicated otherwise.
n = 34 in the bilateral group, n = 38 in the unilateral group and n = 40 in the sham group.
n = 21 in the bilateral group, n = 21 in the unilateral group and n = 18 in the sham group.
Significant difference among the groups (p = 0.037).
At 6 weeks, data on the primary outcome were available for 105 (86.7%) participants. Those who were lost to follow-up did not differ from those retained on any of the baseline demographic or clinical variables. Of the 121 participants who were asked to guess whether they were randomized to the rTMS or sham condition, 76 (62.3%) guessed correctly: 25 (62.5%) in the bilateral group, 21 (52.5%) in the unilateral group and 30 (73.1%) in the sham group. These proportions did not differ significantly (χ22 = 3.7; p = 0.16).
Primary outcome: remission
The remission rates differed significantly among the 3 groups: 8 of 40 (20%) participants in the bilateral group, 3 of 40 (7.5%) in the unilateral group and 1 of 41 (2.4%) in the sham group (p = 0.027). The remission rate in the bilateral group was significantly higher than in the sham group (p = 0.014). The remission rate did not differ between the bilateral and unilateral groups (p = 0.20) or between the unilateral and the sham groups (p = 0.27). In the completer analysis, the significant difference in remission rate across groups persisted (p = 0.031). The remission rate was significantly higher in the bilateral group (8 of 36, 22.2%) than in the sham group (1 of 36, 2.8%, p = 0.028), but did not differ between the bilateral and the unilateral groups (3 of 34, 8.8%, p = 0.19) or between the unilateral and the sham group (p = 0.35). As the mean ATHF score differed among the groups, we conducted a logistic regression analysis and the main effect remained unchanged (p = 0.031). In addition, a logistic regression analysis was conducted to see if the degree of intensity adjustment based on the AdjRMT on both the left (p = 0.027) and right sides (p = 0.022) was related to remission, and it did not affect the remission findings in these models.
Secondary outcomes
Rate of response based on HAMD-17
The proportions of responders did not differ significantly among the 3 groups: 9 of 40 (22.5%) in the bilateral group, 6 of 40 (15.0%) in the unilateral group and 2 of 41 (4.9%) in the sham group (χ22 = 5.25, p = 0.07). The proportion of responders was significantly higher in the bilateral group than in the sham group (p = 0.026). The proportion of responders did not differ between the unilateral and the sham groups (p = 0.16) or between the unilateral and bilateral groups (p = 0.57). In the completer analysis, the overall findings remained unchanged. The proportions of responders did not differ significantly among the 3 groups: 9 of 36 (25.0%) in the bilateral group, 6 of 34 (17.6%) in the unilateral group and 2 of 36 (5.6%) in the sham group (χ22 = 5.15, p = 0.08). Dichotomous comparisons between the bilateral and unilateral (p = 0.56) or unilateral and sham groups (p = 0.15) did not demonstrate significant differences. However, the response rate among completers was higher in the bilateral group than in the sham group (p = 0.046).
Rate of remission based on the BDI-II
Based on the BDI-II, the remission rates differed significantly among the 3 groups: 7 of 40 (17.5%) in the bilateral group, 1 of 40 (2.5%) in the unilateral group and 1 of 41 (2.4%) in the sham group (p = 0.016). The remission rate in the bilateral group was significantly higher than in the sham group (p = 0.029) but not in the unilateral group (p = 0.06). There was no significant difference between the unilateral and the sham groups (p > 0.99).
Rate of response based on the BDI-II
The proportions of responders did not differ significantly among the 3 groups: 11 of 40 (27.5%) in the bilateral group, 6 of 40 (15%) in the unilateral group and 5 of 41 (12.2%) in the sham group (χ22 = 3.60, p = 0.17).
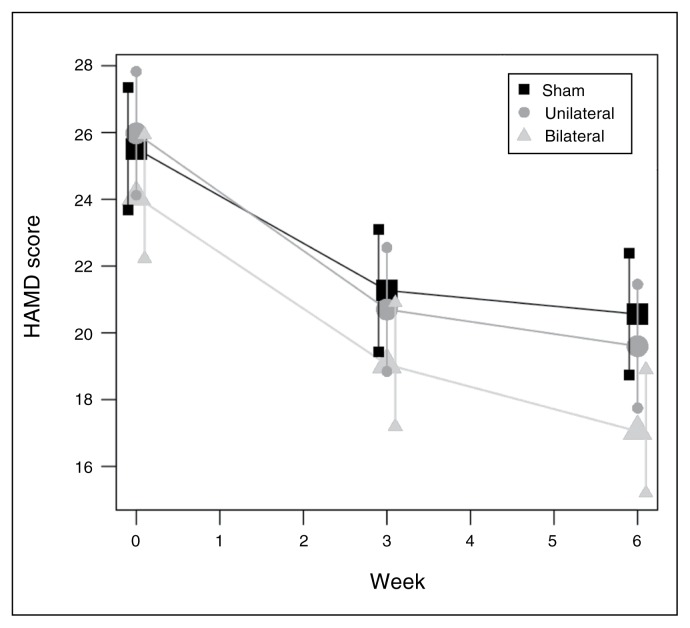
HAMD-17 change
Change in HAMD-17 scores over time is shown in Fig. 2. As there were no significant differences among the groups in baseline HAMD-17 scores, we compared change in absolute HAMD-17 scores from baseline to end point among the groups. The mean decrease in HAMD-17 score was −6.8 ± 7.2 in the bilateral group, −6.4 ± 7.0 in the unilateral group and −5.0 ± 4.8 in the sham group. There was no overall group effect (F118,2 = 0.90, p = 0.40). This lack of an overall effect of treatment group was also observed in the completer analysis (F109,2 = 0.95, p = 0.39). A linear mixed model with HAMD-17 scores as the outcome was fitted to the data for weeks 3 and 6 using a heterogeneous compound symmetric covariance structure. This model included group (bilateral v. unilateral v. sham), time and group × time interaction with baseline HAMD-17 score (centred on its mean) as predictors. Both the F test for the interaction (F236,2 = −2.07, p = 0.10) and the group × time parameter estimates (F236,2 = −1.42, p = 0.25) for the sham and HFL-rTMS groups indicated that the rates of change in mean HAMD-17 score in these 2 groups did not differ significantly from the rate in the bilateral group.
Fig. 2.
Change in Hamilton Rating Scale for Depression (HAMD) scores over time. Shown are estimates of the mean value (large symbols, horizontal lines) and 95% confidence intervals (small symbols, vertical lines) produced from a mixed-effects regression model.
Dropouts and adverse effects
With respect to dropouts for all causes, 16 of 121 participants (13.2%) did not complete an end point assessment for the primary outcome: 4 of 40 (10.0%) in the bilateral group, 7 of 40 (17.5%) in the unilateral group and 5 of 41 (12.1%) in the sham group (Fig. 1). Reasons for not achieving an end point assessment were as follows. One participant in the bilateral group withdrew after being admitted to hospital for anxiety and subsequently receiving medication prohibited by the study protocol. Three participants in the unilateral group withdrew owing to an increase in anxiety. Two participants in the bilateral group and 2 participants in the unilateral group withdrew owing to inability to tolerate the treatment. As the intensity adjustment was less than 1.5% in all participants, these dropouts suggest that intensity adjustment for coil-to-cortex distance was not associated with the tolerability of the procedure. One participant in the sham group withdrew owing to an unrelated serious adverse event after being admitted to hospital for surgery. The remaining reasons for withdrawal included lack of commitment to complete the treatment protocol and missed treatments (1 participantn in the bilateral group, 2 in the unilateral group and 4 in the sham group). Individuals who missed 1 treatment were reminded that missing more than the allowed 2 consecutive scheduled treatments would result in cessation from the treatment.
With respect to adverse effects, headache was most frequently reported (7 participants in each of the 3 groups), followed by pain (7 participants in the bilateral group, 8 in the unilateral group and 2 in the sham group). Adverse effects are summarized in Table 3.
Table 3.
Adverse effects of rTMS among study participants
| Group; % | |||
|---|---|---|---|
|
|
|||
| Adverse effect | Bilateral | Unilateral | Sham |
| Headache | 17.5 | 17.5 | 17.0 |
| Pain | 17.5 | 20 | 4.9 |
| Fatigue | 5 | 5 | 2.4 |
| Difficulty sleeping | 5 | 5 | 2.4 |
| Racing thoughts | 2.5 | 0 | 2.4 |
| Worsening mood | 2.5 | 0 | 0 |
| Suicidal thoughts | 0 | 0 | 2.4 |
| Nightmares | 2.5 | 0 | 0 |
| Anger | 0 | 2.5 | 0 |
| Tremor | 0 | 0 | 2.4 |
| Lightheadedness | 2.5 | 0 | 0 |
| Confusion | 0 | 2.5 | 0 |
| Sore hip | 0 | 0 | 2.4 |
| Tinnitus | 2.5 | 0 | 0 |
| Flu | 2.5 | 2.5 | 2.4 |
| Scraping feeling | 0 | 2.5 | 0 |
| Metallic taste | 2.5 | 0 | 0 |
| Neck stiffness | 0 | 2.4 | 0 |
| Lactation | 2.5 | 0 | 0 |
| Vomiting | 0 | 2.4 | 0 |
| Anxiety | 2.5 | 7.5 | 0 |
rTMS = repetitive transcranial magnetic stimulation.
Discussion
To our knowledge this is the first randomized sham-controlled trial comparing sequential bilateral and unilateral rTMS using cortical coregistration, adjusting intensity for coil-to-cortex distance and providing up to 6 weeks of treatment. We did not observe a statistically significant difference in overall depression change scores. In addition, we did not see enhanced efficacy rates compared with those reported in previous studies using the enhanced techniques of adjusting MT for coil-to-cortex distance or MRI targeting of the DLFPC. However, our findings supported 1 of our a priori hypotheses — that sequential bilateral rTMS is superior to sham rTMS — as the remission rate was higher in the bilateral group than in the sham group. This result is consistent with the rates of response and with the findings of previous studies that have demonstrated the efficacy of bilateral rTMS compared with sham stimulation.17,18,20 Unilateral HFL-rTMS was associated with a lower rate of remission than sequential bilateral rTMS and a higher rate of remission than sham rTMS. However, these differences did not reach significance. As our sample size was small, with a post hoc calculation showing a limited power (29%) for these comparisons, this lack of significance should be interpreted as inconclusive.
Overall both treatment protocols were reasonably well tolerated, with only 2 participants in the bilateral group and 2 in the unilateral group withdrawing owing to an inability to tolerate the treatment. None of these individuals had a marked increase in the AdjRMT, suggesting that stimulation intensity was not the cause for the inability to tolerate the procedure. As with other rTMS studies, 15%–20% of participants experienced headache and scalp discomfort. No participants experienced any serious adverse effects that were attributed to the treatment. These findings are consistent with the existing literature, indicating that rTMS is a well-tolerated treatment with low dropout rates in clinical trials.
The strengths of this study include a focus on inclusion of participants with difficult to treat depression with stage 2 or higher treatment resistance,28 use of sham rTMS as a control condition, localization of the DLPFC stimulation site using cortical coregistration techniques,24 adjustment of stimulation intensity according to coil-to-cortex distance,26 treatment duration up to 6 weeks and use of remission rates as a primary outcome. Furthermore, the inclusion of participants aged 18–85 years is another strength of the study as it broadens the clinical applicability of findings. In our previous study we included patients up to age 85 years and did not observe worse outcomes in those older than 60 years using an intensity of 120% MT.20 Another study that used coil-to-cortex distance adjustment demonstrated that a mean intensity of 114% MT was required to stimulate the cortex in a sample of older adults.41 Furthermore, a predictor analysis from one of the largest rTMS trials that used stimulation intensity of 120% MT did not find age to be a negative predictor of outcome.42 Taken together, it appears that as long as intensities are above 110%, older age does not preclude response to rTMS.
The observed rates of remission and response with bilateral rTMS are clinically meaningful given the broad range and degree of treatment resistance (mean ATHF score of 7.4, range 0–36) in the sample: the remission rate of 20% observed in this study is similar to that observed in previous sham-controlled rTMS studies.10 The lack of statistical difference in remission and response between the unilateral and bilateral groups is in keeping with previous findings suggesting the difference between the 2 is modest.14 However, our data suggest the possibility that in more treatment-resistant samples a sequential bilateral approach may be more beneficial as efficacy persisted despite a higher degree of treatment resistance in the bilateral group. A recent meta-analysis comparing bilateral and sham rTMS found an overall remission rate of 19% in the bilateral group.17 By contrast, a more recent meta-analysis comparing bilateral and unilateral rTMS reported a remission rate of 35.1% for bilateral stimulation.23
The lack of difference between the unilateral HFL-rTMS and sham conditions is somewhat unexpected given the MRI targeting of the DLPFC and the intensity adjustment for coil-to-cortex distance. Most (but not all43,44) studies to date have shown a significant effect of HFL-rTMS over sham.6,7 However, in more recent trials, the difference appears to be modest.10–12 Given this modest difference, our study lacked power. In addition, our failure to show a superior efficacy of HFL-rTMS over sham may be related to a lower number of total pulses delivered than reported in the positive trials10,11 or to the relatively high level of treatment resistance in our sample, as no upper resistance level was specified (maximum ATHF score for the current episode was 36) and this has been a strong predictor of poor outcome.42
Limitations
A potential limitation of this study is the concurrent use of antidepressants by most participants, which may have obscured the findings owing to the effects of specific medication combinations. However, previous work has shown similar findings when medication doses were stable. Furthermore, this approach is in keeping with the clinical practice of maintaining medications in patients receiving rTMS.45 Additionally, participants taking medication had been on stable doses for at least 4 weeks before starting the trial and they had a high degree of treatment resistance, with a mean ATHF score for the current episode of 7.4 for all participants. Though we used a sham control condition, our technique may not have been optimal and may have led to some cortical activation.10 However, the equipment used in this study (Magventure RX 100, B65 Coil) was not capable of automating active versus sham stimulation at the time the study was conducted. Other studies that have used the same equipment have used the same sham technique.6,18,20 Despite the technique, our rates of sham remission and response were similar to those reported in studies that used an active sham condition.10 The possibility exists that the blinding was compromised, as participants were able to guess correctly at a rate better than chance the treatment to which they were allocated; however, no statistical differences in ability to identify the treatment was found among the 3 groups. Participants were asked to guess their allocation at the study end; asking them to do so earlier during treatment may decrease the chance that the response is based on eventual response to treatment,17 but it may lead participants to pay more attention to the blinding and to try harder to figure out their treatment condition. Another limitation of the design is a lack of data on durability of response.
Conclusion
Our findings suggest that sequential bilateral rTMS is a safe and effective treatment for patients with TRD. While sequential bilateral rTMS was superior to sham stimulation, HFL-rTMS was not superior to sham stimulation in our trial. Despite the use of MRI targeting and coil-to-cortex intensity adjustment to optimize efficacy, the remission rates were not greater than those seen with rTMS studies that have used approximation to target the DLPFC.20,22,46 This finding also has important implications for clinical practice and suggests that MRI targeting and adjusting for coil-to-cortex distance is unlikely to yield higher remission rates than optimal methods of approximation and a stimulation intensity of 120% MT.47,48 Future research to identify new methods to improve the efficacy of rTMS for TRD are needed. We propose that identifying and engaging biological targets of treatment response49 may be a more productive method of enhancing efficacy. However, the identification of biomarkers is a long and difficult process. Methods to improve the efficiency of rTMS delivery using θ burst stimulation50 may hold potential in clinical practice.
Footnotes
Contributors: D. Blumberger, B. Mulsant, T. Rajji, P. Brown and Z. Daskalakis designed the study. D. Blumberger, T. Rajji, M. Maher and Z. Daskalakis acquired the data, which D. Blumberger, J. Maller, L. Thomson, B. Mulsant, T. Rajji, P. Brown, J. Downar, F. Vila-Rodriguez, P. Fitzgerald and Z. Daskalakis analyzed. D. Blumberger, J. Maller, L. Thomson, T. Rajji, M. Maher and Z. Daskalakis wrote the article, which all authors reviewed and approved for publication.
Competing interests: This work was funded by a Grant from the Ontario Mental Health Foundation (OMHF). D. Blumberger receives research support from the Canadian Institutes of Health Research (CIHR), Brain Canada, National Institutes of Health (NIH), Temerty Family through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Family Research Institute. He receives nonsalary operating funds and in-kind equipment support from Brainsway Ltd. for an investigator-initiated study. He is the site principal investigator for several sponsor-initiated clinical trials from Brainsway Ltd. He receives in-kind equipment support from Tonika/Magventure for an investigator-initiated study. B. Mulsant receives research support from CIHR, NIH, Brain Canada, the CAMH Foundation, Bristol-Myers Squibb (medications for a NIH-funded clinical trial) and Pfizer (medications for a NIH-funded clinical trial). He owns stocks of General Electric (less than $5,000). Within the past five years, he has also received some travel support from Roche. T. Rajji receives research support from Brain Canada, the Brain and Behavior Research Foundation, the Canadian Foundation for Innovation, CIHR, the Ontario Ministry of Health and Long-Term Care, the Ontario Ministry of Research and Innovation, NIH, and the W. Garfield Weston Foundation. J. Downar has received research support from CIHR, NIH, the Klarman Family Foundation, the Buchan Family Foundation, and the Toronto General and Western Hospital Foundation. He has also received a travel stipend from Lundbeck and from ANT Neuro, and in-kind equipment support for an investigator-initiated study from Tonika/Magventure. P. Fitzgerald is supported by an NHMRC Practitioner Fellowship (606907). He has received equipment for research from MagVenture A/S, Medtronic Ltd, Cervel Neurotech and Brainsway Ltd and funding for research from Cervel Neurotech. Z. Daskalakis has received external funding through Brainsway Ltd and a travel allowance through Pfizer and Merck. He has also received speaker funding through Sepracor Inc and AstraZeneca and has served on the advisory board for Hoffmann-La Roche Limited. He has received funding from OMHF, CIHR, the Brain and Behaviour Research Foundation and the Temerty Family and Grant Family and through the CAMH Foundation and the Campbell Institute. No other competing interests declared.
References
- 1.Patten SB, Kennedy SH, Lam RW, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. I. Classification, burden and principles of management. J Affect Disord. 2009;117(Suppl 1):S5–14. doi: 10.1016/j.jad.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Greden JF. The burden of recurrent depression: causes, consequences, and future prospects. J Clin Psychiatry. 2001;62(Suppl 22):5–9. [PubMed] [Google Scholar]
- 3.Pincus HA, Pettit AR. The societal costs of chronic major depression. J Clin Psychiatry. 2001;62(Suppl 6):5–9. [PubMed] [Google Scholar]
- 4.Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–52. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 5.Little A. Treatment-resistant depression. Am Fam Physician. 2009;80:167–72. [PubMed] [Google Scholar]
- 6.Avery DH, Holtzheimer PE, III, Fawaz W, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2006;59:187–94. doi: 10.1016/j.biopsych.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Rossini D, Serretti A, Franchini L, et al. Sertraline versus fluvoxamine in the treatment of elderly patients with major depression — a double-blind, randomized trial. J Clin Psychopharmacol. 2005;25:471–5. doi: 10.1097/01.jcp.0000177548.28961.e7. [DOI] [PubMed] [Google Scholar]
- 8.Klein E, Kolsky Y, Puyerovsky M, et al. Right prefrontal slow repetitive transcranial magnetic stimulation in schizophrenia: a double-blind sham-controlled pilot study. Biol Psychiatry. 1999;46:1451–4. doi: 10.1016/s0006-3223(99)00182-1. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald PB, Brown TL, Marston NA, et al. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003;60:1002–8. doi: 10.1001/archpsyc.60.9.1002. [DOI] [PubMed] [Google Scholar]
- 10.George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507–16. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 11.O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–16. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Berlim MT, Van den Eynde F, Daskalakis ZJ. High-frequency repetitive transcranial magnetic stimulation accelerates and enhances the clinical response to antidepressants in major depression: a meta-analysis of randomized, double-blind, and sham-controlled trials. J Clin Psychiatry. 2013;74:e122–9. doi: 10.4088/JCP.12r07996. [DOI] [PubMed] [Google Scholar]
- 13.Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38:543–51. doi: 10.1038/npp.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgerald PB, Hoy K, Gunewardene R, et al. A randomized trial of unilateral and bilateral prefrontal cortex transcranial magnetic stimulation in treatment-resistant major depression. Psychol Med. 2011;41:1187–96. doi: 10.1017/S0033291710001923. [DOI] [PubMed] [Google Scholar]
- 15.Rossini D, Lucca A, Magri L, et al. A symptom-specific analysis of the effect of high-frequency left or low-frequency right transcranial magnetic stimulation over the dorsolateral prefrontal cortex in major depression. Neuropsychobiology. 2010;62:91–7. doi: 10.1159/000315439. [DOI] [PubMed] [Google Scholar]
- 16.Stern WM, Tormos JM, Press DZ, et al. Antidepressant effects of high and low frequency repetitive transcranial magnetic stimulation to the dorsolateral prefrontal cortex: a double-blind, randomized, placebo-controlled trial. J Neuropsychiatry Clin Neurosci. 2007;19:179–86. doi: 10.1176/jnp.2007.19.2.179. [DOI] [PubMed] [Google Scholar]
- 17.Berlim MT, Van den Eynde F, Daskalakis ZJ. A systematic review and meta-analysis on the efficacy and acceptability of bilateral repetitive transcranial magnetic stimulation (rTMS) for treating major depression. Psychol Med. 2012;43:2245–54. doi: 10.1017/S0033291712002802. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald PB, Benitez J, de Castella A, et al. A randomized, controlled trial of sequential bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression. Am J Psychiatry. 2006;163:88–94. doi: 10.1176/appi.ajp.163.1.88. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald PB, Hoy KE, Singh A, et al. Equivalent beneficial effects of unilateral and bilateral prefrontal cortex transcranial magnetic stimulation in a large randomized trial in treatment-resistant major depression. Int J Neuropsychopharmacol. 2013;16:1975–84. doi: 10.1017/S1461145713000369. [DOI] [PubMed] [Google Scholar]
- 20.Blumberger DM, Mulsant BH, Fitzgerald PB, et al. A randomized double-blind sham-controlled comparison of unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant major depression. World J Biol Psychiatry. 2012;13:423–35. doi: 10.3109/15622975.2011.579163. [DOI] [PubMed] [Google Scholar]
- 21.Pallanti S, Bernardi S, Di Rollo A, et al. Unilateral low frequency versus sequential bilateral repetitive transcranial magnetic stimulation: Is simpler better for treatment of resistant depression? Neuroscience. 2010;167:323–8. doi: 10.1016/j.neuroscience.2010.01.063. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald PB, Hoy KE, Herring SE, et al. A double blind randomized trial of unilateral left and bilateral prefrontal cortex transcranial magnetic stimulation in treatment resistant major depression. J Affect Disord. 2012;139:193–8. doi: 10.1016/j.jad.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Chen JJ, Liu Z, Zhu D, et al. Bilateral vs. unilateral repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomized controlled trials. Psychiatry Res. 2014;219:51–7. doi: 10.1016/j.psychres.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Herwig U, Padberg F, Unger J, et al. Transcranial magnetic stimulation in therapy studies: examination of the reliability of “standard” coil positioning by neuronavigation. Biol Psychiatry. 2001;50:58–61. doi: 10.1016/s0006-3223(01)01153-2. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald PB, Hoy K, McQueen S, et al. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology. 2009;34:1255–62. doi: 10.1038/npp.2008.233. [DOI] [PubMed] [Google Scholar]
- 26.Kozel FA, Nahas Z, deBrux C, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci. 2000;12:376–84. doi: 10.1176/jnp.12.3.376. [DOI] [PubMed] [Google Scholar]
- 27.Daskalakis ZJ, Levinson AJ, Fitzgerald PB. Repetitive transcranial magnetic stimulation for major depressive disorder: a review. Can J Psychiatry. 2008;53:555–66. doi: 10.1177/070674370805300902. [DOI] [PubMed] [Google Scholar]
- 28.Thase ME, Rush AJ. Treatment resistant depression. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press Ltd; 1995. pp. 1081–97. [Google Scholar]
- 29.Mulder RT, Joyce PR, Frampton C. Relationships among measures of treatment outcome in depressed patients. J Affect Disord. 2003;76:127–35. doi: 10.1016/s0165-0327(02)00080-0. [DOI] [PubMed] [Google Scholar]
- 30.Pascual-Leone A, Rubio B, Pallardo F, et al. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–7. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 31.Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 32.Stokes MG, Chambers CD, Gould IC, et al. Distance-adjusted motor threshold for transcranial magnetic stimulation. Clin Neurophysiol. 2007;118:1617–25. doi: 10.1016/j.clinph.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Mosimann UP, Marre SC, Werlen S, et al. Antidepressant effects of repetitive transcranial magnetic stimulation in the elderly: correlation between effect size and coil-cortex distance. Arch Gen Psychiatry. 2002;59:560–1. doi: 10.1001/archpsyc.59.6.560. [DOI] [PubMed] [Google Scholar]
- 34.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–22. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- 35.Chen R, Gerloff C, Classen J, et al. Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol. 1997;105:415–21. doi: 10.1016/s0924-980x(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 36.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 37.Lisanby SH, Gutman D, Luber B, et al. Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol Psychiatry. 2001;49:460–3. doi: 10.1016/s0006-3223(00)01110-0. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 39.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 40.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–7. [PubMed] [Google Scholar]
- 41.Nahas Z, Li X, Kozel FA, et al. Safety and benefits of distance-adjusted prefrontal transcranial magnetic stimulation in depressed patients 55–75 years of age: a pilot study. Depress Anxiety. 2004;27:90. doi: 10.1002/da.20015. [DOI] [PubMed] [Google Scholar]
- 42.Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34:522–34. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- 43.Berman RM, Narasimhan M, Sanacora G, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry. 2000;47:332–7. doi: 10.1016/s0006-3223(99)00243-7. [DOI] [PubMed] [Google Scholar]
- 44.Loo C, Mitchell P, Sachdev P, et al. Double-blind controlled investigation of transcranial magnetic stimulation for the treatment of resistant major depression. Am J Psychiatry. 1999;156:946–8. doi: 10.1176/ajp.156.6.946. [DOI] [PubMed] [Google Scholar]
- 45.Connolly KR, Helmer A, Cristancho MA, et al. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73:e567–73. doi: 10.4088/JCP.11m07413. [DOI] [PubMed] [Google Scholar]
- 46.Fitzgerald PB, Herring S, Hoy K, et al. A study of the effectiveness of bilateral transcranial magnetic stimulation in the treatment of the negative symptoms of schizophrenia. Brain Stimul. 2008;1:27–32. doi: 10.1016/j.brs.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Rusjan PM, Barr MS, Farzan F, et al. Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp. 2010;31:1643–52. doi: 10.1002/hbm.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mir-Moghtadaei A, Caballero R, Fried P, et al. Concordance between BeamF3 and MRI-neuronavigated target sites for repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex. Brain Stimul. 2015;8:965–73. doi: 10.1016/j.brs.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silverstein WK, Noda Y, Barr MS, et al. Neurobiological predictors of response to dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation in depression: a systematic review. Depress Anxiety. 2015;32:871–91. doi: 10.1002/da.22424. [DOI] [PubMed] [Google Scholar]
- 50.Prasser J, Schecklmann M, Poeppl TB, et al. Bilateral prefrontal rTMS and theta burst TMS as an add-on treatment for depression: a randomized placebo controlled trial. World J Biol Psychiatry. 2015;16:57–65. doi: 10.3109/15622975.2014.964768. [DOI] [PubMed] [Google Scholar]