Abstract
Blood ammonia and glutamine levels are used as biomarkers of control in patients with urea cycle disorders (UCDs). This study was undertaken to evaluate glutamine variability and utility as a predictor of hyperammonemic crises (HACs) in UCD patients.
Methods
The relationships between glutamine and ammonia levels and the incidence and timing of HACs were evaluated in over 100 adult and pediatric UCD patients who participated in clinical trials of glycerol phenylbutyrate.
Results
The median (range) intra-subject 24-hour coefficient of variation for glutamine was 15% (8–29%) as compared with 56% (28%–154%) for ammonia, and the correlation coefficient between glutamine and concurrent ammonia levels varied from 0.17 to 0.29. Patients with baseline (fasting) glutamine values >900 µmol/L had higher baseline ammonia levels (mean [SD]: 39.6 [26.2] µmol/L) than patients with baseline glutamine ≤900 µmol/L (26.6 [18.0] µmol/L). Glutamine values >900 µmol/L during the study were associated with an approximately 2-fold higher HAC risk (odds ratio [OR]=1.98; p=0.173). However, glutamine lost predictive significance (OR=1.47; p=0.439) when concomitant ammonia was taken into account, whereas the predictive value of baseline ammonia ≥ 1.0 upper limit of normal (ULN) was highly statistically significant (OR=4.96; p=0.013). There was no significant effect of glutamine >900 µmol/L on time to first HAC crisis (hazard ratio [HR]=1.14; p=0.813), but there was a significant effect of baseline ammonia ≥ 1.0 ULN (HR=4.62; p=0.0011).
Conclusions
The findings in this UCD population suggest that glutamine is a weaker predictor of HACs than ammonia and that the utility of the predictive value of glutamine will need to take into account concurrent ammonia levels.
INTRODUCTION
Urea cycle disorders (UCDs) are inborn errors of metabolism involving deficiencies of enzymes or transporters involved in the conversion of ammonia to urea, which result in the accumulation of toxic levels of ammonia in affected patients. Medical management of UCDs is aimed at reducing ammonia levels to within normal limits through the restriction of protein intake and the use of alternate pathway drugs to enhance waste nitrogen excretion.
Blood ammonia and glutamine levels are widely used as biomarkers of disease control in UCD patients. However, blood ammonia levels exhibit considerable daily variability, even among comparatively stable and well-controlled UCD patients [1], and can be affected by blood collection techniques. Plasma glutamine is less affected than ammonia by blood sampling procedures, but glutamine levels also vary over 24 hours, reportedly being highest after fasting [2–7]. Fasting ammonia levels have been shown to correlate strongly with total daily ammonia exposure and to be a strong predictor of hyperammonemic crises (HACs) [1]. Glutamine levels exceeding 900 or 1000 µmol/L are commonly taken as indicative of inadequate disease control and a harbinger of HACs [2–6]. However, a recent study by Lee et al suggested that glutamine appears a weaker predictor of HACs than ammonia [1].
The objective of this study was to extend the work of Lee et al [1] to compare the 24-hour variability of glutamine and ammonia, and to evaluate the utility of glutamine compared with ammonia as an independent predictor of HACs.
1 METHODS
1.1 Clinical Trials
We performed a post-hoc pooled analysis of data from clinical trials of glycerol phenylbutyrate (GPB, HPN-100, RAVICTI®; Horizon Therapeutics, Brisbane, CA) in pediatric and adult UCD patients. The clinical trials have been described in detail elsewhere [8–11]. Blood samples for 24-hour ammonia and glutamine levels were collected during steady-state dosing with GPB or sodium phenylbutrate (NaPBA) in a Phase 2, open-label, crossover study in 10 adult UCD patients [11]. Blood samples for evaluating the comparative utility of glutamine vs. ammonia in predicting HACs were collected from 100 stable adult and pediatric UCD patients during GPB dosing in one of three 12-month safety extension studies [8–10].
All study protocols and informed consents were reviewed and approved by the Investigational Review Board of each participating institution prior to study initiation. Informed consent was obtained from all patients prior to being included in the study. For all studies, eligible patients had a confirmed or clinically suspected UCD and had been receiving NaPBA prior to enrollment. Major exclusion criteria included liver transplant, hypersensitivity to PBA, and laboratory abnormalities or ECG findings viewed as clinically significant by the Investigator. In all studies, patients received GPB three times daily (or more frequently in small children to match their prior NaPBA schedule) at a daily dose equivalent to their previously prescribed NaPBA dose.
1.2 Ammonia and Glutamine Measurements
During the crossover study, serial venous blood samples for ammonia and glutamine analyses were collected over 24 hours after the patient had received 1 to 2 weeks of steady-state dosing with either NaPBA or GPB. During the 12-month studies, fasting blood samples for ammonia and glutamine analyses were collected monthly or quarterly and information on HACs was recorded. Baseline values were defined as the screening or month 0 value when the patient was on NaPBA prior to receiving GPB. An HAC was defined as compatible clinical symptoms associated with one or more ammonia levels ≥ 100 µmol/L. Ammonia and HAC data were also collected retrospectively for up to 12 months prior to enrollment in the GPB studies while patients were receiving NaPBA. Ammonia and glutamine concentrations were measured by an accredited hospital laboratory at each study site and ammonia values were normalized to a standard range of 9 to 35 µmol/L.
1.3 Statistical Methods
Glutamine and ammonia levels over 24 hours following dosing with GPB or NaPBA in the crossover study were summarized using descriptive statistics to compare overall and intra-subject variability in glutamine as compared with ammonia. We summarized baseline subject characteristics including HAC frequency, baseline glutamine and ammonia levels and demographics for patients enrolled in the 12-month studies
Correlations between baseline ammonia and glutamine levels in the 12-month studies were calculated using the Spearman’s rank correlation method, which is robust to outliers and doesn’t assume normality of data. The percentage of patients experiencing an HAC was determined based on categorical glutamine levels at baseline (≤ 900 µmol/L vs > 900 µmol/L) and baseline ammonia levels (< 0.5, 0.5–0.99, and ≥ 1.0 times the upper limit of normal [ULN]) and evaluated by a test of association based on a Fisher’s exact test. We evaluated the longitudinal effect of varying glutamine over time on the odds of having an HAC event within the next 3 months through logistic general estimating equation (GEE) regression models12 using the same glutamine categories and separate models using continuous glutamine (with beta regression transformations to represent odds ratios in terms of increases of 100 or 200 µmol/L in glutamine). The same regression models were repeated with the inclusion of baseline ammonia categories (< 0.5, 0.5 – 0.99, and ≥ 1.0 ULN) and patient demographic criteria (categorical age, gender, race) to evaluate changes in the association between glutamine and HAC events controlling for ammonia and patient demographics. Similar analyses were performed including only patients with baseline ammonia > 0.5 ULN. Lastly, the time to first HAC was evaluated through Kaplan-Meier analyses and Cox proportional hazards regression models with independent predictors of glutamine and ammonia categories similar to above.
2 RESULTS
The population for the 12-month studies included 51 adult and 49 pediatric UCD patients. Mean (SD) baseline glutamine was 740.1 (234.6) µmol/L (ULN=746) and 18% of patients had glutamine values > 900 µmol/L. Mean (SD) baseline ammonia was 28.8 (19.9) µmol/L and 27% of patients had ammonia levels above the ULN (Table 1).
Table 1.
Baseline Characteristics for Patients Enrolled in 12-Month Studies (N=100)*
| Gender: (%) | |
| Male | 33.0 |
| Female | 67.0 |
| Race: (%) | |
| White | 81.0 |
| Nonwhite | 19.0 |
| Age (years): mean (SD) | 19.6 (15.9) |
| Age group: (%) | |
| Pediatric: < 18 years | 49.0 |
| Adult: ≥ 18 years | 51.0 |
| Baseline Glutamine (μmol/L): (n=96) | |
| Mean (SD) | 740.1 (234.6) |
| Median (25th, 75th percentiles) | 704.5 (586.5, 829.5) |
| Patients with baseline glutamine > 900 μmol/L: % | 18.0 |
| Baseline ammonia (μmol/L) | |
| Mean (SD) | 28.8 (19.9) |
| Median (25th, 75th percentiles) | 28.9 (11.0, 37.5) |
| Patients with baseline ammonia: % | |
| 0–0.49 ULN | 39.0 |
| 0.5–0.99 ULN | 34.0 |
| ≥ 1.0 ULN | 27.0 |
| Number of HACs | |
| Pre-study | 54 |
| During study | 27 |
| Patients with ≥1 HAC: % | |
| Pre-study | 30.0 |
| During study | 19.0 |
Baseline values for ammonia and glutamine represent the values at the time of enrollment into the glycerol phenylbutyrate clinical trials, at which time patients had been taking sodium phenylbutyrate for months to years. HAC: hyperammonemic crisis; ULN: upper limit of normal
2.1 Association between Glutamine and Ammonia Levels
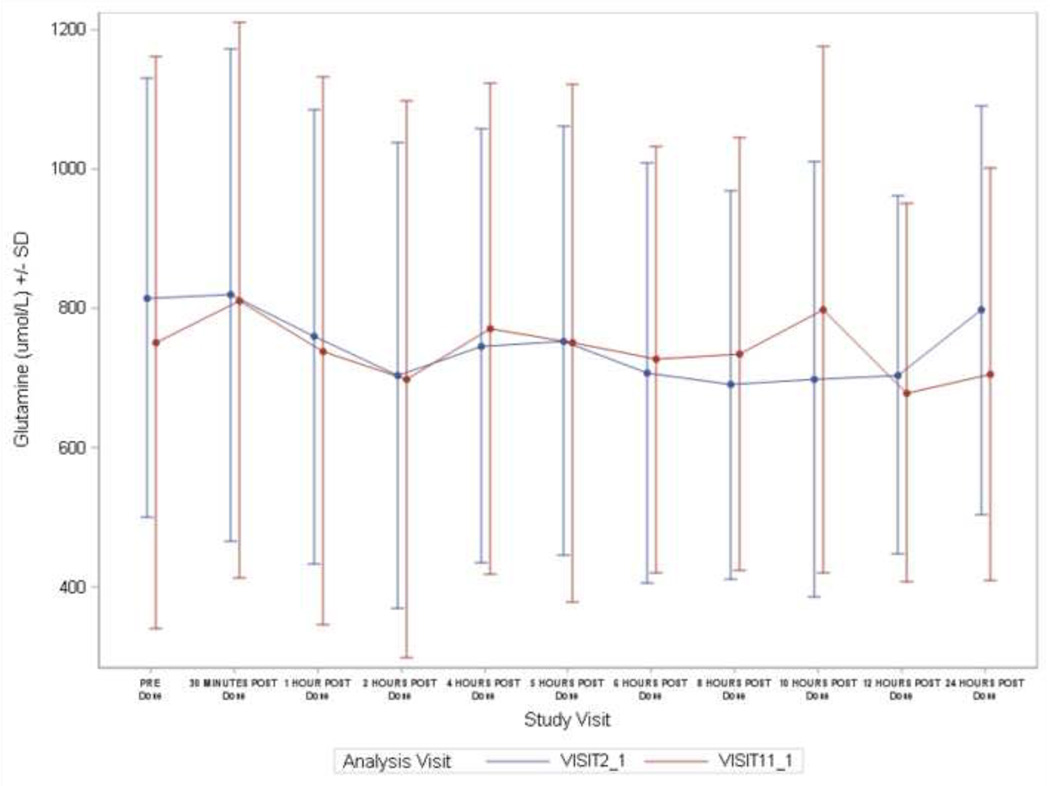
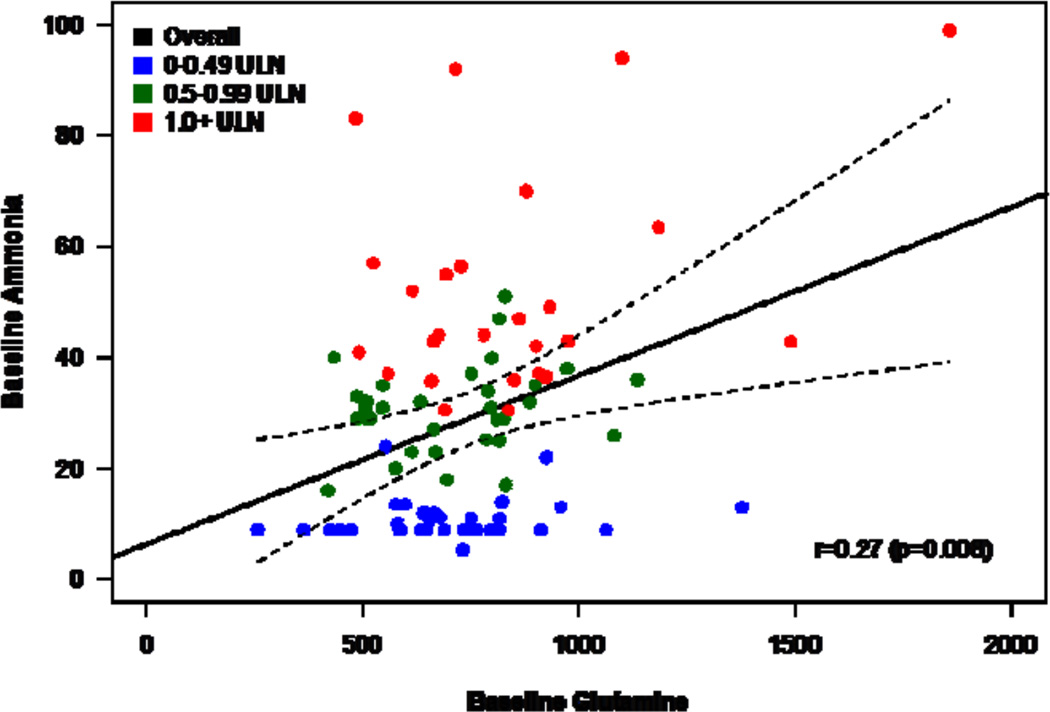
In 10 adult subjects with 24-hour data, glutamine levels exhibited a 24-hour pattern different from ammonia with less daily variability. The median (range) of intra-subject coefficient of variation over 24 hours for glutamine was 15% (8–29%) compared with 56% (28%–154%) for ammonia (Figure 1A). Furthermore there was no clear diurnal pattern in glutamine levels compared with ammonia that showed significant fluctuation during the day (Figure 1B). In the 12-month studies, patients with baseline (fasting) glutamine values > 900 µmol/L had higher mean baseline ammonia levels than patients with baseline glutamine ≤ 900 µmol/L (mean [SD]: 39.6 [26.2] vs. 26.6 [18.0] µmol/L). About 35% of patients with baseline ammonia ≥ ULN had a baseline glutamine of > 900 µmol/L, compared with 9% and 13% of patients with baseline ammonia levels 0.5 - 0.99 ULN and < 0.5 ULN, respectively. During the 12-month studies, the correlation coefficient between monthly glutamine and concurrent ammonia levels varied from 0.17 to 0.32 (baseline correlation shown in Figure 2).
Figure 1.
A: Coefficient of variation for 24-hour ammonia and glutamine sampling in 10 adult patients. The data during NaPBA and GPB treatment have been combined. Whiskers represents 5% and 95% percentiles and box represents 25%–75% percentiles
B: Twenty-four hour variability in glutamine (upper panel) and ammonia (lower panel) levels during treatment with NaPBA (blue lines; study visit 2-1) or glycerol phenylbutyrate (GPB) (red lines; study visit 11-1) in 10 adult patients.
Figure 2.
Scatterplot of baseline (fasting) glutamine and ammonia. Ammonia values are shown for upper limit of normal (ULN) categories: < 0.5 ULN (blue), 0.5 to < 1.0 ULN (green), and ≥ 1.0 ULN (red). Spearman correlation and regression line with 95% confidence bands are shown.
2.2 Risk of Hyperammonemic Crises
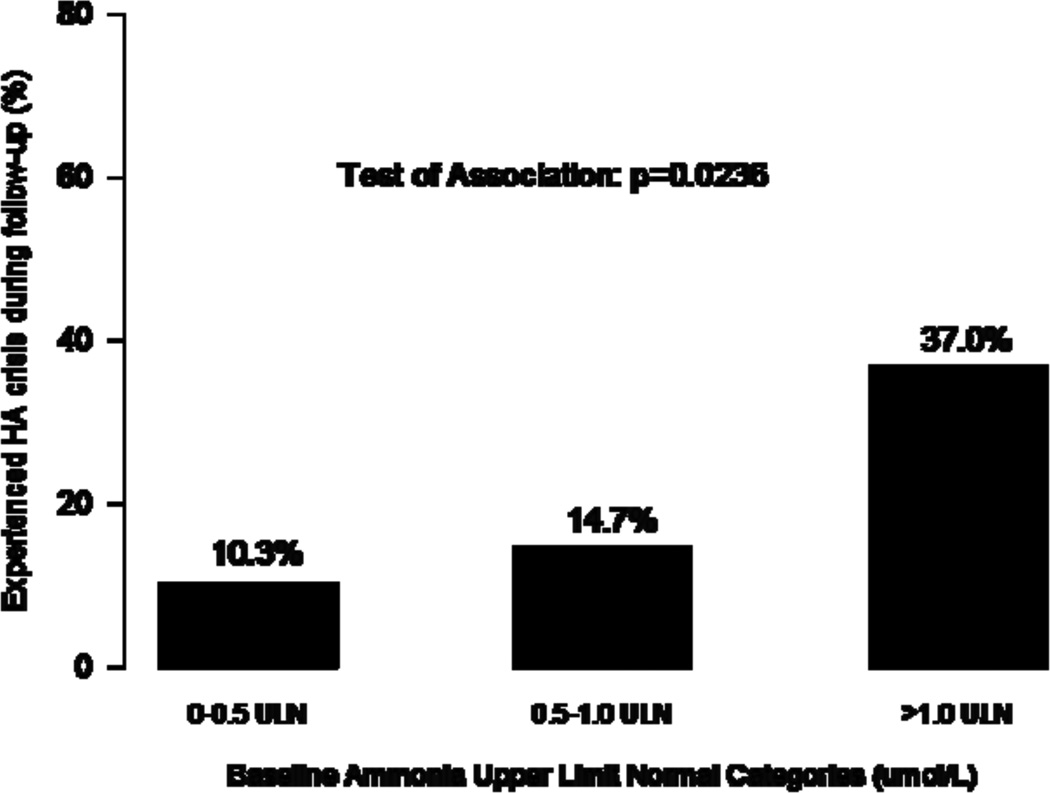
Baseline ammonia levels had a significant association with the occurrence of an HAC over the following 12 months (p=0.0236), but baseline glutamine levels did not (p=0.739) (Figure 3). In the model with binary glutamine by itself, glutamine levels > 900 µmol/L at any time during the 12-month studies were associated with an approximately 2-fold higher risk of an HAC over the next 3 months compared with glutamine levels ≤ 900 µmol/L (odds ratio [OR]=1.98; p=0.173) (Table 2). When the analysis added baseline ammonia to the glutamine model, the association between glutamine and HAC crisis was diminished (OR=1.47; p=0.439), while the predictive value of ammonia ≥ 1.0 ULN was statistically significant with approximately 5 fold increase in the odds of an HAC over the next 3 months (OR =4.96; p=0.013). We observed similar associations when patient demographics were included in the model.
Figure 3.
Percentage of patients having an HAC event by baseline glutamine levels (top panel) and baseline ammonia levels (bottom panel). Test of association based on Fisher’s exact test.
TABLE 2.
Odds of experiencing an HAC within 3 Months in Relation to Glutamine with or without Ammonia*
| Risk Factor | Odds Ratio | p-value | |
|---|---|---|---|
| MODEL 1: Categorical Glutamine Alone | |||
| Glutamine (μmol/L) | |||
| ≤ 900 | Ref | ||
| > 900 | 1.98 | 0.173 | |
| MODEL 2: Continuous Glutamine Alone | |||
| Glutamine | |||
| For a 100 μmol/L increase | 1.14 | 0.088 | |
| For a 200 μmol/L increase | 1.30 | ||
| MODEL 3: Categorical Glutamine + Baseline Ammonia ULN categories | |||
| Glutamine (μmol/L) | |||
| ≤ 900 | Ref | ||
| > 900 | 1.47 | 0.439 | |
| Baseline Ammonia (in relation to ULN) | |||
| 0–0.49 ULN | Ref | ||
| 0.5–0.99 ULN | 0.86 | 0.837 | |
| ≥1 ULN | 4.96 | 0.013 | |
| MODEL 4: Baseline Glutamine + Ammonia + Patient Characteristics | |||
| Baseline Glutamine (μmol/L) | |||
| ≤ 900 | 79 | Ref | |
| > 900 | 17 | 0.85 | 0.830 |
| Baseline Ammonia (in relation to ULN) | |||
| 0–0.49 ULN | 37 | Ref | |
| 0.5–0.99 ULN | 33 | 1.45 | 0.622 |
| ≥1 ULN | 26 | 6.17 | 0.013 |
| Dichotomous Age (no. of subjects) | |||
| Pediatric: < 18 years | 47 | Ref | |
| Adult: ≥ 18 years | 49 | 0.40 | 0.114 |
| Gender | |||
| Male | 32 | 2.01 | 0.236 |
| Female | 64 | Ref | |
| Race | |||
| White | 78 | 1.16 | 0.834 |
| Non-white | 18 | Ref | |
Based on logistic generalized estimating equation regression models using categorical glutamine levels over the study period (n=100).
We replaced binary glutamine with continuous glutamine and observed that glutamine increases of 100 or 200 µmol/L only slightly increased the odds of HAC (OR=1.14 for 100 µmol/L; OR=1.30 for 200 µmol/L; p=0.088). Inclusion of baseline ammonia in the analyses gave similar results as the binary glutamine models (i.e. the odds ratio of glutamine are attenuated towards the null and ammonia is still a significant predictor of HAC).
To determine whether the predictive utility of glutamine was potentially greater if patients with the lowest ammonia levels were excluded, the analyses were repeated for patients with baseline ammonia levels ≥ 0.5 ULN (n=61) and the results were similar. Using binary glutamine, the OR was 2.06 (p=0.153) for glutamine alone and 1.81 (p=0.275) when baseline ammonia was included. In the continuous glutamine model, glutamine increases of 100 or 200 µmol/L only slightly increased the odds of HAC (OR=1.15 for 100 µmol/L; OR=1.32 for 200 µmol/L; p=0.104); inclusion of baseline ammonia in the model gave similar results. In all the models including glutamine and ammonia, patient age (< 18, ≥ 18 years), gender, and race had no significant effect on the odds of experiencing an HAC.
2.3 Time to Hyperammonemic Crises
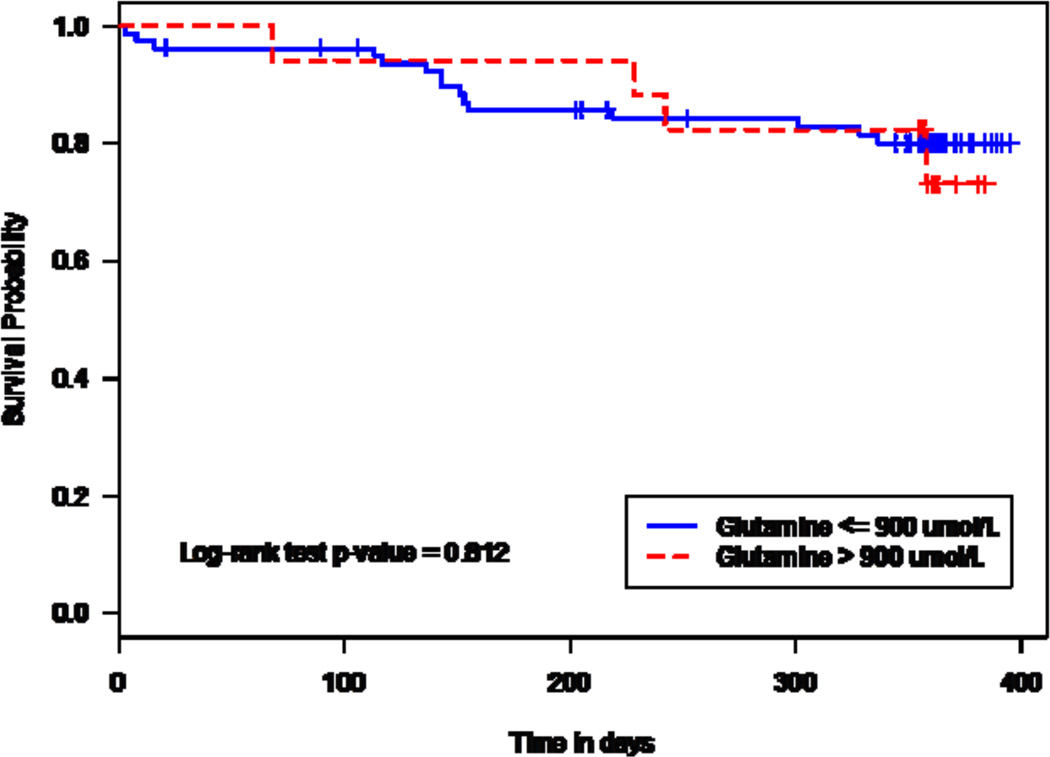
Based on Kaplan-Meier analyses, there was no significant difference between baseline glutamine levels ≤ 900 µmol/L and > 900 µmol/L for time to first HAC crisis (log-rank p=0.812). However, the time to first HAC was significantly shorter in patients with baseline ammonia ≥ 1.0 ULN than for those with baseline ammonia < 0.5 ULN (p=0.008) (Figure 4). Based on a Cox proportional hazards model that included baseline binary glutamine, baseline categorical ammonia and patient demographics, the hazard ratio [HR] for time to first HAC was statistically significant for ammonia ≥ 1.0 ULN vs. < 0.5 ULN (HR=5.52; p=0.007), but not for glutamine > 900 µmol/L vs. ≤ 900 µmol/L (HR=0.75; p=0.648). Similar results were observed with models using standardized baseline continuous glutamine for all patients (glutamine HR=1.02; p=0.913) and for patients with baseline ammonia levels ≥ 0.5 ULN (glutamine HR=0.96; p=0.882). Patient age (< 18, ≥ 18 years), gender, and race had no significant effect on the time to first HAC.
Figure 4.
Kaplan-Meier analysis of time to first HAC by baseline glutamine categories (upper panel) and baseline ammonia categories (lower panel). Log-rank p-values: glutamine, p=0.812; ammonia, p=0.008
3 DISCUSSION
Plasma glutamine is widely used as a biomarker of control in patients with UCDs. Not only can glutamine be considered a depot form of ammonia as the two are interconvertible via glutamine synthetase, but it also shows less diurnal variability, is less prone to sample handling variability, and glutamine levels exceeding 900 to 1000 µmol/L are thought to be associated HACs [2–6]. However, glutamine levels reportedly change with feeding status, being highest after fasting [7], and the evidence for an association between glutamine levels and HACs is based on data from a limited number of subjects. A study by Wilson et al suggested that the relationship between ammonia and glutamine is not a simple linear one and may depend on the type of UCD [3]. More recently, Lee et al showed that while fasting ammonia levels were a strong predictor of HACs, glutamine appeared to be a weaker predictor of these events [1].
The present analyses indicate that plasma glutamine shows notably less 24-hour variability than ammonia, consistent with it being a more stable biomarker. In contrast to ammonia levels which are highest post-prandially and in contrast to a prior report suggesting that glutamine levels are highest with fasting, no clear feeding-fasting or diurnal pattern was apparent with respect to plasma glutamine among the 10 adult patients studied. Patients with elevated fasting glutamine levels had higher mean ammonia levels and patients with fasting ammonia above the ULN were more likely to have elevated glutamine levels. However, the correlation between glutamine and ammonia over the 12-month study period was comparatively weak.
Consistent with prior reports, glutamine levels > 900 µmol/L were associated with an approximately 2-fold increase in the risk of having an HAC as compared with levels ≤ 900 µmol/L. However, this relationship was not statistically significant and weaker than for ammonia; fasting ammonia levels ≥ 1.0 ULN were, as previously reported, associated with a more than 4-fold higher risk of HAC [1]. Importantly, the relationship between glutamine levels > 900 µmol/L and HAC attenuated when baseline ammonia values were taken into account.
When glutamine was analyzed in increments rather than as a dichotomous variable, increases of 100 or 200 µmol/L were associated with only a slightly increased risk of HAC, regardless of whether or not ammonia was included in the analysis. The results were similar when the analyses were restricted to patients with baseline ammonia levels > 0.5 ULN, a population that may be considered more likely to experience an HAC. Thus, unlike baseline ammonia, which correlated strongly with the time to first HAC, glutamine levels did not, regardless of whether they were analyzed in 100 or 200 µmol/L increments or in relation to a 900 µmol/L cutoff.
Limitations of this study include the fact that analyses were not examined in relation to specific UCD subtypes and 24-hour analyses of glutamine variability, for ethical reasons related to blood sampling, were performed in adults and not in children. In addition, clinical experience suggests that patients with elevated ammonia vary considerably in their apparent susceptibility to HAC, which is why the results are expressed in terms of relative risk or rate of HAC rather than biochemical cutoff values. Indeed, this variability in patient susceptibility underscores the necessity of a large dataset when examining relationships between HAC and ammonia or glutamine. Perhaps the most important limitation of the presented analyses is that they are based on post-hoc analyses from several different studies, none of which were specifically designed to examine the utility of ammonia vs. glutamine as predictors of HAC. However, the ammonia and glutamine samples were collected prospectively, and the size and precision of the dataset, which included over 1000 timed ammonia and glutamine samples from over 100 adult and pediatric UCD patients, most of whom were evaluated for up to 12 months, is without precedent.
Collectively these analyses indicate that plasma glutamine does correlate with ammonia and exhibits less variability over 24 hours than does ammonia. However, the correlation is weak, likely reflecting the variety of ‘metabolic choices’ that ammonia-derived nitrogen has other than conversion to glutamine. Moreover, glutamine correlates more weakly with the risk of HAC than does ammonia, and the independent predictive value of glutamine is largely lost when concurrent ammonia values are taken into account. These findings suggest that blood ammonia responds more rapidly than glutamine to changes in protein digestion or catabolism and represents a better ‘real time’ read out of HAC risk. Therefore, use of plasma glutamine as a marker of metabolic control in patients with UCD needs to take ammonia into account. What remains unanswered is how chronic elevations of glutamine correlate with glutamine within CSF and the brain, as dysregulation of glutamine-glutamate cycle has been hypothesized in many context to contribute to neuropathology as well as the relationship between HAC and the rate of production of ammonia or glutamine. Future studies focused on these issues would be important in elucidating the neuropsychological features associated with UCD independent of HACs.
Acknowledgments
The authors gratefully efforts of the staff at the clinical study who made these analyses possible, including N. Schrager (Icahn School of Medicine at Mount Sinai), A. Donovan, J. Crawford, Pediatric TRU Staff, K. Defouw, J. Balliet (The Medical College of Wisconsin), M. Keuth, N. O’Donnell (Long Beach Memorial Hospital), M. Hussain, E. Bailey, A. Orton, M. Ambreen (The Hospital for Sick Children, University of Toronto, ON, Canada), C. Bailey, M.J. Dunkley(The University of Utah), J. Perry, V. de Leon, A. Niemi, K. Cusmano (Stanford University), T. Carlson, J. Parker (University of Minnesota), S. Burr (Children’s Hospital Colorado), K. Simpson (Children’s National Medical Center), K. Regis (Nationwide Children’s Hospital), A. Behrend, T. Marrone (Oregon Health & Sciences University), N. Dorrani (University of California, Los Angeles), C. Heggie (Case Western Reserve University), S. Mortenson (Maine Medical Center), S. Deward (Children’s Hospital of Pittsburgh), K. Bart, C. Duggan (SNBL), K. Murray, C. Dedomenico (Tufts Medical Center), C. Gross (University of Florida), L. Brody (Seattle Children’s Hospital), M. Mullins, S. Carter, A. Tran, J. Stuff, TCH General Clinical Research Center nursing staff (Baylor), Antonia Martinez, Tristen Moors (Hyperion), as well as the Clinical and Translational Science Awards/General Clinical Research Center Grants (Baylor College of Medicine, M01RR00188; Case Western Reserve University, NIDDK 1K08DK074573; Clinical and Translational Science Institute at Children’s National Medical Center NIH/NCRR, UL1RR31988; Medical College of Wisconsin, UL1RR31973; Mount Sinai School of Medicine, UL1RR29887; Oregon Health & Science University, UL1RR24140; Stanford University, UL1RR25744; Tufts University, UL1RR25752; University of California, Los Angeles, UL1RR33176; University of Colorado, UL1RR25780; University of Florida, UL1RR29890; University of Minnesota, UL1RR33183; University of Pittsburgh, NIH UL1TR000005; University of Utah, UL1RR25764; University of Washington,UL1TR000423), the Urea Cycle Disorders Consortium (NIH Grant U54RR019453) and grants from the O’Malley Foundation, Kettering Fund, and Rotenberg Fund which provided support. SCS Nagamani is an awardee of the National Urea Cycle Disorders Foundation Research Fellowship and is supported by the Clinical Scientist Development Award by the Doris Duke Charitable Foundation.
List of Abbreviations
- GPB
glycerol phenylbutyrate (generic name for glyceryl tri (4-phenylbutyrate), also referred to as HPN-100 or RAVICTI®)
- NaPBA
sodium phenylbutyrate
- UCD
urea cycle disorder
Footnotes
Conflict of Interest Statement: D.F. Coakley, M. Marino, M. Mokhtarani, R. Rowell and B.F. Scharschmidt are/were employees of, or are/were consultants to, Horizon Pharma (formerly Hyperion Therapeutics). None of the other authors have a financial interest in Horizon, although payments were made to Baylor College of Medicine (B. Lee, S. Nagamani, PIs), Mt. Sinai (G. Diaz, PI), Medical College of Wisconsin (W. Rhead, PI), Children’s National Medical Center (U. Lichter-Konecki, PI), the Univ. of Toronto (A. Feigenbaum, A. Schulze, PIs), the Univ. of Minnesota (S. Berry, PI), Miller Children’s Hospital (J. Bartley, PI), University of Utah (N. Longo, PI), Stanford University (W. Berquist, PI), Univ. of CO (R. Gallagher, PI), Oregon Health & Sciences Univ. (C.O. Harding, PI), Case Western Reserve Univ. (S. McCandless, PI), Maine Medical Center (W. Smith, PI) and for services provided in the conduct of the study.
ClinicalTrials.gov Identifiers: NCT00551200, NCT00947544, NCT00947297, NCT01347073
Prior Presentation: These data were presented as a poster at the annual meeting of the Society for the Study of Inborn Errors of Metabolism (SSIEM), Lyon, France, September 1–4, 2015 and have been published in abstract form (J Inherit Metab Dis (2015) 38 (Suppl 1):S1–S34).
REFERENCES
- 1.Lee B, Diaz GA, Rhead W, Lichter-Konecki U, Feigenbaum A, Berry SA, Le Mons C, Bartley JA, Longo N, Nagamani SC, Berquist W, Gallagher R, Bartholomew D, Harding CO, Korson MS, McCandless SE, Smith W, Cederbaum S, Wong D, Merritt JL, 2nd, Schulze A, Vockley J, Kronn D, Zori R, Summar M, Milikien DA, Marino M, Coakley DF, Mokhtarani M, Scharschmidt BF. Blood ammonia and glutamine as predictors of hyperammonemic crises in patients with urea cycle disorder. Genet. Med. 2015;17:561–568. doi: 10.1038/gim.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maestri NE, McGowan KD, Brusilow SW. Plasma glutamine concentration: a guide in the management of urea cycle disorders. J. Pediatr. 1992;121:259–261. doi: 10.1016/s0022-3476(05)81200-4. [DOI] [PubMed] [Google Scholar]
- 3.Wilson CJ, Lee PJ, Leonard JV. Plasma glutamine and ammonia concentrations in ornithine carbamoyltransferase deficiency and citrullinaemia. J. Inher. Metab. Dis. 2001;24:691–695. doi: 10.1023/a:1012995701589. [DOI] [PubMed] [Google Scholar]
- 4.Feillet F, Leonard JV. Alternative pathway therapy for urea cycle disorders. J. Inher. Metab. Dis. 1998;21(Suppl 1):101–111. doi: 10.1023/a:1005365825875. [DOI] [PubMed] [Google Scholar]
- 5.Ahrens M, Barsotti R, Batshaw ML, Berry GT, Cederbaum JA, Jopling M, Lee B, Le Mons C, Leonard JV, Markowitz D. Consensus statement from a conference for the management of urea cycle disorders. J. Pediatr. 2001;138:S1–S5. doi: 10.1067/mpd.2001.111830. [DOI] [PubMed] [Google Scholar]
- 6.Haberle J, Boddaert N, Burlina A, Chakrapani A, Dixon M, Huemer M, Karall D, Martinelli D, Crespo PS, Santer R, Servais A, Valayannopoulos V, Lindner M, Rubio V, Dionisi-Vici C. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J. Rare Dis. 2012;7:32. doi: 10.1186/1750-1172-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brusilow SW. Phenylacetylglutamine may replace urea as a vehicle for waste nitrogen excretion. Ped. Res. 1991;29:147–150. doi: 10.1203/00006450-199102000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Diaz GA, Krivitzky LS, Mokhtarani M, Rhead W, Bartley J, Feigenbaum A, Longo N, Berquist W, Berry SA, Gallagher R, Lichter-Konecki U, Bartholomew D, Harding CO, Cederbaum S, McCandless SE, Smith W, Vockley G, Bart SA, Korson MS, Kronn D, Zori R, Merritt JL, 2nd, S CSN, Mauney J, Lemons C, Dickinson K, Moors TL, Coakley DF, Scharschmidt BF, Lee B. Ammonia control and neurocognitive outcome among urea cycle disorder patients treated with glycerol phenylbutyrate. Hepatology. 2013;57:2171–2179. doi: 10.1002/hep.26058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mokhtarani M, Diaz GA, Rhead W, Berry SA, Lichter-Konecki U, Feigenbaum A, Schulze A, Longo N, Bartley J, Berquist W, Gallagher R, Smith W, McCandless SE, Harding C, Rockey DC, Vierling JM, Mantry P, Ghabril M, Brown RS, Jr, Dickinson K, Moors T, Norris C, Coakley D, Milikien DA, Nagamani SC, Lemons C, Lee B, Scharschmidt BF. Elevated phenylacetic acid levels do not correlate with adverse events in patients with urea cycle disorders or hepatic encephalopathy and can be predicted based on the plasma PAA to PAGN ratio. Mol. Genet. Metab. 2013;110:446–453. doi: 10.1016/j.ymgme.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry SA, Lichter-Konecki U, Diaz GA, McCandless SE, Rhead W, Smith W, Lemons C, Nagamani SC, Coakley DF, Mokhtarani M, Scharschmidt BF, Lee B. Glycerol phenylbutyrate treatment in children with urea cycle disorders: pooled analysis of short and long-term ammonia control and outcomes. Mol. Genet. Metab. 2014;112:17–24. doi: 10.1016/j.ymgme.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee B, Rhead W, Diaz GA, Scharschmidt BF, Mian A, Shchelochkov O, Marier JF, Beliveau M, Mauney J, Dickinson K, Martinez A, Gargosky S, Mokhtarani M, Berry SA. Phase 2 comparison of a novel ammonia scavenging agent with sodium phenylbutyrate in patients with urea cycle disorders: safety, pharmacokinetics and ammonia control. Mol. Genet. Metab. 2010;100:221–228. doi: 10.1016/j.ymgme.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988:1049–1060. [PubMed] [Google Scholar]