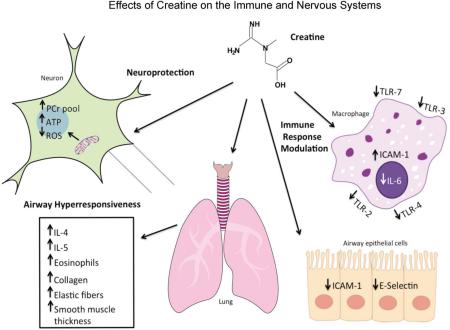
Abstract
Creatine is widely used by both elite and recreational athletes as an ergogenic aid to enhance anaerobic exercise performance. Older individuals also use creatine to prevent sarcopenia and, accordingly, may have therapeutic benefits for muscle wasting diseases. Although the effect of creatine on the musculoskeletal system has been extensively studied, less attention has been paid to its potential effects on other physiological systems. Because there is a significant pool of creatine in the brain, the utility of creatine supplementation has been examined in vitro as well as in vivo in both animal models of neurological disorders and in humans. While the data are preliminary, there is evidence to suggest that individuals with certain neurological conditions may benefit from exogenous creatine supplementation if treatment protocols can be optimized. A small number of studies that have examined the impact of creatine on the immune system have shown an alteration in soluble mediator production and the expression of molecules involved in recognizing infections, specifically toll-like receptors. Future investigations evaluating the total impact of creatine supplementation are required to better understand the benefits and risks of creatine use, particularly since there is increasing evidence that creatine may have a regulatory impact on the immune system.
Keywords: creatine, immune response, immunomodulation, neuroprotection
Graphical Abstract
1. Introduction
Creatine is naturally synthesized in the liver, kidney, and pancreas of vertebrates from the amino acids arginine, methionine, and glycine [1–3]. In vivo, creatine is a product of the arginine biosynthesis pathway and metabolizes to creatinine [3,4]. Individuals who eat meat and/or fish obtain approximately 1 g d−1 of creatine from the diet [5], and approximately 1 g d−1 is synthesized endogenously. Vegetarians have significantly lower muscle creatine stores and lower creatinine levels as compared to those who eat meat and/or fish products [6,7]. The average creatine pool for a 70 kg individual ranges from 120-140 g and approximately 2 g d−1 is lost in the urine in the form of creatinine [1]. Given that daily intake and excretion are approximately equal, the most efficient way to increase creatine stores in the body is through dietary supplementation. Creatine enters the muscle cells via a sodium- and chloride-dependent creatine transporter [3,8,9] and is primarily stored in the skeletal muscle as free creatine or phosphocreatine, which is a major source of energy to the host [3,8,10].
While the majority of creatine in the body is stored in skeletal muscles [3], there is also a significant pool of creatine in the brain [11], which may provide some protection against neurological disorders and trauma. For example, several studies using animal models have shown that oral creatine supplementation provides neuroprotective effects in a variety of neurological conditions including traumatic brain injury [12], Huntington's Disease [13], amyotrophic lateral sclerosis [14], ischemia [15], and Parkinson's Disease [16]. There have also been a small number of studies that suggest that pro-inflammatory responses are reduced following creatine supplementation [17–24]. However, the mechanism of how creatine acts in modulating inflammation remains unclear, although recent work [25] demonstrating that European sea bass fed a diet supplemented with arginine (a precursor of creatine) had reduced disease resistance suggests that the mechanism may be evolutionarily conserved. The potential of creatine as an immunomodulator may have important implications for individuals with certain pro-inflammatory diseases such as arthritis.
The purpose of this review is to examine the potential of dietary creatine supplementation to modulate disease, as well as to discuss potential mechanisms of action of creatine in its ability to function as a neuroprotective or immunomodulatory agent.
2. Creatine use by athletes
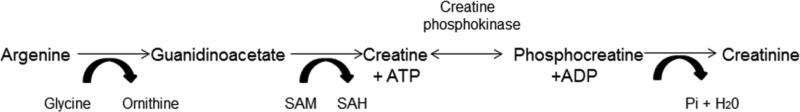
Creatine was first discovered as a constituent of meat in the 1800's; however, it was not until the 1970's that it was used as a potential ergogenic aid by athletes in the Soviet Union and Eastern bloc countries, and then gained wide research interest in the 1990's [26]. The phosphagen energy system is the metabolic system that produces ATP most rapidly, as compared to glycolysis or the aerobic system [27]. Inside the cell, creatine phosphokinase catalyzes a reversible reaction between the γ-phosphate group of ATP to the guanidino group of creatine resulting in ADP and phosphocreatine (Figure 1). Cellular stores of phosphocreatine can reach concentrations of up to 40 mM [3,8,28–30] and is essential for replenishing ATP stores that are immediately used during high intensity exercise.
Figure 1. The arginine biosynthesis pathway.
Creatine is generated as part of the arginine biosynthesis pathway. Creatine and phosphocreatine exist in equilibrium in the cell. Creatine phosphokinase catalyzes the reaction between creatine and phosphocreatine, which results in energy (ATP) generation. Hydrolysis of phosphocreatine yields the end product of the pathway, creatinine. SAM, S-adenosyl-methionine; SAH, S-adenosyl-homocysteine
Dietary creatine supplementation increases the phosphocreatine stores in the muscles, and has been shown to enhance performance during high-intensity, short duration activities or repeated bouts of high-intensity exercise with short rest periods such as jumping, sprinting, and strength training [26,31–39]. It is estimated that 27-78% of all college athletes have used creatine supplements [40–43] and the proportion of individuals using creatine is likely much higher in athletes participating in sports such as football, track, wrestling, and soccer [26,31–34,44]. A recent study of body builders in Iran reported that creatine was the most common nutritional supplement used by men (60.8%) [35]. Initially, creatine was primarily utilized by elite athletes; but its use has become increasingly widespread among older adults, recreational athletes, and high school athletes [1,45–51].
The most widely used form of creatine by athletes is creatine monohydrate [41–43]. Oral bioavailability of creatine monohydrate is low due to the rapid conversion of creatine to creatinine in acidic environments, as would be encountered in the stomach [52,53]. While there has been little study examining how creatine crosses the intestine, the small intestine does express the Na+/Cl− creatine transporter [54] which is also expressed in other organs including the brain, kidney, and heart [55,56]. However, some work has suggested that creatine may move across the jejunum by paracellular movement [57]. The contributions of each of these potential mechanisms of transport is unclear although it has been shown that creatine supplementation of individuals deficient in the creatine transporter does improve muscular, but not cognitive and psychiatric symptoms of the condition [58], indicating that paracellular transport may be a sufficient to increase creatine stores in the muscles.
Athletes normally engage in the practice of loading that consists of ingesting 20 g d−1 of creatine for five days administered over several (usually four) doses followed by 1-10 g d−1 for several weeks or months [3,34]. The loading phase increases muscle stores of phosphocreatine 15 to 40% [59,60]. Minimal side effects as a result of the creatine loading phase have been reported and include cramping, nausea, fluid retention, and diarrhea [43,61]. Although it is typically recommended that individuals creatine load for 4-7 days, it has been reported that creatine uptake into muscle is greatest during the first 2 days of loading [62]. Hultman et al. [63] have also reported that a dose of 3 g d−1 for 28 days is as effective as creatine loading for increasing total muscle creatine stores. Therefore, ‘slowly loading’ the muscle with creatine may result in significant increases in performance and alleviate side effects that are sometimes associated with a 4-7 day loading regimen.
Very few individuals (~20% of users) reach maximal creatine saturation of their skeletal muscles (160 mmol/kg dry mass [10]), thus there is significant interest in developing formulations that have enhanced bioavailability. One currently available form of creatine, creatine ethyl ester, is reported to have a greater degree of stability and bioavailability than creatine monohydrate [53]. It is postulated that because the carboxyl group is no longer available, creatine ethyl ester is not converted to creatinine in the stomach, but can be absorbed in the intestine where the creatine ethyl ester enters the blood. Esterases in the intestinal cells and blood convert the creatine ethyl ester to creatine, which is then stored in the muscle cells as phosphocreatine. Of particular note, creatine ethyl ester is more stable than creatine monohydrate at a lower pH (as would be encountered in the stomach) [52,53]. In addition, in vitro studies utilizing Caco-2 cell monolayers have demonstrated increased permeability of creatine ethyl ester compared to creatine monohydrate [53]. Together, these studies [52,53] suggest that creatine ethyl ester may be more bioavailable than creatine monohydrate.
There are multiple mechanisms by which creatine functions to enhance athletic performance. As shown in Figure 1, creatine is the substrate for the creatine kinase reaction, resulting in the generation of phosphocreatine, which comprises 60% of the creatine in skeletal muscle (~ 60% of muscle creatine is stored as phosphocreatine and 40% as free creatine) [2]. As previously mentioned, phosphocreatine is responsible for the re-phosphorylation of ADP to ATP during bursts of high intensity movements, thus resulting in an increased availability of energy during short periods of explosive exercise [35–39,64]. As phosphocreatine levels decline due to the re-phosphorylation of ADP, phosphofructokinase production is stimulated, thereby increasing the rate of glycolysis [39]. Creatine can also function to buffer the pH changes that occur due to the accumulation of lactate and hydrogen ions by using the hydrogen ions in the creatine kinase reaction [65,66]. Individuals with creatine or phosphocreatine deficiencies due to genetic defects in proteins involved in creatine synthesis (L-arginine-glycine amidinotransferase or guanidinoacetate methyltransferase) or transport (creatine transporter [SLC6A8]) have reduced levels of ATP in the brain resulting in developmental delays and mental retardation [67–69]. While individuals with defects in creatine synthesis can be treated with exogenous creatine, no treatment exists for individuals with deficits in the creatine transporter [67–69].
3. Creatine as a mediator of neuroprotection
3.1 The mitochondrial permeability transition pore
Because of the high levels of creatine in the central nervous system [11], a considerable number of studies have focused on the potential neuroprotective effects of oral creatine supplementation in a variety of neurological conditions including traumatic brain injury (TBI) [12,70,71], Huntington's Disease (HD) [13,72–76], amyotrophic lateral sclerosis (ALS) [14,77–80], cerebral ischemia [15,81], and Parkinson's Disease (PD) [82–88]. One of the key focuses as to how creatine may work to reduce neuropathology in the central nervous system (CNS) has been the effect of creatine on the mitochondrial permeability transition pore [14,89]. In the CNS, the mitochondrial permeability transition pore is induced in the conditions mentioned above including stroke [90], PD [91,92], HD [93], TBI [94,95], and ALS [14,96,97]. The mitochondrial permeability transition pore is also induced by reactive oxygen species (ROS) [98] and, correspondingly, ROS are released as a result of development of the pore [99]. Conversely, high ATP and ADP levels prevent the mitochondrial pore from being induced [98,100,101].
Excitotoxicity is a major cause of neuronal death that is the result of an influx of calcium into the cell as a result of glutamate receptor overactivation [102–107]. The mitochondrial permeability transition pore permits the influx of molecules smaller than 1.5kDa (including calcium), which ultimately results in altered osmotic balance in the organelle resulting in damage to the mitochondria and the release of cytochrome c [108]. ATP generation is also dysregulated by the development of the mitochondrial permeability transition pore as ATP synthase hydrolyzes ATP under these conditions [109]. It has been hypothesized that neuroprotection via creatine supplementation may occur via inhibition of the mitochondrial permeability transition pore by stabilizing mitochondrial creatine kinase and stimulating the production of phosphocreatine; which stabilizes energy (ATP) levels in the cell [110]. The inhibition of the mitochondrial permeability transition pore ultimately inhibits cell death from occurring by prohibiting proapoptotic proteins from being released into the cytosol [105].
3.2. Traumatic Brain Injury
TBI due to accidents and sports is a significant contributor of cognitive impairment to individuals in the United States and Canada [111,112]. While the primary injury results from the disruption of tissue at the time of impact, the secondary injuries occur minutes to days later. A component of the secondary injury is the result of alterations in calcium disruption that results in mitochondrial dysfunction and an inadequate supply of ATP to the neuron [113–117]. In 2000, Sullivan et al. [12] examined the ability of creatine supplementation to reduce the level of tissue damage in mice and rats in a model of TBI. Mice intraperitoneally injected with creatine for three and five days prior to TBI had reduced lesion volumes than control injected mice at day seven post injury. Similar results were observed in rats treated with creatine prior to TBI [12]. The authors then determined that rats fed a creatine-supplemented diet for seven days after TBI experienced significantly smaller cortical lesions than animals fed the control diet. Additionally, diet-based supplementation of rats for four weeks post TBI determined that there was reduced reactive oxygen intermediates production, increased ATP levels, and increased mitochondrial membrane potentials [12]. In humans, pilot data has examined both the short-term [70] and long-term [71] effect of creatine supplementation on TBI in children and adolescents. Individuals receiving 0.4 g kg−1 oral creatine for 6 months following TBI had significant improvement in communication, cognition, personality/behavior, and self-care as compared to individuals who did not receive creatine. While not statistically significant, the length of stay in the intensive care unit was shortened which would result in decreased hospitalization costs [70]. A follow-up of these patients at 6 months post-injury indicated that the creatine-treated individuals had less dizziness, headaches, and fatigue than the control patients [71]. While there appear to be no other studies that have examined the potential benefit of creatine supplementation in TBI patients, the results of these studies [70,71] are promising and future large-scale studies on the utility of creatine are warranted, particularly since no negative side-effects were noted in these reports.
3.3. Huntington's Disease
HD is an autosomal dominant, progressive neurological disorder that is ultimately fatal. The mean survival from the time of diagnosis is 15 to 20 years and symptoms include both cognitive disorders and progressive motor impairment [118]. Chemical lesioning of animals has been used to induce pathology similar to that observed in HD patients [110,119–122]. Malonate is a reversible succinate dehydrogenase inhibitor that when injected intrastriatially in rats, induces neuropathology similar to that observed in HD patients [119]. Similarly, 3-nitropropionic acid (3-NPA), an irreversible succinate dehydrogenase inhibitor that is a metabolite of the fungus Arthrinium sp. [123], induces similar pathology to malonate [124]. Both malonate and 3-NPA impair mitochondrial respiration resulting in membrane depolarization and eventually triggering apoptotic pathways and production of ROS leading to cell death [121]. In rat models of brain lesioning with malonate or 3-NPA, oral supplementation with either creatine or cyclocreatine resulted in smaller lesion volumes in the malonate lesioning model, while only creatine was effective in reducing lesion volume in the 3-NPA model of lesion induction [120].
The mutation in HD patients is an extended (>35) CAG (glutamine) repeat [125] and disease onset and severity correlates with the repeat length [126–128]. Identification of this mutation permitted the development of a transgenic mouse model of HD with CAG repeats of 141-157 [129]. In 2000, studies by Ferrante et al. [13] demonstrated that mice fed a diet supplemented with 1-3% creatine had increased survival and reduced brain atrophy compared to controls. In addition, the mice had better rotarod performance (a measure of coordination) as compared to mice fed the control diet. An interesting finding of this study [13] was that mice fed 1% creatine had better rotarod performance than mice fed 3% creatine, suggesting that the range of effectiveness (at least in regard to motor performance in this model) is very narrow. In a similar study [73], HD transgenic mice fed a diet of 2% creatine had increased survival and reduced brain atrophy compared to control mice. Work by Dedeoglu et al. [74] examined the impact of creatine feeding on HD transgenic mice after clinical signs of HD were apparent (6 and 8 weeks of age). In these studies, rotarod performance was significantly improved compared to the performance of control mice. Brain weight was higher in mice receiving 2% oral creatine supplementation. Consistent with the increased brain weights, the supplemented mice also had less atrophy of the striatal neurons. Starting creatine supplementation at a later point in the disease (10 weeks) did not result in improvements [74], indicating that there may be an ideal window in which to begin supplementation to observe improvements in HD patients.
These studies provided the basis to test creatine in a pilot study in HD patients [76]. HD patients were given either creatine (5 g d−1) or placebo for one year. Muscular and cognitive function was assessed at 6 and 12 months. No differences were observed between groups using the Unified Huntington's Disease Rating Scale to assess cognitive and motor function and functional ability. Additional tests included a maximal incremental exercise test using a cycle ergometer and assessment of elbow muscle strength using an isokinetic dynamometer [76]. Of note, this study was small (n=26 in treatment group, n=15 in placebo) and utilized patients with stage I, II, and III HD [76]. Bender et al. [130] reported that in HD patients undergoing creatine supplementation (20 g d−1× 5 d, then 6 g d−1 for remainder of the study) had a decrease in brain glutamate concentrations following 8-10 weeks of creatine ingestion, but no change on the clinical rating scales. When considering the dose of creatine fed to mice and how that would translate into humans, much higher doses of creatine (40-50 g d−1) [131] would be required in humans than have been utilized in most clinical studies (5-10 g d−1).
A recent study utilized higher doses of creatine (30 g d−1) in a study designed to test both the safety and tolerability of creatine in patients at risk of developing HD [132]. The PRECREST (Creatine Safety and Tolerability in Premanifest HD) trial enrolled individuals who were at 50% risk of developing HD who had affected first degree relatives, as well as individuals who had undergone genetic testing and had the HD mutation. At 6 and 18 months of creatine or placebo, neuroimaging was performed to assess brain atrophy. Individuals in the creatine group had significantly less cortical and striatal atrophy compared to that observed in control group [132], suggesting that creatine may be helpful in slowing disease progression. It is important to note that this study was performed on individuals prior to the onset of clinical signs. There can be minor changes in clinical signs or brain atrophy for many years prior to a diagnosis of HD [133–136]. Together, these data suggest that creatine may be most effective in HD treatment prior to the development of clinical signs.
3.4. Amyotrophic Lateral Sclerosis
ALS is an extremely rare (estimated incidence of 2 in 100,000 per year) [137,138] progressive neurodegenerative disease wherein 90 to 95% of the cases have no known family history [139–141]. In addition to the loss of motor neurons in the spinal cord and cortex, muscle weakness and atrophy are also hallmarks of the disease [142,143]. Proposed causes of the disease include neuroinflammation, oxidative stress, and mitochondrial dysfunction [142–144] and many have speculated that oral creatine supplementation may slow the progression of this universally fatal condition [14,77]. Hereditary causes of ALS have been linked to a mutation in copper zinc superoxide dismutase, which is responsible for reducing free radicals in the cell [139]. The identification of this mutation made it possible to develop a mouse model of hereditary ALS [145] which mimics the key pathology associated with the disease in humans, particularly the profound loss of large ventral horn neurons [139,140,142,143]. The mouse model is representative of approximately 10% of the cases of human ALS, as the cause of sporadic ALS is unknown [138,140–144]. The impact of oral creatine treatment on the transgenic mouse model of ALS was examined and it was found that creatine provided significant neuroprotection to the mice and that these mice were indistinguishable from control wildtype mice [14]. Further studies in this mouse model determined both survival and rotarod performance was enhanced in mice fed a diet supplemented with 2% creatine [77]. Human trials with ALS patients were initiated based upon the results of animal studies. In contrast to the animal studies, creatine supplementation had no effect on survival, motor function, or respiratory function of patients [78–80]. While disappointing, it should not be surprising that the human trials did not parallel the mouse studies, since only a small percentage of the human cases have the same genetic defect found in the transgenic mice, and the trials were not specifically targeted using individuals with a mutation in copper zinc superoxide dismutase. In addition, the amount of creatine used for supplementation in the human trials was significantly less than the amount used in the mouse studies.
3.5. Cerebral Ischemia
In the United States, stroke is the fifth leading cause of death, and a significant cause of disability [146]. As a result of reports of the neuroprotective effects of creatine, Zhu et al. [15] examined the ability of creatine to provide protection in a mouse model of stroke. In these studies, animals were fed a 2% creatine diet for 4 weeks prior to occlusion of the middle cerebral artery for 2 hours. Animals were evaluated 24 hours post-reperfusion. Lesion volume and neurological impairment were significantly reduced in the creatine fed mice compared to the controls. Brain creatine and ATP levels were depressed in lesioned brain hemispheres of animals that did not receive the creatine-supplemented diets compared to levels in the contralateral hemisphere [15]. One of the main mediators of ischemic injury in the brain has been reported to be the activation of caspases [147–150]. Levels of caspase-3 activation and cytochrome c release in mice fed creatine were decreased compared to control fed mice [15]. The authors [15] stated that the effectiveness of creatine feeding was similar to that observed in mice and that the anti-apoptotic protein, Bcl-2, was overexpressed in neurons [148,149,151].
In a study examining the effectiveness of creatine on newborn mice with cerebral hypoxia-ischemia, carotid ligation was performed at post-natal day 10 for 25 minutes [81]. At post-natal day 20 (weaning), mice were placed in either control or creatine-fed groups. Creatine was supplemented at either 1% or 3% for 3 weeks. Creatine fed mice demonstrated increased performance on the rotarod and the Morris water maze (a measure of memory) (p < 0.05) as compared to control-fed animals. Additionally, mice fed a diet of 3% creatine had increased performance on both tests as compared with animals fed the 1% creatine diet [81]. Lesion size measurements were also reduced in creatine fed mice. While no clinical trials examining the impact of creatine supplementation were identified, the findings described herein suggest that it may be advisable to supplement stroke patients with creatine, in addition to the standard medical care.
3.6. Parkinson's Disease
PD is a neurodegenerative disorder characterized by progressive loss of dopaminergic neurons in the substantia nigra and the development of Lewy bodies in the brain. Many studies of PD utilize the loss of tyrosine hydroxylase-positive neurons as an indicator of the level of disease [152–157], as tyrosine hydroxylase is the rate-limiting enzyme in the generation of L-3,4-dihydroxyphenylalanine(L-DOPA) [158], the precursor to the neurotransmitter dopamine and norepinephrine [158]. Tremors, rigidity, and gait disorders are common clinical signs of the disease [159] and are correlated with the loss of dopaminergic neurons [159]. In 2013, direct and indirect costs for the population of PD patients in the United States exceeded $20 billion per year [160].
Systemic administration of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to mice [16,161–166] or non-human primates [167–169] is a well-accepted model of PD. Matthews et al. [16] examined the neuroprotective effects of creatine in the mouse model of MPTP-induced PD. In this study, oral creatine Nissil and tyrosine hydroxylase immunostaining demonstrated that creatine significantly reduced the level of dopaminergic neurons lost following MPTP administration. Furthermore, there was no significant difference in the neuronal counts in the substantia nigra between control (no MPTP) mice and creatine fed MPTP-lesioned mice, indicating the effectiveness of creatine as a neuroprotective agent [16]. Protection against dopamine depletion and loss of tyrosine hydroxylase positive neurons in the substantia nigra was further enhanced when co-administered with the cyclooxygenase 2 (COX-2) inhibitor rofecoxib [170]. A related study examined a combination treatment using creatine and coenzyme Q10 (CoQ10). In this study, α-synuclein levels (a key neuronal protein that serves as a marker for staging PD pathology) were examined [171,172] in MTPT-treated rats. In PD, α-synuclein forms aggregates that have been compared to the β-amyloid aggregates observed in Alzheimer's Disease [173]. Because α-synuclein downregulates tyrosine hydroxylase, elevated α-synuclein levels are associated with decreased dopamine levels [173]. Rats receiving the combination creatine and CoQ10 treatment had reduced lesion volumes and reduced α-synuclein levels in the brain [172], as well as a reduced loss of tyrosine hydroxylase-positive neurons as compared with singly-treated rats [172].
In vitro test systems have also demonstrated the effectiveness of creatine in a variety of culture systems [174–176]. Andres et al. [174] examined the ability of creatine to protect dopaminergic neurons from a variety of insults including serum and glucose starvation, 1-methyl-4-phenyl pyridinium ion (MPP+) and 6-hydroxydopamine (6-OHDA). Primary neuronal cultures from embryonic rats were treated with 5 mM creatine monohydrate and then exposed to MPP+ or 6-OHDA. In both instances, creatine-treated neuronal cultures were significantly protected as compared to non-creatine treated neurons. In addition, neurons that experienced serum and glucose starvation had enhanced survival when cultured with creatine [174]. Similar results have been obtained in an in vitro slice culture system using striatal slices from adult rats and 6-OHDA lesioning [175].
One complication of PD is the development of L-DOPA-induced dyskinesia (LID) which is characterized by abnormal involuntary movements (AIMs) and is the result of prolonged use of levodopa (L-DOPA) to manage the disease [177,178]. To study the effects of creatine on the development of LID, lesions were induced in rats using 6-OHDA and 21 days later rats were placed on either a 2% creatine-supplemented diet or a normal diet. After 32 days on the diet, LDOPA treatment began and for 3 weeks animals were observed for the development of AIMs [179]. The results showed that AIMs were significantly reduced in the rats on the creatine diet. In addition, markers associated with LID – prodynorphin and FosB/ ΔFos [180–183] – were reduced in the brains of mice fed a creatine-supplemented diet indicating that creatine supplementation had significant positive effects even after the development of pathology in this model [179].
On the basis of the animal and tissue culture studies, a number of clinical trials have examined the potential of creatine as a possible therapy for PD [82–88,184]. A randomized, double-blind futility trial was undertaken with 200 participants who received creatine (10 g d−1), minocycline (200 mg d−1), or placebo. Creatine reduced disease progression in PD patients diagnosed within 5 years and could not be rejected as futile [88]. Another study determined that creatine (20 g·d−1 for 6 days, then 2 g d−1 for 6 months, and then 4 g d−1 for up to 2 years) reduced the level of dopaminergic therapy but did not alter PD clinical scores [87]. Long-term creatine (4 g·d−1 for 2 years) has been shown to be safe in patients with PD [184], although one study associated high caffeine intake with faster progression in creatine-supplementing patients [83]. A double-blind, multicenter, long-term efficacy trial recruited 1,741 PD patients who were randomly treated with either creatine monohydrate (10 g d−1) or placebo. Because no differences were noted between creatine- and placebo-treated groups after 5 years, the trial was concluded early [82]. While the studies in humans have been disappointing, the doses of creatine used in humans do not reflect those used in the mouse and rat studies, but rather doses similar to those used by athletes. Therefore, future studies in humans involving creatine should be designed to more closely utilize doses that are consistent with rodent studies.
4. Creatine as an anti-inflammatory agent
4.1. Early studies
Although the available literature is limited, there are a few reports that have supported the use of creatine as an anti-inflammatory agent [17–19,22,24,185]. Creatine was first reported as an anti-inflammatory by Madan and Khana in 1976 who tested a series of amino acids in a rat model of carrageenan-induced acute inflammation [186]. In this widely-used model for testing anti-inflammatory agents [187–194], carrageenan is injected into the paw, and a number of potent inflammatory mediators are induced both locally and systemically, with maximum edema normally occurring within 3 hours of injection, due to activation of the cyclo-oxgenase pathway [187]. Mediators induced include histamine, bradykinin, serotonin, prostaglandins, tumor necrosis factor-α (TNF-α), interleukin -1 (IL-1), and IL-6 [188–190]. Recent data suggests that carrageenan-induced inflammation is the result of activation of nuclear factor-κB (NF-κB) via a toll-like receptor-4 (TLR-4)-dependent mechanism [195]. Using this model, Madan and Khana [186] found that intraperitoneal injection of creatine was a potent reducer of edema [186]. As a follow-up to this initial study, they tested the efficacy of oral creatine as a mediator of acute and chronic inflammation in rats, as well as the ability of creatine to function as an analgesic [19]. In a model of carrageenan-induced acute inflammation of the foot paw, creatine suppressed inflammation as effectively as the control drug phenylbutazone [19], a nonsteroidal anti-inflammatory drug (NSAID) used in animals [196,197]. Because serotonin (also referred to as 5-HT) plays a significant role in the early stages of inflammation in the carrageenan model [189,192], the authors utilized the serotonin-induced model of paw edema to verify the utility of oral creatine as an anti-inflammatory agent [198] and found that creatine-fed animals had reduced inflammation compared to control-treated animals [198]. Table 1 summarizes the in vivo animal studies that have been performed using oral creatine to impact inflammatory responses.
Table 1.
Summary of in vivo studies examining the effects of oral creatine on inflammation
| Species | Model | Dose | Results | Reference |
|---|---|---|---|---|
| Rat | Carrageean-induced edema Nystatin-induced edema 5-HT-induced edema Formaldehyde-induced arthritis Granuloma pouch |
50-500 mg kg−1 500 mg kg−1 500 mg kg−1 100-500 mg kg−1 100 mg kg−1 ×7d |
Reduced swelling Reduced swelling Reduced paw volume Reduced paw volume Reduced exudate, reduced pouch weight |
[19] |
| Mouse | OVA sensitization | 0.5 g kg−1d−1 | Increased allergic inflammation, airway responsiveness, airway remodeling | [24] |
| Mouse | OVA sensitization ± exercise | 0.5 g kg−1d−1 | Low intensity exercise reduced negative effects of creatine | [220] |
| Mouse | OVA sensitization | 0.5 g kg−1d−1 | Increased allergic inflammation and airway remodeling | [221] |
To ensure that the observed effect was not model-specific, an additional model of inflammation was tested. Oral creatine was found to be effective for decreasing paw swelling in a nystatin model of inflammation [19]. The nystatin model of paw edema induces chronic inflammation, which can last up to 15 days [199]. Nystatin triggers TLR-2 and induces secretion of TNF-α, IL-8, and IL-1β [200]. Based on more recent data from our laboratory [20], we would suggest that the reduced inflammation in this model was the result of creatine reducing expression of TLR-2 in vivo.
To determine if oral creatine treatment could induce similar results in chronic inflammatory conditions, two animal models of arthritis were used [198]. Using a formaldehyde-induced arthritis model, formaldehyde was injected into the plantar fascia to induce a biphasic localized response, with the first phase characterized by release of serotonin, histamine, and kinins, and the second phase characterized by the release of prostaglandins [192,201–203]. The second model utilized in these experiments was the granuloma pouch model [198]. In this model, air was injected between the shoulder blades of the rat and an irritant injected into the air pouch and after 8 days, the animals were sacrificed and the exudate collected. In this experiment, 2% croton oil in a vehicle of ground nut oil was utilized as the irritant [198]. Both creatine and phenylbutazone caused a significant reduction of formaldehyde-induced arthritis and granuloma pouch weight (p≤0.001) [19], indicating that creatine was effective at reducing chronic inflammation. The authors [18] also examined the impact of creatinine on a variety of edema models including those induced by carrageenan, serotonin, nystatin, and formaldehyde-induced arthritis. In these studies, the authors also demonstrated that creatinine was effective at reducing edema in these models. While these studies [18,19] were purely descriptive in nature, they served as the impetus to further examine the immune-modulating properties of creatine and its alleged biologically inert breakdown product – creatinine.
4.2. Effect of creatine on the airways
In a study by Vieira et al. (2007), the authors used a mouse model of airway inflammation and remodeling to examine the impact of aerobic exercise on airway health [21]. Mice were sensitized to ovalbumin (OVA) and were exercised on a treadmill at either low or moderate intensity and showed reduced levels of eosinophils in their broncheoalveolar lavage fluids compared with non-exercisers. Additionally, reduced levels of IL-4 and IL-5, as well as decreased thickening of the airway walls were observed in the exercised animals as compared with the non-exercised animals. These findings reflect reports in humans that showed exercise improved cardiorespiratory fitness in individuals with asthma [204], and that individuals who trained at moderate intensity were more likely to demonstrate improvements in their asthma symptoms than those who exercised at a low intensity [205–210]. Based upon these data, it seems reasonable to suggest that athletes would have a lower incidence of asthma compared to the general public; however, in reality, the opposite may be true [211–214]. While there could be several factors to explain this, strenuous exercise increases the levels of T helper 2 (Th2) cytokines [215–218] along with other factors that also impact airway remodeling. While the factors that impact the severity of asthma are not completely defined, the intensity of exercise appears to be one factor, thus the casual exerciser may experience improvements in their asthma, while elite athletes may have a deterioration of their condition.
A related study focused on the impact of creatine supplementation on allergic inflammation and airway remodeling in a mouse model of allergic asthma [24]. This investigation was based upon the observation that 1) the rate of asthma in athletes is higher than that observed in the general population; and 2) athletes are more likely to use creatine supplementation than the general population. The impact of creatine supplementation on female BALB/c mice with OVA-induced airway hyper-responsiveness was examined [24]. Under normal circumstances (that is, in the absence of creatine treatment), this particular model of airway hyper-responsiveness was characterized by a strong Th2 response in mice with high levels of IL-4, IL-5, and insulin-like growth factor-1 (IGF-1) [219]. The mice also developed thickened airway smooth muscles and collagen and elastic fiber deposition along with eosinophil infiltration of the airways. After creatine monohydrate treatment of the OVA-sensitized animals, mice experienced increased levels of IL-4, IL-5, IGF-1, eosinophil infiltration of the airways, increased smooth muscle thickness, and increased levels of collagen and elastic fibers in the lungs. Interestingly, the control group that was not OVA sensitized, but received creatine also experienced significant alterations in airway physiology and immune responses consistent with allergic inflammation [24], demonstrating that creatine, in and of itself, may be a risk factor for the development of asthma. The data also demonstrated a skewing of the immune response towards a more profound Th2-like response. As a follow-up to these studies, the impact of exercise on the creatine-induced changes in OVA-sensitized mice was examined [220]. The data demonstrated that exercise can reduce the effects of creatine in the model, since mice that completed a low intensity exercise regimen showed reduced numbers of eosinophils, numbers of IL-5 and IL-4 positive cells, and other pathological changes in the lungs indicative of airway hypo-responsiveness compared to animals that did not exercise [220]. Collectively, the data leads to the intriguing possibility that individuals with Th2-mediated diseases (i.e., allergy, asthma, lupus) could exacerbate their disease if they supplement their diet with creatine monohydrate. The results of these studies also lead to the question of whether creatine has the potential to alleviate Th1-mediated immunopathology.
The ability of creatine to impact various respiratory disease states is not limited to its effects on cells of the immune system. Ferreira et al. (2010) examined the effects of creatine supplementation on the airway epithelium in the mouse model of OVA-induced allergic asthma [221]. Creatine supplementation of the OVA-sensitized mice had decreased NF-κB activation in epithelial cells compared to the level observed in the non-creatine supplemented, OVA-sensitized mice [221]. Since this transcription factor acts as a master regulator for the immune response [222–224], this alteration of NF-κB activation resulted in increased expression of IL-5, CCL5, CCL2, TIMP-1, matrix metalloproteinase-9 (MMP-9), transforming growth factor-β1 (TGF–β1), IGF-1, EGFR, TIMP-2, and MMP-12 in epithelial cells compared to non-creatine supplemented OVA-sensitized mice. Because of these widespread alterations in immune mediators, the authors examined the levels of NF-κB in the epithelial cells of mice [221]. These mediators are associated not only with inflammation, but also with airway remodeling. The chemokine CCL-5 is involved in recruitment of eosinophils, a key cell type in asthma, while CCL2 recruits dendritic cells, T cells, and monocytes to the site. Together, these data provide insight into why some athletes may have a higher than normal rate of asthma than the general population, and therefore, should be cautioned before beginning a creatine supplementation program.
4.3. Impact of creatine on the innate immune system
Creatine monohydrate and creatine ethyl ester also appear to exhibit immunomodulatory potential. Leland and colleagues (2011) demonstrated that both forms of creatine down regulate expression of TLR-2, TLR-3, TLR-4, and TLR-7 in RAW 264.7 cells, a mouse macrophage cell line [20]. These TLRs interact with constituents of a wide array of pathogen-associated molecular patterns (PAMPs) including lipoteichoic acid (TLR-2), double-stranded RNA (TLR-3), bacterial lipopolysaccharide (TLR-4), and single-stranded RNA (TLR-7) [225]. As TLR down-regulation in the host should reduce the effectiveness of the innate immune response in sensing infection [226], this could result in an immunosuppressive impact and, thus, could be significant in individuals who have compromised immune systems, such as the elderly. The down regulation of TLRs by creatine may also be a mechanism by which creatine could alter PD pathogenesis, as it has been shown that α-synuclein upregulates several TLRs (TLRs-1, 2, 3, 4, and 7), as well as MyD88, a key signaling model involved in TLR activation [227]. In addition, TLR-4 antagonists have been shown to inhibit neuronal death in vitro in an ALS model [228].
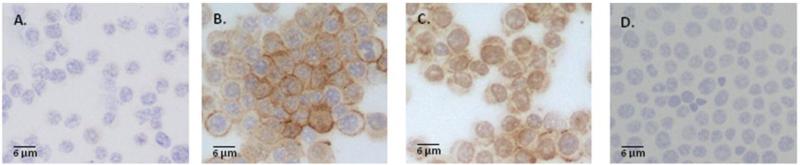
An investigation into the anti-inflammatory effects of creatine on pulmonary epithelial cells in vitro was performed to determine whether creatine altered intercellular adhesion molecule-1 (ICAM-1) and E-selectin expression on the cells [17]. Human pulmonary cells exposed to 0.05 mM creatine had levels of ICAM-1 and E-selectin expression on their surface similar to untreated control cells. In response to the stimulus TNF-α [17], a cytokine that is induced early in an immune response [229], high levels of ICAM-1 and E-selectin were observed [17]. As the levels of creatine increased, the levels of ICAM-1 and E-selectin decreased to control levels at a concentration of 5 mM creatine [17]. In addition to altered surface molecule expression, the study also found that there was a decrease in endothelial permeability and the adhesion of neutrophils to the endothelial cells [17], suggesting that neutrophil extravasation may be affected by creatine use, as adhesion is a key step in this process [230]. These findings would suggest that the immune response may be dampened in the presence of high levels of creatine, despite the presence of pro-inflammatory processes (that is, the production of TNF-α). This effect may be cell type specific, as data from our laboratory demonstrated that when mouse macrophages (RAW 264.7) were incubated with 10 mM creatine monohydrate there was an upregulation of ICAM-1 on the cells after 24 or 48 hours (Figure 2A-D). However, it is important to note that our studies did not expose the macrophages to TNF-α after creatine incubation. Given that ICAM-1 is an integral molecule for antigen presentation, alterations in levels of this protein on antigen presenting cells may indicate that there are reduced or enhanced ability of cells to present antigen to T cells in the presence of creatine.
Figure 2. ICAM-1 expression is upregulated in mouse macrophages following exposure to 10 mM creatine monohydrate.
Immunohistochemical staining revealed an increase in ICAM-1 signal after incubation with 10 mM creatine monohydrate for 24 (B) or 48 (C) hours. Control (A) cells were incubated with culture media alone. Non-specific staining controls received rat IgG (isotype control) in lieu of primary antibody (D). Immunohistochemical staining for ICAM-1 was performed using the Avidin Biotin Complex (ABC) method (Vector Laboratories) as previously described [238] using an antibody to mouse ICAM-1 (BD Pharmingen). Development was performed using the AEC Substrate Kit (Vector Laboratories). Positive staining is indicated by the brown reaction product.
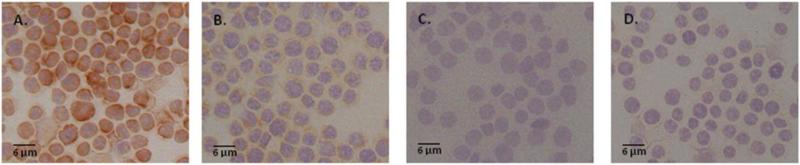
Because cytokines play a key role in both enhancing and downregulating immune responses, we examined whether creatine pretreatment of mouse macrophages altered production of IL-6, a pro-inflammatory mediator that has a key role in the production of acute phase proteins. As shown in Figure 3A-D, reduced immunoreactivity was observed following incubation of macrophages with 10 mM creatine for 24 or 48 hours compared to control-treated cells. The reduction production in this cytokine could be interpreted to that the initial phases of the immune response could be dampened in individuals using creatine. The mechanism by which creatine was able to downregulate receptors, adhesion molecules, and pro-inflammatory cytokines in certain cell types while having the alternative occurring in asthma-induced models is not yet understood. However, it was hypothesized that creatine affected the NF-κB signaling pathway [20,221,231] causing the production of cytokines, receptors, and growth factors to be increased or decreased. Down regulation of pro-inflammatory cytokines by creatine may also contribute to some the neuroprotective effects that have been observed, as inflammation is a common feature of many CNS diseases, including ALS [232] and PD [233,234].
Figure 3. IL-6 expression is downregulated by mouse macrophages following exposure to 10 mM creatine monohydrate.
Immunohistochemical staining demonstrated a decrease in IL-6 expression following incubation with 10 mM creatine monohydrate for 24 (B) or 48 (C) hours. Control (A) cells were incubated with media alone. Non-specific staining controls received rat IgG (isotype control) in lieu of primary antibody (D). Immunohistochemical staining for IL-6 was performed using the Avidin Biotin Complex (ABC) method (Vector Laboratories) as previously described [238] using an antibody to mouse IL-6 (BD Pharmingen). Development was performed using the AEC Substrate Kit (Vector Laboratories). Positive staining is indicated by the brown reaction product.
4.4. Antioxidant properties of creatine
Since oxidants have the ability to negatively impact muscle fatigue and growth, Lawler et al. (2002) [235] examined whether creatine had direct antioxidant activity in vitro. To test this possibility, the effect of creatine on five different ROS was examined in a cell-free system. The ROS studied were hydrogen peroxide, 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) cation radical, peroxynitrate, lipid peroxides (tert-butyl hydroperoxide) and superoxide anions. The findings showed that 40 mM creatine significantly (p<0.05) reduced levels of ABTS+, superoxide anions, and peroxynitrate, but not hydrogen peroxide and lipid peroxides [235].
In a related study [236] that used a lower level of creatine (0.1-10 mM) to examine the antioxidant activity of creatine on in vitro cultured mouse and human cells after oxidative injury, it was found that when human promonocyte cells (U937 cells) were incubated with creatine for 2 hours and then treated with hydrogen peroxide, tert-butyl hydroperoxide, or peroxynitrate, the creatine-treated U937 had increased viability when compared to the control treated cells [236]. Additional cell lines (C2C12 [murine myoblasts]; HUVEC [human endothelial]) were also tested in a similar manner. Creatine reduced cell death following exposure to both hydrogen peroxide and tert-butyl hydroperoxide [236]. However, unlike the U937 cells, C2C12 and HUVEC cells required extended preincubation with creatine (24 hours) to provide maximal cell survival, indicating that all cell types do not respond similarly [236].
To determine whether creatine could protect DNA from ROS-mediated damage, Guidi et al. [237] used a system that generated superoxide anions, hydroxyl radicals, and hydrogen peroxide in the presence of mitochondrial and nuclear DNA. Using a PCR-based assay to identify the level of DNA damage, the authors found that there were increased levels of protection of the DNA in creatine-treated, hydrogen peroxide exposed mitochondrial DNA compared to control-treated, hydrogen peroxide exposed DNA. The levels of protection that creatine afforded approached the levels observed in control DNA that was not exposed to ROS [237]. Further studies utilizing HUVEC cells that were pretreated with creatine for 24 hours then exposed to hydrogen peroxide demonstrated that creatine treated cells had increased viability compared to control cells, and that creatine pretreatment also protected mitochondrial DNA from significant damage compared to the control cells [237]. In addition, the nuclear DNA experienced less damage than the mitochondrial DNA in both control and creatine-treated cells, however, the differences between treatments were not statistically significant. In total, these studies [235–237] suggest that creatine has direct antioxidant activity and that both cell viability and the levels of DNA damage are positively affected by exposure to creatine.
5. Conclusions
Creatine has been used by athletes as an ergogenic aid since the 1970s. Herein, we describe potential uses for creatine in the clinical setting. Of note, creatine is extremely inexpensive compared to most neuroprotective agents and immunomodulatory drugs. It is critical that future studies in humans examining the neuroprotective properties of creatine are designed to ensure that participants are ingesting doses that parallel the doses used in rodent studies. Furthermore, it is of great interest to continue to explore the potential of this ergogenic supplement in animal models of disease to better understand the mechanism(s) of action of creatine in vivo. Given creatine's impact on the immune system studies in models of autoimmune disease may be particularly applicable.
Highlights.
Creatine increases airway hyperresponsiveness in mice.
Soluble mediator production, such as IL-4, IL-5, and IL-6, is altered by creatine.
ICAM-1, E-selectin, and TLR expression are impacted by creatine exposure.
Creatine has neuroprotective and antioxidative effects in mice.
Inadequate creatine dose may explain clinical trial failure in humans.
Acknowledgements
The authors are grateful to Luciano Trevor Holzmer and Sara Pitz for their expertise and assistance in generating the graphical abstract.
This work was performed in a facility supported by Grant Number 1 C06 RR17417-01 from the National Center for Research Resources (KMD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bemben MG, Witten MS, Carter JM, Eliot KA, Knehans AW, Bemben DA. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J Nutr Health Aging. 2010;14:155–159. doi: 10.1007/s12603-009-0124-8. [DOI] [PubMed] [Google Scholar]
- 2.Walker JB. Creatine: biosynthesis, regulation, and function. Adv Enzymol. 1979;50:177–252. doi: 10.1002/9780470122952.ch4. [DOI] [PubMed] [Google Scholar]
- 3.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 4.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 5.Balsom PD, Söderlund K, Ekbolm B. Creatine in humans with special reference to creatine supplementation. Sports Med. 1994;18:268–280. doi: 10.2165/00007256-199418040-00005. [DOI] [PubMed] [Google Scholar]
- 6.Delanghe J, DeSlypere JP, DeBuyzere M, Robbrecht J, Wieme R, Vermeulen A. Normal reference values for creatine, creatinine, and carnitine are lower in vegetarians. Clin Chem. 1989;35:1802–1803. [PubMed] [Google Scholar]
- 7.Shomrat A, Weinstein Y, Katz A. Effect of creatine feeding on maximal exercise performance in vegetarians. Eur J Appl Physiol. 2000;82:321–325. doi: 10.1007/s004210000222. [DOI] [PubMed] [Google Scholar]
- 8.Snow RJ, Murphy RM. Creatine and the creatine transporter: a review. Mol Cell Biochem. 2001;224:169–181. doi: 10.1023/a:1011908606819. [DOI] [PubMed] [Google Scholar]
- 9.Speer O, Neukomm LJ, Murphy RM, Zanolla E, Schlattner U, Henry H, Snow RJ, Wallimann T. Creatine transporters: a reappraisal. Mol Cell Biochem. 2004;256-257:407–424. doi: 10.1023/b:mcbi.0000009886.98508.e7. [DOI] [PubMed] [Google Scholar]
- 10.Greenhaff P. The nutritional biochemistry of creatine. J Nutr Biochem. 1997;8:610–618. [Google Scholar]
- 11.Braissant O, Henry H, Loup M, Eilers B, Bachmann C. Endogenous synthesis and transport of creatine in the rat brain: An in situ hybridization study. Mol Brain Res. 2001;86:193–201. doi: 10.1016/s0169-328x(00)00269-2. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan PG, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against traumatic brain injury. Ann Neurol. 2000;48:723–729. [PubMed] [Google Scholar]
- 13.Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, Mueller G, Wermer M, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nature Medicine. 1999;5:347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- 15.Zhu S, Li M, Figueroa BE, Liu A, Stavrovskaya IG, Pasinelli P, Beal MF, Brown RH, Kristal BS, Ferrante RJ, Friedlander RM. Prophylactic creatine administration mediates neuroprotection in cerebral ischemia in mice. J Neurosci. 2004;24:5909–5912. doi: 10.1523/JNEUROSCI.1278-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, Kaddurah-Daouk R, Beal MF. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol. 1999;157:142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- 17.Nomura A, Zhang M, Sakamoto T, Ishii Y, Morishima Y, Mochizuki M, Kimura T, Uchida Y, Sekizawa K. Anti-inflammatory activity of creatine supplementation in endothelial cells in vitro. Br J Pharm. 2003;139:715–720. doi: 10.1038/sj.bjp.0705316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madan BR, Khanna NK. Effect of creatinine on various experimentally induced inflammatory models. Indian J Physiol Pharmacol. 1979;23:1–7. [PubMed] [Google Scholar]
- 19.Khanna NK, Madan BR. Studies on the anti-inflammatory activity of creatine. Arch Int Pharmacodyn Ther. 1978;231:340–350. [PubMed] [Google Scholar]
- 20.Leland KM, McDonald TL, Drescher KM. Effect of creatine, creatinine, and creatine ethyl ester on TLR expression in macrophages. Int Immunopharm. 2011;11:1341–1347. doi: 10.1016/j.intimp.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vieira RP, Claudino RC, Duarte ACS, Santos ABG, Perini A, Faria Neto HCC, Mauad T, Martins MA, Dolhnikoff M, Carvalho CRF. Aerobic exercise decreases chronic allergic lung inflammation and airway remodeling in mice. Am J Respir Crit Care Med. 2007;176:871–877. doi: 10.1164/rccm.200610-1567OC. [DOI] [PubMed] [Google Scholar]
- 22.Bassit RA, Curi R, Costa Rosa LF. Creatine supplementation reduces plasma levels of pro-inflammatory cytokines and PGE2 after a half-ironman competition. Amino Acids. 2008;35:425–431. doi: 10.1007/s00726-007-0582-4. [DOI] [PubMed] [Google Scholar]
- 23.Taes YE, Marescau B, DeVriese A, DeDeyn PP, Schepers E, Vanholder R, Delanghe JR. Guanidino compounds after creatine supplementation in renal failure patients and their relation to inflammatory status. Nephrol Dial Transplant. 2008;23:1330–1335. doi: 10.1093/ndt/gfm793. [DOI] [PubMed] [Google Scholar]
- 24.Vieira RP, Duarte ACS, Claudino RC, Perini A, Santos ABG, Moriya HT, Arantes-Costa FM, Martins MA, Carvalho CRF, Dolhnikoff M. Creatine supplementation exacerbates allergic lung inflammation and airway remodeling in mice. Am J Respir Cell Mol Biol. 2007;37:660–667. doi: 10.1165/rcmb.2007-0108OC. [DOI] [PubMed] [Google Scholar]
- 25.Azeredo R, Pérez-Sánchex J, Sitjà-Bobadilla A, Fouz B, Tort L, Aragão C, Oliva-Teles A, Costas B. European sea bass (Dicentrarchus labrax immune status and disease resistance are impaired by arfinine dietary supplementation. Plos One. 2015;10:1371. doi: 10.1371/journal.pone.0139967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bemben MG, Lamont HS. Creatine supplementation and exercise performance: recent findings. Sports Med. 2005;35:107–125. doi: 10.2165/00007256-200535020-00002. [DOI] [PubMed] [Google Scholar]
- 27.Gastin PB. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001;31:725–741. doi: 10.2165/00007256-200131100-00003. [DOI] [PubMed] [Google Scholar]
- 28.Field ML. Creatine supplementation in congestive heart failure. Cardiovasc Res. 1996;31:174–175. [PubMed] [Google Scholar]
- 29.Saks VA, Belikova YO, Kuznetsov AV, Khuchua ZA, Branishte TH, Semenovsky ML, Naumov VG. Phosphocreatine pathway for energy transport: ADP diffusion and cardiomyopathy. Am J Physiol. 1991;261:30–38. doi: 10.1152/ajplung.1991.261.4.L30. [DOI] [PubMed] [Google Scholar]
- 30.Kushmerick MJ, Moerland TS, Wiseman RW. Mammalian skeletal muscle fibers distinguished by contents of phosphocreatine, ATP, and Pi. Proc Nat Acad Sci USA. 1992;89:7521–7525. doi: 10.1073/pnas.89.16.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosco C, Tihanyi J, Pucspk J, Kovacs I, Gabossy A, Colli R, Pulvirenti G, Tranquilli C, Foti C, Viru M, Viru A. Effect of oral creatine supplementation on jumping and running performance. Int J Sports Med. 1997;18:369–372. doi: 10.1055/s-2007-972648. [DOI] [PubMed] [Google Scholar]
- 32.Dawson B, Cutler M, Moody A, Lawrence S, Goodman C, Randall N. Effects of oral creatine loading on single and repeated maximal short sprints. Aust J Sci Med Sport. 1995;27:56–61. [PubMed] [Google Scholar]
- 33.Volek JS, Duncan ND, Mazzetti SA, Staron RS, Putukian M, Gómez AL, Pearson DR, Fink WJ, Kraemer WJ. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med Sci Sports Exerc. 1999;31:1147–1156. doi: 10.1097/00005768-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Kreider RB. Effects of creatine supplementation on performance and training adaptations. Mol Cell Biochem. 2003;244:89–94. [PubMed] [Google Scholar]
- 35.Karimian J, Esfahani PS. Supplement consumption in body builder athletes. J Res Med Sci. 2011;16:1347–1353. [PMC free article] [PubMed] [Google Scholar]
- 36.Terjung RL, Clarkson PM, Eichner ER, Greenhaff PL, Hespel PJ, Israel RG, Kraemer WJ, Meyer RA, Spriet LL, Tarnopolsky MA, Wagenmakers AJ, Williams MH. American college of sports medicine roundtable. The physiological and health effects of oral creatine supplementation. Med Sci Sports Exerc. 2000;32:706–717. doi: 10.1097/00005768-200003000-00024. [DOI] [PubMed] [Google Scholar]
- 37.Mihic S, MacDonald JR, McKenzie S, Tarnopolsky MA. Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Med Sci Sports Exerc. 2000;32:291–296. doi: 10.1097/00005768-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Grindstaff PD, Kreider R, Bishop R, Wilson M, Wood L, Alexander C, Almada A. Effects of oral creatine supplementation on repetitive spring performance and body composition in competitive swimmers. Int J Sport Nutr. 1997;7:330–346. doi: 10.1123/ijsn.7.4.330. [DOI] [PubMed] [Google Scholar]
- 39.Volek JS, Kraemer WJ. Creatine supplementation: its effect on human muscular performance and body composition. J Strength Cond Res. 1996;10:200–210. [Google Scholar]
- 40.Burns RD, Schiller MR, Merrick MA, Wolf KN. Intercollegiate student athlete use of nutritional supplements and the role of athletic trainers and dietitians in nutrition counseling. J Amer Diet Assoc. 2004;104:246–249. doi: 10.1016/j.jada.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Calfee R, Fadale P. Popular ergogenic drugs and supplements in young athletes. Pediatrics. 2006;117:577–589. doi: 10.1542/peds.2005-1429. [DOI] [PubMed] [Google Scholar]
- 42.Metzl JD, Small E, Levine SR, Gershel JC. Creatine use among young athletes. Pediatrics. 2001;108:421–425. doi: 10.1542/peds.108.2.421. [DOI] [PubMed] [Google Scholar]
- 43.Bamberger M. The magic potion. Sports Illus. 1998;88:58–61. [Google Scholar]
- 44.Poortmans JR, Francaux M. Adverse effects of creatine supplementation: Fact or fiction? Sports Med. 2000;30:155–170. doi: 10.2165/00007256-200030030-00002. [DOI] [PubMed] [Google Scholar]
- 45.Pearlman JP, Fielding RA. Creatine monohydrate as a therapeutic aid in muscular dystrophy. Nutr Rev. 2006;64:80–88. doi: 10.1301/nr.2006.feb.80-88. [DOI] [PubMed] [Google Scholar]
- 46.McGuine TA, Sullivan JC, Bernhardt DA. Creatine supplementation in Wisconsin high school athletes. Wisc Med J. 2002;101:25–30. [PubMed] [Google Scholar]
- 47.Mason MA, Giza M, Clayton L, Lonning J, Wilkerson RD. Use of nutritional supplements by high school football and volleyball players. Iowa Orthop J. 2001;21:43–48. [PMC free article] [PubMed] [Google Scholar]
- 48.McGuine TA, Sullivan JC, Bernhardt DT. Creatine supplementation in high school football players. Clin J Sports Med. 2001;11:247–253. doi: 10.1097/00042752-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Smith J, Dahm DL. Creatine use among a select population of high school athletes. Mayo Clin Proc. 2000;75:1257–1263. doi: 10.4065/75.12.1257. [DOI] [PubMed] [Google Scholar]
- 50.Neves M, Jr, Gualano B, Roschel H, Lima FR, Lúcia de Sá-Pinto A, Seguro AC, Shimizu MH, Sapienza MT, Fuller R, Lancha AH, Jr, Bonfá E. Effect of creatine supplementation on measured glomerular filtration in postmenopausal women. Appl Physiol Nutr Metab. 2011;36:419–422. doi: 10.1139/h11-014. [DOI] [PubMed] [Google Scholar]
- 51.Rawson ES, Venezia AC. Use of creatine in the elderly and evidence for effects on cognitive function in young and old. Amino Acids. 2011;40:1349–1362. doi: 10.1007/s00726-011-0855-9. [DOI] [PubMed] [Google Scholar]
- 52.Gufford BT, Sriraghavan K, Miller NJ, Miller DW, Gu X, Vennerstrom JL, Robinson DH. Physicochemical characterization of creatine N-methylguanidinium salts. J Diet Suppl. 2010;7:240–252. doi: 10.3109/19390211.2010.491507. [DOI] [PubMed] [Google Scholar]
- 53.Gufford B, Ezell E, Robsinson D, Miller D, Miller N, Gu X, Vennerstrom J. pH-dependent stability of creatine ethyl ester: relevance to oral absorption. J Diet Suppl. 2013;10:241–251. doi: 10.3109/19390211.2013.822453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peral MJ, García-Delgado M, Calonge ML, Durán JM, De La Horra MC, Wallimann T, Speer O, Ilundáin AA. Human, rat, and chicken small intestinal Na+/Cl−/creatine transporter: functional, molecular characterization, and localization. J Physiol. 2002;545:133–144. doi: 10.1113/jphysiol.2002.026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.García-Delgado M, Peral MJ, Cano M, Calonge ML, Ilundáin AA. Creatine transport in brush-border membrane vesicles isolated from rat kidney cortex. J Am Soc Nephrol. 2001;12:1819–1825. doi: 10.1681/ASN.V1291819. [DOI] [PubMed] [Google Scholar]
- 56.Guimbal C, Kilimann MW. A Na+-dependent creatine transporter in rabbit brain, muscle, heart and kidney. cDNA cloning and functional expression. J Biol Chem. 1993;268:8418–8421. [PubMed] [Google Scholar]
- 57.Orsenigo MN, Faelli A, De Biasi S, Sironi C, Laforenza U, Paulmichl M, Tosco M. Jejunal creatine absorption: what is the role of the basolateral membrane? J Membrane Biol. 2005;207:183–195. doi: 10.1007/s00232-005-0813-0. [DOI] [PubMed] [Google Scholar]
- 58.Valayannopoulos V, Boddaert N, Chabli A, Barbier V, Desguerre I, Philippe A, Afenjar A, Mazzuca M, Cheillan D, Munnich A, de Keyzer Y, Jakobs C, Salomons GS, de Lonlay P. Treatment by oral creatine, L-arginine and L-glycine in six severely affected patients with creatine transporter defect. J Inherit Metab Dis. 2012;35:151–157. doi: 10.1007/s10545-011-9358-9. [DOI] [PubMed] [Google Scholar]
- 59.Kreider RB. Creatine supplementation in exercise and sport. In: Driskell J, Wolinsky J, editors. Energy-yielding macronutrients and energy metabolism in sports nutrition. CRC Press LLC; Boca Raton, FL: 1999. pp. 213–242. [Google Scholar]
- 60.Kreider R. Creatine supplementation: analysis of ergogenic value, medical safety, and concerns. J Exerc Physiol Online. 1998;1:7–18. [Google Scholar]
- 61.Juhn MS, Tarnopolsky M. Potential side effects of oral creatine supplementation: A critical review. Clin J Sports Med. 1998;8:298–304. doi: 10.1097/00042752-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Harris RC, Söderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci. 1992;83:367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- 63.Hultman E, Söderlund K, Timmons JA, Cederblad G, Greenhaff PL. Muscle creatine loading in men. J Appl Physiol. 1996;81:232–237. doi: 10.1152/jappl.1996.81.1.232. [DOI] [PubMed] [Google Scholar]
- 64.Burke DG, Candow DG, Chilibeck PD, MacNeil L, Roy BD, Tarnopolsky M, Ziegenfuss T. Effect of creatine supplementation and resistance-exercise training on muscle insulin-like growth factor in young adults. Int J Sport Nutr Exerc Metab. 2008;18:389–398. doi: 10.1123/ijsnem.18.4.389. [DOI] [PubMed] [Google Scholar]
- 65.Demant TW, Rhodes FC. Effects of creatine supplementation on exercise performance. Sports Med. 1999;28:49–60. doi: 10.2165/00007256-199928010-00005. [DOI] [PubMed] [Google Scholar]
- 66.Lefavi RG, Mcmillan JL, Kahn PJ, Crosby JF, DiGioacchino RF, Streater JA. Effects of creatine monohydrate on performance of collegiate baseball and basketball players. J Strength Cond Res. 1998;12:275–279. [Google Scholar]
- 67.Schulze A. Creatine deficiency syndromes. Mol Cell Biochem. 2003;244:143–150. [PubMed] [Google Scholar]
- 68.Stöckler-Ipsiroglu S, Mercimek-Mahmutoglu S, Salomons GS. Creatine deficiency syndromes. In: Saudubray M, van d Berghe G, Walter JH, editors. Inborn metabolic diseases. Diagnosis and treatment. Springer; New York City: 2012. pp. 240–247. [Google Scholar]
- 69.Nasrallah F, Feki M, Kaabachi N. Creatine and creatine deficiency syndromes: biochemical and clinical aspects. Pediatr Neurol. 2010;42:163–171. doi: 10.1016/j.pediatrneurol.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 70.Sakellaris G, Kotsiou M, Tamiolaki M, Kalostos G, Tsapaki E, Spanaki M, Spilioti M, Charissis G, Evangeliou A. Prevention of complications related to traumatic brain injury in children and adolescents with creatine administration: an open label randomized pilot study. J Trauma. 2006;61:322–329. doi: 10.1097/01.ta.0000230269.46108.d5. [DOI] [PubMed] [Google Scholar]
- 71.Sakellaris G, Nasis G, Kotsiou M, Tamiolaki M, Charissis G, Evangeliou A. Prevention of traumatic headache, dizziness and fatigue with creatine administration.A pilot study. Acta Paediatr. 2008;97:31–34. doi: 10.1111/j.1651-2227.2007.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryu H, Rosas HD, Hersch SM, Ferrante RJ. The therapeutic role of creatine in Huntington's disease. Pharmacol Ther. 2005;108:193–207. doi: 10.1016/j.pharmthera.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 73.Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, Friedlich A, Browne SE, Schilling G, Borchelt DR, Hersch SM, Ross CA, Beal MF. Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington's disease. Neurobiol Dis. 2001;8:479–491. doi: 10.1006/nbdi.2001.0406. [DOI] [PubMed] [Google Scholar]
- 74.Dedeoglu A, Kubilus JK, Yang L, Ferrante KL, Hersch SM, Beal MF, Ferrante RJ. Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington's disease transgenic mice. J Neurochem. 2003;85:1359–1367. doi: 10.1046/j.1471-4159.2003.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tabrizi SJ, Blamire AM, Manners DN, Rajagopalan B, Styles P, Schapira AHV, Warner TT. Creatine therapy for Huntington's disease: clinical and MRS findings in a 1-year pilot study. Neurology. 2003;61:141–142. doi: 10.1212/01.wnl.0000070186.97463.a7. [DOI] [PubMed] [Google Scholar]
- 76.Verbessem P, Lemiere J, Eijnde BO, Swinnen S, Vanhees L, Van Leemputte M, Hespel P, Dom R. Creatine supplementation in Huntington's disease: a placebo-controlled pilot trial. Neurology. 2003;61:925–930. doi: 10.1212/01.wnl.0000090629.40891.4b. [DOI] [PubMed] [Google Scholar]
- 77.Andreassen OA, Jenkins BG, Dedeoglu A, Ferrante KL, Bogdanov MB, Kaddurah-Daouk R, Beal MF. Increases in cortical glutamate concentrations in transgenic amyotrophic lateral sclerosis mice are attenuated by creatine supplementation. J Neurochem. 2001;77:383–390. doi: 10.1046/j.1471-4159.2001.00188.x. [DOI] [PubMed] [Google Scholar]
- 78.Groeneveld GJ, Veldink JH, van der Tweel I, Kalmijn S, Beijer C, de Visser M, Wokke JH, Franssen H, van den Berg LH. A randomized sequential trial of creatine in amyotrophic lateral sclerosis. Ann Neurol. 2003;53:437–445. doi: 10.1002/ana.10554. [DOI] [PubMed] [Google Scholar]
- 79.Rosenfeld J, King RM, Jackson CE, Bedlack RS, Barohn RJ, Dick A, Phillips LH, Chapin J, Gelinas DF, Lou JS. Creatine monohydrate in ALS: effects on strength, fatigue, respiratory status and ALSFRS. Amyotroph Lateral Scler. 2008;9:266–272. doi: 10.1080/17482960802028890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shefner JM, Cudkowicz ME, Schoenfeld D, Conrad T, Taft J, Chilton M, Urbinelli L, Qureshi M, Zhang H, Pestronk A, Caress J, Donofrio P, Sorenson E, Bradley W, Lomen-Hoerth C, Pioro E, Rezania K, Ross M, Pascuzzi R, Heiman-Patterson T, Tandan R, Mitsumoto H, Rothstein J, Smith-Palmer T, MacDonald D, Burke D, NEALS Consortium A clinical trial of creatine in ALS. Neurol. 2004;63:1656–1661. doi: 10.1212/01.wnl.0000142992.81995.f0. [DOI] [PubMed] [Google Scholar]
- 81.Allah Yar R, Akbar A, Iqbal F. Creatine monohydrate supplementation for 10 weeks mediates neuroprotection and improves learning/memory following neonatal hypoxia ischemia encephalopathy in female albino mice. Brain Res. 2015;1595:92–100. doi: 10.1016/j.brainres.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 82.Writing group for the NINDS exploratory trials in Parkinson disease (NET-PD) investigators Effect of creatine monohydrate on clinical progression in patients with Parkinson disease. JAMA. 2015;313:584–593. doi: 10.1001/jama.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simon DK, Wu C, Tilley BC, Wills A-M, Aminoff MJ, Bainbridge J, Hauser RA, Schneider JS, Sharma S, Singer C, Tanner CM, Truong D, Wong PS. Caffeine and progression of Parkinson disease: a deleterious interaction with creatine. Clin Pharmacol. 2015;38:163–169. doi: 10.1097/WNF.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elm JJ, NINDS NET-PD Investigators Design innovations and baseline findings in a long-term Parkinson's trial: the National Institute of Neurological Disorders and Stroke exploratory trials in Parkinson's disease long-term study-1. Mov Disord. 2012;27:1513–1521. doi: 10.1002/mds.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parashos SA, Luo S, Biglan KM, Bodis-Wollner I, He B, Liang GS, Ross GW, Tilley BC, Shulman LM, NET-PD investigators Measuring disease progression in early Parkinson disease: The National Institutes of Health exploratory trials in Parkinson disease (NET-PD) experience. JAMA Neurol. 2014;71:710–716. doi: 10.1001/jamaneurol.2014.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.NINDS NET-PD Investigators A pilot study clinical trial of creatine and minocycline in early Parkinson disease: 18-month results. Clin Neuropharmacol. 2008;31:141–150. doi: 10.1097/WNF.0b013e3181342f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bender A, Koch W, Elstner M, Schombacher Y, Bender J, Moeschl M, Gekeler F, Müller-Myhsok B, Gasser T, Tatsch K, Klopstock T. Creatine supplementation in Parkinson disease: a placebo-controlled randomized pilot trial. Neurology. 2006;67:1262–1264. doi: 10.1212/01.wnl.0000238518.34389.12. [DOI] [PubMed] [Google Scholar]
- 88.NINDS NET-PD Investigators A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 89.Dolder M, Walzel B, Speer O, Schlattner U, Walliman T. Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J Biol Chem. 2003;278:17760–17766. doi: 10.1074/jbc.M208705200. [DOI] [PubMed] [Google Scholar]
- 90.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Nat Acad Sci USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cassarino DS, Parks JK, Parker WD, Jr, Bennett JP., Jr The parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochem Biophys Acta. 1999;1453:49–62. doi: 10.1016/s0925-4439(98)00083-0. [DOI] [PubMed] [Google Scholar]
- 92.Martin LJ, Semenkow S, Hanaford A, Wong M. Mitochondrial permeability transition pore regulates Parkinson's disease development in mutant α-synuclein transgenic mice. Neurobiol Aging. 2014;35:1132–1152. doi: 10.1016/j.neurobiolaging.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quintanilla RA, Jin YN, von Bernhardi R, Johnson GVW. Mitochondrial permeability transition pore induces mitochondrial injury in Huntington disease. Mol Neurodegen. 2013;8:45. doi: 10.1186/1750-1326-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mazzeo AT, Beat A, Singh A, Bullock MR. The role of mitochondrial transition pore, and its modulation, in traumatic brain injury and delayed neurodegeneration after TBI. Exp Neurol. 2009;218:363–370. doi: 10.1016/j.expneurol.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 95.Veech RL, Valeri CR, VanItallie TB. The mitochondrial permeability transition pore provides a key to the diagnosis and treatment of traumatic brain injury. IUBMB Life. 2012;64:203–207. doi: 10.1002/iub.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin LJ. Mitochondrial pathobiology in ALS. J Bioenerg Biomembr. 2011;43:569–579. doi: 10.1007/s10863-011-9395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martin LJ, Gertz B, Pan Y, Price AC, Molkentin JD, Chang Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp Neurol. 2009;218:333–346. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brustovetsky N, Brustovetsky T, Purl KJ, Capano M, Crompton M, Dubinsky JM. Increased susceptibility of striatal mitochondria to calcium-induced permeability transition. J Neurosci. 2003;23:4858–4867. doi: 10.1523/JNEUROSCI.23-12-04858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochem Biophys Acta. 1998;1366:79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 100.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch Biochem Biophys. 1979;195:468–477. doi: 10.1016/0003-9861(79)90373-4. [DOI] [PubMed] [Google Scholar]
- 101.Beutner G, Rück A, Riede B, Brdiczka D. Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochem Biophys Acta. 1998;1368:7–18. doi: 10.1016/s0005-2736(97)00175-2. [DOI] [PubMed] [Google Scholar]
- 102.Ichas F, Mazat JP. From calcium signaling to cell death: Two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochem Biophys Acta. 1998;1366:33–50. doi: 10.1016/s0005-2728(98)00119-4. [DOI] [PubMed] [Google Scholar]
- 103.Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci. 1996;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.White RJ, Reynolds IJ. Mitochondrial depolarization in glutamate-stimulated neurons: An early signal specific to excitotoxin exposure. J Neurosci. 1996;16:5688–5697. doi: 10.1523/JNEUROSCI.16-18-05688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: A common mechanism in necrosis, apoptosis and autophagy. Biochem Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 106.Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J Neurochem. 2002;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- 107.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 108.Büki A, Okonkwo DO, Wang KK, Povlishock JT. Cytochrome c release and caspase activation in traumatic axonal injury. J Neurosci. 2000;20:2825–2834. doi: 10.1523/JNEUROSCI.20-08-02825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stravrovskaya IG, Kristal BS. The powerhouse takes control of the cell: Is the mitochondrial permeability transition a viable therapeutic target against neuronal dysfunction and death? Free Radic Biol Med. 2005;38:687–697. doi: 10.1016/j.freeradbiomed.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 110.Beal MF. Neuroprotective effects of creatine. Amino Acids. 2011;40:1305–1313. doi: 10.1007/s00726-011-0851-0. [DOI] [PubMed] [Google Scholar]
- 111.McNair ND. Traumatic brain injury. Nurs Clin North Amer. 1999;34:637–659. [PubMed] [Google Scholar]
- 112.Tibbs RE, Jr, Haines DE, Parent AD. The child as a projectile. Anat Rec. 1998;253:167–175. doi: 10.1002/(SICI)1097-0185(199812)253:6<167::AID-AR5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 113.Xiong Y, Gu Q, Peterson PL, Muizelaar JP, Lee CP. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J Neurotrauma. 1997;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]
- 114.Montal M. Mitochondria, glutamate neurotoxicity and the death cascade. Biochem Biophys Acta. 1998;1366:113–126. doi: 10.1016/s0005-2728(98)00124-8. [DOI] [PubMed] [Google Scholar]
- 115.Sullivan PG, Keller JN, Mattson MP, Scheff SW. Traumatic brain injury alters synaptic homeostasis: implications for impaired mitochondrial and transport function. J Neurotrauma. 1998;15:789–798. doi: 10.1089/neu.1998.15.789. [DOI] [PubMed] [Google Scholar]
- 116.Sullivan PG, Thompson MB, Scheff SW. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp Neurol. 1999;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- 117.Young L, Rule GT, Bocchieri RT, Walilko TJ, Burns JM, Ling G. When physics meets biology: low and high-velocity penetration, blunt impact, and blast injuries to the brain. Front Neurol. 2015;6:1–13. doi: 10.3389/fneur.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hersch SM, Ferrante RJ. Neuropathology and pathophysiology of Huntington's disease. In: Watts RL, Koller WC, editors. Movement Disorders: Neurologic Principles and Practice. McGraw-Hill; New York: 1997. pp. 503–518. [Google Scholar]
- 119.Ferger B, Eberhardt O, Teismann P, de Groote C, Schulz JB. Malonate-induced generation of reactive oxygen species in rat striatum depends on dopamine release but not on NMDA receptor activation. J Neurochem. 1999;73:1329–1332. doi: 10.1046/j.1471-4159.1999.0731329.x. [DOI] [PubMed] [Google Scholar]
- 120.Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington's disease. J Neurosci. 1998;18:156–163. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ikonomidou C, Turski L. Neurodegenerative disorders: Clues from glutamate and energy metabolism. Crit Rev Neurobiol. 1996;10:239–263. doi: 10.1615/critrevneurobiol.v10.i2.50. [DOI] [PubMed] [Google Scholar]
- 122.Nony PA, Scallet AC, Rountree RL, Ye X, Binienda Z. 3-nitropropionic acid (3-NPA) produces hypothermia and inhibits histochemical labeling of succinate dehydrogenase (SDH) in rat brain. Metabolic Br Dis. 1999;14:83–94. doi: 10.1023/a:1020753629477. [DOI] [PubMed] [Google Scholar]
- 123.Ming L. Moldy sugarcane poisoning - a case report with a brief review. J Toxicol Clin Toxicol. 1995;33:363–367. doi: 10.3109/15563659509028924. [DOI] [PubMed] [Google Scholar]
- 124.Greene JG, Porter RH, Eller RV, Greenamyre JT. Inhibition of succiniate dehydrogenase by malonic acid produces an ‘excitotoxic’ lesion in rat striatum. J Neurochem. 1993;61:1151–1154. doi: 10.1111/j.1471-4159.1993.tb03634.x. [DOI] [PubMed] [Google Scholar]
- 125.The Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 126.Andrews SE, Goldberg YP, Kremer B, Telenius H, Theilmann J, Adam S, Starr E, Squitieri F, Lin B, Kalchman MA, Graham RK, Hayden MR. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nature Genetics. 1993;4:398–403. doi: 10.1038/ng0893-398. [DOI] [PubMed] [Google Scholar]
- 127.Snell RG, MacMillan JC, Cheadle JP, Fenton I, Lazarou LP, Davies P, MacDonald ME, Gusella JF, Harper PS, Shaw DJ. Relationship between trinucleotide repeat expansion and phenotypic variation in Huntington's disease. Nature Genetics. 1993;4:393–397. doi: 10.1038/ng0893-393. [DOI] [PubMed] [Google Scholar]
- 128.Duyao M, Ambrose C, Myers R, Novelletto A, Persichetti F, Frontali M, Folstein S, Ross C, Franz M, Abbott M, Gray J, Conneally P, Young A, Penney J, Hollingsworth Z, Shoulson I, Lazzarini A, Falek A, Koroshetz W, Sax D, Bird E, Vonsattel J, Bonilla E, Alvir J, Bickham Gonde J, Cha J-H, Dure L, Gomez F, Ramos M, Sanchez-Ramos J, Snodgrass S, deYoung M, Wexler N, Moscowitcz C, Penchaszadeh G, MacFarlane H, Anderson M, Jenkins B, Srinidhi J, Barnes G, Gusella J, MacDonald M. Trinucleotide repeat length instability and age of onset of Huntington's disease. Nature Genetics. 1993;4:387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- 129.Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 130.Bender A, Auer DP, Merl T, Reilmann R, Saemann P, Yassouridis A, Bender J, Weindl A, Dose M, Gasser T, Klopstock T. Creatine supplementation lowers brain glutamate levels in Huntington's disease. J Neurol. 2005;252:36–41. doi: 10.1007/s00415-005-0595-4. [DOI] [PubMed] [Google Scholar]
- 131.Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Br Res Bull. 2008;76:329–343. doi: 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 132.Rosas HD, Doros G, Gevorkian S, Malarick K, Reuter M, Coutu JP, Triggs TD, Wilkens PJ, Matson W, Salat DH, Hersch SM. PRECREST: A phase II prevention and biomarker trial of creatine in at-risk Huntington disease. Neurology. 2014;82:850–857. doi: 10.1212/WNL.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Matsui JT, Vaidya JG, Wassermann D, Kim RE, Magnotta VA, Johnson HJ, Paulsen JS, PREDICT-HD Investigators and Coordinators of the Huntington Study Group Prefrontal cortex white matter tracts in prodromal Huntington disease. Hum Brain Mapp. 2015;36:3717–3732. doi: 10.1002/hbm.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Williams JK, Kim JI, Downing N, Farias S, Harrington DL, Long JD, Mills JA, Paulsen JS, PREDICT-HD Investigators and Coordinators of the Huntington Study Group Everyday cognition in prodromal Huntington disease. J Neuropsychol. 2015;29:255–267. doi: 10.1037/neu0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koenig KA, Lowe MJ, Harrington DL, Lin J, Durgerian S, Mourany L, Paulsen JS, Rao SM, PREDICT-HD Investigators of the Huntington Study Group Functional connectivity of primary motor cortex is dependent on genetic burden in prodromal Huntington disease. Brain Connect. 2014;4:535–546. doi: 10.1089/brain.2014.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tabrizi SJ, Reilmann R, Roos RA, Durr A, Leavitt B, Owen G, Jones R, Johnson H, Craufurd D, Hicks SL, Kennard C, Landwehrmeyer B, Stout JC, Borowsky B, Scahill RI, Frost C, Langbehn DR, RACK-HD Investigators Potential endpoints for clinical trials in premanifest and early Huntington's disease in the TRACK-HD study: analysis of 24 month observational data. Lancet. 2012;11:42–53. doi: 10.1016/S1474-4422(11)70263-0. [DOI] [PubMed] [Google Scholar]
- 137.Mandrioli J, Faglioni P, Merelli E, Sola P. The epidemiology of ALS in Modena, Italy. Neurol. 2003;60:683–689. doi: 10.1212/01.wnl.0000048208.54755.78. [DOI] [PubMed] [Google Scholar]
- 138.Worms PM. The epidemiology of motor neuron diseases: a review of recent studies. J Neurol Sci. 2001;191:3–9. doi: 10.1016/s0022-510x(01)00630-x. [DOI] [PubMed] [Google Scholar]
- 139.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng XT. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 140.Siddique T, Hentati A. Familial amyotrophic lateral sclerosis. Clin Neurosci. 1995;3:338–347. [PubMed] [Google Scholar]
- 141.Li TM, Alberman E, Swash M. Comparison of sporadic and familial disease amongst 580 cases of motor neuron disease. J Neurol Neurosurg Pysch. 1988;51:778–784. doi: 10.1136/jnnp.51.6.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goodall EF, Morrison KE. Amyotrophic lateral sclerosis (motor neuron disease): proposed mechanisms and pathways to treatment. Expert Rev Mol Med. 2006;8:1–22. doi: 10.1017/S1462399406010854. [DOI] [PubMed] [Google Scholar]
- 143.Rowland LP, Schneider NA. Amyotrophic lateral sclerosis. N Eng J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 144.Przedborski S, Mitsumoto H, Rowland LP. Recent advances in amyotrophic lateral sclerosis research. Curr Neurol Neurosci Rep. 2003;3:70–77. doi: 10.1007/s11910-003-0041-x. [DOI] [PubMed] [Google Scholar]
- 145.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 146.Kochanek KD, Xu JQ, Murphy SL, Arias E. Mortality in the United States, 2013. NCHS Data Brief National Center for Health Statistics, Centers for Disease Control and Prevention, US Dept of Health and Human Services. 2014;178:1–8. [Google Scholar]
- 147.Hara H, Friedlander RM, Gagliardini V, Ayata C, Fink K, Huang Z, Shimizu-Sasamata M, Yuan J, Moskowitz MA. Inhibition of interleukin-1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Nat Acad Sci USA. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Friedlander RM, Gagliardini V, Hara H, Fink KB, Li W, MacDonald G, Fishman MC, Greenberg AH, Moskowitz MA, Yuan J. Expression of a dominant negative mutant of interleukin-1 beta converting enzyme in transgenic mice prevents neuronal cell death induced by trophic factor withdrawal and ischemic brain injury. J Exp Med. 1997;185:933–940. doi: 10.1084/jem.185.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hara H, Fink K, Endres M, Friedlander RM, Gagliardini V, Yuan J, Moskowitz MA. Attenuation of transient focal cerebral ischemic injury in transgenic mice expressing a mutant ICE inhibitory protein. J Cereb Blood Flow Metab. 1997;17:370–375. doi: 10.1097/00004647-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 150.Zhang WH, Wang X, Narayanan M, Zhang Y, Huo C, Reed JC, Friedlander RM. Fundamental role of the Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proc Nat Acad Sci USA. 2003;100:16012–16017. doi: 10.1073/pnas.2534856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, Huarte J. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring death and experimental ischemia. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 152.Jing X, Wei X, Ren M, Wang L, Zhang X, Lou H. Neuroprotective Effects of Tanshinone I Against 6-OHDA-Induced Oxidative Stress in Cellular and Mouse Model of Parkinson's Disease Through Upregulating Nrf2. Neurochem Res. 2015 doi: 10.1007/s11064-015-1751-6. E pub ahead of print Nov 4. [DOI] [PubMed] [Google Scholar]
- 153.Chung YC, Kim YS, Bok E, Yune TY, Maeng S, Jin BK. MMP-3 contributes to nigrostriatal dopaminergic neuronal loss, BBB damage, and neuroinflammation in an MPTP mouse model of Parkinson's disease. Mediators Inflamm. 2013;2013:370526. doi: 10.1155/2013/370526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Salama M, Ellaithy A, Helmy B, El-Gamal M, Tantawy D, Mohamed M, Sheashaa H, Sobh M, Arias-Carrión O. Colchicine protects dopaminergic neurons in a rat model of Parkinson's disease. CNS Neurol Disord Drug Targets. 2012;11:836–843. doi: 10.2174/1871527311201070836. [DOI] [PubMed] [Google Scholar]
- 155.Mouatt-Prigent A, Agid Y, Hirsch EC. Does the calcium binding protein calretinin protect dopaminergic neurons against degeneration in Parkinson's disease. Brain Res. 1994;668:62–70. doi: 10.1016/0006-8993(94)90511-8. [DOI] [PubMed] [Google Scholar]
- 156.Gai WP, Vickers JC, Blumbergs PC, Blessing WW. Loss of non-phosphorylated neurofilament immunoreactivity, with preservation of tyrosine hydroxylase, in surviving substantia nigra neurons in Parkinson's disease. J Neurol Neurosurg Pysch. 1994;57:1039–1046. doi: 10.1136/jnnp.57.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guan J, Krishnamurthi R, Waldvogel HJ, Faull RL, Clark R, Gluckman P. N-terminal tripeptide of IGF-1 (GPE) prevents the loss of TH positive neurons after 6-OHDA induced nigral lesion in rats. Brain Res. 2000;859:286–292. doi: 10.1016/s0006-8993(00)01988-0. [DOI] [PubMed] [Google Scholar]
- 158.Tabrez S, Jabir NR, Shakil S, Greig NH, Alam Q, Abuzenadah AM, Damanhouri GA, Kamal MA. A synopsis on the role of tyrosine hydroxylase in Parkinson's Disease. CNS Neurol Disord - Drug Targets. 2012;11:395–409. doi: 10.2174/187152712800792785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.van der Brug MP, Singleton A, Gasser T, Lewis PA. Parkinson's disease: from human genetics to clinical trials. Science Trans Med. 2015;7:205ps20. doi: 10.1126/scitranslmed.aaa8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson's disease in the United States. Mov Disord. 2013;28:311–318. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- 161.Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010;1341C:72–83. doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim SN, Doo AR, Park JY, Bae H, Chae Y, Shim I, Lee H, Moon W, Lee H, Park HJ. Acupuncture enhances the synaptic dopamine availability to improve motor function in a mouse model of Parkinson's disease. Plos One. 2011;6:e27566. doi: 10.1371/journal.pone.0027566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vuckovic MG, Wood RI, Holschneider DP, Abernathy A, Togaski DM, Smith A, Petzinger GM, Jakowec MW. Memory, mood, dopamine, and serotonin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Neurobiol Dis. 2008;32:319–327. doi: 10.1016/j.nbd.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bian M, Liu J, Hong X, Yu M, Huang Y, Sheng Z, Fei J, Huang F. Overexpression of parkin ameliorates dopaminergic neurodegeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Plos One. 2012;7:e39953. doi: 10.1371/journal.pone.0039953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dean ED, Mexas LM, Cápiro NL, McKeon JE, DeLong MR, Pennell KD, Doorn JA, Tangpricha V, Miller GW, Evatt ML. 25-Hydroxyvitamin D depletion does not exacerbate MPTP-induced dopamine neuron damage in mice. Plos One. 2012;7:e39227. doi: 10.1371/journal.pone.0039227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kadoguchi N, Okabe S, Yamamura Y, Shono M, Fukano T, Tanabe A, Yokoyama H, Kasahara J. Mirtazapine has a therapeutic potency in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MTPT)-induced mice model of Parkinson's disease. BMC Neurosci. 2014;15:79. doi: 10.1186/1471-2202-15-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McCormack AL, Mak SK, Shenasa M, Langston WJ, Forno LS, DiMonte DA. Pathological modifications of α-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated squirrel monkeys. J Neuropathol Exp Neurol. 2008;67:793–802. doi: 10.1097/NEN.0b013e318180f0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fifel K, Vezoli J, Dzahini K, Claustrat B, Leviel V, Kennedy H, Procyk E, Dkhissi-Benyahya O, Gronfier C, Cooper HM. Alteration of daily and circadian rhythms following dopamine depletion in MPTP treated non-human primates. Plos One. 2014;9:e86240. doi: 10.1371/journal.pone.0086240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tian L, Xia Y, Flores HP, Campbell MC, Moerlein SM, Perlmutter JS. Neuroimaging analysis of the dopamine basis for apathetic behaviors in an MPTP-lesioned primate model. Plos One. 2015;10:e0132064. doi: 10.1371/journal.pone.0132064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Klivenyi P, Gardian G, Calingasan NY, Yang L, Beal MF. Additive neuroprotective effects of creatine and a cyclooxygenase 2 inhibitor against dopamine depletion in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. J Mol Neurosci. 2003;21:191–198. doi: 10.1385/jmn:21:3:191. [DOI] [PubMed] [Google Scholar]
- 171.Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson's disease (preclinical and clinical stages). J Neurol. 2002;249(Suppl 3):III, 1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- 172.Yang L, Calingasan NY, Wille EJ, Cormier K, Smith K, Ferrante RJ, Beal MF. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson's and Huntington's diseases. J Neurochem. 2009;109:1427–1439. doi: 10.1111/j.1471-4159.2009.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stefanis L. α-synuclein in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a009399. doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Andres RH, Huber AW, Schlattner U, Pérez-Bouza A, Krebs SH, Seiler RW, Wallimann T, Widmer HR. Effects of creatine treatment on the survival of dopaminergic neurons in cultured fetal ventral mesencephalic tissue. Neuroscience. 2005;133:701–713. doi: 10.1016/j.neuroscience.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 175.Cunha MP, Martín-de-Saavedra MD, Romero A, Egea J, Ludka FK, Tasca CI, Farina M, Rodrigues ALS, López MG. Both creatine and its product phosphocreatine reduce oxidative stress and afford neuroprotection in an in vitro Parkinson's model. ASN Neuro. 2014;6:1–16. doi: 10.1177/1759091414554945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Andres RH, Ducray AD, Pérez-Bouza A, Schlattner U, Huber AW, Krebs SH, Seiler RW, Wallimann T, Widmer HR. Creatine supplementation improves dopaminergic cell survival and protects against MPP+ toxicity in an organotypic tissue culture system. Cell Transplant. 2005;14:537–550. doi: 10.3727/000000005783982756. [DOI] [PubMed] [Google Scholar]
- 177.Gerlach M, Riederer P, Scheller D. Mechanisms underlying and medical management of L-Dopa-associated motor complications. J Neural Transm. 2011;118:1659–1660. doi: 10.1007/s00702-011-0728-0. [DOI] [PubMed] [Google Scholar]
- 178.Cenci MA. Presynaptic mechanisms of L-DOPA-induced dyskinesia: the findings, the debate, and the therapeutic implications. Front Neurol. 2014;5:1–15. doi: 10.3389/fneur.2014.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Valastro B, Dekundy A, Danysz W, Quack G. Oral creatine supplementation attenuates L-DOPA-induced dyskinesia in 6-hydroxydopamine-lesioned rats. Behav Brain Res. 2009;197:90–96. doi: 10.1016/j.bbr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 180.Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson's disease. Neurobiol Dis. 1999;6:461–474. doi: 10.1006/nbdi.1999.0259. [DOI] [PubMed] [Google Scholar]
- 181.Westin JE, Andersson M, Lundblad M, Cenci MA. Persistent changes in striatal gene expression induced by long-term L-DOPA treatment in a rat model of Parkinson's disease. Eur J Neurosci. 2001;14:1171–1176. doi: 10.1046/j.0953-816x.2001.01743.x. [DOI] [PubMed] [Google Scholar]
- 182.Stefanova N, Lundblad M, Tison F, Poewe W, Cenci MA, Wenning GK. Effects of pulsatile L-DOPA treatment in the double lesion rat model of striatonigral degeneration (multiple system atrophy). Neurobiol Dis. 2004;15:630–639. doi: 10.1016/j.nbd.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 183.Sgambato-Faure V, Buggia V, Gilbert F, Lévesque D, Benabid AL, Berger F. Coordinated and spatial upregulation of arc in striatonigral neurons correlates with L-dopa-induced behavioral sensitization in dyskinetic rats. J Neuropathol Exp Neurol. 2005;64:936–947. doi: 10.1097/01.jnen.0000186922.42592.b7. [DOI] [PubMed] [Google Scholar]
- 184.Bender A, Samtleben W, Elstner M, Klopstock T. Long-term creatine supplementation is safe in aged patients with Parkinson disease. Nutr Res. 2008;28:172–178. doi: 10.1016/j.nutres.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 185.Santos RV, Bassit RA, Caperuto EC, Costa Rosa LF. The effect of creatine supplementation upon inflammatory and muscle soreness markers after a 30km race. Life Sci. 2004;75:1917–1924. doi: 10.1016/j.lfs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 186.Madan BR, Khanna NK. Effect of aminoacids on the carrageenan-induced paw oedema in rats: a preliminary report. Indian J Physiol Pharmacol. 1976;8:227–229. [Google Scholar]
- 187.Di Rosa M, Willoughby DA. Screens for anti-inflammatory drugs. J Pharm Pharmcol. 1971;23:297–298. doi: 10.1111/j.2042-7158.1971.tb08661.x. [DOI] [PubMed] [Google Scholar]
- 188.Macêdo SB, Ferreira LR, Perazzo FF, Carvalho JC. Anti-inflammatory activity of Arnica montana 6cH: preclinical study in animals. Homeopathy. 2004;93:84–87. doi: 10.1016/j.homp.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 189.Necas J, Bartosikova L. Carrageenan: a review. Veterinarni Medicina. 2013;58:187–205. [Google Scholar]
- 190.Xu Z, Zhou J, Cai J, Zhu Z, Sun X, Jiang C. Anti-inflammation effects of hydrogen saline in LPS activated macrophages and carrageenan induced paw oedema. J Inflamm (London) 2012;9:2. doi: 10.1186/1476-9255-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dhawan BN, Srimal RC. Anti-inflammatory and some other pharmacological effects of 3,4-trans-2,2-dimethyl-3-phenyl-4-[p-(β-pyrrolidinoethoxy)-phenyl]-7-methoxy-chroman (Centchroman). Br J Pharmacol. 1973;49:64–73. doi: 10.1111/j.1476-5381.1973.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Eddouks M, Chattopadhyay D, Zeggwagh NA. Animal models as tools to investigate antidiabetic and anti-inflammatory plants. Evid Bas Complement Alt Med. 2012;2012:142087. doi: 10.1155/2012/142087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Guay J, Bateman K, Gordon R, Mancini J, Riendeau D. Carrageenan-induced paw edema in rat elicits a predominant prostaglandin E2 (PGE2) response in the central nervous system associated with the induction of microsomal PGE2 synthase-1. J Biol Chem. 2004;279:24866–24872. doi: 10.1074/jbc.M403106200. [DOI] [PubMed] [Google Scholar]
- 194.Pires AF, Rodrigues NV, Soares PM, Ribeiro Rde A, Aragão KS, Marinho MM, da Silva MT, Cavada BS, Assreuy AM. A novel N-acetyl-glucosamine lectin of Lonchocarpus araripensis attenuates acute cellular inflammation in mice. Inflamm Res published on line. 2015 Nov 6; doi: 10.1007/s00011-015-0889-7. 2015. [DOI] [PubMed] [Google Scholar]
- 195.Borthakur A, Bhattacharyya S, Anbazhagan AN, Kumar A, Dudeja PK, Tobacman JK. Prolongation of carrageenan-induced inflammation in human colonic epithelial cells by activation of an NFκB-BCL10 loop. Biochem Biophys Acta. 2012;1822:1300–1307. doi: 10.1016/j.bbadis.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.MacKay RJ, French TW, Nguyen HT, Mayhew IG. Effects of large doses of phenylbutazone administration to horses. Am J Vet Res. 1983;44:774–780. [PubMed] [Google Scholar]
- 197.Lees P, Higgins AJ. Clinical pharmacology and therapeutic uses of non-steroidal anti-inflammatory drugs in the horse. Equine Vet J. 1985;17:83–96. doi: 10.1111/j.2042-3306.1985.tb02056.x. [DOI] [PubMed] [Google Scholar]
- 198.LeFloc'h N, Melchior D, Obled C. Modifications of protein and amino acid metabolism during inflammation and immune system activation. Livestock Prod Sci. 2004;87:37–45. [Google Scholar]
- 199.Schiatti P, Selva D, Arrigoni-Martelli E. L'edema localizzato da nystatin come modello di inflammazione sperimentale. Boll Chim Farm. 1970;109:33–38. [PubMed] [Google Scholar]
- 200.Razonable RR, Henault M, Watson HL, Paya CV. Nystatin induces secretion of interleukin (IL)-1β, IL-8,and tumor necrosis factor alpha by a toll-like receptor-dependent mechanism. Antimicrob Agents Chemother. 2005;49:3546–3549. doi: 10.1128/AAC.49.8.3546-3549.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kaithwas G, Majumdar DK. Therapeutic effect of Linum usitatissimum (flaxseed/linseed) fixed oil on acute and chronic arthritic models in albino rats. Inflammopharmacol. 2010;18:127–136. doi: 10.1007/s10787-010-0033-9. [DOI] [PubMed] [Google Scholar]
- 202.Kaithwas G, Gautam R, Jachak SM, Saklani A. Antiarthritic effects of Ajuga bracteosa wall ex benth. in acute and chronic models of arthritis in albino rats. Asian Pac J Trop Biomed. 2012;2:185–188. doi: 10.1016/S2221-1691(12)60039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Choudhary M, Kumar V, Gupta P, Singh S. Investigation of antiarthritic potential of Plumeria alba L. leaves in acute and chronic models of arthritis. Biomed Res Intl. 2014;2014:474616. doi: 10.1155/2014/474616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ram FS, Robinson SM, Black PN, Picot J. Physical training for asthma. Cochrane Database Syst Rev. 2005;4:CD001116. doi: 10.1002/14651858.CD001116.pub2. [DOI] [PubMed] [Google Scholar]
- 205.Szentágothai K, Gyene I, Szócska M, Osváth P. Physical exercise program for children with bronchial asthma. Pediatr Pulmonol. 1987;3:166–172. doi: 10.1002/ppul.1950030310. [DOI] [PubMed] [Google Scholar]
- 206.Fanelli A, Cabral AL, Neder JA, Martins MA, Carvalho CR. Exercise training on disease control and quality of life in asthmatic children. Med Sci Sports Exerc. 2007;39:1474–1480. doi: 10.1249/mss.0b013e3180d099ad. [DOI] [PubMed] [Google Scholar]
- 207.Nickerson BG, Bautista DB, Namey MA, Richards W, Keens TG. Distance running improves fitness in asthmatic children without pulmonary complications or changes in exercise-induced bronchospasm. Pediatrics. 1983;71:147–152. [PubMed] [Google Scholar]
- 208.Arborelius M, Jr., Svenonius E. Decrease of exercise-induced asthma after physical training. Eur J Respir Dis Suppl Acta Paediatr. 1984;136:25–31. [PubMed] [Google Scholar]
- 209.Matsumoto I, Araki H, Tsuda K, Odajima H, Nishima S, Higaki Y, Tanaka M, Shindo M. Effects of swimming training on aerobic capacity and exercise induced bronchoconstriction in children with bronchial asthma. Thorax. 1999;54:196–201. doi: 10.1136/thx.54.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Henriksen JM, Nielsen TT. Effect of physical training on exercise-induced bronchoconstriction. Acta Paediatr Scand. 1983;72:31–36. doi: 10.1111/j.1651-2227.1983.tb09659.x. [DOI] [PubMed] [Google Scholar]
- 211.Weiler JM, Metzger WJ, Donnelly AL, Crowley ET, Sharath MD. Prevalence of bronchial hyperresponsiveness in highly trained athletes. Chest. 1986;90:23–28. doi: 10.1378/chest.90.1.23. [DOI] [PubMed] [Google Scholar]
- 212.Weiler JM, Layton T, Hunt M. Asthma in United States olympic athletes who participated in the 1996 summer games. J Allergy Clin Immunol. 1998;102:722–726. doi: 10.1016/s0091-6749(98)70010-7. [DOI] [PubMed] [Google Scholar]
- 213.Helenius I, Haahtela T. Allergy and asthma in elite summer sport athletes. J Allergy Clin Immunol. 2000;106:444–452. doi: 10.1067/mai.2000.107749. [DOI] [PubMed] [Google Scholar]
- 214.Weiler JM, Ryan EJ. Asthma in United States olympic athletes who participated in the 1998 winter games. J Allergy Clin Immunol. 2000;106:267–271. doi: 10.1067/mai.2000.108605. [DOI] [PubMed] [Google Scholar]
- 215.Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol. 1999;515:287–291. doi: 10.1111/j.1469-7793.1999.287ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pedersen BK, Toft AD. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. 2000;34:246–251. doi: 10.1136/bjsm.34.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tilz GP, Domej W, Diez-Ruiz A, Weiss G, Brezinschek R, Brezinschek HP, Huttl E, Pristautz H, Wachter H, Fuchs D. Increased immune activation during and after physical exercise. Immunobiology. 1993;188:194–202. doi: 10.1016/s0171-2985(11)80497-3. [DOI] [PubMed] [Google Scholar]
- 218.Moldoveanu AI, Shephard RJ, Shek PN. The cytokine response to physical activity and training. Sports Med. 2001;31:115–144. doi: 10.2165/00007256-200131020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.MacKenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J Immunol. 2001;167:3146–3155. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- 220.Vieira RP, Duarte AC, Santos AB, Medeiros MC, Mauad T, Martins MA, Carvalho CR, Dolhnikoff M. Exercise reduces effects of creatine on lung. Int J Sports Med. 2009;30:684–690. doi: 10.1055/s-0029-1224176. [DOI] [PubMed] [Google Scholar]
- 221.Ferreira SC, Toledo AC, Hage M, Santos AB, Medeiros MC, Martins MA, Carvalho CR, Dolhnikoff M, Vieira RP. Creatine activates airway epithelium in asthma. Int J Sports Med. 2010;31:906–912. doi: 10.1055/s-0030-1267160. [DOI] [PubMed] [Google Scholar]
- 222.Brasier AR. The NF-kB regulatory network. Cardiovasc Toxicol. 2006;6:111–130. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- 223.Gilmore TD. Introduction to NF-kB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 224.Khalaf H, Jass J, Olsson P-E. Differential cytokine regulation by NF-kB and AP-1 in Jurkat T-cells. BMC Immunol. 2010;11:26–38. doi: 10.1186/1471-2172-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Beutler B. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Degraaf AJ, Zaslona Z, Bourdonnay E, Peters-Golden M. Prostaglandin E2 reduces Toll-like receptor 4 expression in alveolar macrophages by inhibition of translation. Am J Respir Cell Mol Biol. 2014;51:242–250. doi: 10.1165/rcmb.2013-0495OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Béraud D, Maguire-Zeiss KA. Misfolded α-synuclein and toll-like receptors: therapeutic targets for Parkinson's disease. Parkin Rel Disorders. 2012;18S1:S17–S20. doi: 10.1016/S1353-8020(11)70008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Paola M, Sestito SE, Mariani A, Memo C, Fanelli R, Freschi M, Bendotti C, Calabrese V, Peri F. Synthetic and natural small molecule TLR4 antagonists inhibit motoneuron death in cultures from ALS mouse model. Pharmacol Res. 2015;S1043-6618:30004–30009. doi: 10.1016/j.phrs.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 229.Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222–225. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lyck R, Enzmann G. The physiological roles of ICAM-1 and ICAM-2 in neutrophil migration into tissues. Curr Opin Hematol. 2015;22:53–59. doi: 10.1097/MOH.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 231.Silva LA, Tromm CB, Da Rosa G, Bom K, Luciano TF, Tuon T, De Souza CT, Pinho RA. Creatine supplementation does not decrease oxidative stress and inflammation in skeletal muscle after eccentric exercise. J Sports Sci. 2013:1–13. doi: 10.1080/02640414.2013.773403. [DOI] [PubMed] [Google Scholar]
- 232.Berjaoui S, Povedano M, Garcia-Esparcia P, Carmona M, Aso E, Ferrer I. Complex inflammation mRNA-related response in ALS is region dependent. Neural Plast. 2015;2015:573784. doi: 10.1155/2015/573784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mosley RL, Hutter-Saunders JA, Stone DK, Gendelman HE. Inflammation and adaptive immunity in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a009381. doi: 10.1101/cshperspect.a009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Alam Q, Alam MZ, Mushtaq G, Damanhouri GA, Rasool M, Kamal MA, Haque A. Inflammatory Process in Alzheimer and Parkinson's Diseases: Central Role of Cytokines. Curr Pharm Des Epub ahead of print. 2015 Nov 24; doi: 10.2174/1381612822666151125000300. 2015. [DOI] [PubMed] [Google Scholar]
- 235.Lawler JM. Direct antioxidant properties of creatine. Biochem Biophys Res Comm. 2002;290:47–52. doi: 10.1006/bbrc.2001.6164. [DOI] [PubMed] [Google Scholar]
- 236.Sestili P, Martinelli C, Bravi G, Piccoli G, Curci R, Battistelli M, Falcieri E, Agostini D, Gioacchini AM, Stocchi V. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med. 2006;40:837–849. doi: 10.1016/j.freeradbiomed.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 237.Guidi C, Potenza L, Sestili P, Martinelli C, Guescini M, Stocchi L, Zeppa S, Polidori E, Annibalini G, Stocchi V. Differential effect of creatine on oxidatively-injured mitochondrial and nuclear DNA. Biochem Biophys Acta. 2008;1780:16–26. doi: 10.1016/j.bbagen.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 238.Pavelko KD, Howe CL, Drescher KM, Gamez JD, Johnson AJ, Wei T, Ransohoff RM, Rodriguez M. Interleukin-6 protects anterior horn neurons from lethal virus-induced injury. J Neurosci. 2003;23:481–492. doi: 10.1523/JNEUROSCI.23-02-00481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]