Abstract
Objective
Despite evidence for detrimental effects of alcohol on sleep quality in laboratory studies, alcohol is commonly used as a self-prescribed sleep aid. This study examined the within-person associations of alcohol use with sleep duration and quality in everyday life to gain insight into the ecological validity of laboratory findings on the association between sleep and alcohol.
Method
A sample of 150 adults (age 19–89 years) were followed for 60+ days as part of an intensive experience sampling study wherein participants provided daily reports of their alcohol use, sleep duration, and sleep quality. Within-person and between-person associations of daily sleep duration and quality with alcohol use were examined using multilevel models.
Results
A significant, negative within-person association was observed between sleep quality and alcohol use. Sleep quality was lower on nights following alcohol use. Sleep duration did not vary as a function of within-person variation in alcohol use.
Conclusions
In line with laboratory assessments, alcohol use was associated with low sleep quality but was not associated with sleep duration, suggesting that laboratory findings generalize to everyday life. This examination of individuals’ daily lives suggests that alcohol does not systematically improve sleep quality or duration in real life.
Keywords: alcohol, sleep, experience sampling, substance use, longitudinal analysis
1. INTRODUCTION
Despite the use of alcohol as a self-prescribed sleep aid (Johnson et al., 1998; Taylor & Bramoweth, 2010), alcohol may disrupt sleep. Laboratory examinations of the effects of alcohol on humans’ sleep suggest that alcohol may act as a sedative early in the night, reducing both sleep-onset latency (Rundell et al., 1972) and number of awakenings after sleep onset (Feige et al., 2006). However, later in the night, alcohol appears to disrupt sleep, canceling out the initial benefits (for review see Ebrahim et al., 2013). The purpose of the present study is to investigate the ecological validity of laboratory findings by examining whether there is evidence of a within-person association between alcohol use and sleep duration and quality in situ during daily life.
1.2 Laboratory Assessments of the Effect of Alcohol on Sleep
The most consistent findings across laboratory studies are that alcohol shortens sleeponset latency (Williams et al., 1983; Rundell et al., 1972), but does not generally affect total sleep time (MacLean & Cairns, 1982; Yules et al., 1966; although see Arnedt et al., 2011 for exception observed in women only). As well, participants subjectively characterize their sleep as being of poorer quality and more superficial after consuming alcohol compared to placebo (Landolt et al., 1996; Rohsenow et al., 2010). These differences in subjective sleep quality reflect objective differences in sleep efficiency (percentage of time in bed spent asleep) and depth of sleep (Keklund & Akerstedt, 1997). In particular, alcohol consumption decreases the proportion of total sleep-time spent in rapid eye movement (REM) sleep and increases the proportion of time spent in Stage 1 (light non-REM) sleep (Sagawa et al., 2011; Van Reen et al., 2006). Examined in parcels of time roughly corresponding to when the body is metabolizing alcohol (first half of night) and eliminating alcohol (second half of night), increases in amount of Stage 3 (deep non-REM) sleep in the first 3 hours of sleep-time are accompanied by decreases in Stage 3 sleep later in the night (Stone, 1980; Williams et al., 1983).
Of note, the amount of alcohol consumed is linearly related to the extent of decrease in sleep-onset latency and the extent of increase in Stage 3 (deep non-REM) during the first 3 hours of sleep (Roehrs et al., 2003; Williams et al., 1983), but not related to total sleep time (duration). The more alcohol consumed, the greater the early night increase in Stage 3 (deep non-REM) and corresponding decrease in late night Stage 3 sleep (MacLean & Cairns, 1982; Williams et al., 1983). The shift to a lighter sleep during the second half of the sleep period, as well as more awakenings (Landolt et al., 1996), may partially explain why participants perceive their sleep as being of poorer quality after alcohol consumption. Indeed, sleep efficiency during the second and third quarters of the night are the main drivers of subjective sleep quality (Rosipal et al., 2013). In sum, laboratory studies suggest that when individuals drink alcohol they are likely to experience poorer quality but not shorter sleep, and as alcohol consumption increases their sleep quality worsens.
1.3. Sleep Outside of the Laboratory
Although laboratory studies have increased our understanding of the associations between alcohol consumption and sleep quality, it is unclear whether the processes observed in the laboratory generalize to real-world settings (Mitchell, 2012). In situ intensive repeated measures study designs can examine whether the laboratory-based findings generalize into the real world (e.g., Larson & Csikszentmihalyi, 1983; Shiffman et al., 2008). These study designs collect intensive repeated measures data in everyday settings, as participants go about their lives. The proximal (in place and time) nature of the measurement provides for ecological validity while also reducing retrospective biases often introduced in questionnaires that ask participants to recall and aggregate information about longer periods of time (e.g., previous 30 days; Schwarz, 2007). Further, intensive repeated measures allow for the separation of between-person and within-person associations among predictor and outcome variables (Ram & Gerstorf, 2009). Repeated measures data simultaneously contain information about between-person and within-person differences (Raudenbush & Bryk, 2002). To avoid confounding between- and within-person sources of variability, between- and within-person effects must be disaggregated (see Curran & Bauer, 2011).
Few studies to date have used in situ designs to examine associations between alcohol and sleep. Galambos et al. (2009), using 14-days of data provided by 191 Canadian undergraduate students (age 17 to 19 years), found that self-reported sleep quality was lower on alcohol use days than on no alcohol use days. Self-reported sleep duration was shorter on alcohol use days than on no alcohol use days. Similarly,Geoghegan et al. (2012), in a 7-day study of 47 Irish college students (age 20 to 25 years) found that sleep-onset latency and sleep duration were shorter on alcohol use days than on no-alcohol use days, but did not find any differences in self-reported reported sleep quality. Patrick et al. (2016), in a 56-day study of 667 U.S. college students, and the only study to separate within- and between-associations between alcohol and sleep, found that sleep quality was lower and sleep quantity was shorter after binge drinking, defined as 4+/5+ drinks in a 2-hour period for women/men. These existing studies have not consistently replicated laboratory results and the discrepancy may stem at least partially from the use of college student samples given findings that greater alcohol use is associated with later sleep schedules in student populations (Singleton & Wolfson, 2009).
1.4. The Present Study
To bridge laboratory and existing in situ studies, the present study used a U.S. sample of adults who were observed for many (60+) days. To reflect laboratory studies (e.g., Roehrs et al., 2003; Williams et al., 1983), the present study considered day-to-day differences in alcohol dose, hypothesizing that alcohol use would have a negative, linear relationship with sleep quality, but not with sleep duration.
2. METHOD
2.1. Participants
The Intraindividual Study of Affect, Health, and Interpersonal Behavior (iSAHIB; Ram et al., 2014) worked with 150 adults (51% women) recruited from the Pennsylvania State University and surrounding community, and purposively stratified by gender and across the adult life span in 18–24 (n=22), 25–34 (n=27), 35–49 (n=30), 50–64 (n=41), 65+ (n=30) year age-bins. Participants were between 19 and 89 years of age (M= 47.10, SD= 18.76), had obtained between 2 and 24 years of formal education (M= 16.36, SD= 3.90), and had between 0 and 6 children (M= 1.5, SD= 1.41). Participants self-identified as White (91%), African American (4%), Asian American (1%), and Mixed or Other (4%) ethnicity, and had yearly family income ranging from ‘under $20,000’ to ‘$200,000 and over’ (Median= ‘$50,000 – $74,999’), with 8.7% declining to answer. Of the 150 participants, 136 (90.7%) completed the entire protocol, and 14 withdrew (relocation, health, loss of interest) after completing between a third (n= 11, 7.3%) and two-thirds (n= 3; 2.0%) of the total protocol. Participants who withdrew did not differ systematically from those who completed the entire protocol with respect to the measured demographics (ps> .05). For the present analysis, tethered sleep and alcohol data span 8381 days (93.1% of possible days) nested within 150 persons.
2.2. Procedure
After being recruited, learning about the intensive nature of the assessments, and self-selecting into the study, participants completed a series of three 21-day “measurement bursts” spaced at approximately 4.5-month intervals (M= 5.25 months between bursts 1 and 2; 4.25 months between bursts 2 and 3). During each burst, individuals reported about their social interactions as they went about their daily lives, and provided end-of-day reports about their feelings, thoughts, and behaviors using a study-provided smartphone (Verizon XV6900). Prior to and after each 21-day burst, individuals visited the laboratory to receive training or debriefing and complete web-based batteries of questionnaires. Participants were compensated $500 for completing the entire protocol.
2.3. Measures
2.3.1. Daily Sleep
Daily sleep was measured at the end of each day using items adapted from The Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989). Typically used as a previous-month recall measure, two items were configured for daily assessment as previous-night recalls. Sleep duration was measured as responses to the question, “How many hours of sleep did you get last night (This may be different from # of hours spent in bed)? Please round to the nearest hour.” Sleep quality was measured as responses to the question, “Last night, how would you rate your quality of sleep?” on a touch point continuum (slider-type interface) with anchors at 0= Very Bad and 100= Very Good (numbers not visible to participants). Such single-item, daily self-report measures are more strongly correlated with actigraphy based measures of sleep than retrospective, previous month reports (Lauderdale et al., 2008), are practical when measurements are taken frequently to reduce participant burden, and have demonstrated favorable psychometric properties (e.g., Cappelleri et al., 2009). On average, participants slept 6.78 hours per night (SD= 1.52, Range= 0 to 14) with quality of 65.72 (SD= 21.50, Range= 0 to 100).
2.3.2. Day’s Alcohol Consumption
Each day’s alcohol consumption was measured at the end of the day using three items of the form, “How many of the following did you consume today?” followed with prompts and definitions of standard servings for “beer” (12 fl oz), “wine” (5 fl oz), and “shots of liquor” (1.5 fl oz). Responses given on a 0, 1, 2, 3, 4, 5+ response scale for each beverage category were summed to obtain the total servings of alcohol consumed for each day (see Conroy et al., 2015 for other analyses of these alcohol data). On average, participants consumed 0.67 drinks per day (SD= 1.33; range= 0 to12). On drinking days (29.67% of all days and with 86% of participants having at least one drinking day), participants drank an average of 2.24 drinks (SD= 1.55; range= 1 to 12). To reduce potential outlier effects, days with >5 total drinks consumed (1.63% of days) were recoded as 5+.
2.3.4. Covariates
Participants reported each day’s caffeine use by indicating the number of caffeinated drinks (0,1,2,3,4, 5+) they consumed (with instructions for reporting relative to caffeine in a standard cup of coffee). Day of week was derived from the date of response and, following previous literature (e.g., Galambos et al., 2009), split into three categories: Friday & Saturday, Sunday, and other weekdays (reference category). Gender and age were obtained from a demographics questionnaire.
2.4. Data Analysis
The intensive repeated measures data (8381 days nested within 150 persons) were analyzed using multilevel models (Snijders & Bosker, 2012) that were parameterized to separate within-person and between-person associations by splitting predictors into time-invariant (between-person) and time-varying (within-person) components (see Bolger & Laurenceau, 2013). Time-invariant person-level variables for usual alcohol use, usual caffeine use, usual sleep quality, and usual sleep duration were calculated as the arithmetic mean across each individual’s repeated measures. Time-varying, day-level variables are typically calculated as deviations from those person-level means. However, to facilitate interpretation of the intercept as sleep duration or sleep quality on a typical, no alcohol use weekday and to facilitate interpretation of the association between day’s alcohol use and sleep as differences from zero to one drink, the day’s alcohol use variable retained a 0 (no alcohol use) to 5+ (highest level of alcohol consumption) coding (see Patrick et al., 2016 for a similar centering approach in the context of binge drinking in college students, and Enders & Tofighi, 2007 for a discussion of centering predictor variables in the case of non-normally distributed Level 1 predictor variables); day’s caffeine use, previous day’s sleep quality, and previous day’s sleep duration were calculated as daily deviations from person-specific means; day of week was dummy coded such that 0 represented Monday to Thursday; and age and gender were sample-mean centered.
Models were constructed as
| (1) |
where Sleepit is the sleep variable of interest (sleep duration and quality were examined separately) for person i on day t; β0i indicates the expected sleep duration or quality on a weekday with no alcohol use and typical caffeine use; β1i indicates within-person differences in sleep associated with the differences in day’s alcohol use; β2i and β3i indicate differences in sleep associated with Friday and Saturday and with Sunday relative to other days of the week (Monday through Thursday), respectively; β4i indicates differences in sleep associated with the day’s caffeine use; β5i and β6i indicate differences in sleep associated with the previous day’s sleep quality and duration, respectively, and eit are day-specific residuals that were allowed to autocorrelate (AR1).
Person-specific intercepts and associations (from the Level 1 model) were specified (at Level 2) as
| (2) |
where the γs are sample-level parameters and the us are residual between-person differences that may be correlated, but are uncorrelated with eit. Parameters γ11 through γ15 indicate how between-person differences in the within-person associations of day’s alcohol use and sleep were moderated by usual alcohol use, usual caffeine use, usual sleep duration or quality (with usual sleep duration included in the sleep quality model and usual sleep quality included in the sleep duration model), age, and gender. Quadratic and cubic age and other interaction terms (e.g., day’s caffeine use × Usual caffeine use) were also tested but were not significant and thus not included in the more parsimonious final models.
All models were fit using the nlme package in R (Pinheiro et al., 2015) using maximum likelihood estimation, with incomplete data (< 1%) treated using missing at random assumptions. Statistical significance was evaluated at α= .05.
3. RESULTS
3.1. Associations between Alcohol Use and Sleep Duration
Results from the multilevel model examining associations between alcohol use and sleep duration are shown in the left columns of Table 1. In line with the hypothesis that the day’s alcohol use would not affect sleep duration, the within-person association between sleep duration and day’s alcohol use was not significant (γ10= −0.02, p= .32). This within-person association was not moderated by individuals’ usual alcohol use, usual caffeine use, usual sleep quality, age, or gender (γ11 to γ15, ps> .05). The between-person association between sleep duration and usual alcohol use was positive and significant (γ01= 0.33, p< .001). That is, participants who drank more during the course of the study tended to sleep for longer.
Table 1.
Results of Multilevel Models Examining Associations Between Alcohol Use and Sleep Duration and Quality
| Sleep Duration | Sleep Quality | |||||
|---|---|---|---|---|---|---|
| Fixed Effects | Est. | SE | p-value | Est. | SE | p-value |
| Intercept (γ00) | 6.67* | 0.06 | <.001 | 64.81* | 1.00 | <.001 |
| Day’s Alcohol Use (γ10) | −0.02 | 0.02 | .32 | −0.71* | 0.32 | .03 |
| Friday/Saturday (γ20) | 0.36* | 0.04 | <.001 | 1.83* | 0.47 | < .001 |
| Sunday (γ30) | 0.12* | 0.04 | .004 | 1.13* | 0.51 | .03 |
| Day’s Caffeine Use (γ40) | −0.08* | 0.02 | <.001 | −0.75* | 0.23 | .001 |
| Previous Day’s Sleep Quality (γ50) |
0.001 | 0.001 | .51 | −0.23* | 0.01 | <.001 |
| Previous Day’s Sleep Duration (γ60) |
−0.22* | 0.01 | <.001 | −0.04 | 0.15 | .77 |
| Usual Alcohol Use (γ01) | 0.33* | 0.08 | <.001 | −3.06* | 1.36 | .03 |
| Usual Caffeine Use (γ02) | 0.02 | 0.05 | .65 | −1.06 | 0.87 | .23 |
| Age (γ03) | −0.01 | 0.003 | .12 | 0.11* | 0.05 | .04 |
| Gender (γ04) | −0.10 | 0.12 | .41 | 2.85 | 2.00 | .16 |
| Usual Sleep Duration/Quality (γ05) |
0.03* | 0.005 | <.001 | 7.50* | 1.25 | <.001 |
| Day’s Alcohol Use X Usual Alcohol Use (γ11) |
−0.01 | 0.02 | .91 | 0.44 | 0.33 | .18 |
| Day’s Alcohol Use X Usual Caffeine Use (γ12) |
−0.01 | 0.02 | .69 | −0.0001 | 0.26 | .99 |
| Day’s Alcohol Use X Age (γ13) |
−0.001 | 0.001 | .47 | −0.002 | 0.02 | .87 |
| Day’s Alcohol Use X Gender (γ14) |
−0.01 | 0.04 | .76 | −0.004 | 0.55 | .99 |
| Day’s Alcohol Use X Usual Sleep Duration/Quality (γ15) |
0.002 | 0.002 | .24 | −0.11 | 0.34 | .74 |
| Random Effects | Est. | CI | Est. | CI | ||
| Intercept () | 0.65 | 0.57 – 0.75 | 11.14 | 9.76 – 12.72 | ||
| Day’s Alcohol Use () | 0.10 | 0.06 – 0.16 | 1.65 | 1.15 – 2.36 | ||
| Correlation (ru0u1) | 0.11 | −0.11 – 0.32 | −0.10 | −0.34 – 0.15 | ||
| AR(1) | 0.35 | 0.32 – 0.39 | 0.44 | 0.41 – 0.50 | ||
| Residual () | 1.37 | 1.34 – 1.40 | 18.72 | 18.28 – 19.17 | ||
| Fit Indices | ||||||
| AIC | 28433.83 | 71520.80 | ||||
| BIC | 28588.52 | 71675.49 | ||||
Note: Nobservations = 8381 days nested within 150 persons; AIC = Akaike information criteria; BIC = Bayesian information criteria; SE = standard error; CI = confidence intervals.
p < .05.
Looking at the covariates, we found the expected effects of day’s caffeine use. On days that participants consumed more caffeine than usual, they slept less (γ40= −0.08, p< .001). However, there was no evidence of a between-person association. Individual differences in usual caffeine use were not related to sleep duration (γ02= 0.02, p= .65). Relative to weekdays, individuals’ sleep duration was significantly longer on Friday & Saturday (γ20= 0.36, p< .001) and Sunday nights (γ30= 0.12, p< .01), relative to other days of the week. Previous day’s sleep duration (γ60= −0.22, p< .001), but not previous day’s sleep quality (γ50= 0.001, p= .51), was negatively associated with sleep duration. As would be expected, higher usual sleep quality was associated with longer sleep duration (γ05= 0.03, p< .001). However, neither age nor gender were significantly related to sleep duration (ps> .05).
3.2. Associations between Alcohol Use and Sleep Quality
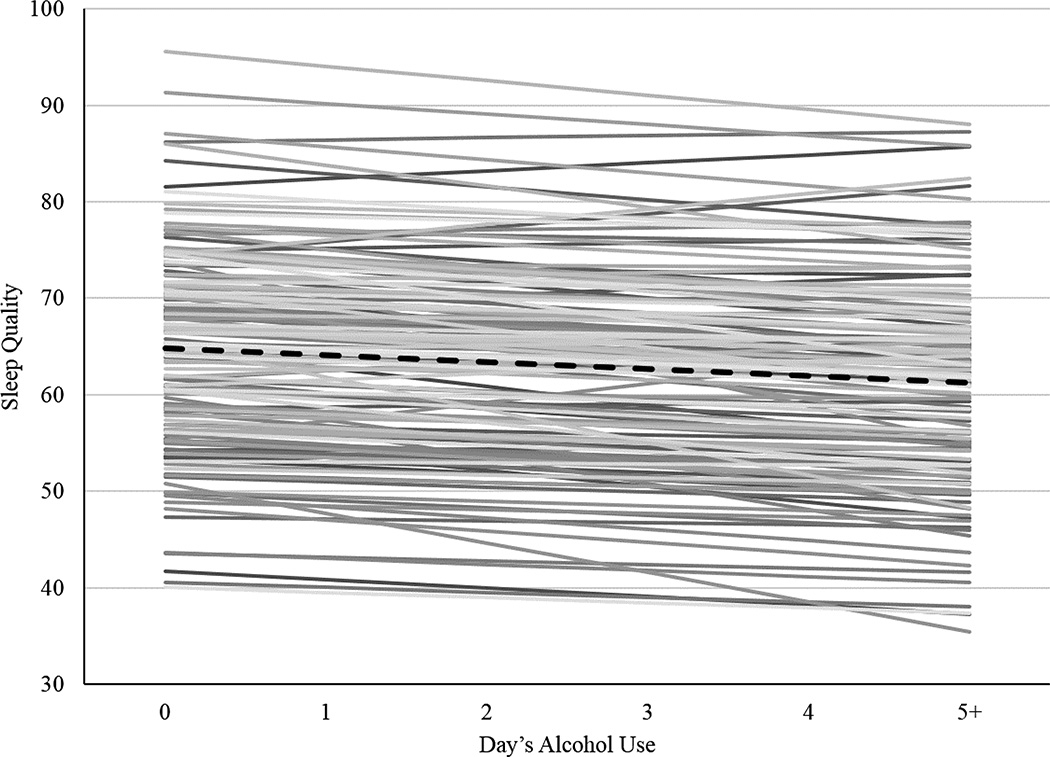
Results from the multilevel model examining associations between alcohol use and sleep quality are shown in the right columns of Table 1. In line with the hypothesis that alcohol use reduces sleep quality, we found a significant, negative within-person association between sleep quality and day’s alcohol use (γ10= −0.71, p= .03). Shown for the prototypical individual by the dashed black line in Figure 1, each additional drink was associated with a 0.71 decrease in sleep quality. Although a relatively small effect on average with an effect size of 0.16 (estimate for the within-person association divided by the within-person residual standard deviation, γ10/σe = 0.71/sqrt(18.72), this within-person association ranged widely, from β1i =−3.75 to 1.91 across persons (thin gray lines). Notably, the extent of the within-person association was not related to individuals’ usual alcohol use, usual caffeine use, usual sleep duration, age, or gender (ps> .05). The between-person association between usual alcohol use and sleep quality was negative and significant (γ01= −3.06, p= 0.03). That is, participants who drank more alcohol during the study tended to have lower overall sleep quality.
Figure 1.
Prototypical within-person (dashed black line, γ10 = −0.71, p < .05) and person-specific (thin lines; N = 150; β1i = −3.75 to 1.91) associations between daily sleep quality (0 to 100 ratings) and the day’s alcohol use (0 to 5+ drinks).
Looking at the covariates, we found the expected association between day’s caffeine use and sleep quality (γ40= −0.75, p <.01). That is, on days that participants consumed more caffeine than usual, they reported lower quality sleep. However, there was no between-person association; usual caffeine use was not related to sleep quality (γ02= −1.06, p= .23). Consistent with duration, sleep quality was significantly higher on Friday & Saturday (γ20= 1.82, p< .001) and Sunday nights (γ30= 1.13, p= .03), relative to other days of the week. As expected, previous day’s sleep quality was associated with sleep quality (γ05= −0.23, p< .001), and although previous day’s sleep duration was not related to current sleep quality, (γ06= −0.04, p= .77), individuals with higher usual sleep duration tended to have higher overall sleep quality (γ05= 7.50, p<.001) As in prior studies, higher age was associated with higher overall sleep quality (γ13= 0.11, p= .04), and gender was not related to overall sleep quality (γ04= 2.85, p= .16).
4. DISCUSSION
4.1. Within-Person Effects of Alcohol on Sleep
The present study demonstrated that the within-person association between alcohol use and sleep quality observed in the laboratory extends to daily life. We observed a negative within-person association between alcohol use and sleep quality. On days with greater alcohol use, sleep quality was lower, consistent with laboratory research demonstrating a dose-related association between alcohol use and sleep disturbance (e.g., Landolt et al., 1996; MacLean & Cairns, 1982). Even though small, it is notable that this association can be detected in the real world in the midst of all the noise that exists outside the laboratory. Sleep duration was not associated with alcohol use. This is also consistent with laboratory findings demonstrating that alcohol use impacts the distribution of sleep stages but not sleep duration (e.g., Williams et al., 1983). The finding for sleep duration is inconsistent with previous in situ studies that observed shorter sleep duration following alcohol (e.g., Galambos et al., 2009) but all existing in situ studies used college samples, only one separated between- and within-person associations (Patrick et al., 2016), and none tested for a linear association between increasing amounts of alcoholic drinks (focusing instead on dichotomized alcohol vs. no alcohol use days or binge drinking vs no binge drinking days) and sleep parameters.
4.2. Between-Person Associations of Alcohol Use and Sleep
While not the focus of the current study, individuals with higher levels of usual alcohol use tended to have longer average sleep duration. This between-person association is unlikely to be driven by the acute effects of alcohol. It is perhaps more likely that participants exhibiting greater rates of alcohol use had greater capacity to extend sleep duration throughout the study due to fewer time use obligations. Consistent with this interpretation, level of usual alcohol use was negatively correlated with participants’ reports of average time spent at work (r= −0.24, p< .01). In line with cross-sectional survey studies (e.g., Popovici & French, 2013), individuals with higher levels of usual alcohol use tending to report lower sleep quality.
4.3. Associations Between Sleep and Covariates
Other findings of interest include that caffeine use was negatively associated with sleep duration and quality, in line with previous laboratory research noting that the effects of caffeine include reductions in total sleep time and perceived sleep quality (Hindmarch et al., 2000). The inclusion of caffeine adds to the ecological validity of the current study given the common practice of consuming caffeinated drinks to promote wakefulness and to enhance the experience of alcohol intoxication (Ishak et al., 2012). Also in line with previous findings (Hale, 2005), greater sleep duration and quality was observed on Friday and Saturday and Sunday nights relative to week nights.
Sleep quality was positively associated with age and sleep duration was not linearly associated with age. While sleep duration and quality have been reported to decline with age across adulthood (Floyd et al., 2007), robust associations have not consistently been observed (Reyner et al., 1995) and may depend on sampling factors such as the health status of study participants (Bliwise, 1993). Indeed, recent studies controlling for health factors have observed increases in sleep quality across adulthood into old age (Grander et al., 2012; Luca et al., in press). Consistent with some existing experience sampling studies (e.g., Galambos et al., 2009) but not others (Patrick et al., 2016), no gender effects were observed in the present study.
4.4. Limitations and Outlook
It is important to consider the findings in light of the study’s limitations. The sample was drawn from a mostly White population in central Pennsylvania with moderate and low-risk alcohol use. As such, care should be taken when attempting to generalize the findings to more diverse, and/or high risk, heavy alcohol use populations. This study sample is unique in that it includes adults between ages 18 and 89 years and allowed, with 60+ days of reporting, for assessment of the naturally-occurring variation and co-variation of a fluctuating behavior (alcohol use).
Future in situ studies may be improved through collection of more refined sleep data, either by more fine-grained self-report (e.g., 15 minute increments) or actigraphy-based, objective measures of sleep duration, especially when these devices can provide information on sleep onset latency (which is more difficult to obtain through self-report). Similarly, more precise data on how much alcohol individuals are ingesting (e.g., using response scales that do not include a 5+ ceiling) and when they start and stop drinking will allow greater consideration of the biological processes through which alcohol effects sleep and examination of time course on which different dosages of alcohol influence sleep (e.g., Leffingwell et al., 2014; Roehrs & Roth, 2001; Thakkar et al., 2015). Theories of alcohol’s sleep disrupting effects are associated with the elimination of alcohol within 4 to 5 hours of sleep onset. In particular, alcohol may exert stimulating effects when blood alcohol concentration is rising but sedative effects when blood alcohol concentration peaks and begins to decline (see Hendler et al., 2013 for review). Thus, it may be especially important to track timing of both ingestion of alcohol and other foods and beverages (e.g., those containing caffeine) that change the concentration of alcohol. In terms of the measure of caffeine intake, a standard definition of the amount of caffeine intake may be preferable in future studies rather than using number of caffeinated drinks as in the present study as caffeine content may differ across drink types (Heckman et al., 2010).
4.5. Synopsis
In summary, the current study extends previous in situ examinations of the association between sleep and alcohol using an adult sample that was observed for many days, and by considering how day-to-day differences in alcohol dose were linked to sleep duration and quality. The current study demonstrated that the within-person coupling between alcohol use and sleep quality observed in the laboratory can also be found in the real world using intensive measurements of daily life. Although often used as a self-prescribed sleep aid, our examination of individuals’ daily lives provides confirmation that alcohol does not systematically improve sleep quality or duration in real life. As such, multi-component prevention programs (e.g., Thadani et al., 2014) aiming to promote responsible alcohol use may benefit from addressing knowledge deficits about alcohol’s effects on sleep quality and duration.
Highlights.
Alcohol use days were associated with decreased sleep quality
Greater alcohol use throughout the study was associated with decreased sleep quality
Sleep duration did not differ between alcohol use and non-alcohol use days
Acknowledgments
Thanks very much to the study participants for providing a detailed glimpse of their daily lives for such extended periods of time, and to the research staff that helped obtain such rich data.
Role of Funding Sources
This work was supported by the National Institute on Health (RC1 AG035645, R01 HD076994, R24 HD041025, UL TR000127) and the Penn State Social Science Research Institute. DML was supported by T32 DA017629 from the National Institute on Drug Abuse and an ISSBD-JJF Mentored Fellowship for Early Career Scholars. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
DEC, ALP, and NR designed the original study and oversaw data collection. DML, JLM, NR aided in the interpretation of the data. DML and NR performed the statistical analyses. All authors contributed to writing of and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- Arnedt JT, Rohsenow DJ, Almeida AB, Hunt SK, Gokhale M, Gottlieb DJ, Howland J. Sleep following alcohol intoxication in healthy, young adults: effects of sex and family history of alcoholism. Alcoholism: Clinical & Experimental Research. 2011;35:870–878. doi: 10.1111/j.1530-0277.2010.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Bolger N, Laurenceau J-P. Intensive longitudinal methods: An introduction to diary and experience sampling research. New York, NY: Guilford Press; 2013. [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, McDermott AM, Sadosky AB, Petrie CD, Martin S. Psychometric properties of a single-item scale to assess sleep quality among individuals with fibromyalgia. Health Quality of Life Outcomes. 2009;7:54. doi: 10.1186/1477-7525-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy DE, Ram N, Pincus AL, Coffman DL, Lorek AE, Rebar AL, Roche MJ. Daily physical activity and alcohol use across the adult lifespan. Health Psychology. 2015;34:653–660. doi: 10.1037/hea0000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Bauer DJ. The disaggregation of within-person and between-person effects in longitudinal models of change. Annual Review of Psychology. 2011;62:583. doi: 10.1146/annurev.psych.093008.100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: effects on normal sleep. Alcoholism: Clinical & Experimental Research. 2013;37:539–549. doi: 10.1111/acer.12006. [DOI] [PubMed] [Google Scholar]
- Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychological Methods. 2007;12:121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Feige B, Gann H, Brueck R, Hornyak M, Litsch S, Hohagen F, Riemann D. Effects of alcohol on polysomnographically recorded sleep in healthy subjects. Alcoholism Clinical & Experimental Research. 2006;30:1527–1537. doi: 10.1111/j.1530-0277.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- Floyd JA, Janisse JJ, Jenuwine ES, Ager JW. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30:829–836. doi: 10.1093/sleep/30.7.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos NL, Dalton AL, Maggs JL. Losing sleep over it: daily variation in sleep quantity and quality in Canadian students’ first semester of university. Journal of Research on Adolescence. 2009;19:741–761. [Google Scholar]
- Geoghegan P, O’Donovan MT, Lawlor BA. Investigation of the effects of alcohol on sleep using actigraphy. Alcohol & Alcoholism. 2012;47:538–544. doi: 10.1093/alcalc/ags054. [DOI] [PubMed] [Google Scholar]
- Grander MA, Martin JL, Patel NP, Jackson NJ, Gehrman PR, Pien G, Gooneratne NS. Age and sleep disturbances among American men and women: data from the U.S. Behavioral Risk Factor Surveillance System. Sleep. 2012;35:395–406. doi: 10.5665/sleep.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L. Who has time to sleep? Journal of Public Health. 2005;27:205–211. doi: 10.1093/pubmed/fdi004. [DOI] [PubMed] [Google Scholar]
- Heckman MA, Weil J, Mejia D, Gonzalez E. Caffeine (1,3,7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. Journal of Food Science. 2010;75:R77–R87. doi: 10.1111/j.1750-3841.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- Hendler RA, Ramchandani VA, Gilman J, Hommer DW. Stimulant and sedative effects of alcohol. Current Topics in Behavioral Neurosciences. 2013;13:489–509. doi: 10.1007/7854_2011_135. [DOI] [PubMed] [Google Scholar]
- Hindmarch I, Rigney U, Stanley N, Quinlan P, Rycroft J, Lane J. A naturalistic investigation of the effects of day-long consumption of tea, coffee and water on alertness, sleep onset and sleep quality. Psychopharmacology. 2000;149:203–216. doi: 10.1007/s002130000383. [DOI] [PubMed] [Google Scholar]
- Ishak WW, Ugochukwu C, Bagot K, Khalili D, Zaky C. Energy drinks: psychological effects and impact on well-being and quality of life—a literature review. Innovations in Clinical Neuroscience. 2012;9(1):25. [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, Roehrs T, Roth T, Breslau N. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep. 1998;21:178–186. doi: 10.1093/sleep/21.2.178. [DOI] [PubMed] [Google Scholar]
- Keklund G, Akerstedt T. Objective components of individual differences in subjective sleep quality. Journal of Sleep Research. 1997;6:217–220. doi: 10.1111/j.1365-2869.1997.00217.x. [DOI] [PubMed] [Google Scholar]
- Landolt H-P, Roth C, Dijk D-J, Borbely AA. Late-afternoon ethanol intake affects nocturnal sleep and the sleep EEG in middle-aged men. Journal of Clinical Psychopharmacology. 1996;16:428–436. doi: 10.1097/00004714-199612000-00004. [DOI] [PubMed] [Google Scholar]
- Larson R, Csikszentmihalyi M. The experience sampling method. In: Reis HT, editor. New Directions for Methodology of Social and Behavioral Sciences. Vol. 15. San Francisco, CA: Jossey-Bass; 1983. pp. 41–56. [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffingwell TR, Cooney NJ, Murphy JG, Luczak S, Rosen G, Dougherty DM, Barnett NP. Continuous objective monitoring of alcohol use: 21st century measurement using transdermal sensors. Alcoholism: Clinical and Experimental Research. 2014;37:16–22. doi: 10.1111/j.1530-0277.2012.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca G, Rubio JH, Andries D, Tobback N, Vollenweider P, Waeber G, Tafti M. Age and gender variations of sleep in subjects without sleep disorders. Annals of Medicine. doi: 10.3109/07853890.2015.1074271. (in press) [DOI] [PubMed] [Google Scholar]
- MacLean AW, Cairns J. Dose-response effects of ethanol on the sleep of young men. Journal of Studies on Alcohol. 1982;43:434–444. doi: 10.15288/jsa.1982.43.434. [DOI] [PubMed] [Google Scholar]
- Mitchell G. Revisiting truth or triviality: the external validity of research in the psychological laboratory. Perspectives on Psychological Science. 2012;7:109–117. doi: 10.1177/1745691611432343. [DOI] [PubMed] [Google Scholar]
- Patrick ME, Griffin J, Huntley ED, Maggs JL. Energy drinks and binge drinking predict college students’ sleep quantity, quality, and tiredness. Behavioral Sleep Medicine. doi: 10.1080/15402002.2016.1173554. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. 2015. R Package version 3.1-120. http://CRAN.R-project.org/package=nlme. [Google Scholar]
- Popovici I, French MT. Binge drinking and sleep problems among young adults. Drug & Alcohol Dependence. 2013;132:207–215. doi: 10.1016/j.drugalcdep.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram N, Conroy DE, Pincus AL, Lorek A, Rebar A, Roche MJ, Coccia M, Gerstorf D. Examining the interplay of processes across multiple time-scales: Illustrating with the Intraindividual Study of Affect, Health, and Interpersonal Behavior (iSAHIB) Research in Human Development. 2014;11:142–160. doi: 10.1080/15427609.2014.906739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram N, Gerstorf D. Time structured and net intraindividual variability: Tools for examining the development of dynamic characteristics and processes. Psychology and Aging. 2009;24:778–791. doi: 10.1037/a0017915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. Vol. 1. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Reyner LA, Horne JA, Reyner A. Gender- and age-related differences in sleep determined by home-recorded sleep logs and actimetry from 400 adults. Sleep. 1995;18:127–134. [PubMed] [Google Scholar]
- Roehrs T, Buruvali E, Bonahoom A, Drake C, Roth T. Ethanol and sleep lose: A “dose” comparison of impairing effects. Sleep. 2003;26:891–895. doi: 10.1093/sleep/26.8.981. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, and alcohol use. Alcohol Research & Health. 2001;25:101–109. [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Arnedt JT, Almeida AB, Greece J, Minsky S, Kempler CS, Sales S. Intoxication with bourbon versus vodka: effects on hangover, sleep, and next-day neurocognitive performance in young adults. Alcoholism: Clinical & Experimental Research. 2010;34:509–518. doi: 10.1111/j.1530-0277.2009.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosipal R, Lewandowski A, Dorffner G. In search of objective components of sleep quality indexing in normal sleep. Biological Psychology. 2013;94:210–220. doi: 10.1016/j.biopsycho.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell OH, Lester BK, Griffiths WJ, Williams HL. Alcohol and sleep in young adults. Psychopharmacologia. 1972;26:201–218. doi: 10.1007/BF00422697. [DOI] [PubMed] [Google Scholar]
- Sagawa Y, Kondo H, Matsubuchi N, Takemura T, Kanayama H, Kaneko Y, Kanbayashi T, Hishikawa Y, Shimizu T. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcoholism: Clinical & Experimental Research. 2011;35:2093–2100. doi: 10.1111/j.1530-0277.2011.01558.x. [DOI] [PubMed] [Google Scholar]
- Schwarz N. Retrospective and concurrent self-reports: the rationale for real-time data capture. In: Stone A, Shiffman S, Atienza A, Nebeling L, editors. The science of real-time data capture: self-reports in health research. New York, NY: Oxford University Press; 2007. pp. 11–26. [Google Scholar]
- Shiffman S, Stone A, Hufford M. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Singleton RA, Wolfson AR. Alcohol consumption, sleep, and academic performance among college students. Journal of Studies on Alcohol and Drugs. 2009;70:355–363. doi: 10.15288/jsad.2009.70.355. [DOI] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ. Multilevel analysis: an introduction to basic and advanced multilevel modeling. 2nd. London, UK: Sage Publishers; 2012. [Google Scholar]
- Stone BM. Sleep and low doses of alcohol. Electroencephalography & Clinical Neurophysiology. 1980;48:706–709. doi: 10.1016/0013-4694(80)90427-7. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Bramoweth AD. Patterns and consequences of inadequate sleep in college students: substance use and motor vehicle accidents. Journal of Adolescent Health. 2010;46:610–612. doi: 10.1016/j.jadohealth.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Thadani V, Hutching K, LaBrie J. Alcohol-related information in multi-component interventions and college students’ drinking behavior. Journal of Alcohol & Drug Education. 2014;53:31–51. [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Sharma R, Sahota P. Alcohol disrupts sleep homeostasis. Alcohol. 2015;49:299–310. doi: 10.1016/j.alcohol.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reen E, Jenni OG, Carskadon MA. Effects of alcohol on sleep and the sleep electroencephalogram in healthy young women. Alcoholism: Clinical & Experimental Research. 2006;6:974–981. doi: 10.1111/j.1530-0277.2006.00111.x. [DOI] [PubMed] [Google Scholar]
- Willams DL, MacLean AW, Cairns J. Dose-response effects of ethanol on the sleep of young women. Journal of Studies on Alcohol. 1983;44:515–523. doi: 10.15288/jsa.1983.44.515. [DOI] [PubMed] [Google Scholar]
- Yules RB, Freedman DX, Chandler KA. The effect of ethyl alcohol on man’s electroencephalographic sleep cycle. Electroencephalograpy & Clinical Neurophysiology. 1966;20:109–111. doi: 10.1016/0013-4694(66)90153-2. [DOI] [PubMed] [Google Scholar]