Abstract
The potent estrogen 17β-Estradiol (E2) plays a critical role in mediating hippocampal function, yet the precise mechanisms through which E2 enhances hippocampal memory remain unclear. In young adult female rodents, the beneficial effects of E2 on memory are generally attributed to ovarian-synthesized E2. However, E2 is also synthesized in the adult brain in numerous species, where it regulates synaptic plasticity and is synthesized in response to experiences such as exposure to females or conspecific song. Although de novo E2 synthesis has been demonstrated in rodent hippocampal cultures, little is known about the functional role of local E2 synthesis in mediating hippocampal memory function. Therefore, the present study examined the role of hippocampal E2 synthesis in hippocampal memory consolidation. Using bilateral dorsal hippocampal infusions of the aromatase inhibitor letrozole, we first found that blockade of dorsal hippocampal E2 synthesis impaired hippocampal memory consolidation. We next found that elevated levels of E2 in dorsal hippocampus observed 30 min after object training were blocked by dorsal hippocampal infusion of letrozole, suggesting that behavioral experience increases acute and local E2 synthesis. Finally, aromatase inhibition did not prevent exogenous E2 from enhancing hippocampal memory consolidation, indicating that hippocampal E2 synthesis is not necessary for exogenous E2 to enhance hippocampal memory. Combined, these data are consistent with the hypothesis that hippocampally-synthesized E2 is necessary for hippocampus-dependent memory consolidation in rodents.
Keywords: aromatase, letrozole, estradiol, object recognition
Introduction
In recent years, the sex steroid hormone 17β-estradiol (E2) has been shown to play a vital role in mediating hippocampal function. Although much has been learned in recent years about how E2 regulates hippocampal memory (Daniel, 2006; Fortress and Frick, 2014; Frick, 2012; Luine and Frankfurt, 2012; Srivastava and Evans, 2013; Woolley, 2007), many important questions remain. Historically, the beneficial effects of E2 on memory and hippocampal function have been attributed to ovarian estrogens acting on the brain via a traditional endocrine mechanism. However, E2 can also be synthesized de novo in cognitive regions of the brain from cholesterol or androgen precursors, challenging the long-held dogma that these brain regions are merely targets for peripheral endocrine glands. For example, In vitro data suggests that rodents are able to synthesize E2 de novo in hippocampal slice cultures (Hojo et al., 2004; Kretz et al., 2004; Prange-Kiel et al., 2003). Additionally, data from zebra finches demonstrates that E2 can be rapidly synthesized in the cortex during behavioral experiences including exposure to female conspecifics or conspecific song (Remage-Healey et al., 2010; Remage-Healey et al., 2012; Remage-Healey et al., 2008). This capacity for local steroidogenesis in the brain suggests that neurosteroids like E2 may acutely and precisely modulate neural circuitry by acting on a rapid timescale similar to traditional neurotransmitters (Balthazart and Ball, 2006; Saldanha et al., 2011). Despite these findings, little is known about the behavioral significance of de novo E2 synthesis in the rodent brain, particularly in regions critical for regulating learning and memory, such as the hippocampus.
In vitro work conducted over the past few decades has been instrumental to expanding our understanding of neurosteriodogenesis in the adult vertebrate brain. This progress is due to the discovery that aromatase and all other necessary precursors for E2 synthesis (Abdelgadir et al., 1994; Sanghera et al., 1991; Wehrenberg et al., 2001) are expressed in several regions of the adult brain in a multitude of species, including songbirds, rodents, non-human primates, and humans (Azcoitia et al., 2011; Ivanova and Beyer, 2000; Roselli et al., 1985; Roselli and Resko, 1989; Vockel et al., 1990). At the cellular level, aromatase, the enzyme responsible for converting androgen precursors to estrogens, can be found throughout the hippocampal formation, including granular cells of dentate gyrus and pyramidal cells in CA1 and CA3 in humans and rodents (Azcoitia et al., 2011; Hojo et al., 2011; Yague et al., 2010). Further, intracellular localization studies have documented aromatase throughout the soma and dendrites of hippocampal pyramidal cells, as well as at pre- and post-synaptic terminals (Hojo et al., 2004; Hojo et al., 2011; Lephart, 1996). The presence of aromatase in hippocampal synaptic terminals suggests the potential for local E2 synthesis to rapidly mediate hippocampal plasticity and memory consolidation.
De novo E2 synthesis was first demonstrated in adult rodent hippocampal neurons in vitro (Prange-Kiel et al., 2003), and subsequent work has shown that levels of E2 are substantially higher in the hippocampus than in circulating plasma in both male and female rats (Hojo et al., 2004). In vitro, hippocampal E2 synthesis is markedly reduced by the aromatase inhibitor letrozole (Fester et al., 2012; Hojo et al., 2004; Kretz et al., 2004; Prange-Kiel et al., 2003). The letrozole-mediated reduction in hippocampal E2 levels is accompanied by decreased spine synapse density and pre-synaptic bouton number in rats and mice, suggesting that the loss of local E2 may reduce synaptic connectivity (Kretz et al., 2004; Zhou et al., 2010; Fester et al., 2012). This notion is supported by other in vitro studies demonstrating that aromatase inhibition decreases the expression of the presynaptic membrane protein synaptophysin and the postsynaptic protein spinophillin, key components of spine formation in rat hippocampal neurons (Kretz et al., 2004; Fester et al., 2012). Consistent with these findings, systemic injections of letrozole decrease hippocampal synaptic protein levels in both intact and ovariectomized female mice (Zhou et al., 2010), and impair hippocampal long-term potentiation (LTP) in gonadally-intact male and female mice, as well as ovariectomized female mice (Vierk et al., 2012).
Despite evidence that de novo E2 regulates hippocampal synaptic plasticity, little is known about the role of hippocampal E2 synthesis in mediating memory formation. One recent study showed that systemic treatment with the aromatase inhibitor fadrozole prior to fear extinction training impaired the consolidation of extinction memories in male rats (Graham and Milad, 2014). Interestingly, male rats receiving fadrozole four hours after extinction training exhibited no deficits in later fear extinction recall, suggesting that de novo synthesis is particularly important for fear memory consolidation within the first four hours. However, the systemic injections used in this study did not permit conclusions to be drawn about the role of hippocampal E2 synthesis in memory consolidation. Therefore, the present study sought to address the questions of whether hippocampally-synthesized E2 is essential for either hippocampus-dependent memory consolidation or the memory enhancing effects of exogenous E2. Using bilateral dorsal hippocampal infusions of letrozole, we found that hippocampal E2 synthesis is necessary for both object recognition and spatial memory consolidation in ovariectomized female mice. We further found that letrozole blocked what appeared to be a transient increase in hippocampal E2 synthesis after object training. Finally, our data suggest that hippocampal E2 synthesis is not necessary for exogenous E2 to enhance hippocampal memory consolidation, at least at the doses used here. To our knowledge, this is the first demonstration that hippocampal E2 synthesis is essential for memory consolidation in rodents.
Methods
Subjects
All experiments used young (8–12 week old) female C57BL/6 mice (Taconic, Cambridge City, IN) as subjects. Mice were housed in groups of up to five until surgery, after which they were singly housed. Mice were maintained on a 12 h light/dark cycle with ad libitum access to food and water. All experimental procedures were approved by the University of Wisconsin-Milwaukee Institutional Animal Care and Use Committee and are in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals.
General experimental design
Approximately 4 days after arrival in the lab, mice were implanted with guide cannulae and ovariectomized within a single surgical session. At least one week after surgery, mice underwent behavioral training and testing in an object recognition (OR) task to measure object recognition memory and/or an object placement (OP) task to measure spatial memory. Each task used unique sets of objects to maintain novelty and prevent interference from one task to the other. When, as stipulated below, mice participated in both OR and OP, the order of testing varied within each experiment and testing was separated by two weeks to allow the hippocampus to fully recover from each infusion. For a subset of mice (see below), dorsal hippocampi were collected bilaterally two weeks after completing object recognition testing for measurement of E2 levels.
Surgery
All surgeries were conducted at least one week prior to behavioral testing as described previously (Boulware et al., 2013; Fortress et al., 2013). Mice were anesthetized with isoflurane (5% for induction, 2% for maintenance) in 100% oxygen and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). Female mice underwent ovariectomy and cannula implantation in the same surgical session as described previously (Boulware et al., 2013; Fortress et al., 2013). After completion of ovariectomy, mice were implanted with stainless steel bilateral guide cannulae (Plastics One, Roanoke, VA) aimed at the dorsal hippocampus (DH; C232GC, 22 gauge; −1.7 mm AP, ±1.5 mm ML, and −2.3 mm DV (injection site)) or a triple cannula that consisted of a single cannula aimed at the dorsal third ventricle (intracerebroventricular; ICV; C232GC, 22 gauge; −0.9 mm AP, ± 0.0 mm ML, and −2.8 mm DV (injection site)) in addition to the bilateral dorsal hippocampal cannulae. This triple infusion protocol is routinely used by our lab to prevent potential damage to the DH from two infusions into the DH in rapid succession (Fernandez et al., 2008; Fan et al., 2010; Zhao et al., 2010; Zhao et al., 2012; Boulware et al., 2013; Fortress et al., 2013). Here, we used triple cannulae to administer E2 adjacent to the DH via the dorsal third ventricle while infusing letrozole bilaterally into the DH. This protocol avoids potential damage to the DH from infusions of both compounds, and precludes possible interactions between the drugs within the DH and reduced drug efficacy that could result from the dilution of each drug in the larger ICV infusion volume. Cannulae were fixed to the skull with dental cement (Darby Dental Supply, New York, NY) that served to close the wound. Dummy cannulae (C232DC; Plastics One) were inserted into each cannula to prevent clogging of the cannula tracts. Mice received 10% ibuprofen in their drinking water for 5 days after surgery, and were allowed a minimum of one week to recover before behavioral testing.
Drugs and Infusions
The aromatase inhibitor letrozole (Selleckchem, Houston, TX) was dissolved in sterile 0.9% saline and 2% dimethyl sulfoxide (DMSO) to concentrations of 0.01, 0.05, and 0.1 μg/μl. A volume of 0.5 μl was infused bilaterally into each side of the DH immediately after training. Vehicle-infused controls received infusions of sterile 0.9% saline and 2% DMSO at the same rate and total volume. Hippocampal infusions were conducted at a rate of 0.5 μl/min for 1 min per hemisphere as described previously (Fernandez et al., 2008; Fortress et al., 2013; Zhao et al., 2012; Zhao et al., 2010b), resulting in letrozole doses of 0.005, 0.025, and 0.05 μg/hemisphere. For experiments also involving ICV infusion of E2, cyclodextrin-encapsulated E2 (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile 0.9% saline to a concentration of 10 μg/μl. The vehicle consisted of 2-hydroxypropyl-β-cyclodextrin (HBC; Sigma-Aldrich, St. Louis, MO) dissolved in saline to the same concentration of cyclodextrin present in the cyclodextrin-encapsulated E2 solution. ICV infusions were conducted at the same rate as DH infusions (0.5 μl/min) for 2 min total, to allow for infusion of the same total volume at the same rate as DH infusions.
Behavioral Testing
Object recognition (OR) and object placement (OP) were used to measure object recognition and spatial memory as we have previously described (Boulware et al., 2013; Fortress et al., 2013). Both tasks involve the DH (Baker and Kim, 2002; Cohen et al., 2013; Fernandez et al., 2008). In ovariectomized mice, immediate post-training bilateral infusion of 5 μl E2 into the dorsal hippocampus enhances OR tested 48 h after infusion (Boulware et al., 2013; Fan et al., 2010; Fernandez et al., 2008; Fortress et al., 2013; Fortress et al., 2014; Pereira et al., 2014; Zhao et al., 2012; Zhao et al., 2010a) and OP tested 24 h after infusion (Boulware et al., 2013; Fortress et al., 2014). Mice were first handled for one min/day for three days prior to habituation. After the first day of handling, a Lego was placed in each home cage to habituate the mice to objects during the remaining handling days and habituation period. After three days of handling, mice were habituated to the apparatus for two consecutive days by allowing them to explore the empty white arena (60 cm x 60 cm x 47 cm) for 5 min/day. For the OR task, mice were then returned to the arena and allowed to accumulate 30 sec exploring two identical objects placed about 5 cm from the upper left and right corners of the arena, or until 20 min had elapsed. Immediately after training, mice received DH or DH + ICV drug infusions. Such post-training infusions allowed us to pinpoint effects of aromatase inhibition specifically to the memory consolidation period, while also minimizing the confounding effects of hormones on performance factors (e.g., motivation, anxiety) during training or retention testing (Frick and Gresack, 2003; McGaugh, 1989). Object recognition memory was then tested 24 or 48 hours later by measuring the amount of time spent with the novel and familiar object. A 24-hour delay was used to measure the potential memory-impairing effects of letrozole because vehicle-infused ovariectomized females show intact OR memory consolidation at this time point (Fortress et al., 2013, Boulware et al., 2013). A 48-hour delay was used to measure whether letrozole blocked the memory-enhancing effects of E2 because vehicle-infused females show impaired OR memory consolidation, whereas E2-infused females show intact OR memory consolidation, at this time point (Fortress et al., 2013, Boulware et al., 2013). Moreover, E2-infused females spend significantly more time with the novel object than vehicle-infused females 48 hours after infusion (Kim, 2016). Because most mice inherently prefer novelty, those that remember the training objects spend more time than chance (15 sec) exploring the novel object during testing. Chance was set at 15 sec because this is the value at which mice spend exactly the same amount of time with each object. Therefore, chance levels of exploration indicate that mice do not remember the training objects.
Training and testing for OP was identical to OR, except that testing was conducted four hours after training, and involved moving one of the identical training objects to a new location in the arena (lower right or lower left corner). Spatial memory consolidation was demonstrated if the mice spent more time than chance with the moved object. At this four-hour delay, vehicle-infused ovariectomized females show intact OP memory (Boulware et al., 2013), thereby permitting observation of letrozole-induced memory impairments. A 24-hour delay was used to determine whether letrozole could block the memory-enhancing effects of E2 on OP memory consolidation. At this time point, E2-infused, but not vehicle-infused, mice spend more time than chance with the moved object and E2-infused mice spend significantly more time with the moved object than vehicle-infused mice (Boulware et al., 2013; Kim, 2016).
E2 Measurement
E2 levels were measured in the DH using an EIA assay following dual liquid-solid phase extraction (Chao et al., 2011). Briefly, frozen DH tissue was first homogenized in 0.1 M phosphate buffer, followed by three rounds of ether extraction. The final organic phase was dried under air in a 50 C water bath, followed by re-suspension in 250 μl of 0.1 M phosphate buffer prior to solid phase extraction. Re-suspended samples were then eluted through C18 columns under vacuum pressure and washed with ddH2O to elute hydrophilic polar compounds. Hydrophobic compounds, including steroids, were then eluted with a series of washes with 100% methanol, followed by an evaporation step under air in a 50 C water bath. After drying, samples were suspended in EIA buffer. E2 concentrations were then measured from EIA plates per the manufacturer’s instructions using an Epoch Microplate Spectrophotometer plate reader (Biotek) with a 450 nm filter and Gen5 Software (Chao et al., 2011). The EIA assay used to measure brain estrogen levels in this experiment is highly specific for E2 (cross-reactivity: 14% for E2-3-glucuronide; 12% for estrone; 1% for E2-17-glucuronide; < 0.10% for other major steroids including testosterone; Cayman chemical). Data are expressed in pg E2 per mg wet weight of DH tissue, after correcting for recovery (recovery was 60.6% and intrassay CV was 2.17%).
Data analysis
All statistical analyses were conducted using GraphPad Prism 6 (La Jolla, CA). To determine whether each group demonstrated intact memory for each task, OR and OP data were first analyzed using one sample t-tests to determine if the time spent with the novel or moved object differed significantly from chance (15 seconds; Fortress et al., 2013; Boulware et al., 2013). This analysis is used because time spent with the objects is not independent; time spent with one object reduces time spent with the other object (Frick and Gresack, 2003). To then measure between-group treatment differences for each task, one-way ANOVAs with treatment as the independent variable were conducted for each behavioral experiment followed by Tukey’s post hoc tests. EIA data were analyzed using a two-way ANOVA, followed by Tukey’s post hocs when appropriate, to determine effects of post-training drug treatments over time. Statistical significance for all analyses was determined as p ≤ 0.05. Finally, effect sizes were calculated using eta-squared for ANOVAs (η2 = sum of squares between/sum of squares total) and Cohen’s d (d = mean of differences/standard deviation of differences) for pair-wise comparisons.
Results
Blocking dorsal hippocampal E2 synthesis immediately after training impairs object recognition and object placement memory consolidation
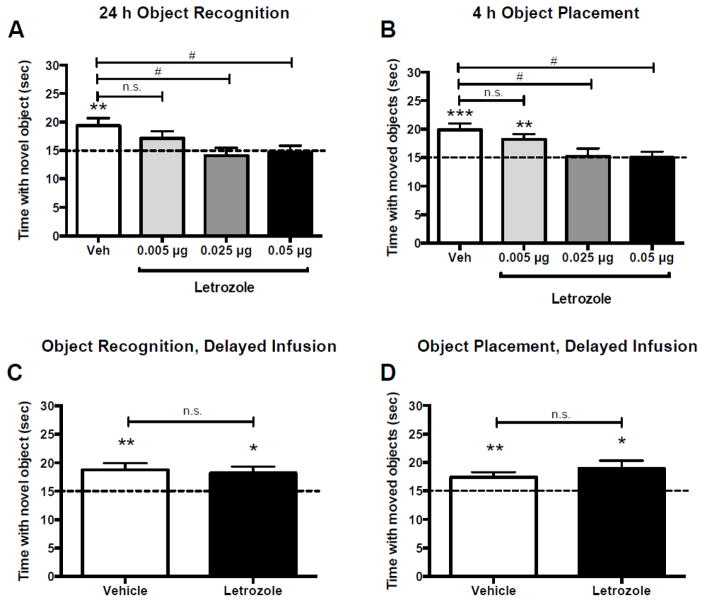
To determine if hippocampal E2 synthesis is necessary for the consolidation of object memories, young female mice were ovariectomized and then bilaterally implanted with cannulae aimed at the DH one week prior to the start of behavioral training. Immediately after OR training, mice received bilateral DH infusion of vehicle or one of three doses of the aromatase inhibitor letrozole (0.005, 0.025, or 0.05 μg/hemisphere; n=9–13/group). Object recognition memory was tested 24 hours later, a time point at which young ovariectomized vehicle-treated mice remember the familiar object (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Zhao et al., 2010b). Vehicle-infused mice spent significantly more time than chance with the novel object (t(12) = 3.35, p = 0.006; Fig 1A), demonstrating intact memory for the familiar training object. However, mice infused with 0.005, 0.025, or 0.05 μg/hemisphere letrozole spent similar amounts of time with the familiar and novel objects (0.005 μg: t(8) = 1.64, p = 0.14; 0.025 μg: t(9) = 0.67, p = 0.52; 0.05 μg: t(11) = 0.26, p = 0.80; Fig. 1A), indicating impaired OR memory. These effects were supported by a one-way ANOVA, which demonstrated a significant main effect of treatment (F(3,40) = 3.81, p = 0.017, η2 = 0.22), and Tukey’s post-hoc tests which revealed that mice infused with vehicle spent significantly more time with the novel object than mice infused with 0.025 or 0.05 μg letrozole (ps < 0.05; Fig. 1A). Elapsed time to accumulate 30 s of exploration did not differ among the groups during testing (F(3,40) = 1.02, p = 0.39; Vehicle = 649.7 ± 84.61; 0.005 μg = 821.4 ± 87.07; 0.025 μg = 669.7 ± 46.51; 0.05 μg = 714.4 ± 57.07).
Figure 1. Letrozole impairs object recognition and object placement memory consolidation.
(A) Mice receiving bilateral dorsal hippocampal infusion of vehicle (n=13), but not 0.005 μg (n=9), 0.025 μg (n=10), or 0.05 μg (n=12) letrozole, immediately after training spent significantly more time with the novel object 24 h after training relative to chance (dashed line at 15 sec). Vehicle-infused mice also spent significantly more time with the novel object than mice infused with 0.025 or 0.05 μg letrozole. These data suggest that the 0.025 and 0.05 μg doses of letrozole blocked object recognition memory consolidation. (B) Mice receiving bilateral dorsal hippocampal infusions of vehicle (n=12) or 0.005 μg (n=9) letrozole, but not 0.025 μg (n=9) or 0.05 μg (n=9) letrozole, immediately after training spent significantly more time than chance (*p < 0.05) with the moved object 4 h after training. Vehicle-infused mice also spent significantly more time with the moved object than mice infused with 0.025 or 0.05 μg letrozole, suggesting that the 0.025 and 0.05 μg doses of letrozole blocked also spatial memory consolidation. This effect was limited to within the first 2–3 hours after training, as mice receiving a bilateral infusion of vehicle (n=11) or 0.025 μg letrozole (n=10) 3 h after OR training (C), or 2 h after OP training (vehicle: n=13; letrozole: n=9) (D), spent significantly more time than chance with the novel object (*p < 0.05), suggesting that the memory-impairing effects of letrozole are specific to the consolidation period immediately after training. *p < 0.05, **p < 0.01, ***p < 0.001 relative to chance; #p < 0.05 for between-group differences measured by Tukey’s post hoc tests
Next, to determine if local E2 is also essential for spatial memory consolidation, a different set of mice received bilateral DH infusion of vehicle or one of the same three doses of letrozole (n=9–12/group) immediately after OP training. Spatial memory was tested four hours later, a time point at which young ovariectomized vehicle-treated mice remember the unmoved object (Boulware et al., 2013). Mice infused with either vehicle (t(11) = 4.40, p = 0.001) or 0.005 μg/hemisphere letrozole (t(8) = 3.53, p = 0.008) spent significantly more time than chance with the moved object (Fig. 1B), demonstrating intact memory, whereas those infused with 0.025 or 0.05 μg/hemisphere letrozole exhibited impaired OP memory (0.025 μg: t (8) = 0.18, p = 0.86; 0.05 μg: t (8) = 0.02, p = 0.98; Fig. 1B). As with OR, one-way ANOVA demonstrated a significant main effect of treatment (F(3,35) = 4.60, p = 0.008, η2 = 0.28) and Tukey’s post-hoc tests revealed that mice infused with vehicle spent significantly more time with the moved object than mice infused with 0.025 or 0.05 μg letrozole (ps < 0.05; Fig. 1B). Elapsed time to accumulate 30 s of exploration did not differ among the groups during testing (F(3,35) = 2.12, p = 0.12; Vehicle = 569.8 ± 61.30; 0.005 μg = 408.8 ± 43.65; 0.025 μg = 442.3 ± 50.43; 0.05 μg = 564.0 ± 63.16). Together, these data provide the first evidence that local E2 synthesis in the DH is necessary for the consolidation of hippocampus-dependent object recognition and spatial memories in young female mice.
To demonstrate that the post-training effects of letrozole on memory are specific to the memory consolidation period immediately after training, we next infused new mice with vehicle or 0.025 μg letrozole three hours after OR training or two hours after OP training. These delays were chosen because the memory-enhancing effects of post-training systemic or intrahippocampal E2 treatment are restricted to between 1–3 hours after training (Fernandez et al., 2008; Frye et al., 2007; Packard and Teather, 1997; Walf et al., 2006). In our own hands, post-training intrahippocampal infusion of E2 delayed 3 hours after training did not enhance OR memory consolidation in ovariectomized mice (Fernandez et al., 2008). We used this same delay for OR in the present study, and used a 2-hour delay for OP based on our experience that OP memory decays faster than OR memory (Boulware et al., 2013). In the present study, memory was tested 24 hours later in OR, or 4 hours later in OP (as in Fig. 1A and B). We found that mice infused with either vehicle (t(10) = 3.16, p = 0.01) or 0.025 μg/hemisphere letrozole (t(9) = 2.83, p = 0.02) three hours after OR training spent significantly more time than chance with the novel object (Fig. 1C). Moreover, mice infused with vehicle or letrozole did not differ in the time spent with the novel object (t(19) = 0.33, p = 0.74, d = 0.15). Together, these results demonstrate that letrozole infusion did not block OR memory consolidation when delayed three hours after training. Similarly, we found that mice infused with either vehicle (t(12) = 2.86, p = 0.01) or 0.025 μg/hemisphere letrozole (t(8) = 2.91, p = 0.02) two hours after OP training spent significantly more time than chance with the moved object, and the groups again did not differ from each other (t(20) = 0.99, p = 0.33, d = 0.15), demonstrating that a delayed infusion of letrozole also failed to block OP memory consolidation. Together, these findings support the notion that hippocampal de novo synthesis is particularly critical for memory formation during the consolidation period that occurs immediately after training.
Letrozole reduces dorsal hippocampal E2 levels 30 minutes after object training
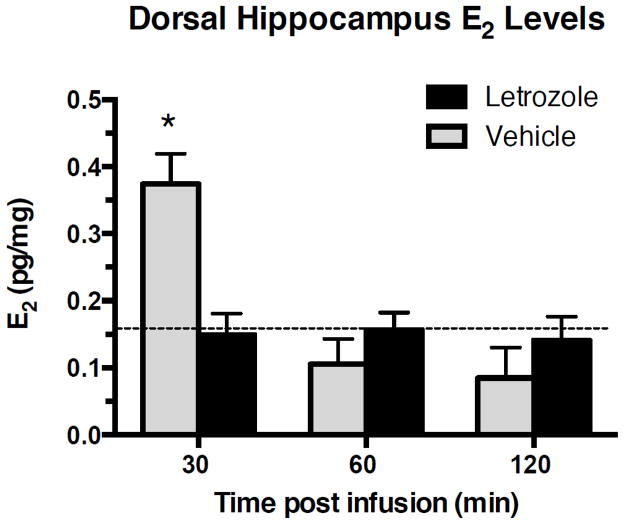
We next measured levels of E2 in the DH at various points after object training to determine: 1) the extent to which letrozole reduced E2 levels within the DH, and 2) if letrozole could block a learning-induced increase in DH E2 levels. Two weeks after the completion of OR testing, 28 mice included in Fig. 1A were trained with two new identical objects and then immediately received a DH infusion of vehicle or the lowest dose of letrozole shown to impair memory in both OR and OP (0.025 μg/hemisphere). The DH was then dissected 30, 60, and 120 min later (30 min: n=3 Veh, n=6 Let; 60 min: n=3 Veh, n=7 Let; 120: n=3 Veh, n=5 Let) on wet ice and stored at −80 °C prior to dual liquid and solid phase extraction for quantification of DH E2 content by EIA. A two-way ANOVA revealed a significant interaction between treatment and time (F(2, 21) = 8.55, p = 0.002, η2 = 0.303; Fig. 2) and a main effect of treatment (F(2, 21) = 8.58, p = 0.002, η2 = 0.025). Post hoc analyses revealed that DH E2 levels were significantly higher 30 min after training in mice infused with vehicle relative to those infused with letrozole (t(7) = 4.06, p = 0.005, d = 2.87; Fig. 2). DH E2 levels in vehicle-treated mice dropped substantially by 60 min after training, and were similar to those of letrozole-treated mice at the 60 min and 120 min time points (60 min: t(8) = 1.11, p = 0.31, d = 0.75; 120 min: t(6) = 0.82, p = 0.44, d = 0.60; Fig. 2). DH E2 levels were similar in absolute quantity to those reported in other studies of hippocampal and brain DH E2 content in mice (Overk et al., 2013) and songbirds (Fokidis et al., 2015; Heimovics et al., 2016), although most studies, including the current study, focus on relative quantity of brain E2 content between regions or time periods. These data suggest that letrozole suppresses an increase in DH E2 levels 30 min after infusion at a dose shown to impair OR and OP memory consolidation in vivo.
Figure 2. Letrozole reduces hippocampal E2 levels at a dose that impairs spatial and object recognition memory consolidation.
Mice receiving bilateral dorsal hippocampal infusion of 0.025 μg letrozole had significantly lower dorsal hippocampal E2 levels than vehicle-treated mice 30 min after infusion (*p < 0.05; vehicle n=3; letrozole n=6). Dorsal hippocampal E2 levels in vehicle-treated mice dropped to the level of those in letrozole-treated mice by 60 min after training, and were similar to that of letrozole-treated mice at the 60 min (vehicle n=3; letrozole n=7) and 120 min (vehicle n=3; letrozole n=5) time points. Dashed horizontal line indicates the average background estradiol concentration as reported by EIA for control wells; all estradiol levels above this concentration are validated as detectable levels on the EIA.
Dorsal hippocampal E2 synthesis is not necessary for exogenous E2 to enhance hippocampal memory consolidation
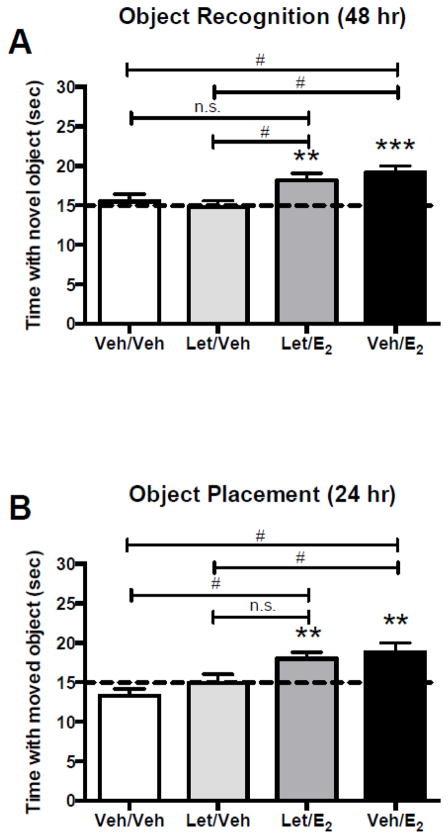
Previous work from our laboratory has demonstrated that 5 μg E2 infused bilaterally into the dorsal hippocampus or 10 μg infused into the dorsal third ventricle (ICV) enhances the consolidation of OR and OP memories in young ovariectomized female mice in an ERK-dependent manner (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Zhao et al., 2010b). However, these studies did not take into account the potential contribution of local E2 synthesis to the observed memory enhancements. In the hypothalamus of ovariectomized monkeys, exogenous estradiol benzoate infusion increases the release of local E2 (Kenealy et al., 2013), suggesting potentially important interactions between exogenous and local E2. To determine if local and exogenous E2 may synergistically facilitate memory consolidation, we next investigated the role of local E2 synthesis in the memory-enhancing effects produced by exogenous E2. Mice were ovariectomized and then implanted with bilateral DH cannulae and a unilateral ICV cannula as in our previous work (e.g., Boulware et al., 2013; Fortress et al., 2013). Immediately after object training, mice received a DH infusion of 2% DMSO in sterile saline vehicle or letrozole (0.025 μg/hemisphere), followed immediately by an ICV infusion of HBC vehicle or 10 μg E2. OR and OP memory were then tested 48 or 24 hours later, respectively, because exogenous E2 enhances memory in these tasks at these time points (e.g., Boulware et al., 2013; Fortress et al., 2013). The same mice were used in both tasks, with the order of presentation of OR and OP varied within each group. Mice receiving vehicle infusion into the DH, followed by ICV infusion of vehicle or letrozole, did not spend more time than chance with the novel object in the OR task (Veh/Veh: t(9) = 0.51, p = 0.62; Let/Veh: t(10) = 0.27, p = 0.79; Fig. 3A), or the moved object in the OP task (Veh/Veh: t(9) = 1.91, p = 0.09; Let/Veh: t(10) = 0.10, p = 0.93; Fig. 3B). In contrast, mice who were bilaterally infused with vehicle or letrozole into the DH, followed by ICV infusion of E2, spent more time than chance with the novel object 48 h after OR training (Veh/E2: t(11) = 4.75, p = 0.0006; Let/E2: t (13) = 3.40, p = 0.005; Fig. 3A), and with the moved object 24 h after OP training (Veh/E2: t(11) = 3.20, p = 0.01; Let/E2: t(11) = 3.73, p = 0.003; Fig. 3B). For OR, one-way ANOVA demonstrated a significant main effect of treatment (F(3,43) = 5.32, p = 0.003, η2 = 0.27), and Tukey’s post-hoc tests revealed that Veh/E2 mice spent significantly more time with the novel object than Veh/Veh or Let/Veh mice (ps < 0.05; Fig. 3A). Let/E2 mice spent significantly more time with the novel object than Let/Veh mice (p < 0.05; Fig. 3A). Similarly, for the OP task, one-way ANOVA demonstrated a significant main effect of treatment (F(3,41) = 6.28, p = 0.001, η2 = 0.32), and Tukey’s post-hoc tests revealed that Let/E2 mice spent significantly more time with the moved object than Veh/Veh mice (ps < 0.05; Fig. 3B). Veh/E2 mice also spent significantly more time with the moved object relative to Veh/Veh or Let/Veh mice. Elapsed time to accumulate 30 s of exploration did not differ among the groups during OP testing (F(3,41) = 0.36, p = 0.783; Veh/Veh = 746.1 ± 67.01; Let/Veh = 748.8 ± 69.77; Let/E2 = 785.0 ± 78.24; Veh/E2 = 837.2 ± 67.40). Elapsed time to accumulate 30 s of exploration in OR did vary among the groups, seemingly due to the increased time taken by Veh/E2 mice (F(3,43) = 4.33, p = 0.01; Veh/Veh = 649.4 ± 65.93; Let/Veh = 588.0 ± 62.02; Let/E2 = 767.5 ± 72.10; Veh/E2 = 901.1± 55.86). However, this increase is unlikely to have significantly contributed to the time spent with the novel object because time spent with the objects and elapsed time were not correlated in any behavioral experiment reported here or in previous publications (e.g., Fernandez et al., 2008). Together, these data suggest that inhibition of local E2 synthesis in the DH does not prevent exogenous E2 from enhancing object recognition or spatial memory consolidation, and suggest that hippocampal de novo E2 synthesis is not necessary for exogenous E2 to enhance memory, at least at the 10 μg dose.
Figure 3. Dorsal hippocampal E2 synthesis is not necessary for exogenous DH infusion of E2 to enhance object recognition or spatial memory consolidation.
Mice receiving infusions of vehicle or 0.025 μg letrozole into the dorsal hippocampus, followed by ICV infusion of vehicle (Veh/Veh, Let/Veh) did not spend significantly more time than chance with the novel object (A) or moved object (B). In contrast, mice receiving bilateral infusion of vehicle or 0.025 μg letrozole into the dorsal hippocampus, followed by ICV infusion of E2, (Veh/E2, Let/E2) spent significantly more time than chance (dashed line at 15 sec, *p < 0.05) with the novel object 48 h after training (A) and the moved object 24 h after training (B). For the OR task, the Veh/E2 group (n=12) spent significantly more time with the novel object than both the Let/Veh (n=11) and the Veh/Veh group (n=10), and the Let/E2 group (n=14) spent more time with the novel object relative to the Let/Veh control group. For the OP task, the Veh/E2 group (n=12) spent significantly more time with the novel object than both the Let/Veh (n=11) and the Veh/Veh groups (n=10), and the Let/E2 group (n=12) spent more time with the novel object relative to the Veh/Veh control group. These data suggest that inhibition of local E2 synthesis in the dorsal hippocampus is not sufficient to prevent exogenous E2 from enhancing object recognition or spatial memory consolidation. **p < 0.01, ***p < 0.001 relative to chance; #p < 0.05 for between-group differences measured Tukey’s post hoc tests
Discussion
Exogenous estrogens have long been known to regulate many types of learning and memory mediated by the hippocampus and other brain regions (see (Bean et al., 2015; Daniel et al., 2015; Duarte-Guterman et al., 2015; Ervin et al., 2015; Foster, 2012; Frick, 2015; Frick et al., 2015; Korol and Pisani, 2015; Luine, 2014) for recent reviews). The current findings provide novel insight into the functional role of brain-derived estrogens on learning and memory in rodents. First, we demonstrated that hippocampal E2 synthesis immediately after training is necessary for object recognition and spatial memory consolidation in young ovariectomized mice. Next, we found that DH E2 levels are elevated 30 min after novel object training relative to later time points, and that this elevation is blocked by DH infusion of an aromatase inhibitor at a dose that impairs recognition and spatial memory consolidation in vivo. Together, these data suggest E2 synthesis may increase acutely in an experience-dependent manner, and that this increase is necessary for the consolidation of recognition and spatial memories. We also found in a subsequent experiment that local E2 synthesis is not necessary for exogenous E2 to enhance object recognition and spatial memory consolidation, at least at the 10 μg dose of E2 we used. Collectively, these findings are the first to demonstrate that de novo E2 synthesis in the dorsal hippocampus is necessary for the consolidation of hippocampus-dependent memories in female rodents. Further, these experiments provide novel insights into the role of dorsal hippocampal E2 synthesis in the memory-enhancing effects of exogenous E2 in rodents.
Our data showing that letrozole blocks OR and OP memory consolidation are consistent with previous in vitro studies suggesting that de novo E2 synthesis regulates the expression of synaptic proteins, synaptic spine density, and LTP (Fester et al., 2012; Kretz et al., 2004; Vierk et al., 2014; Vierk et al., 2012). Our present findings are also consistent with recently published in vivo studies showing that de novo E2 synthesis in the hippocampus is important for spatial memory in male and female zebra finches (Bailey et al., 2013; Rensel et al., 2013), and a recent study conducted in male rats demonstrating the necessity of de novo E2 synthesis for the extinction of fear memories (Graham and Milad, 2014). The latter study administered the aromatase inhibitor fadrozole systemically, precluding definitive conclusions about the key locus of E2 synthesis in the brain for extinction. However, given the important role of the basolateral amygdala in fear extinction (Zeidan et al., 2011), de novo E2 synthesis in this brain region may play a role. Other recent data supports the importance of local E2 synthesis by showing that lentiviral delivery of estrogen receptor α (ERα) to the hippocampus ofovariectomized ERα knockout mice or middle-aged rats reverses spatial memory deficits and increases ERK activation in the absence of exogenous E2 (Foster et al., 2008; Witty et al., 2012). These data suggest that endogenously synthesized E2 may have bound to the virally-introduced ERα to facilitate spatial memory formation. Together with the present data, the findings from zebra finches and rodents suggest that regulation of memory by de novo E2 synthesis may be a general property of both male and female adult vertebrate brains, and that this process is particularly important during the consolidation phase of memory in male and female rodents. Although numerous rodent studies have demonstrated that exogenous E2 treatment drives p42 ERK activation (Boulware et al., 2013; Fernandez et al., 2008; Srivastava et al., 2008), the mechanisms through which de novo E2 facilitates memory consolidation in rodents are currently unknown. Therefore, future studies should address whether local E2 synthesis is necessary and/or sufficient to activate the MAPK/ERK pathway and its downstream targets, which would ultimately influence the expression of genes that support learning and memory.
The current study also provides the first evidence that a learning experience may increase local E2 synthesis in the rodent hippocampus. The fact that the elevated E2 levels in vehicle-treated mice observed 30 min after training was not observed at 60 or 120 min later, suggests a transient increase in hippocampal E2 synthesis driven by object training. Although the high E2 levels at 30 min cannot be definitively attributed to learning in the absence of samples collected at 0 min, the fact that hippocampal E2 levels were suppressed following 30 min of letrozole infusion into the DH infusion suggests that aromatase inhibition blocked an acute elevation due to learning or methodological parameters (e.g., infusion). The nature of such a putative change must therefore be tested in future experiments, and the role of DH E2 synthesis, degradation, and conjugation vis-à-vis our quantification of dynamic DH E2 levels are still unclear. Nevertheless, the 30-min data serve to confirm that DH infusion of letrozole suppresses local E2 synthesis in the DH at a dose that impairs recognition and spatial memory consolidation in vivo. The putative experience-induced increase in hippocampal E2 levels observed here is consistent with avian studies reporting experience-induced changes in the male zebra finch forebrain after social interactions with female conspecifics or exposure to various auditory stimuli (Remage-Healey et al., 2010; Remage-Healey et al., 2011; Remage-Healey et al., 2008). Also, our finding that DH delivery of the aromatase inhibitor letrozole suppresses local E2 synthesis is consistent with zebra finch studies showing suppression of acute changes in E2 in the caudomedial nidopallium (NCM) of male zebra finches after retrodialysis of the aromatase inhibitor fadrozole (Remage-Healey et al., 2010; Remage-Healey et al., 2008). In the zebra finch forebrain, it has also been established that rapid changes in E2 synthesis are dependent upon Ca2+ influx, much like classical neurotransmitters (Remage-Healey et al., 2011). Evidence from zebra finches also shows that blocking de novo E2 synthesis in the NCM disrupts neuronal response properties, such as spike rate and burst firing activity, during the processing of auditory stimuli (Remage-Healey et al., 2010). Furthermore, suppressing de novo E2 synthesis in the NCM changes firing rate and stimulus selectivity in the higher vocal center (HVC), a structure that receives indirect afferent input from the NCM. Thus, suppressing the ability of one region to synthesize E2 impacts auditory processing in other downstream target regions that receive input from the NCM (Remage-Healey and Joshi, 2012). Although the present study did not measure the electrophysiological consequences of blocking local E2 in female mice in vivo, the avian data and ex vivo rodent data suggest that such investigations may provide valuable additional insight into the mechanisms through which local E2 synthesis influences cognitive function.
Finally, the present findings also suggest that local E2 synthesis is not essential for exogenous E2 to exert its beneficial effects on object recognition and spatial memory consolidation. These findings do not align with previously reported in vitro data showing that treatment of hippocampal cells with an aromatase inhibitor prevents exogenous E2 from increasing mRNA and protein expression of synaptic plasticity markers PSD-95 and Arc (Chamniansawat and Chongthammakun, 2012), or of the presynaptic marker synaptophysin in hippocampal slice cultures (Kretz et al., 2004). There are several possible reasons for this discrepancy. First, the previous studies were conducted in vitro and the duration of exposure to the aromatase inhibitor was chronic (4 – 12 days), instead of the single acute infusion used in the current study. It is possible that chronic delivery in our study would have prevented exogenous E2 from enhancing hippocampal memory consolidation. Second, the synaptic proteins measured in the in vitro studies discussed above may not be necessary for E2 to enhance object recognition or spatial memory consolidation, as the necessity of these specific proteins for E2-induced memory enhancement has not been directly evaluated in our behavioral paradigms. Third, the dose of exogenous E2 used in the present study may have been too high for local inhibition to matter, as much lower doses of E2 were used in the aforementioned in vitro studies (10−7 – 10−12 M E2 compared to 10 μg E2 in this study). Finally, the timing of aromatase inhibitor and E2 administration may have played an important role in our findings. That is, local E2 synthesis may not have been sufficiently suppressed by the time exogenous E2 was administered. Thus, exogenous E2 may have activated the cell-signaling cascades (i.e., ERK, PI3K, mTOR) necessary for E2 to enhance memory (Boulware et al., 2013; Fortress et al., 2013) before letrozole had time to suppress local E2 levels. Delaying the infusion of E2, perhaps by 30–60 min, may have better allowed for an observation of interactions between local and exogenous E2.
In conclusion, the present study provides novel insights into the role of hippocampally-synthesized E2 in mediating hippocampal memory consolidation in female mice. This is the first study to demonstrate that dorsal hippocampal E2 synthesis is necessary for the consolidation of object recognition and spatial memories in rodents. Moreover, our finding that E2 levels are elevated in the DH within 30 min of object training suggests that rodents synthesize E2 in the hippocampus in an experience-dependent manner. However, the mechanisms that may regulate dorsal hippocampal E2 synthesis remain unclear at the present time. Finally, our data also suggest that dorsal hippocampal E2 synthesis is not necessary for exogenous DH infusion of E2 to enhance object recognition and spatial memory consolidation, at least at the dose of E2 used here. Collectively, these findings shed new light into the contribution of dorsal hippocampal E2 synthesis to learning and memory, and build a foundation for future studies to investigate the molecular mechanisms through which de novo E2 mediates memory in rodents.
Highlights.
Hippocampal estradiol synthesis is necessary for object recognition memory
Letrozole blocks elevated estradiol levels observed after object learning
Hippocampal estradiol is not necessary for exogenous estradiol to enhance memory
Acknowledgments
This project was supported by the University of Wisconsin-Milwaukee, a UWM Foundation Research Growth Initiative award to K.M.F, NIH R00NS066179 and NSF IOS1354906 to L.R.H, and a UWM Advanced Opportunity Program Fellowship and Department of Psychology Summer Research Fellowship to J.J.T. We would also like to thank the Office of Undergraduate Research at the University of Wisconsin-Milwaukee for their support of J.S.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–147. doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Ma C, Soma KK, Saldanha CJ. Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinology. 2013;154:4707–4714. doi: 10.1210/en.2013-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn Mem. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Bean LA, Kumar A, Rani A, Guidi M, Rosario AM, Cruz PE, Golde TE, Foster TC. Re-Opening the Critical Window for Estrogen Therapy. J Neurosci. 2015;35:16077–16093. doi: 10.1523/JNEUROSCI.1890-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamniansawat S, Chongthammakun S. A priming role of local estrogen on exogenous estrogen-mediated synaptic plasticity and neuroprotection. Exp Mol Med. 2012;44:403–411. doi: 10.3858/emm.2012.44.6.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A, Schlinger BA, Remage-Healey L. Combined liquid and solid-phase extraction improves quantification of brain estrogen content. Front Neuroanat. 2011;5:57. doi: 10.3389/fnana.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdottir HN, Stackman RW., Jr The rodent hippocampus is essential for nonspatial object memory. Curr Biol. 2013;23:1685–1690. doi: 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: What have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Witty CF, Rodgers SP. Long-term consequences of estrogens administered in midlife on female cognitive aging. Horm Behav. 2015;74:77–85. doi: 10.1016/j.yhbeh.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Guterman P, Yagi S, Chow C, Galea LA. Hippocampal learning, memory, and neurogenesis: Effects of sex and estrogens across the lifespan in adults. Horm Behav. 2015;74:37–52. doi: 10.1016/j.yhbeh.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Ervin KS, Lymer JM, Matta R, Clipperton-Allen AE, Kavaliers M, Choleris E. Estrogen involvement in social behavior in rodents: Rapid and long-term actions. Horm Behav. 2015;74:53–76. doi: 10.1016/j.yhbeh.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fester L, Prange-Kiel J, Zhou L, Blittersdorf BV, Bohm J, Jarry H, Schumacher M, Rune GM. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. J Steroid Biochem Mol Biol. 2012;131:24–29. doi: 10.1016/j.jsbmb.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Fokidis HB, Adomat HH, Kharmate G, Hosseini-Beheshti E, Guns ES, Soma KK. Regulation of local steroidogenesis in the brain and in prostate cancer: lessons learned from interdisciplinary collaboration. Front Neuroendocrinol. 2015;36:108–129. doi: 10.1016/j.yfrne.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in dorsal hippocampus. Learn Mem. 2013;20:147–155. doi: 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Frick KM. Epigenetic regulation of estrogen-dependent memory. Front Neuroendocrinol. 2014;35:530–549. doi: 10.1016/j.yfrne.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Kim J, Poole RL, Gould TJ, Frick KM. 17beta-Estradiol regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice. Learn Mem. 2014;21:457–467. doi: 10.1101/lm.034033.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22:656–669. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL. Viral vector-mediated delivery of estrogen receptor-alpha to the hippocampus improves spatial learning in estrogen receptor-alpha knockout mice. Molecular Therapy. 2008;16:1587–1593. doi: 10.1038/mt.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Building a better hormone therapy? How understanding the rapid effects of sex steroid hormones could lead to new therapeutics for age-related memory decline. Behav Neurosci. 2012;126:29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 2015;74:4–18. doi: 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Frick KM, Kim J, Tuscher JJ, Fortress AM. Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn Mem. 2015;22:472–493. doi: 10.1101/lm.037267.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Inhibition of estradiol synthesis impairs fear extinction in male rats. Learn Mem. 2014;21:347–350. doi: 10.1101/lm.034926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Prior NH, Ma C, Soma KK. Rapid effects of an aggressive interaction on dehydroepiandrosterone, testosterone and oestradiol levels in the male song sparrow brain: A seasonal comparison. J Neuroendocrinol. 2016:28. doi: 10.1111/jne.12345. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Higo S, Kawato S, Hatanaka Y, Ooishi Y, Murakami G, Ishii H, Komatsuzaki Y, Ogiue-Ikeda M, Mukai H, Kimoto T. Hippocampal synthesis of sex steroids and corticosteroids: essential for modulation of synaptic plasticity. Front Endocrinol (Lausanne) 2011;2:43. doi: 10.3389/fendo.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova T, Beyer C. Ontogenetic expression and sex differences of aromatase and estrogen receptor-alpha/beta mRNA in the mouse hippocampus. Cell Tissue Res. 2000;300:231–237. doi: 10.1007/s004410000199. [DOI] [PubMed] [Google Scholar]
- Kenealy BP, Kapoor A, Guerriero KA, Keen KL, Garcia JP, Kurian JR, Ziegler TE, Terasawa E. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci. 2013;33:19051–19059. doi: 10.1523/JNEUROSCI.3878-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Szinte JS, Boulware MI, Frick KM. 17β-estradiol and agonism of G-protein Coupled Estrogen Receptor (GPER) enhance hippocampal memory via different cell-signaling mechanisms. J Neurosci. 2016 doi: 10.1523/JNEUROSCI.0257-15.2016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, Pisani SL. Estrogens and cognition: Friends or foes?: An evaluation of the opposing effects of estrogens on learning and memory. Horm Behav. 2015;74:105–115. doi: 10.1016/j.yhbeh.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Brain Res Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- Luine VN. Estradiol and cognitive function: Past, present and future. Horm Behav. 2014;66:602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Front Neuroendocrinol. 2012;33:388–402. doi: 10.1016/j.yfrne.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Dissociating learning and performance: Drug and hormone enhancement of memory storage. Brain Res Bull. 1989;23:339–345. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- Overk CR, Perez SE, Ma C, Taves MD, Soma KK, Mufson EJ. Sex steroid levels and AD-like pathology in 3xTgAD mice. J Neuroendocrinol. 2013;25:131–144. doi: 10.1111/j.1365-2826.2012.02374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 1997;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Pereira LM, Bastos CP, de Souza JM, Ribeiro FM, Pereira GS. Estradiol enhances object recognition memory in Swiss female mice by activating hippocampal estrogen receptor alpha. Neurobiol Learn Mem. 2014;114C:1–9. doi: 10.1016/j.nlm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13:226–234. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci. 2011;31:10034–10038. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107:1621–1631. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci. 2012;32:8231–8241. doi: 10.1523/JNEUROSCI.1114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensel MA, Salwiczek L, Roth J, Schlinger BA. Context-specific effects of estradiol on spatial learning and memory in the zebra finch. Neurobiol Learn Mem. 2013;100:41–47. doi: 10.1016/j.nlm.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117:2471–2477. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. Testosterone regulates aromatase activity in discrete brain areas of male rhesus macaques. Biol Reprod. 1989;40:929–934. doi: 10.1095/biolreprod40.5.929. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev. 2011;32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghera MK, Simpson ER, McPhaul MJ, Kozlowski G, Conley AJ, Lephart ED. Immunocytochemical distribution of aromatase cytochrome P450 in the rat brain using peptide-generated polyclonal antibodies. Endocrinology. 1991;129:2834–2844. doi: 10.1210/endo-129-6-2834. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Evans PD. G-protein oestrogen receptor 1: trials and tribulations of a membrane oestrogen receptor. J Neuroendocrinol. 2013;25:1219–1230. doi: 10.1111/jne.12071. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci U S A. 2008;105:14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierk R, Brandt N, Rune GM. Hippocampal estradiol synthesis and its significance for hippocampal synaptic stability in male and female animals. Neuroscience. 2014;274:24–32. doi: 10.1016/j.neuroscience.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, Wilkars W, Bender RA, Lewerenz M, Gloger S, Graser L, Schwarz J, Rune GM. Aromatase inhibition abolishes LTP generation in female but not in male mice. J Neurosci. 2012;32:8116–8126. doi: 10.1523/JNEUROSCI.5319-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vockel A, Prove E, Balthazart J. Sex- and age-related differences in the activity of testosterone-metabolizing enzymes in microdissected nuclei of the zebra finch brain. Brain Res. 1990;511:291–302. doi: 10.1016/0006-8993(90)90174-a. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrenberg U, Prange-Kiel J, Rune GM. Steroidogenic factor-1 expression in marmoset and rat hippocampus: co-localization with StAR and aromatase. J Neurochem. 2001;76:1879–1886. doi: 10.1046/j.1471-4159.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- Witty CF, Foster TC, Semple-Rowland SL, Daniel JM. Increasing hippocampal estrogen receptor alpha levels via viral vectors increases MAP kinase activation and enhances memory in aging rats in the absence of ovarian estrogens. PLoS One. 2012;7:e51385. doi: 10.1371/journal.pone.0051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Ann Rev Pharm Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Yague JG, Azcoitia I, DeFelipe J, Garcia-Segura LM, Munoz A. Aromatase expression in the normal and epileptic human hippocampus. Brain Res. 2010;1315:41–52. doi: 10.1016/j.brainres.2009.09.111. [DOI] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry. 2011;70:920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci. 2012;32:2344–2351. doi: 10.1523/JNEUROSCI.5819-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci U S A. 2010a;107:5605–5610. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate the estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci U S A. 2010b;107:5605–5610. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Fester L, von Blittersdorff B, Hassu B, Nogens H, Prange-Kiel J, Jarry H, Wegscheider K, Rune GM. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology. 2010;151:1153–1160. doi: 10.1210/en.2009-0254. [DOI] [PubMed] [Google Scholar]