Summary
The protein kinases Mst1 and Mst2 have tumor suppressor activity, but their mode of regulation is not well established. Mst1 and Mst2 are broadly expressed and may have certain overlapping functions in mammals, as deletions of both Mst1 and Mst2 together are required for tumorigenesis in mouse models [1–3]. These kinases act via a three-component signaling cascade comprising Mst1/2, the protein kinase Lats1/2, and the transcriptional coactivators Yap and Taz [4–6]. Mst1/2 contain C-terminal SARAH domains that mediate their homodimerization as well as heterodimerization with other SARAH-domain containing proteins, which may regulate Mst1/2 activity. Here, we show that, in addition to forming homodimers, Mst1 and Mst2 heterodimerize in cells, that this interaction is mediated by their SARAH domains and is favored over homodimers, and that these heterodimers have much reduced protein kinase activity compared to Mst1 or Mst2 homodimers. Mst1/Mst2 heterodimerization is strongly promoted by oncogenic H-ras, and this effect requires activation of the Erk pathway. Cells lacking Mst1, in which Mst1/Mst2 heterodimers are not possible, are resistant to H-ras-mediated transformation and maintain active hippo pathway signaling compared to wild-type cells or cells lacking both Mst1 and Mst2. Our results suggest that H-ras, via an Erk-dependent mechanism, down-regulates Mst1/2 activity by inducing the formation of inactive Mst1/Mst2 heterodimers.
Graphical Abstract

eTOCS Blurb
Mst1 and Mst2 are protein kinases that activate the hippo tumor suppressor pathway, yet their mode of regulation is poorly understood. Rawat et al. show that H-ras V12 induces Mst1/Mst1 and Mst2/Mst2 homodimers to switch partners to form Mst1/Mst2 heterodimers. These heterodimers are inactive, promoting YAP activation and oncogenic transformation.
Results and Discussion
Mst1 and Mst2 heterodimerize in cells
In a co-immunoprecipitation screen for Mst1 interactors [7], we found an abundance of Mst2-derived peptides (Table S1). As Mst1/Mst2 heterodimers have not been previously reported, this finding prompted us to determine if Mst1 and Mst2 formed a complex in cells. HEK293 cells were transfected either with expression vectors for Myc-tagged Mst1 alone or with Myc-tagged Mst1 and HA-tagged Mst2 together, and co-immunoprecipitations were performed. We found that HA-immunoprecipitates contained Myc-tagged Mst1 (Figure 1A). Similar to the results using exogenous Mst1 and Mst2, we found that HA-SBP-tagged Mst2 co-precipitated endogenous Mst1 (Figure 1B). Antibodies against murine Mst1 and Mst2 do not cross-react and thus can be used to distinguish Mst1 from Mst2 (Figure 1C). We used these antibodies to perform proximity ligation assay (PLA) in HEK293 cells (Supplementary Information). Our results suggested that endogenous Mst1 and Mst2 form heterodimers in cells (Figure 1D and 1F). To test the specificity of the assay, we repeated the PLA using Mst1+/+Mst2+/+ and Mst1−/−Mst2f/− MEFs. We found heterodimer formation only in Mst1+/+Mst2+/+ cells but not in Mst1−/−Mst2f/−, suggesting that there is not significant cross reactivity between the secondary antibodies, and consistent with the idea that Mst1 and Mst2 can heterodimerize in cells expressing both Mst isoforms (Figure 1E and 1F). To further confirm heterodimer formation, we generated a tetracycline-inducible HA-SBP-Mst2 expressing stable cell line (Mst2-Flp-In 293). We induced Mst2-Flp-In 293 cells with tetracycline to express SBP-Mst2 at approximately endogenous levels [7] and then used streptavidin beads to pull down SBP-Mst2 and probed the protein complex with anti-Mst1 antibodies. Detection of endogenous Mst1 in the protein complex further confirmed heterodimer formation (Figure 1G).
Figure 1. Mst1 interacts with Mst2.
(A) HEK-293 cells were transfected with the indicated combinations of plasmids. Cell lysates were immunoprecipitated with anti-HA antibodies. The resulting immunoprecipitated proteins were immunobloted with anti-Myc antibodies. (B) HEK-293 cells were transfected either with empty vector (EV) or HA-SBP-Mst2. Cell lysates were immunoprecipitated with streptavidin beads. The immunoprecipitated proteins were immunoblotted with anti-Mst1 antibodies. (C) 10 ng of recombinant GST-Mst1 and GST-Mst2 protein were resolved on a gel and probed with different Mst1 and Mst2 antibodies. (D) Proximity ligation assay (PLA), using Mst1 antibodies (control), antibodies against total ERK and pERK, or antibodies against Mst1 and Mst2. (E) PLA using antibodies against Mst1 and Mst2 in WT or Mst1−/− cells. (F) Mst2-Flp-In 293 cells were treated with 1 μg/ml of tetracycline for 4 hours. The cell lysates were immunoprecipitated with streptavidin beads, and resulting immunoprecipitated proteins were probed with Mst1 antibodies. See also Figure S1.
As both the kinase and SARAH domains might contribute to the homodimerization of Mst1 and Mst2 proteins [8], we asked what domains mediate the heterodimerization of Mst1 and Mst2. We constructed four forms of Myc-Mst1 – full-length (1–487), Mst1 lacking the SARAH domain (1–430), Mst1 kinase domain only (1–330), and Mst1 minus the kinase domain (276–487)(Figure 2A) – and tested each form for binding to full-length HA-Mst2. We found that full-length Mst1 as well as Mst1 comprising only the autoinhibitory domain plus the SARAH domain (276–487) were able to bind Mst2, whereas Mst1 kinase-domain only (1–330) and Mst1 lacking the SARAH domain (1–430) were not able to bind Mst2 (Figure 2B). These results indicate that the SARAH domain is required for Mst1/Mst2 heterodimerization. To further characterize this interaction, we also tested if kinase activity and T-loop phosphorylation of Mst1 or Mst2 play a role in heterodimerization. We co-tranfected HEK293 cells with HA-Mst1 along with wild-type Myc-Mst2 (WT) or Myc-Mst2 K56R (KR), a kinase dead form of Mst2, or Myc-Mst2 T180D (TD), an activation loop mutant of Mst2. Co-immunoprecipitation experiments revealed that both the kinase-dead and the activation loop mutant of Mst2 were able to bind Mst1, suggesting that kinase activity and T-loop phosphorylation of Mst2 are not required for heterodimeric binding to Mst1 (Supplemental Figure S1A). As with Mst2, mutation of the Mst1 catalytic Lys residue (K59R), or substitution at its T-loop phosphorylation site (T183A), also did not affect heterodimerization (Supplemental Figure S1B). These experiments suggest that kinase activity is not required for heterodimer formation between Mst1 and Mst2.
Figure 2. Analysis of Mst dimers.
(A) Schematic representation of wt-Mst1 and different deletion mutants of Mst1. (B) HEK-293 cells were transfected with the indicated combinations of plasmids encoding HA-Mst2, Myc-wt-Mst1, Myc-Mst1-1-430aa, Myc-Mst1-1-330aa and Myc-Mst1-276-487aa. Cell lysates were immunoprecipitated with anti-HA antibodies. The resulting immunoprecipitated proteins were immunoblotted with anti-Myc antibodies. (C) HEK-293 cells were transfected with the indicated combination of plasmids encoding HA-Mst1, Myc-Mst1, HA-Mst2 and Myc-Mst2. Cell lysates were immunoprecipitated with anti-HA antibody and resulting immunoprecipitated proteins were immunoblotted with anti-Myc antibody. (D) Relative levels of Mst1 homodimer, Mst2 homodimer and Mst1-Mst2 Heterodimer were quantified from (C). (E) Mst1 SARAH domain homodimer was diluted to different concentrations and fluorescence spectra were recorded at each concentration. Representative “molar” fluorescence spectra are shown, obtained by dividing the raw emission spectra (excitation at 290 nm) by the respective protein concentration. (F) Determination of Mst1 homodimer dissociation constant. The C-terminus of the Mst1 SARAH domain was labeled with AEDANS (AED) to serve as an acceptor, while the N-terminal Trp (W439) served as a donor. The ratio of the acceptor (483 nm) and donor (340 nm) emission bands is plotted as a function of Mst1-AED concentration. The curve represents a least-squares fit of a homodimer dissociation model. The inset shows a ribbon diagram of the Mst1 SARAH homodimer structure determined by NMR [9], using a blue to red N- to –C rainbow coloring scheme. The Trp donors in each monomer are labeled D/D’, and A/A’ indicates the approximate location of the AEDANS acceptors. (G) FRET evidence for Mst1-Mst2 heterodimer formation. Fluorescence emission spectra are shown for 3 μM solutions of Mst1 (green), Mst2-AED (blue) and a 1:1 mixture of Mst1 and Mst2-AED (red). The inset shows a ribbon diagram of a (hypothetical) heterodimer of the Mst1 and Mst2 SARAH domains. The Trp343 FRET donor (blue) and the C-terminal AEDANS acceptor (red) are labeled. (H) Different concentrations of Mst1 were titrated into a 1 μM solution of Mst2-AED and fluorescence spectra (excitation at 290 nm) were recorded at each concentration. Corrected spectra were obtained by subtracting matching concentrations of Mst1. The normalized intensity of the emission maximum at 488 nm represents the fraction of dimer formed, and is plotted vs. Mst1 concentration. The curve represents a least-squares fit of a 1:1 binding model. AEDENS labeling and methods for fluorescence measurements of dimer dissociation are presented in Supplementary Information. See also Figure S2.
To assess the relative levels of homodimers versus heterodimers, we transfected HEK293 cells with expression vectors encoding full-length HA or Myc-tagged Mst1 and Mst2, as indicated (Figure 2C). Mst1 and Mst2 were expressed at nearly equal levels. Under these conditions, co-immunoprecipitation revealed a substantial level (~60%) of Mst1/2 heterodimers relative to Mst1/1 and Mst2/2 homodimers (Figure 2C and D). The relatively high levels of Mst1/Mst2 heterodimers suggested a strong affinity of these proteins for one another. We therefore determined the dissociation constants for homo- and heterodimers of the Mst1 and Mst2 SARAH domains using a FRET-based approach. Variants of the Mst1, Mst2 and Mst2-LW SARAH domains containing a C-terminal Cys were expressed in bacteria and purified (see Methods). Modification with the thiol-reactive IAEDANS (Methods) yielded the AEDANS-labeled derivatives Mst1-AED, Mst2-AED and Mst2-LW-AED. Since SARAH domains associate in an antiparallel orientation [9, 10], a unique Trp residue at the N-terminal end of the Mst1 SARAH domain can be used as a FRET donor while the AEDANS label at the C-terminus of the adjacent subunit in the dimer serves as an acceptor (inset Figure 2F). For Mst2, an equivalent Trp residue was introduced by site-directed mutagenesis (Mst2-LW). On the basis of NMR and crystal structures of SARAH domain homodimers [11], we estimate a distance of 12 Å for the inter-subunit donor-acceptor pair, whereas the distance between the Trp donor and the acceptor on opposite ends of the helical monomer is approximately 57 Å. Thus, we expect efficient energy transfer for the inter-subunit Trp-AEDANS pair (Förster distance R0 ~18 Å) and a negligible contribution from intra-subunit FRET. To confirm this, we recorded fluorescence spectra for the Mst1 (Supplemental Figure S2) and Mst2 SARAH domains under native and denaturing (9 M urea) conditions. Upon excitation of the Trp, we observed a partially quenched donor (Trp) emission band compared to an unlabeled sample and a pronounced acceptor (AEDANS) emission band for each of the homodimers. For Mst1, we estimated a donor-acceptor distance, RDA, of 15 Å, which closely matches that observed in the NMR structure [9]. In the presence of 9 M urea, which results in unfolded monomers, donor quenching and acceptor enhancement were less pronounced, consistent with an increase in average donor-acceptor distance for the unfolded monomer (RDA~16 Å). To determine the dissociation constant, KD, for the Mst1 homodimer, we recorded fluorescence spectra at protein concentrations from 40 down to 0.6 μM (Figure 2E). Least-squares fitting of a homodimer dissociation model (Methods, Equ. 1) [12] to the ratio between acceptor and donor emission maxima (which serves as a proxy for the FRET efficiency) vs. protein concentration (Figure 2F) yielded a KD of 6.5 ± 2 μM for the Mst1 homodimer. To determine the Kd of the Mst2 SARAH homodimer, we measured the change in AEDANS emission for Mst2-AED, which contains no tryptophan, upon addition of the unlabeled Mst2-LW variant (Figure 2H); least-squares fitting of a 1:1 binding model yielded a Kd of 4.3 ± 0.3 μM.
In Figure 2G, we compare the fluorescence spectra for equimolar (3 μM) solutions of unlabeled Mst1 (which has a Trp near the N-terminus, Trp439), Mst2-AED (which has no tryptophan, but a C-terminal AEDANS label) and a 1:1 mixture of the two SARAH domains. In the mixed sample, the donor band is ~2-fold lower than that of Mst1 while the acceptor band is strongly enhanced compared to Mst2-AED, which is consistent with efficient FRET from Trp439 in Mst1 to the AEDANS at the C-terminus of Mst2-AED. After accounting for labeling efficiency (18%), we calculate a FRET efficiency of 0.91, which corresponds to a donor-acceptor distance of 12.1 Å, similar to that obtained for the Mst1 homodimer. These results provide unambiguous evidence for formation of an antiparallel heterodimer (inset, Figure 2G). To determine the KD value for the Mst1/Mst2 heterodimer, unlabeled Mst1 SARAH domain was titrated against Mst2-AED (1 μM), and the observed increase in acceptor fluorescence was plotted vs. Mst1 concentration (Figure 2H). The KD obtained by least-squares fitting of a 1:1 binding model was 0.72 ± 0.03 μM. This value is substantially lower than that of the corresponding homodimers (6.5 and 4.3 μM, respectively), indicating that heterodimer formation is favored over self-association of either SARAH domain.
Mst1/2 heterodimers have impaired kinase activity
Having confirmed heterodimer formation between Mst1 and Mst2, we tested the effect of heterodimer formation on kinase activity. To compare the activities of homo- and heterodimers, we isolated Mst1/Mst2 heterodimers and corresponding Mst1/Mst1 and Mst2/Mst2 homodimers using a sequential immunoprecipitation strategy (Figure 3A). Mst1/2 double KO HEK-293 cells [13] were co-transfected with HA-SBP-Mst1 and Myc-Mst2. To isolate Mst1/Mst2 heterodimers, HA-SBP-Mst1 was immunoprecipitated using streptavidin beads and complexes were eluted from the beads with 2 mM biotin. This eluate was then immunoprecipitated with anti-Myc-agarose beads to isolate HA-SBP-Mst1/Myc-Mst2 heterodimers. Myc-Mst2 homodimers and monomers were isolated by immunoprecipitating HA-SBP-Mst1 depleted lysates with anti-Myc-agarose beads. Similarly, HA-SBP-Mst1 homodimers were immunoprecipitated with streptavidin beads from Myc-Mst2 depleted lysates. Mst1/Mst1 and Mst2/Mst2 homodimers and Mst1/Mst2 heterodimers were adjusted to similar levels and assessed for purity using SDS-PAGE (Figure 3B). To measure the kinase activities of each of the three species, we took equal amounts of each species and performed in vitro kinase assays using MBP as a substrate. The kinase activity of Mst1/Mst2 heterodimers was much lower compared to that of Mst1/Mst1 or Mst2/Mst2 homodimers as shown by p-MBP and p-Mst levels (Figure 3C and Supplemental Figure S3A), or p-Mob levels, a highly specific Mst1/Mst2 substrate (Figure 3D). Interestingly, Mst2 homodimers showed more robust activation compared to Mst1 homodimers (Figure 3E). To confirm that the signals in phospho-blots reflect protein kinase activity, we also performed these experiments in the absence of ATP, which resulted in loss of the signals (Figure 3F). Mutants of Mst1 and Mst2 that cannot dimerize due to excision of the C-terminal SARAH domain fail to exhibit cross-inhibition when incubated together (Supplemental Figure S3B).
Figure 3. Activity of Mst1/Mst2 heterodimers.
(A) Scheme for Isolation of Mst1 homodimer, Mst2 homodimer and Mst1/Mst2 heterodimer from cells using sequential immunoprecipitation strategy described in experimental procedures. Mst1 is doubly epitope tagged at the N-terminus with HA-SBP, whereas Mst2 is epitope tagged at the N-terminus with Myc. (B) Mst1/2 double KO HEK293 cells [13] were transfected with Mst1 and Mst2. Homodimers and heterodimers were isolated as described in experimental procedures and were adjusted to similar levels and separated by SDS-PAGE followed by coomassie blue staining. (C) Homodimers and heterodimers were subjected to in vitro kinase assay using MBP as a substrate in the presence of [γ-32P] ATP, or (D) were subjected to in vitro kinase assay using Mob1 as a substrate, with subsequent western blot analysis using anti-phospho Mob1 antibodies. (E) Mst1/Mst2 homodimers and heterodimers were subjected to in vitro kinase assay for the indicated time points using Mob1 as a substrate. (F) Mst1/Mst2 homodimers and heterodimers were subjected to in vitro kinase assay using Mob1 as a substrate in the absence and presence of ATP. (G) HEK-293 cells were co-transfected with WT-HA-Mst1 along with WT-Myc-Mst2 or Myc-Mst2 K59R or Myc-Mst2 T183D. Corresponding Mst1/Mst2 homodimers and heterodimers were isolated and subjected to in vitro kinase assay using Mob1 as a substrate. See also Figure S3.
To determine if the suppressive effect of heterodimerization on enzyme activity required the kinase activities of Mst1 and/or Mst2, we performed kinase assays using Mst1/Mst2 homodimers or heterodimers in which one or both partners were inactive. As expected, homodimers of kinase-dead Mst2 and heterodimers of kinase-dead Mst2 with wild-type Mst1 displayed lower activity compared to their wild-type counterparts (Figure 3G). Interestingly, homodimers of T-loop mutants of Mst2 and heterodimers of T-loop mutants of Mst2 with wild-type Mst1 showed activity similar to their wild-type counterparts (Figure 3G). Similar results were obtained when kinase-dead and T-loop mutants of Mst1 were tested (data not shown). These results suggest that T-loop phosphorylation is uncoupled from enzyme activity in the Mst1/Mst2 heterodimers.
Mst1/2 heterodimerization is promoted by H-ras in an Erk-dependent manner
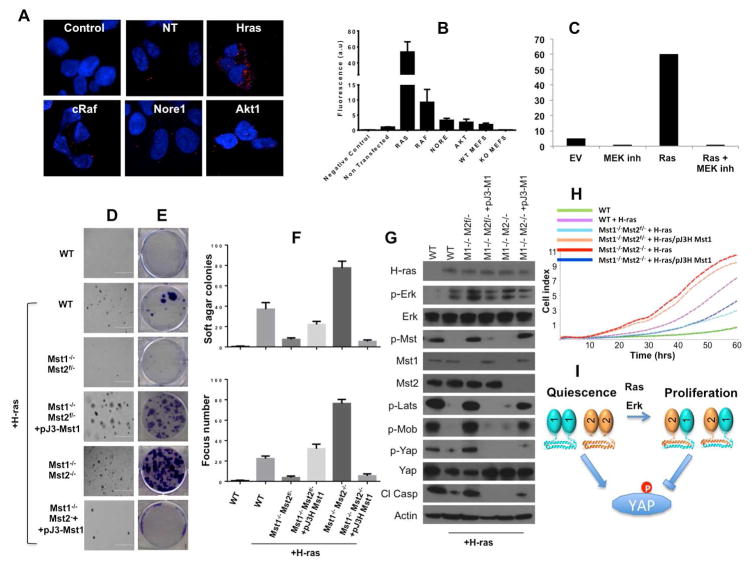
To determine how Mst1/Mst2 heterodimerization is regulated, we asked if oncogenic stimuli alter the levels of Mst1/Mst2 heterodimers. Cells were co-transfected with expression vectors for activated H-ras, c-Raf, Nore1, or Akt1 (Supplemental Figure S4A) and assessed for Mst1/Mst2 heterodimerization using PLA. As shown in Figures 4A and 4B, expression of activated H-ras markedly increased the amount of Mst1/Mst2 heterodimers. c-Raf had a more modest positive effect, whereas Nore1 and Akt only slightly increased heterodimerization. Interestingly, the effect of H-ras on inducing Mst1/Mst2 heterodimers was completely blocked by addition of a Mek inhibitor (Figure 4C), which suggests that a Ras-Mek-Erk pathway regulates Mst1/Mst2 heterodimerization. We also tested if H-ras induced Mst1/Mst2 heterodimer formation is accompanied by loss of hippo signaling. Western blot analysis confirmed that cells expressing H-ras had lower Mst1/Mst2 activity and decreased downstream signaling, as pMst1/2, pLats, pMob1 and pYap levels were lower in H-ras transfected cells compared to non-transfected cells (Figure 4G and Supplemental Figure S4B). As with its effect on Mst1/Mst2 heterodimerization, treatment with a Mek inhibitor blocked the effect of H-ras on Mst1/Mst2 activity and hippo pathways signaling (Supplemental Figure S4B).
Figure 4. Regulation of Mst heterodimerization.
(A) HEK293 were transfected with the indicated plasmids. PLA results are shown. (B) Quantitation of PLA data from (A). (C) HEK293 cells were transfected with a control plasmid or a H-ras plasmid as indicated. Cells were then treated with vehicle or 1 μM Mek inhibitor U0126 for 2 h. PLA was performed to assess the degree of Mst heterodimerization. (D) MEFs of the indicated genotypes were electroporated with a control or H-ras expression vector, with or without pJ3H-Mst1 [33] as indicated, and GFP-H-ras expressing cells were selected for soft agar assay. Cells were grown in soft agar for 3 weeks and colonies were stained with 0.5% crystal violet. (E) MEFs of the indicated genotypes were electroporated with a control or H-ras expression vector and GFP-H-ras expressing cells were selected and used for focus formation assay. “+ pJ3-Mst1” indicates that the cells were transfected with a Mst1 expression vector. (F) Number of colonies quantified from (D) and (E). The results are presented as mean ± s.e. for three independent experiments. (G) MEFs of the indicated genotypes were electroporated with a control or H-ras expression vector. Cell lysates were collected and analysed by western-blot using H-ras, p-Erk, Erk, p-Mst, Mst1, Mst2, p-Lats, p-Mob, p-Yap, Yap, cleaved caspase-3 and actin antibodies. (H) MEFs of the indicated genotypes were electroporated with a control or H-ras expression vector. Proliferation assay was then performed using xCELLigence system as described in ‘Experimental Procedure’. (I) Model of Mst dimer regulation by H-ras. See also Figure S4.
Cells lacking Mst1 resist transformation by activated H-ras
If Mst1/Mst2 heterodimerization reflects an important means for downregulating the tumor-suppressive activity of the hippo pathway, then cells lacking one of the Mst partners should be unable to limit Mst1/2 activity and therefore, despite losing a putative tumor suppressor protein, be harder to transform than wild-type cells. To test this seemingly paradoxical idea, we transfected WT (Mst1+/+Mst2+/+), heterozygous (Mst1−/−Mst2f/−) and homozygous null (Mst1−/−Mst2−/−) MEFs with activated H-ras and performed three assays – cell proliferation, soft agar colony formation, and focus formation - to compare the transforming ability of this oncoprotein in these cells. As expected, transfection of WT MEFs with H-ras promoted increased focus formation, anchorage-independent growth, and proliferation (Figures 4D-F, 4H). Interestingly, H-ras lacked this effect in heterozygous Mst1−/−Mst2f/− MEFs, but regained it in homozygous null Mst1−/−Mst2−/− MEFs. Expression of exogenous Mst1 in the heterozygous Mst1−/−Mst2f/− MEFs or in the homozygous null Mst1−/−Mst2−/− MEFs reversed these effects; i.e., the former cells now became more susceptible to transformation, whereas the latter became more resistant to transformation. These results are consistent with the idea that H-ras-induced Mst1/Mst2 heterodimer formation in WT cells inhibits the tumor suppressor function of the hippo pathway, and that this activity is lost in cells lacking either Mst1 or Mst2, or both Mst1 and Mst2. These results are also consistent with those of Romano et al., who reported that H-ras inactivates Mst1/2 in HeLa cells [14]. Signaling analysis was consistent with the growth and transformation effects: in Mst1−/−Mst2f/− MEFs only, H-ras failed to inactivate Mst2 signaling, as evidenced by elevated p-Mst1/2, p-Lats, p-Mob, p-Yap, and cleaved caspase-3 (Figure 4G). Exogenous expression of Mst1 in Mst1−/−Mst2f/− MEFs reversed the H-ras induced signaling profile; i.e., these cells displayed low levels of p-Mst1/2, p-Lats, p-Mob, p-Yap, and cleaved caspase-3, and proliferated faster (Figure 4G, H). Similarly, exogenous expression of Mst1 in Mst1−/−Mst2−/− MEFs converted the H-ras induced signaling profile to a growth-inhibited state characterized by elevated hippo pathway activity (Figure 4G, H).
The role of Mst1/Mst2 homodimerization in activation is controversial. Some studies suggest that Mst1/Mst2 homodimerization is required for autophosphorylation and activation of these kinases [8, 15, 16] while others indicate that homodimer formation is not required for its activity [8, 17]. In addition to forming homodimers, Mst1 and Mst2 have also been shown to heterodimerize with other SARAH domain containing proteins, such as, RASSF family of proteins [8, 18–20]. Interaction with RASSF proteins has been shown both to positively and negatively regulate Mst1/Mst2 activity; however, an association of Mst1 to Mst2 to form Mst1/Mst2 heterodimers has not been previously reported.
There are relatively few cytosolic protein kinases known to form stable heterodimers. Perhaps the best understood heterodimerization event is provided by the B-Raf and c-Raf proteins, which dimerize in response to mitogenic stimuli or upon exposure to B-Raf inhibitors [21–23]. Unlike the Mst1/Mst2 heterodimer reported here, B-Raf/c-Raf heterodimers have higher protein kinase activity than the corresponding homodimers [24]. Interestingly, another set of STE20-family kinases, p21-activated kinase (Pak)1 and Pak3, have also been shown to heterodimerize. Like the Mst1/Mst2 pair, Pak1/Pak3 heterodimers also show low kinase activity [25].
It was recently shown that Mst1 homodimers in cardiac tissue are disrupted via TORC2-catalyzed phosphorylation at S438 within the Mst1 SARAH domain [16]. While the effects of TORC2 on Mst2 homodimerization and Mst1/Mst2 heterodimerization were not studied in this work, it is interesting to note that the Mst2 SARAH domain lacks the equivalent serine residue found in Mst1. It has also been reported to Rassf5 binding to Mst2 disrupts Mst2 homodimers and inhibits their ability to become activated [8]. In this setting, displacement of one of the two Mst2 proteins in the dimer by Rassf5 is expected to prevent trans-autophosphorylation of the activation loop. The mechanism underlying loss of activity in Mst1/Mst2 heterodimers is presumably based on different structural grounds, as two kinase domains would still be present, as in homodimers. It is possible that the heterodimer adopts a geometry that prevents productive interactions required for transautophosphorylation of the kinase domains. This mechanism is analogous to that reported for Raf/Mst heterodimers, where binding of c-Raf or B-Raf to a C-terminal domain of Mst2 or Mst1, respectively, results in Mst1/2 inhibition [26, 27]. Conversely, Abl-mediated phosphorylation of Mst2 at Y81 disrupts the c-Raf/Mst2 heterodimer and enhances Mst2 homodimerization and activity [28].
Both K-ras and H-ras have previously been shown to regulate the Hippo-Yap pathway. For example, mutant K-ras has been shown to positively regulate Mst2 activity via binding to Rassf1A, which in turn binds and activates Mst2. [14, 29]. Also, it has been reported that withdrawal of K-ras G12D expression in murine pancreatic ductal adenocarcinoma (PDAC) tumors in time leads to Yap1 gene amplification and overexpression, enabling tumor growth and maintenance, suggesting that Yap1 can substitute for oncogenic K-ras in PDAC and other malignancies [30, 31]. Unlike K-ras, H-ras-Erk signaling has previously been shown to impede the Hippo pathway via phosphorylation of the Ajuba homolog WTIP, which then binds to the LATs/WW45 complex and thereby inhibits phosphorylation of Yap [32]. Our results suggest a second mechanism for Erk-mediated inhibition of the Hippo pathway through the formation of low activity Mst1/Mst2 heterodimers. Presumably, this latter mechanism would be inoperative in cells lacking either Mst1 or Mst2 due to mutational loss of epigenetic silencing, as suggested by our studies of H-ras transformation in Mst1-null MEFs (Figure 4D and 4E).
Experimental Procedures
Experimental procedures are described in the Supplemental Experimental Procedures.
Supplementary Material
Highlights.
Mst and Mst2 form heterodimers in cells
Mst1/2 heterodimers have low kinase activity compared to homodimers
Mst1/2 heterodimerization is promoted by H-ras via an Erk-dependent mechanism
Cells lacking Mst1, in which Mst1/2 heterodimers are not possible, resist transformation by H-ras
Acknowledgments
We thank Mark Phillips for the GFP-Hras-V12 plasmid and Jeffrey Peterson for assistance with preparation of Figures 3 and 4. This work was supported by grants from the NIH to JC (R01 CA58836 and R01 CA098830) and to HR (R01 GM056250) and to the Fox Chase Cancer Center (P30 CA006927), as well as by an appropriation from the state of Pennsylvania.
Footnotes
Author Contributions
S.J.R. designed and executed the screen for Mst1 interactors, the isolation of Mst1/2 homo and heterodimers, protein kinase assays, affinity measurements, and writing the manuscript. L.E.A.-R. and O.V.-C. performed the PLA assays, D.A.-O. assisted with soft agar assays, T.Y.P. assisted with cell culture. H.R. assisted with the design and performance of SARAH domain FRET assays, and J.C. helped design the project and co-wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kodaka M, Hata Y. The mammalian Hippo pathway: regulation and function of YAP1 and TAZ. Cell Mol Life Sci. 2015;72:285–306. doi: 10.1007/s00018-014-1742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawat SJ, Chernoff J. Regulation of mammalian Ste20 (Mst) kinases. Trends Biochem Sci. 2015 doi: 10.1016/j.tibs.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawat SJ, Creasy CL, Peterson JR, Chernoff J. The tumor suppressor Mst1 promotes changes in the cellular redox state by phosphorylation and inactivation of peroxiredoxin-1 protein. The Journal of biological chemistry. 2013;288:8762–8771. doi: 10.1074/jbc.M112.414524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni L, Li S, Yu J, Min J, Brautigam CA, Tomchick DR, Pan D, Luo X. Structural Basis for Autoactivation of Human Mst2 Kinase and Its Regulation by RASSF5. Structure. 2013 doi: 10.1016/j.str.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang E, Ryu KS, Paakkonen K, Guntert P, Cheong HK, Lim DS, Lee JO, Jeon YH, Cheong C. Structural insight into dimeric interaction of the SARAH domains from Mst1 and RASSF family proteins in the apoptosis pathway. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9236–9241. doi: 10.1073/pnas.0610716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makbul C, Constantinescu Aruxandei D, Hofmann E, Schwarz D, Wolf E, Herrmann C. Structural and thermodynamic characterization of Nore1-SARAH: a small, helical module important in signal transduction networks. Biochemistry. 2013;52:1045–1054. doi: 10.1021/bi3014642. [DOI] [PubMed] [Google Scholar]
- 11.Hwang E, Cheong HK, Ul Mushtaq A, Kim HY, Yeo KJ, Kim E, Lee WC, Hwang KY, Cheong C, Jeon YH. Structural basis of the heterodimerization of the MST and RASSF SARAH domains in the Hippo signalling pathway. Acta crystallographica Section D, Biological crystallography. 2014;70:1944–1953. doi: 10.1107/S139900471400947X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber G. Energetics of ligand binding to proteins. Advances in protein chemistry. 1975;29:1–83. doi: 10.1016/s0065-3233(08)60410-6. [DOI] [PubMed] [Google Scholar]
- 13.Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nature communications. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romano D, Maccario H, Doherty C, Quinn NP, Kolch W, Matallanas D. The differential effects of wild-type and mutated K-Ras on MST2 signaling are determined by K-Ras activation kinetics. Molecular and cellular biology. 2013;33:1859–1868. doi: 10.1128/MCB.01414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anand R, Kim AY, Brent M, Marmorstein R. Biochemical analysis of MST1 kinase: elucidation of a C-terminal regulatory region. Biochemistry. 2008;47:6719–6726. doi: 10.1021/bi800309m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sciarretta S, Zhai P, Maejima Y, Del Re DP, Nagarajan N, Yee D, Liu T, Magnuson MA, Volpe M, Frati G, et al. mTORC2 regulates cardiac response to stress by inhibiting MST1. Cell Rep. 2015;11:125–136. doi: 10.1016/j.celrep.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creasy CL, Ambrose DM, Chernoff J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. The Journal of biological chemistry. 1996;271:21049–21053. doi: 10.1074/jbc.271.35.21049. [DOI] [PubMed] [Google Scholar]
- 18.Guo C, Zhang X, Pfeifer GP. The tumor suppressor RASSF1A prevents dephosphorylation of the mammalian STE20-like kinases MST1 and MST2. J Biol Chem. 2011;286:6253–6261. doi: 10.1074/jbc.M110.178210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J. 2004;381:453–462. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song H, Oh S, Oh HJ, Lim DS. Role of the tumor suppressor RASSF2 in regulation of MST1 kinase activity. Biochem Biophys Res Commun. 2010;391:969–973. doi: 10.1016/j.bbrc.2009.11.175. [DOI] [PubMed] [Google Scholar]
- 21.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu J, Stites EC, Yu H, Germino EA, Meharena HS, Stork PJ, Kornev AP, Taylor SS, Shaw AS. Allosteric activation of functionally asymmetric RAF kinase dimers. Cell. 2013;154:1036–1046. doi: 10.1016/j.cell.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rushworth LK, Hindley AD, O’Neill E, Kolch W. Regulation and role of Raf-1/B-Raf heterodimerization. Molecular and cellular biology. 2006;26:2262–2272. doi: 10.1128/MCB.26.6.2262-2272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Combeau G, Kreis P, Domenichini F, Amar M, Fossier P, Rousseau V, Barnier JV. The p21-activated kinase PAK3 forms heterodimers with PAK1 in brain implementing trans-regulation of PAK3 activity. The Journal of biological chemistry. 2012;287:30084–30096. doi: 10.1074/jbc.M112.355073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SJ, Lee MH, Kim DW, Lee S, Huang S, Ryu MJ, Kim YK, Kim SJ, Kim SJ, Hwang JH, et al. Cross-regulation between oncogenic BRAF(V600E) kinase and the MST1 pathway in papillary thyroid carcinoma. PloS one. 2011;6:e16180. doi: 10.1371/journal.pone.0016180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Neill E, Rushworth L, Baccarini M, Kolch W. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science. 2004;306:2267–2270. doi: 10.1126/science.1103233. [DOI] [PubMed] [Google Scholar]
- 28.Liu W, Wu J, Xiao L, Bai Y, Qu A, Zheng Z, Yuan Z. Regulation of neuronal cell death by c-Abl-Hippo/MST2 signaling pathway. PloS one. 2012;7:e36562. doi: 10.1371/journal.pone.0036562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matallanas D, Romano D, Al-Mulla F, O’Neill E, Al-Ali W, Crespo P, Doyle B, Nixon C, Sansom O, Drosten M, et al. Mutant K-Ras activation of the proapoptotic MST2 pathway is antagonized by wild-type K-Ras. Mol Cell. 2011;44:893–906. doi: 10.1016/j.molcel.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy BV, Irvine KD. Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Developmental cell. 2013;24:459–471. doi: 10.1016/j.devcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Creasy CL, Chernoff J. Cloning and characterization of a human protein kinase with homology to Ste20. J Biol Chem. 1995;270:21695–21700. doi: 10.1074/jbc.270.37.21695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.