Abstract
Insulin-like growth factor binding protein 2 (IGFBP2) overexpression is common in high-grade glioma and is both a strong biomarker of aggressive behaviour and a well-documented prognostic factor. IGFBP2 is a member of the secreted IGFBP family that functions by interacting with circulating IGFs to modulate IGF-mediated signalling. This traditional view of IGFBP2 activities has been challenged by the recognition of the diverse functions and cellular locations of members of the IGFBP family. IGFBP2 has been previously established as a driver of glioma progression to a higher grade. In this study we sought to determine whether IGFBP2-overexpressing tumours are dependent on continued oncogene expression and whether IGFBP2 is a viable therapeutic target in glioma. We took advantage of the well-characterized RCAS/Ntv-a mouse model to create a doxycycline-inducible IGFBP2 model of glioma and demonstrated that the temporal expression of IGFBP2 has dramatic impacts on tumour progression and survival. Further, we demonstrated that IGFBP2-driven tumours are dependent on the continued expression of IGFBP2, as withdrawal of this oncogenic signal led to a significant decrease in tumour progression and prolonged survival. Inhibition of IGFBP2 also impaired tumour cell spread. To assess a therapeutically relevant inhibition strategy, we evaluated a neutralizing antibody against IGFBP2 and demonstrated that it impaired downstream IGFBP2-mediated oncogenic signalling pathways. The studies presented here indicate that IGFBP2 is not only a driver of glioma progression and a prognostic factor but is required for tumour maintenance and thus represents a viable therapeutic target in the treatment of glioma.
Keywords: IGFBP2, Glioma, RCAS mouse model
Introduction
One of the most consistently overexpressed factors in glioma, the mRNA for insulin-like growth factor binding protein-2 (IGFBP2), is associated with higher grade and poor prognosis in both glioblastoma (GBM) and lower grade glioma (LGG) [1]. Overexpression of IGFBP2 in glioma was identified in early microarray studies and later confirmed in tissue microarray analysis to be overexpressed in more than 80% of GBMs [1, 2]. Since these early observations, IGFBP2 has been found to be one of the strongest biomarkers of aggressive behaviour and is a well-documented prognostic factor [3]. The frequency of IGFBP2 overexpression in glioma suggests that its expression is a key factor in all of the molecular subtypes of GBM. Cooperation between IGFBP2 and platelet-derived growth factor-B (PDGFB) in animal models and the association between IGFBP2 and hypomethylation [4, 5] suggest that it plays an important role in proneural GBM cases lacking the glioma CpG island methylator phenotype (G-CIMP). Further, the poor prognosis associated with the mesenchymal subtype and the frequent association between IGFBP2 and key signature genes (e.g., STAT3 [6] and VEGF [7]) indicate that IGFBP2 is an important factor in mesenchymal GBM.
IGFBP2 is a secreted protein that functions by interacting with circulating IGFs to modulate IGF-mediated signalling [8]. Additional functions for IGFBP2 have recently been identified, many of which are IGF independent and involve intracellular and nuclear IGFBP2 actions. Emerging evidence clearly points to a role for IGFBP2 in tumorigenesis via the modulation of several hallmarks of cancer, indicating that IGFBP2 overexpression is not only a biomarker for glioma but has critical functional consequences for the development and progression of tumour grade in glioma. IGFBP2 is induced under hypoxic conditions, and its overexpression is associated with increased VEGF promoter activation, indicating that it plays a role in tumour angiogenesis [7, 9]. IGFBP2 secreted by metastatic breast cancer cells was demonstrated to mediate endothelial cell recruitment, establishing an environment supportive of metastatic colonization [10]. Overexpression of IGFBP2 also promotes the upregulation of migration- and invasion-enhancing genes and promotes cell migration via interaction with integrins [11, 12]. IGFBP2 may play a role in the ability of tumour cells to resist cell death, and IGFBP2 expression confers resistance to chemotherapy in several tumour types [13, 14]. Finally, our group has demonstrated that overexpression of IGFBP2 cooperates with other growth factors, such as PDGFB, to activate and maintain growth-promoting signalling pathways [5, 15, 16].
Using the RCAS/Ntv-a mouse model of PDGFB-driven gliomas, we first demonstrated that IGFBP2 could cooperate with PDGFB to drive the progression of PDGFB-induced LGG to high-grade glioma (HGG) [5]. This model has proven to be a powerful tool for exploring the initiation and progression of glioma in vivo. With this method, we also elucidated a major pathway of glioma development in which IGFBP2 mediates an ILK-NFκB signalling cascade to drive HGG [15].
Although our earlier report demonstrated that inhibition of at least one major IGFBP2-mediated pathway impairs glioma progression, direct inhibition of IGFBP2 has not yet been assessed. Therefore, we sought to determine whether IGFBP2-overexpressing gliomas are dependent on continued IGFBP2 expression and therefore whether inhibition of IGFBP2 is a viable therapeutic strategy for glioma. We assessed the role of IGFBP2 in tumour progression and survival by developing an inducible model of PDGFB and IGFBP2-driven glioma in mice. We examined the tumour response to IGFBP2 at different stages of tumour development and developed a model with which to examine the in vivo response to oncogene withdrawal. Further, to gain more insight into therapeutically relevant strategies, we examined the inhibition of IGFBP2 using a neutralizing antibody.
The results of this study demonstrate that gliomas that develop under conditions of IGFBP2 expression are dependent on this oncogene to maintain their more aggressive phenotype. Inhibition of IGFBP2 using a clinically relevant neutralizing antibody demonstrated that key tumour-promoting functions that are suspected to be regulated by IGFBP2 can be impaired by IGFBP2 inhibition. This study demonstrates, for the first time, that IGFBP2 may be a valid therapeutic target for glioma.
Methods
RCAS constructs and DF-1 cells
RCAS constructs encoding PDGFB and IGFBP2 have been described [5]. RCANBP(A)TRE and RCASBP(A)-TetON-IRES-Puro were kindly provided by Sheri Holmen and have been described previously [17]. RCANBP(A)TRE-IGFBP2 was generated by Gateway recombination. RCAS/RCAN constructs were transfected into an avian fibroblast cell line, DF-1 (ATCC). DF-1 cells were maintained in DMEM with 10% FBS and cultured at 39°C with 10% CO2.
Animals
All animal studies were performed in accordance with the guidelines of The University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee. Nestin-Tv-a (Ntv-a) mice have been described previously [18]. DF-1 cells producing RCAS/RCAN viral particles were collected, mixed, and resuspended in 1x PBS. Neonatal mice (postnatal day 0.5-2.5) were given an intracranial injection (5μl), in the right hemisphere, of a DF-1 cell suspension using a Hamilton syringe. Doxycycline was administered by replacing standard diet with doxycycline-containing diet (Harlan Teklad). To induce IGFBP2 at birth, doxycycline-containing chow was given to the mothers, as doxycycline can be passed to nursing pups [19]. Mice were placed on standard diet (Birth to 3 wk) or doxycycline-containing diet (Birth and Birth to 6 wk) upon weaning at 3 wk of age. Mice were euthanized upon developing symptoms of tumour, and their brains were collected in OCT and formalin as described previously [5].
Histopathololgy and Statistical Analysis
A section of each mouse brains was snap frozen for further analysis. The remaining tissue was fixed in formalin and paraffin embedded. H&E sections were analysed for the presence and grade of glioma by one author (MD Anderson Neuropathologist, Dr. Gregory N. Fuller). High-grade gliomas were identified by the presence of microvascular proliferation or necrosis. Survival data were analysed by applying the log-rank test and the Kaplan-Meier estimate of survival using GraphPad Prism 6. All other statistical analyses were performed using either GraphPad Prism 6.05 or StatXact 11.0 (Cytel Inc.).
Biomarker analysis and Reverse Transcription (RT)-qPCR
To assess blood based markers, whole blood was collected from mice by cardiac puncture. The serum and remaining blood (RBCs, WBCs, and clotting factors) were separated and stored at −80°C. Nucleated cells were isolated from the blood clot after red blood cell lysis. Total RNA was extracted using the Ambion miRvana kit. To assess IGFBP2 expression in tumour tissue, total RNA was extracted from previously frozen mouse tumour tissue using the Ambion miRvana kit. RT-PCR was performed using Superscript II (ThermoFisher) with random hexamers for reverse transcription and TaqMan primer probe pairs. Samples were run on an ABI Prism 7900. Results were analyzed using SDS 2.3.
ELISA
Mouse serum samples were diluted 1:2 and assessed using a human IGFBP2 ELISA (RayBio cat# ELH-IGFBP2-001) according to the kit instructions.
Immunohistochemisty and Western Blot analyses
Immunohistochemistry and Western blot analyses were performed as described previously [5]. Following antigen retrieval with citrate buffer (pH 6), immunostaining was performed using the EnVision+-system HRP (DAB) kit (Dako). The antibodies used for IHC were IGFBP2 1:100 (Cell Signaling Technology (CST) #3922), Ki-67 1:1000 (Abcam #ab6615), GFAP 1:1000 (Abcam #ab48050), Olig2 1:500 (Millipore #AB9610), Nestin 1:1000 (Abcam #ab6142), and LSD1 1:800 (CST #2184). Western blot analyses were performed using whole cell extracts unless otherwise noted. Histone fractions were isolated using the Histone Extraction Protocol (Abcam). In brief, cell pellets were resuspended in extraction buffer and lysed. After centrifugation, histones were extracted in 0.2N HCL. The antibodies used for Western blot were IGFBP2 C-18 1:500 (Santa Cruz Biotechnology #6001), pEGFRY1068 (CST #3777), EGFR (CST #4267), pSTAT3Y705 (CST #9145), STAT3 (CST #9139), Bcl-xL (CST #2764), LSD1 (CST #2184), Actin I-19 (Santa Cruz Biotechnology #1616), Tubulin 1:2000 (CST #2128), Histone H3 1:5000 (CST #4499), Histone H3K9me2 1:2000 (CST #9753), Histone H3K27me3 1:2000 (CST #9733), pAktS473 (CST # 4051), Akt (CST #9272), and GFP (BD Bioscience #632375). All antibodies were used at a 1:1000 dilution unless otherwise noted above.
Cell culture
U251 and U87MG cells from ATCC were maintained in DMEM:F12 50:50 with 10% FBS at 37°C with 5% CO2. Glial progenitor cells (GPCs) were isolated from Ntv-a mice, as described previously [5], and cultured in DMEM with 10% FBS at 37°C with 5% CO2. For recombinant IGFBP2 treatment, cells were serum-starved overnight prior to the addition of recombinant IGFBP2 (Abcam) in serum-free media for the indicated times. IGFBP2-neutralizing antibody (0.1 – 10 ug/mL) (R&D Systems #AF674) or control goat IgG (2 or 10 ug/mL) were also administered to cells in serum-free media.
Results
IGFBP2 promotes tumour progression in a time-dependent manner
We previously demonstrated that IGFBP2 cooperates with PDGFB to promote tumour progression in the RCAS/Ntv-a model of glioma [5]. These studies demonstrated that the co-delivery of PDGFB with IGFBP2 promoted tumor progression and the development of HGGs that histologically resemble human HGGs and display increased mitosis, microvascular proliferation, and regions of pseudopalisading necrosis. To more thoroughly explore the role IGFBP2 plays in the progression of PDGFB-driven tumours to HGGs, we generated a unique inducible mouse model. We modified the constitutive RCAS-IGFBP2 to place IGFBP2 expression under the control of a tetracycline-responsive element (TRE). In this modified system, PDGFB is constitutively expressed, while IGFBP2 expression is regulated by doxycycline administration (Supplementary Figure 1A).
We first tested this system in glial progenitor cells (GPCs) isolated from neonatal Ntv-a mice. The GPCs were infected with RCAS/RCAN viruses encoding GFP, PDGFB, the reverse tetracycline transactivator (rtTA [Tet-ON]), and tetracycline-inducible IGFBP2 (TRE-IGFBP2). The RCAN construct encodes human IGFBP2, and can be distinguished from endogenous murine IGFBP2 by molecular weight. Upon doxycycline treatment, IGFBP2 levels increased, demonstrating proper induction of IGFBP2 (Supplementary Figure 1B). To induce tumours in mice, newborn pups were injected at birth with DF-1 cells producing the RCAS/RCAN viruses expressing PDGFB, rtTA, and TRE-IGFBP2. Animals were then allocated into groups that received either the standard diet (no doxycycline) or a doxycycline-containing diet. Mice that received doxycycline were further subdivided into groups and placed on the diet at birth, 3 wk of age, or 6 wk of age.
The results of our previous studies indicated that PDGFB-induced gliomas arise as early as 3 wk; therefore, we induced IGFBP2 at birth (concurrently with PDGFB) or at two later time points at which LGG had already begun to develop. To validate the doxycycline-dependent induction of IGFBP2 in vivo, we examined IGFBP2 expression in the resulting tumours by human IGFBP2-specific RT-qPCR (Supplementary Figure 1C), and evaluated human IGFBP2 secretion by serum ELISA (Supplementary Figure 1D).
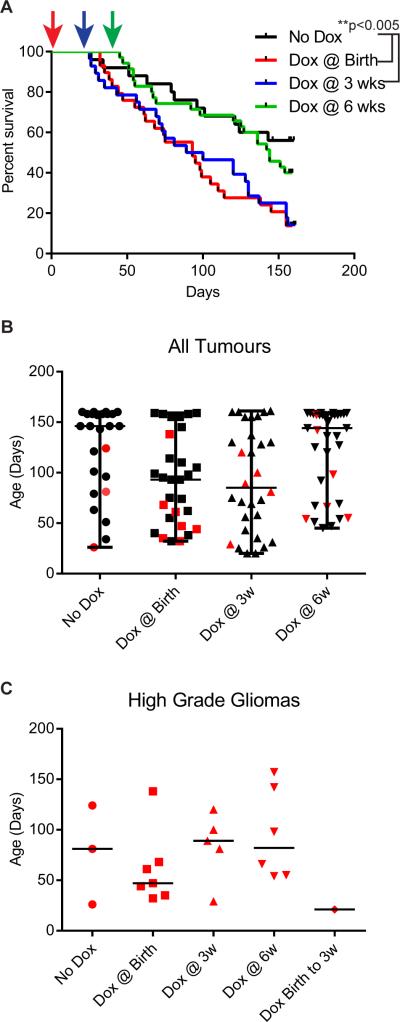
Consistent with the results of our previous studies, PDGFB alone induced primarily LGGs, while concurrent IGFBP2 and PDGFB expression from birth promoted an increased incidence of HGGs and poor survival. A survival analysis demonstrated that induction of IGFBP2 within the first 3 wk of PDGFB exposure resulted in the poorest survival (Figure 1A, Supplementary Table 1). Induction of IGFBP2 expression at 6 wk following tumour initiation by PDGFB resulted in a survival curve similar to that of mice in which IGFBP2 was not induced (PDGFB only). Induction of IGFBP2 at birth (concurrently with PDGFB) resulted in a modest increase in the incidence of HGG (24%) compared to PDGFB alone (12% HGGs). Delayed IGFBP2 induction had a more modest effect (16%-17% HGG) (Table 1). Similar to the findings in our previous studies, mice that did not receive doxycycline primarily developed LGG (88% LGG versus 12% with HGG). We examined the tumour grade relative to the age at which each animal died of disease. Animals died of LGG throughout the study timeframe of 23 wk. Conversely, animals that were determined to harbour HGGs died at an age that was relative to the time of IGFBP2 induction (Figure 1B, 1C). Mice in which IGFBP2 was induced at 3 and 6 wk succumbed to HGG later (median age 89 d and 92 d, respectively) than did those in which IGFBP2 was induced at birth (median age 42 d). The effect of IGFBP2 induction on animal survival was independent of the incidence of HGG, as a survival analysis of only the mice that developed LGG revealed similar survival curves (Supplementary Figure 2A, Supplementary Table 1).
Figure 1.
Early IGFBP2 expression drives HGG. A. Kaplan-Meier survival curves for Ntv-a mice injected with RCAS-PDGFB, RCAN-TRE-IGFBP2, and RCAS-Tet-On. IGFBP2 induction was mediated by doxycycline (dox) administration at the times indicated (arrows). B, C. Age of animals at the time of symptom development in each group. High-grade gliomas are indicated with red symbols. C. Plot showing only high-grade gliomas from each group.
Table 1.
Incidence of High Grade Gliomas upon IGFBP2 induction.
| Time of IGFBP2 Induction | Percent High Grade Gliomas (HGG/Total Tumours) |
|---|---|
| None | 12% (3/25) |
| Birth | 24.1% (7/29) |
| 3 weeks | 17.9% (5/28) |
| 6 weeks | 17.1% (6/35) |
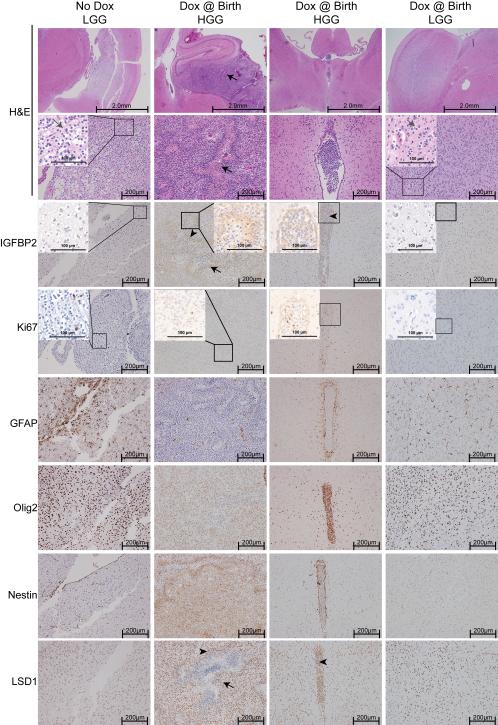
As in our previous studies, tumours that arose in these mice resembled human gliomas. (Figure 2, Supplementary Figure 5) They displayed hypercellularity and varying degrees of proliferation, as determined by Ki-67 immunostaining. LGGs display typical uniform, round nuclei with a “fried egg” appearance (grey arrows). HGGs exhibited microvascular proliferation and pseudopalisading necrosis (black arrows). The PDGFB-induced tumours have been described as a model of the proneural subtype of GBM [20]. Consistent with this, we observed widespread expression of Olig2, whereas Nestin expression varied and GFAP expression was typically restricted to reactive astrocytes (Figure 2). To explore the possibility of circulating tumour cells or other blood-based markers of glioma in our model, we collected whole blood from each animal at the time they were killed. Red blood cells were lysed and RNA was extracted from the remaining nucleated cells. We measured the expression of glioma-associated genes (VEGF and PDGFR-α) and markers of circulating endothelial cells (CD34 and KDR) by RT-qPCR (Supplementary Figure 3). Levels of PDGFR-α were significantly increased in mice with HGG. We also observed a moderate but non-significant increase in expression of CD34, KDR, and VEGF with tumour progression. PDGFRα expression is common biomarker for glioma and a defining feature of the proneural subtype [21, 22], and markers such as CD34, VEGF and KDR have been identified in the circulation of GBM patients [23]. Although we were unable to definitively identify these markers as derived from circulating tumour or endothelial cells, detection of these markers in circulation of tumour-bearing mice further demonstrates that the tumours that develop from this model recapitulate human glioma.
Figure 2.
Inducible IGFBP2-driven tumours resemble human glioma. Representative H&E-stained sections and immunohistochemical staining of sections from mice with and without doxycycline-mediated IGFBP2 induction. Lower grade gliomas (LGGs) display uniform round nuclei and a characteristic “fried egg” appearance (grey arrows). High-grade gliomas (HGGs) display pseudopalisading necrosis (black arrows). Arrowheads indicate regions of high cellularity with intense IGFBP2 and LSD1 concordant staining. Insets represent higher magnification of the outlined regions, scale bar is 100 μm.
IGFBP2-mediated tumour progression is reversible
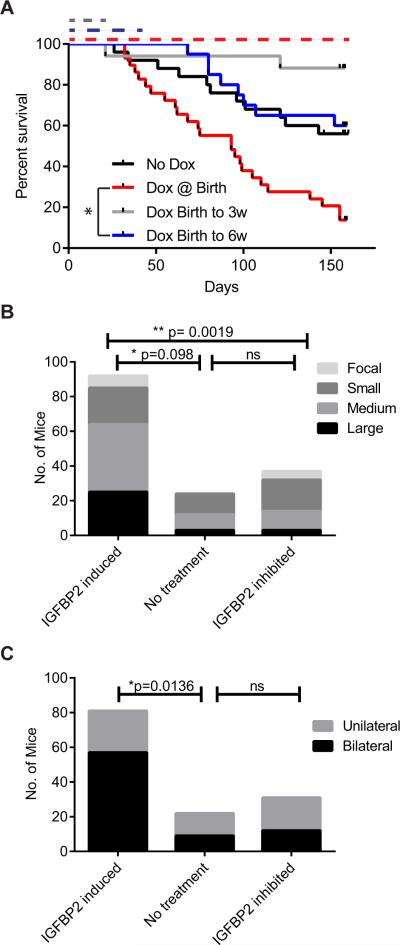
We hypothesized that the effects of IGFBP2 on the progression of tumours to HGGs in our mouse model might be reversible. As in the previous experiments, mice were injected with DF-1 cells that produced RCAS/RCAN viruses encoding PDGFB, TRE-IGFBP2, and rtTA at birth. Concurrently, all mice were placed on a doxycycline-containing diet to induce IGFBP2. At 3 or 6 wk of age, mice were removed from the doxycycline-containing diet and switched to standard feed to inhibit the expression of the RCAN-derived IGFBP2. IGFBP2 levels were decreased in these tumours compared to tumours induced by constitutive RCAS-derived IGFBP2 and induced RCAN-derived IGFBP2 (Supplementary Figure 5). Mice in which IGFBP2 expression was inhibited after glioma initiation had a significant reduction in the incidence of high-grade gliomas (Table 2). The difference in the incidence of HGG in mice in which IGFBP2 expression was inhibited (“birth to 3 wk” and “birth to 6 wk”) compared to mice that received the doxycycline-containing diet for the duration of the experiment (“from birth”) is −21% (95% confidence intervals = (−38%, −5%), p=0.0081)). Consistent with this decreased incidence, inhibition of IGFBP2 by doxycycline removal resulted in improved survival (Figure 3A, Supplementary Table 1). A survival analysis of only the mice that developed LGG revealed similar survival curves (Supplementary Figure 2B, Supplementary Table 1).
Table 2.
Incidence of High Grade Gliomas after IGFBP2 inhibition.
| Duration of IGFBP2 Induction | Percent High Grade Gliomas (HGG/Total Tumours) | P value (vs From Birth) |
|---|---|---|
| None | 12% (3/25) | 0.25 |
| From Birth | 24.1% (7/29) | --- |
| Birth to 3 weeks | 5.9% (1/17) | 0.11 |
| Birth to 6 weeks | 0% (0/20) | 0.0081 |
Figure 3.
IGFBP2 inhibition impairs tumour progression. A. Kaplan-Meier survival curves for Ntv-a mice injected with RCAS-PDGFB, RCAN-TRE-IGFBP2, and RCAS-Tet-On. IGFBP2 induction is mediated by doxycycline administration from birth, and the duration of doxycycline-mediated IGFBP2 expression is indicated by the dotted lines. B. Size of tumours relative to IGFBP2 status. Animals were grouped on the basis of overall IGFBP2 induction. ** p= 0.0019 comparing all three groups by the Kruskal-Wallis test using StatXact 11.0. C. Number of mice exhibiting bilateral tumours representing contralateral spread. Animals were grouped on the basis of overall IGFBP2 induction. * p=0.0136, Fisher Exact test, GraphPad Prism.
Previous studies revealed that IGFBP2 is a major activator of glioma cell invasion and migration. As IGFBP2 inhibition resulted in primarily low-grade and diffuse tumours, we assessed overall tumour size and the tumour's spread from the injection site to the contralateral hemisphere as indicators of tumour cell migration and invasion capacity. All animals in the study were assessed for tumour size, however a few could not be confirmed as unilateral or bilateral and were omitted from this analysis. IGFBP2 inhibition resulted in smaller tumours and significantly fewer bilateral tumours than those that developed in response to consistent PDGFB and IGFBP2 expression (Figure 3B,C). The size and the incidence of bilateral tumours in the mice in which IGFBP2 was inhibited were similar to those of the tumours in mice in which IGFBP2 was not induced (PDGFB only).
IGFBP2 inhibition impairs oncogenic signalling pathways
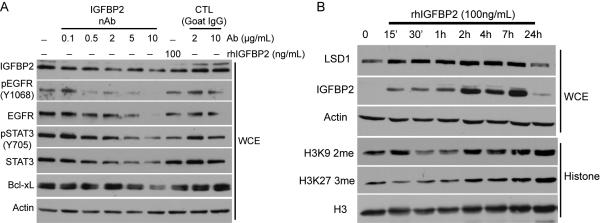
To explore the mechanisms by which IGFBP2 inhibition might impair tumour growth, we used a commercially available neutralizing antibody against IGFBP2 to treat human glioma cell lines. We treated U251 cells (which express endogenous IGFBP2) with the neutralizing antibody against IGFBP2. We recently demonstrated that IGFBP2 overexpression leads to activation of the EGFR/STAT3 signalling pathway in glioma cell lines [6]. Treatment with the IGFBP2-neutralizing antibody led to a reduction in the levels of phosphorylated (activated) EGFR and STAT3, as well as decreased expression of the STAT3 downstream target, Bcl-xL (Figure 4A). Neutralization of IGFBP2 also led to decreased levels of phosphorylated Akt (Supplementary Figure 4). Given recent reports identifying the relationship between IGFBP2 expression and hypomethylation in glioma [4], we determined whether IGFBP2 specifically affected epigenetic factors. As an alternative to the inducible system and the neutralizing antibody, we treated U87MG glioma cells, which do not express detectable endogenous IGFBP2 protein, with recombinant human IGFBP2 (rhIGFBP2), which resulted in transient rhIGFBP2 uptake and response. We examined the key histone -modifying enzymes and histone methylation marks using Western blot analysis (Figure 4B). Treatment with rhIGFBP2 resulted in a rapid increase in levels of the lysine-specific demethylase 1 (LSD1/KDM1A). We also observed a decrease in the levels of methylated histones that have been identified as direct targets of LSD1 (H3K9 and H3K27). These effects were transient: LSD1 protein levels returned to baseline as the recombinant IGFBP2 levels decreased, indicating that the effects of IGFBP2 on the epigenetic state are reversible. Consistent with this observation, treatment of U251 cells with the neutralizing antibody against IGFBP2 also inhibited protein levels of LSD1 (Supplementary Figure 4). We also examined the LSD1 expression level in murine tumours. We observed highly coordinated expression of LSD1 with IGFBP2 surrounding regions of necrosis in the HGGs examined (Figure 2).
Figure 4.
Inhibition of IGFBP2 impairs oncogenic signalling pathways. A. U251 cells were serum starved overnight and incubated with increasing doses of IGFBP2-neutralizing antibody (nAb). A Western blot analysis was performed on whole cell extracts (WCEs). B. U87MG cells were serum starved overnight and incubated with rhIGFBP2 for the indicated times. Western blot analyses were performed on WCEs and isolated histone fractions.
Discussion
Overexpression of IGFBP2 is one of the most consistent and significant events associated with poor prognosis, tumour progression, and tumour recurrence. We sought to demonstrate that IGFBP2 serves not only as a prognostic biomarker for disease, but also as a targetable molecule, the inhibition of which would impair multiple pathways that converge on hallmarks of cancer. Existing therapies that target single pathways or cancer hallmarks have been unsuccessful against glioma. Identifying factors that integrate multiple cancer hallmarks is likely to uncover tumorigenesis network hubs that can be more effectively exploited by targeted therapies.
Previous studies have demonstrated that IGFBP2 is a major driver of glial tumour development and a cooperating factor with PDGFB in mouse models of glioma. In the present study, we extended these findings to uncover the temporal requirements for IGFBP2-driven glioma in a mouse model. We demonstrated that IGFBP2 cooperates with PDGFB to promote HGG progression only when this cooperation occurs in the early stages of tumour initiation. As in our previous animal model studies and in patients, early IGFBP2 expression also negatively impacts survival. Progression to high-grade glioma is directly related to survival, however the negative impact of IGFBP2 induction on survival was apparent even among LGGs. This is consistent with observations in patients, where IGFBP2 levels are associated with poorer survival not only in GBM patients, but also among LGG patients [3, 24]. The mechanisms by which IGFBP2 impacts survival even in the apparent absence of promoting tumour progression are not clearly defined. It is possible that IGFBP2-mediated effects on tumour cell migration, invasion, and aberrant cell signalling cascades imparts a more aggressive nature to these tumours even prior to histological signs of progression to higher grade. We also demonstrated that IGFBP2 expression did not drive HGG progression or have an impact on survival compared to PDGFB alone after development of an established tumour. These findings not only solidify the concept that IGFBP2 is a driver of tumorigenesis but also suggest there is a key window during which IGFBP2 establishes a more aggressive tumour and in which therapeutic intervention may be most effective.
To this end, we further explored the effects of inhibiting IGFBP2 expression after IGFBP2 and PDGFB cooperative tumour initiation. We previously studied the impact of IGFBP2 inhibition in PDGFB-induced gliomas that arose in an INK4a/ARF knockout background [16]. In that study, we inhibited the expression of endogenous murine IGFBP2 in these tumours by using RCAS to deliver antisense oligonucleotides. While the antisense design was imperfect (designed against human IGFBP2), there was a modest yet significant improvement in survival and a slight decrease in the incidence of HGGs. Therefore, in the current study we used the inducible IGFBP2 model as an alternative strategy to better address the effects of IGFBP2 inhibition in vivo. We allowed tumours to initiate with the cooperation of PDGFB and IGFBP2 for 3-6 wk before inhibiting IGFBP2 expression. IGFBP2 inhibition dramatically improved survival and impaired HGG progression compared to that in mice in which IGFBP2 was continuously expressed for the duration of the study. This finding suggests that these tumours are “addicted” to IGFBP2, at least in terms of progression to HGG and survival.
In GBM cases, TCGA protein lysate array data indicate that IGFBP2 levels are significantly decreased in G-CIMP+ cases, although the IGFBP2 promoter itself does not appear to be methylated [3]. The recent TCGA report on the molecular landscape of lower-grade glioma shows that IGFBP2 protein levels are particularly elevated in IDH wild-type cases [24]. Both of these observations are consistent with earlier reports that associate IGFBP2 expression with hypomethylation [4] and are consistent with IGFBP2 being a poor prognostic factor, as IDH mutation and G-CIMP+ confer better prognosis. This suggests the possibility of early events “programming” tumours to develop along a certain pathway. For example, IDH mutation occurs as an early event in tumorigenesis, programming these tumours to develop and progress in a manner that may not require IGFBP2 for progression but also imparting a better overall prognosis. On the other hand, IDH wild-type tumours which overexpress IGFBP2 early in tumour development might be programmed towards a more aggressive nature, but one that is addicted to IGFBP2 and might therefore be a candidate for IGFBP2-targeted therapies.
IGFBP2 levels may serve as a predictive biomarker for therapeutic stratification, and IGFBP2 may serve directly as a targetable molecule in newly developed therapies. In healthy individuals, IGFBP2 levels drop rapidly after birth and are very low in normal adult tissue [25, 26]. Studies using knockout mouse models indicate that IGFBP2 is not required for development, and adult animals lacking IGFBP2 are healthy and fertile [27]. This disease-specific upregulation of IGFBP2 makes it an attractive target for intervention that is likely to only minimally impact normal cells.
Using neutralizing antibodies against secreted factors is a viable therapeutic strategy; several antibodies have been developed and are being evaluated in clinical trials (e.g., bevacizumab against VEGF). Here we demonstrated that inhibition of IGFBP2 using a neutralizing antibody resulted in impairment of IGFBP2-associated oncogenic signalling pathways, as measured by the impaired phosphorylation of key members of the EGFR/STAT3 signalling pathway and downregulation of epigenetic factors, such as LSD1. A recent report described the development of a new neutralizing antibody against IGFBP2 [28]; further studies will be required to test this in a pre-clinical model. We also recently demonstrated that siRNA-based inhibition of IGFBP2 and mutation of the nuclear localization signal of IGFBP2 impaired glioma cell migration and invasion [6], demonstrating that IGFBP2 has nuclear roles in addition to secreted activities, findings that must be considered in assessing the therapeutic impacts of IGFBP2-neutralizing antibodies. Alternative mechanisms for inhibiting IGFBP2 in a clinical setting include microRNA. We recently identified a glioma-associated miRNA that targets IGFBP2, namely miR-491 [18]. MiRNA mimics and sponges are being assessed for their utility as therapeutic agents, and in an orthotopic model of glioma, we demonstrated the ability of miR-491 to inhibit glioma tumour growth [18].
Elevated levels of IGFBP2 are associated with poor prognosis and tumour recurrence following therapy [29]. Inhibition of IGFBP2 in several cancer cells and animal models sensitizes tumour cells to traditional chemotherapeutic agents such as docetaxel, 5-FU, and doxorubicin in prostate and breast cancer cells [14, 30, 31]. IGFBP2 inhibition has also been demonstrated to sensitize some GSCs to temozolomide [32]. Therefore, in addition to the direct therapeutic utility of targeting IGFBP2, these strategies may function as part of combination therapies. Together, the evidence presented here makes a solid case that IGFBP2 is a driver of tumorigenesis and glioma progression and is a viable therapeutic target, given the addictive nature of IGFBP2 expression. One limitation of this study is that tumours were only assessed at the end of the study to allow survival analysis. IGFBP2 inhibition resulted in fewer high-grade tumours, but we were unable to directly determine whether IGFBP2 inhibition resulted in tumour regression. Although inducible models such as this one are very powerful, it does not represent a viable therapeutic method for inhibiting IGFBP2 in patients, therefore, further work will be required to optimize the delivery methods, and additional studies in preclinical models will also be needed.
Supplementary Material
Acknowledgements
We thank Dr. Sheri L. Holmen (University of Utah School of Medicine) for kindly providing the RCAS/RCAN vectors required to generate the inducible IGFBP2 constructs. We also thank Ann Sutton (Scientific Editor, Department of Scientific Publications, MD Anderson) for editorial assistance. This work was supported in part by a postdoctoral fellowship (PF-10-190-01-TBG to L.M.P) from the American Cancer Society, grants from the U.S. National Institutes of Health (CA098503, CA141432 and CA143835 to W.Z and G.N.F and U24 CA143835 to W.Z.), and NIH/NCI grant P30CA016672 to MD Anderson supporting the Research Histology Core Laboratory and the Flow Cytometry and Cellular Imaging Facility.
Footnotes
The authors have no conflicts of interest to disclose.
Author Contributions
LMP and WZ designed the study, analysed the data, and wrote the manuscript. XZ, DEC, and AH carried out the experiments. CYC analysed the data and contributed to writing the manuscript. GNF and KRH analysed the data.
SUPPORTING INFORMATION ON THE INTERNET
Figure S1. Generation of the inducible IGFBP2 RCAS-Ntv-a mouse model.
Figure S2. Kaplan-Meier survival curves.
Figure S3. Tumour-bearing mice display evidence of circulating tumour and endothelial cells.
Figure S4. Inhibition of IGFBP2 impairs oncogenic signalling pathways.
Figure S5. Constitutive RCAS-derived IGFBP2 and inducible RCAN-derived IGFBP2 levels in tumour-bearing mice.
Table S1. Survival Statistics
References
- 1.Fuller GN, Rhee CH, Hess KR, et al. Reactivation of insulin-like growth factor binding protein 2 expression in glioblastoma multiforme: a revelation by parallel gene expression profiling. Cancer Res. 1999;59:4228–4232. [PubMed] [Google Scholar]
- 2.Wang H, Zhang W, Fuller GN. Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol. 2002;12:95–107. doi: 10.1111/j.1750-3639.2002.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan Cameron W, Verhaak Roel GW, McKenna A, et al. The Somatic Genomic Landscape of Glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng S, Houseman EA, Morrison Z, et al. DNA hypermethylation profiles associated with glioma subtypes and EZH2 and IGFBP2 mRNA expression. Neuro Oncol. 2011;13:280–289. doi: 10.1093/neuonc/noq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunlap SM, Celestino J, Wang H, et al. Insulin-like growth factor binding protein 2 promotes glioma development and progression. Proc Natl Acad Sci U S A. 2007;104:11736–11741. doi: 10.1073/pnas.0703145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chua CY, Liu Y, Granberg KJ, et al. IGFBP2 potentiates nuclear EGFR-STAT3 signaling. Oncogene. 2015 doi: 10.1038/onc.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azar WJ, Azar SHX, Higgins S, et al. IGFBP-2 Enhances VEGF Gene Promoter Activity and Consequent Promotion of Angiogenesis by Neuroblastoma Cells. Endocrinology. 2011;152:3332–3342. doi: 10.1210/en.2011-1121. [DOI] [PubMed] [Google Scholar]
- 8.Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278:E967–976. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- 9.Godard S, Getz G, Delorenzi M, et al. Classification of Human Astrocytic Gliomas on the Basis of Gene Expression: A Correlated Group of Genes with Angiogenic Activity Emerges As a Strong Predictor of Subtypes. Cancer Research. 2003;63:6613–6625. [PubMed] [Google Scholar]
- 10.Png KJ, Halberg N, Yoshida M, et al. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2012;481:190–194. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
- 11.Wang GK, Hu L, Fuller GN, et al. An interaction between insulin-like growth factor-binding protein 2 (IGFBP2) and integrin alpha5 is essential for IGFBP2-induced cell mobility. Journal of Biological Chemistry. 2006;281:14085–14091. doi: 10.1074/jbc.M513686200. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Wang H, Shen W, et al. Insulin-like Growth Factor Binding Protein 2 Enhances Glioblastoma Invasion by Activating Invasion-enhancing Genes. Cancer Res. 2003;63:4315–4321. [PubMed] [Google Scholar]
- 13.Biernacka KM, Uzoh CC, Zeng L, et al. Hyperglycaemia-induced chemoresistance of prostate cancer cells due to IGFBP2. Endocrine-Related Cancer. 2013;20:741–751. doi: 10.1530/ERC-13-0077. [DOI] [PubMed] [Google Scholar]
- 14.So AI, Levitt RJ, Eigl B, et al. Insulin-Like Growth Factor Binding Protein-2 Is a Novel Therapeutic Target Associated with Breast Cancer. Clin Cancer Res. 2008;14:6944–6954. doi: 10.1158/1078-0432.CCR-08-0408. [DOI] [PubMed] [Google Scholar]
- 15.Holmes KM, Annala M, Chua CY, et al. Insulin-like growth factor-binding protein 2-driven glioma progression is prevented by blocking a clinically significant integrin, integrin-linked kinase, and NF-kappaB network. Proc Natl Acad Sci U S A. 2012;109:3475–3480. doi: 10.1073/pnas.1120375109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore LM, Holmes KM, Smith SM, et al. IGFBP2 is a candidate biomarker for Ink4a-Arf status and a therapeutic target for high-grade gliomas. Proc Natl Acad Sci U S A. 2009;106:16675–16679. doi: 10.1073/pnas.0900807106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmen SL, Williams BO. Essential Role for Ras Signaling in Glioblastoma Maintenance. Cancer Res. 2005;65:8250–8255. doi: 10.1158/0008-5472.CAN-05-1173. [DOI] [PubMed] [Google Scholar]
- 18.Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci U S A. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie W, Chow LT, Paterson AJ, et al. Conditional expression of the ErbB2 oncogene elicits reversible hyperplasia in stratified epithelia and up-regulation of TGFalpha expression in transgenic mice. Oncogene. 1999;18:3593–3607. doi: 10.1038/sj.onc.1202673. [DOI] [PubMed] [Google Scholar]
- 20.Lei L, Sonabend AM, Guarnieri P, et al. Glioblastoma Models Reveal the Connection between Adult Glial Progenitors and the Proneural Phenotype. PLoS ONE. 2011;6:e20041. doi: 10.1371/journal.pone.0020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verhaak RGW, Hoadley KA, Purdom E, et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karsy M, Neil JA, Guan J, et al. A practical review of prognostic correlations of molecular biomarkers in glioblastoma. Neurosurgical Focus. 2015;38:E4. doi: 10.3171/2015.1.FOCUS14755. [DOI] [PubMed] [Google Scholar]
- 23.Rafat Neysan, Beck Grietje Ch., Schulte J, et al. Circulating endothelial progenitor cells in malignant gliomas. Journal of Neurosurgery. 2010;112:43–49. doi: 10.3171/2009.5.JNS081074. [DOI] [PubMed] [Google Scholar]
- 24.Network CGAR Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. New England Journal of Medicine. 2015;372:2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green BN, Jones SB, Streck RD, et al. Distinct expression patterns of insulin-like growth factor binding proteins 2 and 5 during fetal and postnatal development. Endocrinology. 1994;134:954–962. doi: 10.1210/endo.134.2.7507840. [DOI] [PubMed] [Google Scholar]
- 26.Lee WH, Michels KM, Bondy CA. Localization of insulin-like growth factor binding protein-2 messenger RNA during postnatal brain development: correlation with insulin-like growth factors I and II. Neuroscience. 1993;53:251–265. doi: 10.1016/0306-4522(93)90303-w. [DOI] [PubMed] [Google Scholar]
- 27.Wood TL, Rogler LE, Czick ME, et al. Selective Alterations in Organ Sizes in Mice with a Targeted Disruption of the Insulin-Like Growth Factor Binding Protein-2 Gene. Molecular Endocrinology. 2000;14:1472–1482. doi: 10.1210/mend.14.9.0517. [DOI] [PubMed] [Google Scholar]
- 28.Patil SS, Railkar R, Swain M, et al. Novel anti IGFBP2 single chain variable fragment inhibits glioma cell migration and invasion. J Neurooncol. 2015;123:225–235. doi: 10.1007/s11060-015-1800-7. [DOI] [PubMed] [Google Scholar]
- 29.Lin Y, Jiang T, Zhou K, et al. Plasma IGFBP-2 levels predict clinical outcomes of patients with high-grade gliomas. Neuro-Oncology. 2009;11:468–476. doi: 10.1215/15228517-2008-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foulstone EJ, Zeng L, Perks CM, et al. Insulin-Like Growth Factor Binding Protein 2 (IGFBP-2) Promotes Growth and Survival of Breast Epithelial Cells: Novel Regulation of the Estrogen Receptor. Endocrinology. 2013;154:1780–1793. doi: 10.1210/en.2012-1970. [DOI] [PubMed] [Google Scholar]
- 31.Uzoh CC, Holly JM, Biernacka KM, et al. Insulin-like growth factor-binding protein-2 promotes prostate cancer cell growth via IGF-dependent or -independent mechanisms and reduces the efficacy of docetaxel. Br J Cancer. 2011;104:1587–1593. doi: 10.1038/bjc.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh D, Hsieh A, Stea B, et al. IGFBP2 promotes glioma tumor stem cell expansion and survival. Biochem Biophys Res Commun. 2010;397:367–372. doi: 10.1016/j.bbrc.2010.05.145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.