Summary
Background
We present the largest set of US prevalence data for psoriasis to date, obtained from three prospective cohort studies describing validated clinical phenotypes of psoriasis, including novel data about the prevalence of inverse (intertriginous) psoriasis in these groups. Nonplaque psoriasis phenotypes have been largely unmeasured in observational and interventional studies, and this has led to an under-recognition of this aspect of psoriatic disease.
Aim
To describe the prevalence of nonplaque psoriasis phenotypes in a large prospective cohort.
Methods
We included 3179 women and 646 menin the analysis. Participants in the Nurses Health Study and Health Professionals Follow-up Study with physician-diagnosed psoriasis completed a validated, self-administered questionnaire to assess plaque and nonplaque subsets of psoriasis.
Results
Psoriasis phenotypes were as follows: plaque 55%, scalp 52%, palmar–plantar 14%, nail 23% and inverse 21% in the Nurses Health Study (NHS) (n = 1604); plaque 60%, scalp 56%, palmar–plantar 16%, nail 27% and inverse 24% in the second NHS study (NHS II) (n = 1575); and plaque 55%, scalp 45%, palmar–plantar 12%, nail 27% and inverse 30% in the Health Professionals Follow-up Study (n = 646). Scalp, nail, palmar–plantar and inverse disease represent highly prevalent phenotypes of psoriasis in the USA.
Conclusion
Scalp, nail, palmar–plantar and inverse disease represent highly prevalent phenotypes of psoriasis.
Introduction
Psoriasis is a complex, immune-mediated systemic disease, with both genetic and environmental factors contributing to its development. Psoriasis has a variable clinical presentation manifesting as specific phenotypes, including plaque, scalp, inverse (intertriginous), nail and palmar–plantar.
We present a detailed evaluation of validated phenotypes of psoriasis in three large US prospective cohorts.5,8,7 Of particular interest are new data on the prevalence of the inverse psoriasis phenotype. We further present data on participants who reported multiple (≥ 2) simultaneous psoriasis phenotypes. Most observational studies and interventional trials focussing on plaque psoriasis and other psoriasis phenotypes have been largely unmeasured, and the types of posoriasis under-recognized and, we believe, undertreated.12–15 Recognizing these phenotypes is of increasing importance to the dermatology and rheumatology communities, as increasing evidence indicates that several of these phenotypes are associated with psoriatic arthritis (PsA) and each has a marked impact on quality of life (QoL) measures.16
Methods
This study was approved by the institutional review board of Brigham and Women’s Hospital, with completion and return of the self-administered questionnaires by particpants considered as informed consent.
Study participants were from three cohorts, the Health Professionals Follow-up Study (HPFS), the Nurses’ Health Study (NHS) and the Nurses’ Health Study 2 (NHS II).4,6,10
The HPFS, established in 1986, included 51 529 male health professionals aged 40–75 years, who completed a baseline questionnaire. The NHS was first established in 1976, and contains health information about 121 701 married female registered nurses aged 30–55 years in the US. NHSII was established in 1989, and enrolled 116 430 female registered nurses between the ages of 25 and 42 years. Information on medical history and lifestyle factors has been collected biennially via mailed questionnaires in each cohort since baseline.
In 2005, information on physician-diagnosed psoriasis was asked of participants in NHS2, while in 2008, this same query and the time period of diagnosis were requested of the participants in the HPFS and NHS studies. We contacted those who reported the diagnosis, and confirmed the self-report using the Psoriasis Screening Tool (PST) questionnaire, including type of specialist making the diagnosis and the phenotype(s) of psoriasis.3 A pilot study showed a sensitivity of 99% and a specificity of 94% for PST in psoriasis screening.3 Descriptive statistics were used to present prevalence data regarding the phenotypic subsets of psoriasis among the participants in the respective cohorts.
Results
Detailed demographics of the participants, including daily alcohol use, exercise and prevalent PsA, in each of the cohorts is provided in supplementary Table S1. Participants in the cohorts were overwhelmingly white (> 92%). Regarding weight, 21% of women and 47% of men were overweight, while fewer were obese (8–18%). Past and current smokers made up 64%, 44% and 57% of of NHS I, NHS II and HPFS participants, respectively.
Psoriasis phenotypes were as follows: plaque 55%, scalp 52%, palmar–plantar 14%, nail 23% and inverse 21% in the Nurses Health Study (NHS) (n = 1604); plaque 60%, scalp 56%, palmar–plantar 16%, nail 27% and inverse 24% in the second NHS study (NHS II) (n = 1575); and plaque 55%, scalp 45%, palmar–plantar 12%, nail 27% and inverse 30% in the Health Professionals Follow-up Study (n = 646).
Figure 1 depicts individual and pooled cohort data for the phenotypes. We further examined the prevalence of two nonplaque phenotypes occurring in the same patient. Nail+scalp and inverse+scalp disease were among the more common phenotypes, at around 14–17% prevalence. Figure 2 demonstrates individual and pooled cohort data in individuals with two concurrent phenotypes. Prevalence data considering plaque disease and each non-plaque phenotypic subset is provided in supplementary Figure S1.
Figure 1.
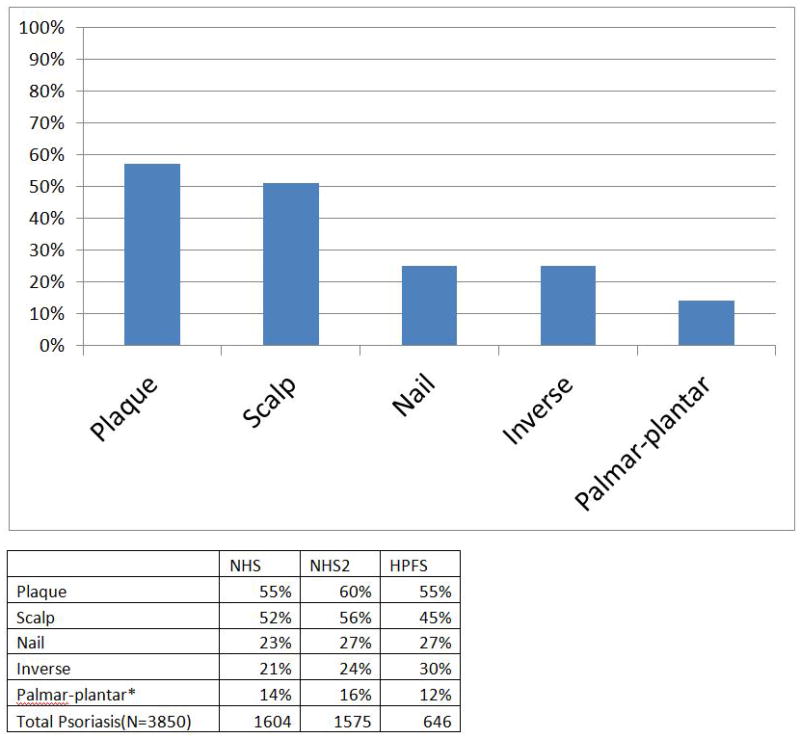
(a) Pooled prevalence data from the Nurses Health Study (NHS), NHS II and Health Professionals Follow-up Study (HPFS) covering 3825 participants with psoriasis. (b) Detailed individual percentages in each cohort presenting with plaque and nonplaque psoriasis phenotypes. *Data from 147 women in NHS II.
Figure 2.
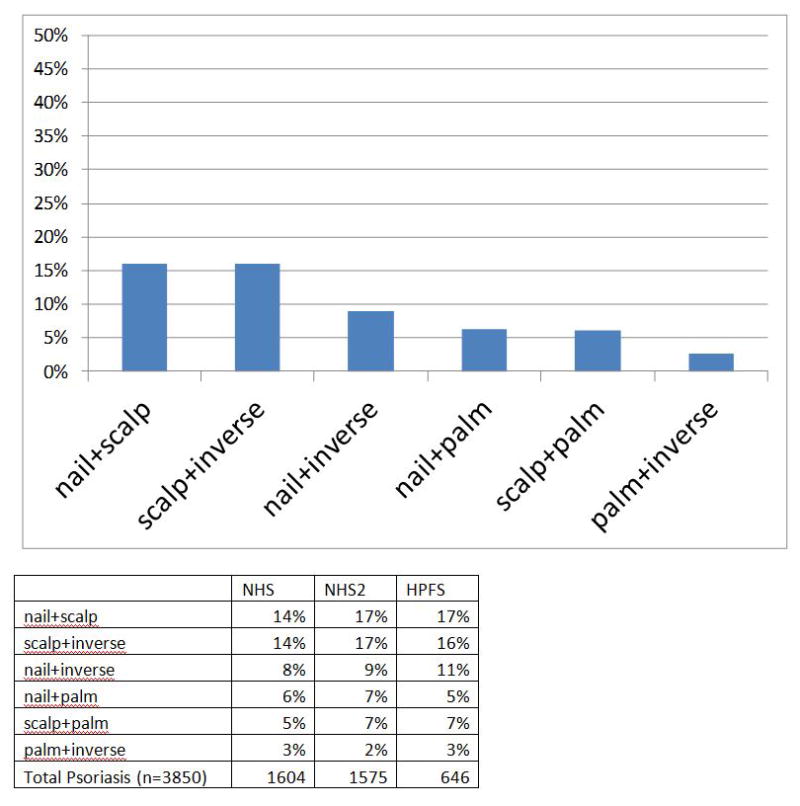
9a) Pooled prevalence data from the Nurses Health Study (NHS), NHS II and Health Professionals Follow-up Study (HPFS), covering 3825 participants with two forms of nonplaque psoriasis. (b) Detailed individual percentages in each cohort presenting two concurrent nonplaque psoriasis phenotypes.
Discussion
In this study, we provide the largest set of US prevalence data describing validated clinical phenotypes of psoriasis to date, including novel data about the prevalence of inverse psoriasis in these groups. Nonplaque psoriasis phenotypes have been largely unmeasured in observational and interventional studies, and this has led to an under-recognition of this aspect of psoriatic disease.
The importance of these phenotypes is emphasized by QoL studies. Patients with psoriasis patients with nail disease was shown to have higher (i.e. worse) mean Dermatology Life Quality Index (DLQI) scores than those without nail involvement,1 with > 50% of patients surveyed specifically reporting nail pain, restriction in daily activities (including housekeeping) and limited ability to work.2 The effect of palmar–plantar disease on QoL has been shown by the Skindex-29 to include significant impairment in work and hobbies,14 and by the Short Form (SF)-36 to have a greater burden of disease than plaque disease alone.15 Patients also report bleeding and discomfort with water exposure.12
Mental health scores on the SF-36 for patients with psoriasis were similar to those seen in psychiatric illnesses, with greater burden of disease among subjects with palmar–plantar disease.15 Using the Scalpdex, patients reported frequently feeling ashamed, self-conscious and embarrassed about their appearance, reporting problematic scalp bleeding and problems with clothing choice, with higher scores reported in men relative to women, and at younger age relative to older age.13
There are limited data on QoL and disease impact of inverse disease, which remains an understudied phenotype of this disease, but in our opinion, it most certainly carries a heavy burden of psychosocial and functional impairment, based on patient feedback alone. It has been our personal experience that many patients do not openly report the burden of inverse disease, owing to the personal and intimate nature of this condition. Inverse disease remains the ‘hidden psoriasis’, and given the large prevalence we newly report, as well as the burden of this disease, clinicians need to consider this aspect of the disease for their patients.
In addition to the individual phenotype prevalence data presented, the prevalence of at least two non-plaque phenotypes present concurrently among individual patients is captured in our analysis. Scalp disease plus another phenotype was present in upwards of 15% of participants. Patients presenting with one nonplaque phenotype should be screened for other subsets, particularly inverse disease, and their increased risk of incident PsA noted.
Nail disease involvement, particularly nail pitting, is associated with increased risk of PsA [hazard ratio (HR) = 2.93]; however, somewhat less known among the dermatology and rheumatology communities is the increased association of scalp disease (HR) = 3.89) and intergluteal/perianal lesions (HR) = 2.35) with PsA.16 For patients presenting with a seronegative inflammatory arthritis, awareness of these subsets is crucial to appropriately examining and diagnosing those patients with PsA, as classic plaque disease might be mild or even nonexistent in a given patient. Raising awareness of PsA screening among patients with these phenotypes is important to capturing early disease and for psoriasis treatment algorithms, which fundamentally hinge upon the presence or absence of inflammatory arthritis in decision-making.
The limitations of our study include reporting bias. Specifically, the reporting of nail disease may be further confounded by the presence of onychomycosis. The average prevalence of onychomycosis among patients with psoriasis is around 18%, increased compared with control groups9,11. This is probably tempered by the fact that psoriatic nail disease often coexists with onychomycosis.
Conclusion
Scalp, nail, palmar–plantar and inverse disease represent highly prevalent phenotypes of psoriasis. We encourage further attention to these potentially debilitating phenotypes in both observational and interventional trials among our colleagues dedicated to studying psoriasis.
Supplementary Material
What’s already known about this topic?
Nonplaque psoriasis has been previously reported as occurring far less commonly than plaque disease and with localized disease (palmar–plantar, inverse/intertiginous) being relatively uncommon among subsets.
QoL for these patients is often disproportionately impacted relative to the affected area of involvement.
These nonplaque phenotypes tend to be under-reported and therefore undertreated.
What does this study add?
Scalp, nail, palmar–plantar and inverse disease are highly prevalent, based upon our evaluation of two large US-based cohorts.
Awareness of these phenotypes has important implications for patients with regard to quality of life and associated risk of PsA.
Inverse (intertriginous) psoriasis is present in a remarkably high percentage (24–30%) of patients, and is an otherwise ‘hidden’ form of psoriasis that goes largely underdiagnosed and therefore undertreated.
Acknowledgments
We thank the participants in the Nurses’ Health Study cohort and the Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA. This work was supported by departmental funding and by grants (CA87969 and CA055075) from the National Institutes of Health.
Footnotes
Conflict of interest: JFM is a consultant for Biogen IDEC, Amgen, Abbvie and Eli Lilly, has received a grant from Biogen IDEC, and has licensed a questionnaire to Abbvie. AAQ has licensed a questionnaire to Merck, Pfizer and Abbvie, has received a grant from Amgen, and has acted as a consultant for Jansen, Novartis and Abbott. None of the other authors has any conflict of interest to report.
References
- 1.Augustin M, Reich K, Blome C, et al. Nail psoriasis in Germany: epidemiology and burden of disease. Br J Dermatol. 2010;163:580–5. doi: 10.1111/j.1365-2133.2010.09831.x. [DOI] [PubMed] [Google Scholar]
- 2.de Jong EM, Seegers BA, Gulinck MK, et al. Psoriasis of the nails associated with disability in a large number of patients: results of a recent interview with 1,728 patients. Dermatology. 1996;193:300–3. doi: 10.1159/000246274. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez PL, Assarpour A, Kuo H, et al. Development and pilot-testing of a psoriasis screening tool. Br J Dermatol. 2009;161:778–84. doi: 10.1111/j.1365-2133.2009.09247.x. [DOI] [PubMed] [Google Scholar]
- 4.Dominguez PL, Han J, Li T, et al. Depression and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1163–7. doi: 10.1111/j.1468-3083.2012.04703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doss N. Psoriasis: different clinical phenotypes. International journal of dermatology. 2014;53:e142–143. doi: 10.1111/j.1365-4632.2012.05652.x. [DOI] [PubMed] [Google Scholar]
- 6.Frankel HC, Han J, Li T, Qureshi AA. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918–24. doi: 10.1001/archdermatol.2012.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffiths CE, Christophers E, Barker JN, et al. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol. 2007;156:258–62. doi: 10.1111/j.1365-2133.2006.07675.x. [DOI] [PubMed] [Google Scholar]
- 8.Guinot C, Latreille J, Perrussel M, et al. Psoriasis: characterization of six different clinical phenotypes. Exp Dermatol. 2009;18:712–19. doi: 10.1111/j.1600-0625.2009.00871.x. [DOI] [PubMed] [Google Scholar]
- 9.Klaassen KM, Dulak MG, van de Kerkhof PC, Pasch MC. The prevalence of onychomycosis in psoriatic patients: a systematic review. J Am Acad Dermatol. 2014;28:533–41. doi: 10.1111/jdv.12239. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Han J, Choi HK, Qureshi AA. Smoking and risk of incident psoriasis among women and men in the United States: a combined analysis. Am J Epidemiol. 2012;175:402–13. doi: 10.1093/aje/kwr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzo D, Alaimo R, Tilotta G, et al. Incidence of onychomycosis among psoriatic patients with nail involvement: a descriptive study. Mycoses. 2013;56:498–9. doi: 10.1111/myc.12042. [DOI] [PubMed] [Google Scholar]
- 12.Sampogna F, Gisondi P, Melchi CF, et al. Investigators IDIMPRoVE Prevalence of symptoms experienced by patients with different clinical types of psoriasis. Br J Dermatol. 2004;151:594–9. doi: 10.1111/j.1365-2133.2004.06093.x. [DOI] [PubMed] [Google Scholar]
- 13.Sampogna F, Linder D, Piaserico S, et al. Quality of Life Assessment of Patients with Scalp Dermatitis Using the Italian Version of the Scalpdex. 2014;94:411–14. doi: 10.2340/00015555-1731. [DOI] [PubMed] [Google Scholar]
- 14.Sampogna F, Tabolli S, Abeni D. Living with psoriasis: prevalence of shame, anger worry, and problems in daily activities and social life. 2012;92:299–303. doi: 10.2340/00015555-1273. [DOI] [PubMed] [Google Scholar]
- 15.Sampogna F, Tabolli S, Soderfeldt B, et al. Measuring quality of life of patients with different clinical types of psoriasis using the SF-36. Br J Dermatol. 2006;154:844–9. doi: 10.1111/j.1365-2133.2005.07071.x. [DOI] [PubMed] [Google Scholar]
- 16.Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. 2009;61:233–9. doi: 10.1002/art.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


