Abstract
Aims
Cigarette smoking has been associated with incident heart failure independent of coronary artery disease (CAD), but the mechanisms linking smoking to cardiac damage are not well understood. This study sought to evaluate the relationship between smoking and N-Terminal Pro-Brain Natriuretic Peptide (NT-ProBNP) and high-sensitivity Troponin T (hs-TnT), respective biomarkers of myocardial wall stress and injury, in a large community-based cohort.
Methods and Results
We examined the association between smoking history and NT-ProBNP and hs-TnT in 9,649 participants free of overt CAD or heart failure from the Atherosclerosis Risk in Communities (ARIC) Study who attended Visit 4 (1996–1998), as well as the association with change in these biomarkers from Visit 4 to Visit 5 (2011–2013) in 3,151 participants. At Visit 4, higher cumulative cigarette exposure, assessed by total pack-years, was associated with elevated levels of NT-ProBNP (p<0.001) and hs-TnT (p=0.01) among ever smokers in multivariable analyses adjusted for potential confounders. After 15 years of follow-up, participants who were active smokers at Visit 4 had greater incidence of elevated NT-ProBNP (adjusted proportion [95%CI] = 48 [41, 54] vs. 35 [32, 39]%; p=0.006] and hs-TnT [adjusted proportion [95%CI] = 32 [26, 38] vs. 23 [20, 26]%; p=0.021) compared to never smokers, adjusting for baseline and follow-up covariates.
Conclusions
In a large community-based cohort free of overt CAD and heart failure, cigarette smoking was associated with biomarkers of myocardial wall stress and injury at baseline as well as with a continued measurable increase in these biomarkers after 15 years of follow-up.
Keywords: Smoking, N-Terminal Brain Natriuretic Peptide, Troponin, Heart
INTRODUCTION
Cigarette smoking is a leading preventable cause of cardiovascular morbidity and mortality (1). Epidemiological studies have suggested that active smokers and to a lesser extent former smokers are at higher risk for developing heart failure (HF) even after adjusting for coronary artery disease (CAD) (2, 3). Animal studies suggested that tobacco smoke may exert direct toxic effects on the myocardium (4), while clinical showed associations between smoking and alterations in cardiac function in subjects without overt cardiovascular disease (5, 6). Altogether, these observations support the notion that tobacco use might directly affect myocardial integrity. However, the potential mechanisms linking smoking to cardiac damage remain unclear.
N-Terminal Pro-Brain Natriuretic Peptide (NT-ProBNP) and high-sensitivity Troponin T (hs-TnT) are recognized as diagnostic and prognostic circulating biomarkers of cardiac dysfunction (7, 8). NT-ProBNP increases in response to hemodynamic stress, which occurs when myocardial chambers are dilated, hypertrophied, and/or subject to increased wall tension, while hs-TnT increases as a consequence of cardiomyocyte injury and/or necrosis (7). Cross-sectional data from community-based cohorts have provided conflicting results regarding the associations between smoking history and NT-ProBNP (9, 10, 11) or hs-TnT (10, 12, 13, 14) levels. Additionally, greater upward trajectories of NT-ProBNP and hs-TnT levels over several years are associated with a heightened risk of developing cardiac dysfunction compared to smaller variations over time (15). Therefore, consideration not only of baseline levels but also of temporal changes in NT-ProBNP and hs-TnT may be useful approaches to assess potential mechanisms involved in myocardial damage. However, the impact of smoking status on the long-term changes of these biomarkers is uncertain.
We analyzed the association between smoking and NT-ProBNP and hs-TnT levels in the large, community-based Atherosclerosis Risk in Communities (ARIC) Study.
METHODS
Study population
The overall ARIC Study is an ongoing, prospective observational study. Detailed study rationale, design, and procedures have been previously published (16). The original cohort included 15,792 participants aged 45 to 64 years recruited between 1987 and 1989 (Visit 1), selected from 4 communities in the United States. The investigation conforms with the principles outlined in the Declaration of Helsinki. Institutional review boards from each site approved the study, and informed consent was obtained from all participants. In this study we considered the 11,656 participants attending Visit 4 (1996–1998). We excluded those with prevalent CAD or HF (n=1,429) and participants whose race was neither black nor white, or with missing NT-ProBNP, hs-TnT or smoking data, resulting in 9,649 individuals eligible for cross-sectional analysis. In addition, we analyzed follow-up data from Visit 4 to Visit 5 (2011–2013). Among the 9,649 participants initially included, 2,043 died during follow-up and 1,151 were excluded because of incident CAD or HF between Visits 4 and 5. Furthermore, participants who did not attend Visit 5 or with missing NT-ProBNP, hs-TnT and smoking data at Visit 5 were excluded. Because we wanted to assess the impact of chronic obstructive pulmonary disease (COPD) as a covariate, participants with missing spirometry at Visit 5 were also excluded, resulting in 3,151 participants for longitudinal analysis (Supplemental Figure 1).
Measurements
Smoking history
Smoking history was ascertained by means of an interviewer-administered questionnaire. Participants were asked if they currently smoked cigarettes or whether they had done so in the past. Participants who stated that they never smoked but referred exposure to environmental tobacco smoke for more than 1 hour per week were classified as passive smokers (17). This approach yielded 4 categories at Visit 4: never smokers, passive smokers, former smokers and current smokers. Smoking pack-years (number of packs per day multiplied by years of smoking) were calculated among current and former smokers at Visit 4. At Visit 5, participants were classified as current, former and never smokers and information about pack-years and exposure to environmental tobacco smoke was not available.
Cardiac Biomarkers
Blood samples were taken at ARIC Visits 4 and 5 and plasma was stored centrally at −80°C. Hs-TnT was measured using a highly sensitive assay (Elecsys Troponin T; Roche Diagnostics, Indianapolis, IN) with a limit of detection of 5 ng/L (18). NT-ProBNP was measured using electrochemiluminescent immunoassay (Roche Diagnostics) with a lower detection limit of 5 pg/mL (8). Participants with NT-ProBNP and hs-TnT levels below the lower limits of detection were assigned values equal to half of the lower limits of detection (8). The variability in NT-proBNP and hsTnT concentrations related to frozen storage and freeze–thaw cycles and the reliability coefficient and interassay coefficient of variation for both biomarkers have been described previously (8).
Measurement of Other Covariates and Outcomes
Information on demographics, clinical history, anthropometric measures, blood pressure, heart rate and lipid levels was obtained at Visits 4 and 5. Definitions for hypertension, diabetes mellitus, current alcohol drinking, CAD and HF were used as previously described in the ARIC study (19). At Visit 4, COPD was defined as a self-report of physician diagnosis of either emphysema or chronic bronchitis. At Visit 5, spirometry was performed and subjects with forced expiratory volume in 1 second/forced vital capacity ratio <0.65 were considered to have COPD (20).
From Visit 4 to December 2012, we assessed the incidence of death, non-fatal CAD, defined as myocardial infarction or coronary revascularization, and non-fatal HF, defined by HF hospitalization (19). Deaths were determined through linkage with the National Death Index.
Statistical methods
Descriptive data are presented as the mean ± standard deviation for normally distributed variables and median [25th–75th percentile] for non-normally distributed variables. Log-transformed variables are also described using geometric mean (95% confidence intervals). Significant pairwise comparisons are shown only for variables in which a significant global difference was detected using one-way ANOVA or Kruskal-Wallis tests. Categorical variables are expressed as proportions and were compared by the chi-square test.
For cross-sectional analyses, NT-ProBNP was modeled continuously using the log transformed value to achieve normality and categorically using a threshold of 125 pg/mL as previously described (21). Hs-TnT was heavily skewed and was modeled as a categorical variable using a threshold of 14 ng/L as formerly reported (13, 18, 22). To assess the association between smoking status and cardiac biomarkers in cross-sectional analysis we used multivariable linear models for NT-ProBNP and logistic models for elevated NT-ProBNP and hs-TnT.
For longitudinal analyses, we calculated the change in NT-ProBNP and hs-TnT values at follow-up (Visit 5), compared to baseline (Visit 4) as the difference on the log scale. The relationship between Visit 4 smoking status and change in cardiac biomarkers was analyzed by linear regression analysis. To illustrate the magnitude of change in cardiac biomarkers, values of adjusted geometric means of NT-ProBNP and hs-TnT at Visits 4 and 5 were presented. The association between Visit 4 smoking status and the incidence of elevated NT-ProBNP or hs-TnT at Visit 5 was assessed by logistic regression analysis, excluding subjects with elevated NT-ProBNP or hs-TnT levels at Visit 4.
To evaluate the smoking-biomarker relationship, we used additive multivariable models. Model covariates were selected based on a priori knowledge. Three regression models were constructed: Model 1 included age and gender; Model 2 additionally adjusted for race, field center, diabetes mellitus, hypertension, body mass index, current alcohol drinking, systolic blood pressure, heart rate, estimated glomerular filtration rate and COPD at Visit 4; Model 3: additionally adjusted for diabetes mellitus, hypertension, body mass index, current alcohol drinking, systolic blood pressure, heart rate, estimated glomerular filtration rate, smoking status and COPD at Visit 5. In cross-sectional analysis, Models 1 and 2 were used, while in longitudinal analysis, all three models were used and the baseline (Visit 4) values of the corresponding outcome variable (NT-ProBNP or hs-TnT) being modeled were also included. The cross-sectional associations between pack-years of smoking and elevated NT-ProBNP and hs-TnT at Visit 4 were assessed in ever smokers using logistic regression analysis adjusted for covariates included in Model 2. To adjust for possible bias due to selective attrition between Visits 4 and 5, we performed a sensitivity analysis incorporating estimated inverse probability weights based on the likelihood that a given patient at Visit 4 would return and have available biomarkers at Visit 5. The likelihood ratio test was used to assess gender and race-based interactions between cardiac biomarkers and smoking categories.
To evaluate the relationship between smoking status, elevated levels of biomarkers and incident HF, CAD or death from Visit 4 to December 2012, we calculated the rate of events per 100 person-years at risk and estimated hazard ratios, based on univariate Cox regression analysis. We also investigated the potential impact of smoking-related competing risk of death on the relationship between smoking status at Visit 4 and incident elevated hs-TnT at Visit 5, by performing sensitivity analyses including participants without elevated hs-TnT at Visit 4, first creating a composite outcome of death or elevated hs-TnT at Visit 5, and also creating an ordinal outcome denoting Visit 5 status as 1) alive without elevated hs-TnT; 2) alive with elevated hs-TnT; and 3) died prior to Visit 5.
Two-sided p values <0.05 were considered significant. Analyses were performed using Stata version 13.1 (Stata Corp., College Station, Texas).
RESULTS
Sample characteristics
Among the 9,649 participants included from Visit 4, never smokers were more likely to be women than the other smoking groups (Table 1). Current smokers were younger, had lower mean blood pressure and body mass index, were more likely to be current alcohol drinkers, had higher heart rate and glomerular filtration rate and higher prevalence of hypertension and COPD than never smokers. When compared to never smokers, passive smokers were younger, had higher body mass index and higher prevalence of diabetes, while former smokers were more frequently black, diabetic and alcohol drinkers, had higher prevalence of COPD and had lower heart rate.
Table 1.
Participants characteristics at Visit 4
| Smoking status | Never (n=2,919) |
Passive (n=1,592) |
Former (n=3,737) |
Current (n=1,401) |
|---|---|---|---|---|
| Male gender (n, %) | 795 (27) | 553 (35)* | 2,077 (56)* | 627 (45)* |
| Age (years) | 62.9 ± 5.7 | 62.0 ± 5.5* | 63.1 ± 5.7 | 61.5 ± 5.5* |
| Black (n,%) | 650 (22) | 381 (24) | 646 (17)* | 347 (25) |
| Body mass index (kg/m2) | 28.7 ± 5.6 | 29.5 ± 5.7* | 28.8 ± 5.2 | 26.7 ± 5.1* |
| Pack-years of smoking | ---- | ---- | 17 [7, 32] | 37 [23, 49] |
| Systolic blood pressure (mmHg) | 128 ± 19 | 128 ± 19 | 127 ± 18 | 125 ± 20* |
| Diastolic blood pressure (mmHg) | 71 ± 10 | 72 ± 10 | 72 ± 10 | 69 ± 11* |
| Hypertension (n,%) | 1337 (46) | 730 (46) | 1642 (44) | 552 (39)* |
| Heart rate (b.p.m.) | 63 ± 10 | 63 ± 10 | 62 ± 10* | 65 ± 11* |
| Diabetes mellitus (n,%) | 391 (13) | 250 (16)* | 590 (16)* | 175 (12) |
| Current alcohol drinkers (n,%) | 1186 (41) | 687 (43) | 2229 (60)* | 802 (57)* |
| COPD (n, %) | 148 (5) | 81 (5) | 312 (8)* | 181 (13)* |
| LDL-cholesterol (mg/dL) | 124 ± 33 | 125 ± 35 | 123 ± 32 | 124 ± 35 |
| HDL-cholesterol (mg/dL) | 53 ± 17 | 52 ± 17* | 49 ± 17* | 49 ± 17* |
| eGFR (mL/min/1.73m2) | 86 ± 16 | 87 ± 16 | 86 ± 15 | 91 ± 16* |
| NT-ProBNP (pg/mL) | 67 [35, 122] | 60 [30, 113]* | 62 [29, 119]* | 66 [33, 133] |
| Geometric Mean (pg/mL) | 62 (60–65) | 56 (53–59)* | 56 (54–59)* | 63 (60–68) |
| Elevated NT-ProBNP (n, %)† | 700 (24) | 335 (21)* | 878 (24) | 373 (27) |
| Hs-TnT (ng/L) | <5 [<5, 7] | <5 [<5, 7] | 5 [<5, 8]* | <5 [<5, 6]* |
| Elevated Hs-TnT (n, %)‡ | 179 (6) | 108 (7) | 327 (9)* | 79 (6) |
p<0.05 compared to never smokers.
Elevated NT-ProBNP was defined as values ≥ 125 pg/mL
Elevated hs-TnT was defined as values ≥ 14 ng/L
Normal-distributed continuous variables are presented as mean ± standard deviation. NT-ProBNP values are presented as median [25th–75th percentile] and geometric mean (95% confidence interval); Pack-years and hs-TnT values are shown as median [25th–75th percentile].
COPD – chronic obstructive pulmonary disease; eGFR - estimated glomerular filtration rate; HDL – high-density lipoprotein; Hs-TnT - high-sensitivity troponin T; LDL – low-density lipoprotein; NT-ProBNP - N-terminal pro–B-type natriuretic peptide.
Cross-sectional analysis of smoking and cardiac biomarkers
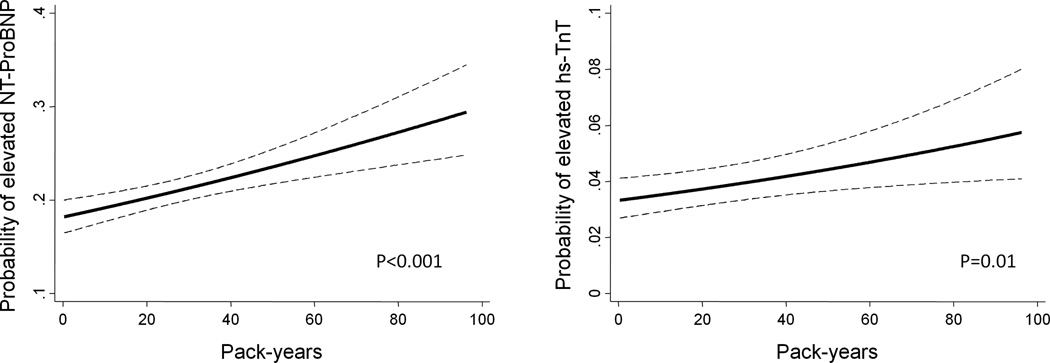
Current smokers showed higher levels of NT-ProBNP compared to never smokers in multivariable analysis (Table 2). In addition, current smokers and to a minor extent former smokers had a higher prevalence of elevated NT–ProBNP in comparison with never smokers. Although we did not find a significant difference in hs-TnT among the smoking groups (Table 2), when using a more comprehensive measure of smoking based on cumulative intensity and duration assessed by pack-years, greater smoking was significantly associated with elevated NT-ProBNP (p<0.001) and hs-TnT (p=0.01) among ever smokers (Figure 1). Since smoking and elevated troponin levels are reported to be associated with higher death rates (22), we hypothesized that survival bias might have influenced our ability to detect differences in hs-TnT among the smoking groups. In accordance with this hypothesis, we found that current smokers with elevated hs-TnT at Visit 4 had the highest rate of death among the studied subgroups (Table 3). Furthermore, the combination of current smoking and elevated hs-TnT was also associated with the highest rate of non-fatal HF and CAD among the studied population (Table 3).
Table 2.
Cross-sectional associations between cardiac biomarkers and smoking status at Visit 4
| Model 1 | p‡ | Model 2 | p‡ | |
|---|---|---|---|---|
| NT-ProBNP, pg/mL | Adjusted geometric mean (95% CI)* |
Adjusted geometric mean (95% CI)* |
||
| Never Smokers | 56 (54, 58) | Ref | 55 (53, 58) | Ref |
| Passive Smokers | 56 (53, 59) | 0.78 | 56 (54, 59) | 0.55 |
| Former Smokers | 59 (57, 62) | 0.027 | 58 (56, 60) | 0.07 |
| Current Smokers | 69 (65, 73) | <0.001 | 73 (69, 77) | <0.001 |
| Elevated NT-ProBNP (≥125 pg/mL) | Adjusted proportion (95% CI), %† |
Adjusted proportion (95% CI), %† |
||
| Never Smokers | 22 (20, 23) | Ref | 21 (20, 23) | Ref |
| Passive Smokers | 21 (19, 23) | 0.63 | 21 (19, 23) | 0.98 |
| Former Smokers | 25 (23, 26) | 0.002 | 24 (23, 26) | 0.003 |
| Current Smokers | 29 (27, 31) | <0.001 | 30 (28, 33) | <0.001 |
| Elevated hs-TnT (≥14 ng/L) | Adjusted proportion (95% CI), %† |
Adjusted proportion (95% CI), %† |
||
| Never Smokers | 8 (7, 9) | Ref | 8 (7, 9) | Ref |
| Passive Smokers | 8 (7, 9) | 0.63 | 8 (7, 9) | 0.87 |
| Former Smokers | 7 (6, 8) | 0.38 | 7 (6, 8) | 0.31 |
| Current Smokers | 6 (5, 7) | 0.06 | 6 (5, 7) | 0.24 |
Results reporting adjusted geometric means and 95% confidence intervals (CI) of NT-ProBNP at Visit 4 obtained from linear regression analysis.
Results reporting the adjusted proportions and 95% CI of elevated NT-ProBNP or hs-TnT at Visit 4 obtained from logistic regression analysis.
p-values regarding the differences in adjusted geometric means or adjusted proportions between never smokers, as the reference (Ref), and the other smoking groups.
hs-TnT – high-sensitivity Troponin T; NT-ProBNP - N-terminal pro–B-type natriuretic peptide.
Model 1: Adjusted for age and gender
Model 2: Further adjusted for race, body mass index, field center, diabetes mellitus, hypertension, chronic obstructive pulmonary disease, current alcohol drinking, systolic blood pressure, heart rate and estimated glomerular filtration rate.
Figure 1.
Cross-sectional association between smoking intensity and duration and cardiac biomarkers. Logistic regression analysis of elevated N-terminal Pro–B-type natriuretic peptide (NT-ProBNP) (≥125 pg/mL) and elevated high sensitivity Troponin T (hs-TnT) (≥14 ng/L), as a function of pack-years of smoking among ever smokers at Visit 4. The 95% confidence intervals are indicated by the dash lines. Models are adjusted for age, gender, race, body mass index, field center, diabetes mellitus, hypertension, current alcohol drinking, systolic blood pressure, heart rate, chronic obstructive pulmonary disease and estimated glomerular filtration rate.
Table 3.
Incidence of death, non-fatal heart failure and non-fatal coronary artery disease from Visit 4 until December 2012 according to smoking status and presence of elevated hs-TnT or NT-ProBNP at Visit4.
| Death | Heart Failure | Coronary artery disease | |||||
|---|---|---|---|---|---|---|---|
| Smoking status |
Elevated hs-TnT* |
Rate per 100 person-years |
HR (95% CI) | Rate per 100 person-years |
HR (95% CI) | Rate per 100 person-years |
HR (95% CI) |
| Never | No | 1.1 (1.0–1.2) | Ref | 0.6 (0.5–0.7) | Ref | 0.6 (0.6–0.7) | Ref |
| Never | Yes | 3.3 (2.6–4.2) | 3.2 (2.5–4.2) | 2.0 (1.4–2.7) | 3.5 (2.5–4.9) | 1.8 (1.3–2.5) | 2.8 (2.0–4.1) |
| Passive | No | 1.1 (1.0–1.3) | 1.0 (0.9–1.2) | 0.7 (0.6–0.8) | 1.1 (0.9–1.4) | 0.7 (0.6–0.9) | 1.2 (0.9–1.4) |
| Passive | Yes | 3.8 (2.9–4.9) | 3.7 (2.8–4.9) | 3.0 (2.2–4.2) | 5.4 (3.8–7.7) | 2.6 (1.8–3.7) | 4.1 (2.8–6.1) |
| Former | No | 1.6 (1.5–1.7) | 1.5 (1.3–1.7) | 0.8 (0.8–0.9) | 1.4 (1.2–1.7) | 1.1 (1.0–1.2) | 1.7 (1.5–2.0) |
| Former | Yes | 4.7 (4.1–5.4) | 4.8 (4.05.7) | 3.3 (2.8–4.0) | 6.1 (4.8–7.7) | 2.7 (2.2–3.4) | 4.3 (3.4–5.5) |
| Current | No | 2.5 (2.3–2.8) | 2.4 (2.1–2.7) | 1.2 (1.1–1.4) | 2.1 (1.7–2.5) | 1.3 (1.1–1.5) | 2.1 (1.7–2.5) |
| Current | Yes | 9.4 (7.3–12.1) | 10.5 (8.0–13.8) | 6.6 (4.6–9.3) | 13.7 (9.5–19.9) | 2.9 (1.8–4.8) | 4.7 (2.8–7.8) |
| Smoking status |
Elevated NT -ProBNP† |
Rate per 100 person-years |
HR (95% CI) | Rate per 100 person-years |
HR (95% CI) | Rate per 100 person-years |
HR (95% CI) |
| Never | No | 0.9 (0.8–1.1) | Ref | 0.5 (0.4–0.6) | Ref | 0.6 (0.6–0.7) | Ref |
| Never | Yes | 2.1 (1.8–2.4) | 2.3 (1.9–2.7) | 1.2 (1.0–1.5) | 2.5 (1.9–3.1) | 0.9 (0.7–1.1) | 1.4 (1.0–1.8) |
| Passive | No | 1.2 (1.0–1.3) | 1.3 (1.1–1.5) | 0.6 (0.5–0.8) | 1.3 (1.0–1.6) | 0.7 (0.6–0.9) | 1.2 (0.9–1.5) |
| Passive | Yes | 1.7 (1.4–2.1) | 1.9 (1.5–2.4) | 1.5 (1.2–2.0) | 3.1 (2.3–4.1) | 1.2 (0.9–1.6) | 1.9 (1.4–2.6) |
| Former | No | 1.5 (1.4–1.6) | 1.6 (1.4–1.8) | 0.7 (0.7–0.8) | 1.5 (1.2–1.8) | 1.1 (1.0–1.2) | 1.7 (1.4–2.0) |
| Former | Yes | 3.0 (2.7–3.3) | 3.3 (2.9–3.9) | 2.0 (1.8–2.3) | 4.1 (3.4–5.1) | 1.6 (1.4–1.9) | 2.5 (2.0–3.1) |
| Current | No | 2.3 (2.0–2.5) | 2.5 (2.1–2.9) | 1.0 (0.8–1.1) | 1.9 (1.5–2.4) | 1.2 (1.0–1.4) | 1.9 (1.5–2.3) |
| Current | Yes | 4.3 (3.7–5.0) | 4.9 (4.1–5.9) | 2.8 (2.3–3.4) | 5.9 (4.6–7.6) | 1.9 (1.5–2.4) | 3.0 (2.3–3.9) |
Elevated NT-ProBNP was defined as values ≥ 125 pg/mL
Elevated hs-TnT was defined as values ≥14 ng/L hs-TnT – high-sensitivity Troponin T; NT-ProBNP - N-terminal pro–B-type natriuretic peptide; HR – hazard ratio; CI – confidence interval.
Longitudinal analysis of smoking and cardiac biomarkers
Subjects included in the follow-up analysis (n=3,151) had similar baseline (Visit 4) clinical characteristics compared to those of the full sample (Supplemental Table 1). The majority of never, passive and former smokers at Visit 4 did not smoke at follow-up (Visit 5), while only 36% of current smokers at Visit 4 were smoking at Visit 5 (Supplemental Table 2). We found that current and former smokers had higher increases in NT-ProBNP levels and higher risk of developing elevated NT-ProBNP at follow-up than never smokers (Table 4). In comparison with never smokers, current smokers and to a marginal degree former smokers at Visit 4 demonstrated higher long-term increases in hs-TnT levels and a higher risk of developing elevated hs-TnT at Visit 5 (Table 4).
Table 4.
Regression analysis between smoking status at baseline (Visit 4) and ajusted long-term change of NT-ProBNP and hs-TnT (from Visit 4 to Visit 5) and adjusted incident elevated NT-ProBNP and hs-TnT at Visit 5
| Model 1 | p‡ | Model 2 | p‡ | Model 3 | p‡ | |
|---|---|---|---|---|---|---|
| GM (95% CI) at Visit 5* |
GM (95% CI) at Visit 5* |
GM (95% CI) at Visit 5* |
||||
| NT-ProBNP, pg/mL | ||||||
| Never Smokers | 113 (108, 119) | Ref | 113 (108, 119) | Ref | 109 (102, 116) | Ref |
| Passive Smokers | 118 (111, 126) | 0.32 | 116 (109, 124) | 0.55 | 116 (107, 124) | 0.14 |
| Former Smokers | 123 (118, 129) | 0.015 | 123 (117, 129) | 0.022 | 128 (120, 136) | 0.004 |
| Current Smokers | 132 (120, 145) | 0.006 | 137 (125, 151) | 0.001 | 132 (119, 148) | 0.006 |
| hs-TnT, ng/L | ||||||
| Never Smokers | 10 (9, 10) | Ref | 10 (9, 10) | Ref | 9 (9, 10) | Ref |
| Passive Smokers | 10 (10, 11) | 0.001 | 10 (10, 11) | 0.004 | 10 (10, 11) | 0.003 |
| Former Smokers | 10 (10, 10) | 0.09 | 10 (10, 10) | 0.07 | 10 (10, 11) | 0.012 |
| Current Smokers | 11 (10, 11) | <0.001 | 11 (10, 12) | <0.001 | 11 (10, 12) | <0.001 |
| Incident rate (95% CI) at Visit 5, %† |
Incident rate (95% CI) at Visit 5, %† |
Incident rate (95% CI) at Visit 5, %† |
||||
| Elevated NT-ProBNP (≥125 pg/mL)# | ||||||
| Never Smokers | 38 (36, 41) | Ref | 38 (35, 41) | Ref | 35 (32, 39) | Ref |
| Passive Smokers | 38 (34, 42) | 0.99 | 38 (34, 42) | 0.87 | 37 (32, 41) | 0.63 |
| Former Smokers | 42 (39, 45) | 0.10 | 42 (39, 45) | 0.08 | 46 (41, 50) | 0.004 |
| Current Smokers | 47 (42, 53) | 0.007 | 49 (43, 55) | 0.001 | 48 (41, 54) | 0.006 |
| Elevated hs-TnT (≥14 ng/L)** | ||||||
| Never Smokers | 24 (22, 27) | Ref | 25 (22, 27) | Ref | 23 (20, 26) | Ref |
| Passive Smokers | 28 (25, 31) | 0.08 | 27 (24, 30) | 0.23 | 25 (22, 29) | 0.26 |
| Former Smokers | 27 (24, 29) | 0.19 | 27 (25, 29) | 0.16 | 29 (26, 32) | 0.038 |
| Current Smokers | 31 (26, 36) | 0.019 | 31 (26, 37) | 0.014 | 32 (26, 38) | 0.021 |
Results reporting adjusted geometric means (GM) and 95% confidence intervals (CI) of NT-ProBNP or hs-TnT at Visit 5 obtained from linear regression analysis. Geometric means (95% CI) of NT-ProBNP and hs-TnT at Visit 4 were 47 (45, 49) pg/mL and 4 (4, 4) ng/L, respectively.
Results reporting the adjusted rates and 95% CI of incident elevated NT-ProBNP or hs-TnT at Visit 5 obtained from logistic regression analysis.
p-values regarding the differences in the change of NT-ProBNP or hs-TnT on the log scale between Visits 4 and 5 or the differences in adjusted rates of incident elevated NT-ProBNP or hs-TnT between never smokers, as the reference (Ref), and the other smoking groups.
In these sub-analyses, participants with NT-ProBNP ≥ 125 pg/mL at Visit 4 (n=525) were excluded.
In these sub-analyses, participants with hs-TnT ≥14 ng/L at Visit 4 (n=102) were excluded.
NT-ProBNP - N-terminal pro–B-type natriuretic peptide; hs-TnT – high-sensitivity Troponin T.
Model 1: Adjusted for age, gender and log NT-ProBNP or median hs-TnT at Visit 4 according to the respective outcome.
Model 2: Further adjusted for race, field center, diabetes mellitus, hypertension, body mass index, current alcohol drinking, systolic blood pressure, heart rate, estimated glomerular filtration rate and chronic obstructive pulmonary disease at Visit 4.
Model 3: Further adjusted for diabetes mellitus, hypertension, body mass index, current alcohol drinking, systolic blood pressure, heart rate, estimated glomerular filtration rate, chronic obstructive pulmonary disease and smoking status at Visit 5.
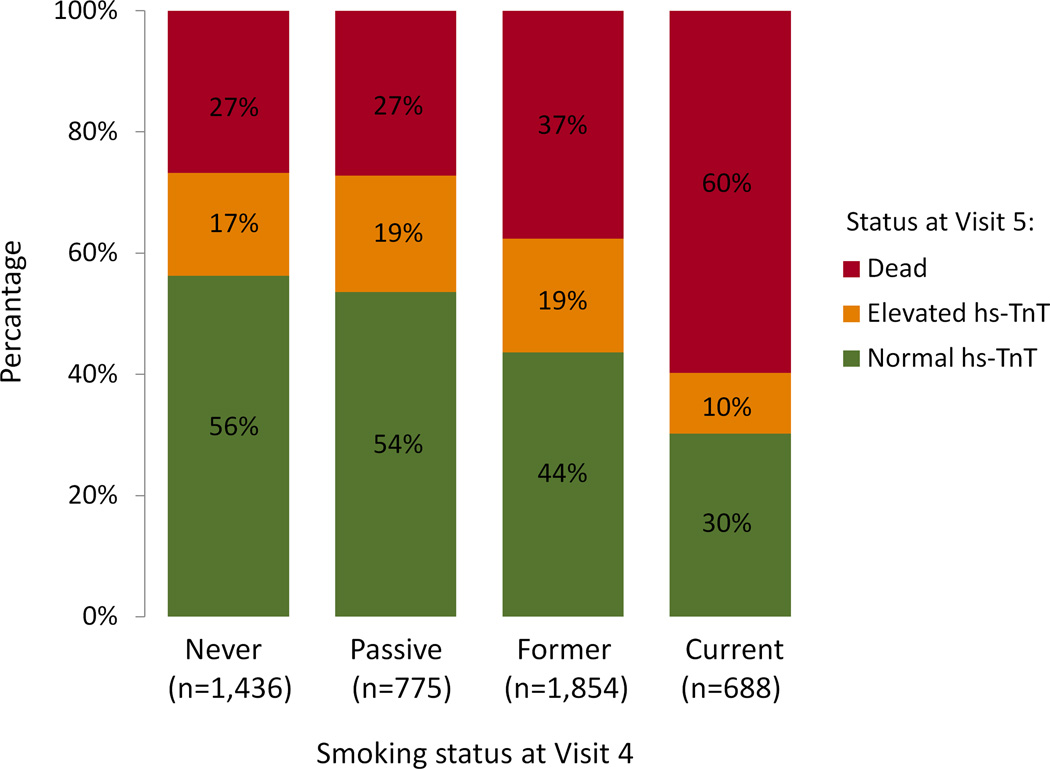
We hypothesized that our initial analysis of the association between smoking status at Visit 4 and incident elevated hs-TnT at Visit 5 was likely to underestimate the true relationship due to smoking-related competing risk of death between Visits 4 and 5. In order to address this, we performed sensitivity analyses including participants without elevated hs-TnT at Visit 4, first creating a composite outcome of death or elevated hs-TnT at Visit 5, and also creating an ordinal outcome denoting Visit 5 status as 1) alive without elevated hs-TnT; 2) alive with elevated hs-TnT; and 3) died prior to Visit 5 (Figure 2 and Supplemental Table 3). These analyses showed that current smoking at Visit 4 was strongly related to the composite outcome of death or elevated hs-TnT at Visit 5 and to Visit 5 status, suggesting that it may be difficult to observe smokers with elevated hs-TnT because of the very high likelihood of death.
Figure 2.
Stacked bar plot regarding the relationship between smoking status at baseline (Visit 4) and hs-TnT status (normal or elevated) or death at Visit 5. Normal and elevated hs-TnT values were considered as values <14 ng/L and ≥14 ng/L, respectively. In these analyses, participants with hs-TnT ≥14 ng/L at Visit 4 (n=441) were excluded. hs-TnT – high-sensitivity Troponin T.
To adjust for possible bias due to selective attrition between Visits 4 and 5, we applied calculated inverse probability weights to the multivariable models (Supplemental Table 4). However, this approach did not change the associations between smoking and long-term change in cardiac biomarkers. Furthermore, there was no significant interaction by gender or race for the relationship between smoking and NT-ProBNP or hs-TnT in cross-sectional and longitudinal analyses.
DISCUSSION
In a large community-based cohort free of CAD and HF, cumulative cigarette exposure, assessed by pack-years of smoking, was associated with higher NT-ProBNP and hs-TnT levels among ever smokers. Furthermore, participants who were current smokers at Visit 4 had greater changes in NT-ProBNP and hs-TnT after 15 years of follow-up, even adjusting for Visit 4 and Visit 5 covariates. Conversely, former smokers had minor long-term changes in NT-ProBNP and hs-TnT in comparison with never smokers.
Previous cross-sectional studies showed conflicting results regarding the relationship between smoking status and NT-ProBNP (9, 10, 11), while the few available studies with longitudinal design did not report an association between smoking and long-term changes in this biomarker in general populations (15, 23). We found that a history of active smoking was associated with higher NT-ProBNP and long-term changes in this biomarker. Moreover, NT-ProBNP levels were directly related to number of pack-years of smoking, suggesting that myocardial wall stress may be related to overall smoking burden. Our larger sample size and longer period of follow-up might have increased the ability to detect significant associations, thus explaining the differences between our findings and those of previous reports (9, 10, 11, 15).
Our data also showed that heavier cumulative cigarette exposure was associated with a higher likelihood of elevated hs-TnT levels among ever smokers, suggesting that subclinical myocardial injury is directly related to the intensity and duration of cigarette exposure. Conversely, we detected no association between smoking status and hs-TnT in cross-sectional analysis. One possible explanation for this latter finding would be survival bias. Given that smoking and elevated troponin levels are associated with higher death rates (22), it can be argued that smokers with elevated hs-TnT were more likely to die prior the cross-sectional visit. In accordance with this assumption, we observed that participants who where current smokers with high hs-TnT at Visit 4 had the highest death rate among all studied participants. Although we were not able to assess whether active smoking coupled with high hs-TnT was associated with higher death rate prior to Visit 4, these results suggest that survival bias might have influenced our ability to detect differences in hs-TnT among the smoking groups. In addition, we showed that current smoking at Visit 4 was associated with greater changes in hs-TnT levels and higher incidence of elevated hs-TnT after 15 years of follow-up. These data support the notion that tobacco might be a risk factor for long-term subclinical myocardial injury.
Prior data from the ARIC cohort and the Health, Aging, and Body Composition Study showed that current smokers and to a minor extent former smokers had higher risk of incident HF, independent of coronary artery events (2, 3). However, the pathophysiological pathways that mediate the long-term deleterious effects of smoking on the myocardium are far from being established. In the present study we shed light on this issue by showing that current and former smoking were independent predictors of soluble biomarkers reflecting increased ventricular wall stress and subclinical myocardial injury after 15 years of follow-up. Furthermore, we observed that the magnitude of long-term association with NT-ProBNP and hs-TnT was stronger in current than in former smokers. Given that these biomarkers are surrogates and predictors of cardiac dysfunction (7, 8, 15), our findings are consistent with the results of epidemiological studies that showed that current smoking was associated with HF to a greater extent than prior smoking (2, 3). It was also noteworthy that among our participants who were current and former smokers at Visit 4, the majority of them were not smoking at Visit 5. This observation indicates that tobacco-associated myocardial alterations may persist even after smoking cessation.
Several mechanisms may be proposed to explain the association between smoking and higher NT-ProBNP and hs-TnT. Smoking could increase these biomarkers by leading to higher blood pressure levels (24, 25) and COPD (26, 27). However, we did not detect elevated blood pressure levels in subjects with a history of smoking. Furthermore, the relationship between smoking and cardiac biomarkers persisted after adjustment for blood pressure levels and COPD, indicating that these factors did not explain our findings. Smoking-induced CAD could also provide an alternative explanation for our results (28). In contrast to this hypothesis, subjects with overt CAD were excluded from the analysis. However, we cannot discard that subclinical myocardial ischemia, especially as a consequence of smoking-induced coronary microvascular dysfunction (29), contributed to justify our findings. Lastly, it is possible that the rise in biomarkers was a consequence of direct effects of tobacco on the myocardium. This hypothesis is supported by experimental evidence showing that tobacco smoke may lead to myocardial damage, by inducing hypoxemia, oxidative stress and activation of matrix-metalloproteinases (4). Nevertheless, further studies are necessary to unveil the precise mechanisms by which smoking influences NT-ProBNP and hs-TnT levels.
A number of limitations should be noted. This is an observational study and cigarette smoking data were self reported in a questionnaire administered by an interviewer, thus participants may have underreported their smoking habits. Data regarding passive smoking were limited to one time point (Visit 4), therefore we could not assess the role of exposure to second-hand smoking before and after Visit 4 in our analyses. Although additive multivariable models were used, residual confounding cannot be excluded. Furthermore, it is possible that smoking-related selective attrition before Visit 5 introduced biased estimates in our longitudinal analysis. However, our results did not substantially change after applying inverse-probability-of-attrition weights to our multivariable analyses. Lastly, NT-ProBNP and hs-TnT were measured from frozen samples and were therefore subject to potential degradation as with any stored sample. Nevertheless, this approach has been standard practice in epidemiological studies. The strengths of this study include its large size, the long period of follow-up and the ability to adjust for cardiovascular risk factors and demographic factors. Moreover, because smoking is associated with CAD and HF, which may in turn lead to changes in NT-ProBNP and hs-TnT, we excluded participants with prevalent CAD or HF in order to focus on biomarker changes not induced through these pathways.
CONCLUSIONS
This study showed that cumulative cigarette exposure was associated with elevated NT-ProBNP and hs-TnT levels in a large community population without overt CAD and HF. Furthermore, current smoking and to a lesser extent former smoking predicted a 15-year progression of NT-ProBNP and hs-TnT. These findings suggest that cigarette smoking might be harmful to the heart beyond stimulating CAD, potentially by increasing ventricular wall stress and inducing subclinical myocardial injury.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding Sources: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This work was also supported by NHLBI cooperative agreement NHLBI-HC-11-08 (S.D.S.), grants R00-HL-107642 (S.C.) and K08-HL-116792 (A.M.S.), and a grant from the Ellison Foundation (S.C.). A.G. was supported by the Portuguese Foundation for Science and Technology Grant HMSP-ICS/007/2012. W.N.J. was supported by the Brazilian National Council for Scientific and Technological Development Grant 249481/2013-8.
Footnotes
Conflict of Interest: None
REFERENCES
- 1.Mozaffarian D, Afshin A, Benowitz NL, Bittner V, Daniels SR, Franch HA, Jacobs DR, Jr, Kraus WE, Kris-Etherton PM, Krummel DA, Popkin BM, Whitsel LP, Zakai NA. American Heart Association Council on Epidemiology and Prevention, Council on Nutrition, Physical Activity and Metabolism, Council on Clinical Cardiology, Council on Cardiovascular Disease in the Young, Council on the Kidney in Cardiovasc. Population approaches to improve diet, physical activity, and smoking habits: a scientific statement from the American Heart Association. Circulation. 2012;126:1514–1563. doi: 10.1161/CIR.0b013e318260a20b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, Quibrera PM, Rosamond WD, Russell SD, Shahar E, Heiss G. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Smith AL, Bauer DC, Newman AB, Kim L, Bibbins-Domingo K, Tindle H, Harris TB, Tang WW, Kritchevsky SB, Butler J. Cigarette smoking exposure and heart failure risk in older adults: the Health, Aging, and Body Composition Study. Am Heart J. 2012;164:236–242. doi: 10.1016/j.ahj.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minicucci MF, Azevedo PS, Polegato BF, Paiva SA, Zornoff LA. Cardiac remodeling induced by smoking: concepts, relevance, and potential mechanisms. Inflamm Allergy Drug Targets. 2012;11:442–447. doi: 10.2174/187152812803589958. [DOI] [PubMed] [Google Scholar]
- 5.Rosen BD, Saad MF, Shea S, Nasir K, Edvardsen T, Burke G, Jerosch-Herold M, Arnett DK, Lai S, Bluemke DA, Lima JA. Hypertension and smoking are associated with reduced regional left ventricular function in asymptomatic: individuals the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;47:1150–1158. doi: 10.1016/j.jacc.2005.08.078. [DOI] [PubMed] [Google Scholar]
- 6.Dalen H, Thorstensen A, Romundstad PR, Aase SA, Stoylen A, Vatten LJ. Cardiovascular risk factors and systolic and diastolic cardiac function: a tissue Doppler and speckle tracking echocardiographic study. J Am Soc Echocardiogr. 2011;24:322–332.e6. doi: 10.1016/j.echo.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 8.Nambi V, Liu X, Chambless LE, de Lemos JA, Virani SS, Agarwal S, Boerwinkle E, Hoogeveen RC, Aguilar D, Astor BC, Srinivas PR, Deswal A, Mosley TH, Coresh J, Folsom AR, Heiss G, Ballantyne CM. Troponin T and N-terminal pro-B-type natriuretic peptide: a biomarker approach to predict heart failure risk--the atherosclerosis risk in communities study. Clin Chem. 2013;59:1802–1810. doi: 10.1373/clinchem.2013.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otsuka T, Kawada T, Seino Y, Ibuki C, Katsumata M, Kodani E. Relation of smoking status to serum levels of N-terminal pro-brain natriuretic peptide in middle-aged men without overt cardiovascular disease. Am J Cardiol. 2010;106:1456–1460. doi: 10.1016/j.amjcard.2010.06.075. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez OA, Duprez DA, Bahrami H, Daniels LB, Folsom AR, Lima JA, Maisel A, Peralta CA, Jacobs DR., Jr The associations between metabolic variables and NT-ProBNP are blunted at pathological ranges: the Multi-Ethnic Study of Atherosclerosis. Metabolism. 2014;63:475–483. doi: 10.1016/j.metabol.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siervo M, Ruggiero D, Sorice R, Nutile T, Aversano M, Stephan BC, Ciullo M. Angiogenesis and biomarkers of cardiovascular risk in adults with metabolic syndrome. J Intern Med. 2010;268:338–347. doi: 10.1111/j.1365-2796.2010.02255.x. [DOI] [PubMed] [Google Scholar]
- 12.Otsuka T, Kawada T, Ibuki C, Seino Y. Association between high-sensitivity cardiac troponin T levels and the predicted cardiovascular risk in middle-aged men without overt cardiovascular disease. Am Heart J. 2010;159:972–978. doi: 10.1016/j.ahj.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 13.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aeschbacher S, Schoen T, Bossard M, van der Lely S, Glättli K, Todd J, Estis J, Risch M, Mueller C, Risch L, Conen D. Relationship Between High-Sensitivity Cardiac Troponin I and Blood Pressure Among Young and Healthy Adults. Am J Hypertens. 2015;28:789–796. doi: 10.1093/ajh/hpu226. [DOI] [PubMed] [Google Scholar]
- 15.Glick D, DeFilippi CR, Christenson R, Gottdiener JS, Seliger SL. Long-term trajectory of two unique cardiac biomarkers and subsequent left ventricular structural pathology and risk of incident heart failure in community-dwelling older adults at low baseline risk. JACC Heart Fail. 2013;1:353–360. doi: 10.1016/j.jchf.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 17.Howard G, Wagenknecht LE, Cai J, Cooper L, Kraut MA, Toole JF. Cigarette smoking and other risk factors for silent cerebral infarction in the general population. Stroke. 1998;29:913–917. doi: 10.1161/01.str.29.5.913. [DOI] [PubMed] [Google Scholar]
- 18.Schneider AL, Rawlings AM, Sharrett AR, Alonso A, Mosley TH, Hoogeveen RC, Ballantyne CM, Gottesman RF, Selvin E. High-sensitivity cardiac troponin T and cognitive function and dementia risk: the atherosclerosis risk in communities study. Eur Heart J. 2014;35:1817–1824. doi: 10.1093/eurheartj/ehu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folsom AR, Yamagishi K, Hozawa A, Chambless LE Atherosclerosis Risk in Communities Study I. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medbø A, Melbye H. Lung function testing in the elderly--can we still use FEV1/FVC<70% as a criterion of COPD? Respir Med. 2007;101:1097–1105. doi: 10.1016/j.rmed.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 21.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 22.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luchner A, Behrens G, Stritzke J, Markus M, Stark K, Peters A, Meisinger C, Leitzmann M, Hense HW, Schunkert H, Heid IM. Long-term pattern of brain natriuretic peptide and N-terminal pro brain natriuretic peptide and its determinants in the general population: contribution of age, gender, and cardiac and extra-cardiac factors. Eur J Heart Fail. 2013;15:859–867. doi: 10.1093/eurjhf/hft048. [DOI] [PubMed] [Google Scholar]
- 24.Feng D, Liu T, Su DF, Wang H, Ding P, He YH, Deng XQ, Hou MJ, Ling WH, Chen WQ. The association between smoking quantity and hypertension mediated by inflammation in Chinese current smokers. J Hypertens. 2013;31:1798–1805. doi: 10.1097/HJH.0b013e328362c21a. [DOI] [PubMed] [Google Scholar]
- 25.Hoshide S, Kario K, Yano Y, Haimoto H, Yamagiwa K, Uchiba K, Nagasaka S, Matsui Y, Nakamura A, Fukutomi M, Eguchi K, Ishikawa J J-HOP Study Group. Association of morning and evening blood pressure at home with asymptomatic organ damage in the J-HOP Study. Am J Hypertens. 2014;27:939–947. doi: 10.1093/ajh/hpt290. [DOI] [PubMed] [Google Scholar]
- 26.Chi SY, Kim EY, Ban HJ, Oh IJ, Kwon YS, Kim KS, Kim YI, Kim YC, Lim SC. Plasma N-terminal pro-brain natriuretic peptide: a prognostic marker in patients with chronic obstructive pulmonary disease. Lung. 2012;190:271–276. doi: 10.1007/s00408-011-9363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neukamm AM, Høiseth AD, Hagve TA, Søyseth V, Omland T. High-sensitivity cardiac troponin T levels are increased in stable COPD. Heart. 2013;99:382–387. doi: 10.1136/heartjnl-2012-303429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Battistoni A, Rubattu S, Volpe M. Circulating biomarkers with preventive, diagnostic and prognostic implications in cardiovascular diseases. Int J Cardiol. 2012;157:160–168. doi: 10.1016/j.ijcard.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 29.Naya M, Morita K, Yoshinaga K, Manabe O, Goto D, Hirata K, Katoh C, Tamaki N, Tsutsui H. Long-term smoking causes more advanced coronary endothelial dysfunction in middle-aged smokers compared to young smokers. Eur J Nucl Med Mol Imaging. 2011;38:491–498. doi: 10.1007/s00259-010-1647-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.