Abstract
Glucocorticoids circulating in breeding birds during egg production accumulate within eggs, and may provide a potent form of maternal effect on offspring phenotype. However, whether these steroids affect offspring development remains unclear. Here, we employed a non-invasive technique that experimentally elevated the maternal transfer of corticosterone to eggs in a wild population of house wrens. Feeding corticosterone-injected mealworms to free-living females prior to and during egg production increased the number of eggs that females produced and increased corticosterone concentrations in egg yolks. This treatment also resulted in an increase in the amount of yolk allocated to eggs. Offspring hatching from these eggs begged for food at a higher rate than control offspring and eventually attained increased prefledging body condition, a trait predictive of their probability of recruitment as breeding adults in the study population. Our results indicate that an increase in maternal glucocorticoids within the physiological range can enhance maternal investment and offspring development.
Keywords: begging, egg, glucocorticoid, house wren, life history, maternal effect
Introduction
Maternal effects arise whenever mothers influence offspring phenotype beyond that which is determined by genetic inheritance (Mousseau and Fox 1998). Such effects are often induced by ecological conditions, and have the potential to prime offspring for conditions they may encounter in the future. Maternal hormones may provide an important source of such effects, and a potentially potent form of effect can arise from maternally derived glucocorticoids. Secreted by the adrenal gland, glucocorticoids are metabolic steroids (primarily cortisol in mammals and fishes, and corticosterone in reptiles and birds) that stimulate gluconeogenesis and promote activity (Sapolsky et al. 2000). Variation in circulating glucocorticoid levels is often affected by ecological conditions, and a number of variables, including resource availability and predation risk, can affect the production of these steroids (Wingfield et al. 1998; Sapolsky et al. 2000).
Effects of corticosterone on behavior can vary widely across taxa and in a context- and dose-dependent manner. In birds, for example, ostensibly stressful events, such as interactions with potential predators, often result in dramatic increases in glucocorticoids that promote self-maintenance at a cost to reproduction (Wingfield 2003; Romero et al. 2009; Lothery et al. 2014; Vitousek et al. 2014; Jones et al. 2016). However, in the absence of imminent threats, increased glucocorticoids can positively affect foraging, allocation to eggs, and parental care (Sinervo and DeNardo 1996; Crossin et al. 2012; Love et al. 2014; Bowers et al. 2015b), and enhance survival in free-living populations (Sinervo and DeNardo 1996; Rivers et al. 2012). There is also evidence that glucocorticoids can affect offspring phenotype (Sinervo and DeNardo 1996; McCormick 1998; Love et al. 2005, 2013; Love and Williams 2008; Sheriff et al. 2010; Giesing et al. 2011; Sheriff and Love 2013). For example, in a recent study, high local density increased maternal glucocorticoids in North American red squirrels (Tamiasciurus hudsonicus), and this increase positively affected offspring growth (Dantzer et al. 2013). Thus, glucocorticoid-mediated maternal effects may prime offspring for the environmental conditions they are likely to encounter (see also Love and Williams 2008; Breuner 2008; Smiseth et al. 2011).
Like other steroids, corticosterone is lipophilic and, in the amniotic egg, accumulates in lipid-rich yolks during egg formation (McCormick 1998; Hayward and Wingfield 2004; Okuliarová et al. 2010; Almasi et al. 2012; Pitk et al. 2012). Thus, egg-yolk corticosterone can serve as an integrated, non-invasive proxy of maternal physiology and may affect offspring development (Bowers et al. 2015b). Moreover, conditions experienced prior to breeding are known to affect investment in eggs (Martin 1987; Nooker et al. 2005; Ruffino et al. 2012), and integrated measures of circulating corticosterone prior to the breeding season, as measured by corticosterone accumulated in feathers, recently have been found to predict egg size (Kouwenberg et al. 2013), a trait that by itself has strong and persistent effects on offspring (Whittingham et al. 2007; Krist 2011; Love and Williams 2011; Rollinson and Hutchings 2013; but see also Giordano et al. 2014). However, effects of experimentally altered corticosterone levels within eggs on offspring development can be dynamic and context-dependent; for example, elevated corticosterone in eggs of the European starling (Sturnus vulgaris) can enhance the development of flight muscles after hatching and improve the flight performance of fledglings (Chin et al. 2009), but also reduce the survival and growth of male offspring (Love et al. 2005). Although this latter effect might suggest a maladaptive consequence of maternal stress, such an effect on brood reduction can be adaptive in certain ecological contexts (e.g., Love and Williams 2008; Sheriff and Love 2013). Thus, effects of in ovo corticosterone on offspring phenotype after hatching will inevitably be context-dependent, and should therefore be studied within a life-history framework.
In this study, we test whether elevated maternal corticosterone during egg production increases corticosterone accumulation in eggs, and whether such an increase affects offspring development. We used a non-invasive manipulation by feeding corticosterone to female house wrens (Troglodytes aedon; Fig. 1). During the early stages of the nesting cycle, we provided corticosterone-injected mealworms to free-living females at doses known to increase circulating corticosterone rapidly and in a physiologically relevant range indicative of acute stress (Breuner et al. 1998). We supplemented females prior to and during egg production, and collected every other egg on the day each was laid to quantify levels of corticosterone that accumulated in egg yolks over this period. We left the remaining eggs in the nest to develop and hatch naturally, and monitored the subsequent development of these nestlings. In a recent cross-fostering experiment in this population, we fostered offspring among nests prior to hatching and found that the concentration of corticosterone in egg yolks produced by females positively predicts the rate at which they deliver food to unrelated foster offspring after hatching; moreover, we found that offspring hatching from eggs with increased corticosterone attain enhanced body condition when reared by foster parents (Bowers et al. 2015b). Thus, if corticosterone in mothers and their eggs modifies offspring behavior according to the environmental conditions they are likely to encounter after hatching, we predicted that offspring hatching from eggs produced by experimental females, with increased yolk-corticosterone concentrations, would beg for food at a higher rate than offspring of control females (sensu Love and Williams 2008; Smiseth et al. 2011; Sheriff and Love 2013), and that these offspring would attain better body condition prior to fledging than those of control females.
Fig. 1.
A female house wren collecting a freshly injected mealworm outside her nestbox.
Materials and methods
STUDY AREA AND SPECIES
House wrens are small, secondary-cavity-nesting songbirds with a widespread distribution across North and South America (biology summarized in Johnson 2014). We studied a population breeding in secondary deciduous forest in McLean County, Illinois, USA (40.665ºN, 88.89ºW). Upon arriving on the study area from spring migration, females select a mate that is defending a nest site and, after completing nest construction, produce one egg per day until their clutch is completed. Only females incubate eggs, but both parents provision offspring with food after hatching, and the length of the nestling period is typically ca. 15 days (Bowers et al. 2013, 2014a). House wrens readily accept nestboxes for nesting, and the nestboxes in this study were spaced 30 m apart along north-south transects separated by 60 m and mounted atop 48.3-cm diameter aluminum predator baffles on 1.5-m poles (Lambrechts et al. 2010 provide further details on nestboxes). During incubation or early in the nestling-rearing stage, we captured adults inside nestboxes or by using mist nets near the box. We measured their body mass (± 0.1 g) and tarsus length (± 0.1 mm), and banded them with a unique U. S. Geological Survey leg band; males received three additional colored bands in a unique combination so that they could be identified using binoculars (males are more difficult to capture than females). We attempt to capture and mark all individuals on the site each year.
Experimental procedures
During this experiment in the 2015 breeding season, we checked nestboxes at least twice weekly for evidence of males defending nest sites, initiating nest construction, and singing to attract females. Males generally initiate nest construction by bringing woody sticks (ca. 1–3-mm diameter) into their nestbox and using them to construct a cup-shaped base. Once paired, the female continues nest construction and lines the cup with soft, insulative materials including grasses, feathers, and other soft materials (e.g., fur, snakeskin, and cellophane). When we detected evidence of female activity at a nest prior to laying eggs, we initiated treatments by attaching a plastic cup to the predator baffle immediately outside the nest entrance and providing five freshly injected mealworms (Fig. 1).
Treatments were randomly assigned, and the initiation of new treatments was alternated with the start of each new nest. Mealworms were injected with peanut oil using a 19-g needle, and any that leaked upon injection were discarded. For the control treatment, mealworms were injected with 20 μL of peanut oil; for the low-corticosterone and high-corticosterone treatments, mealworms were injected with 20 μL of peanut oil containing 0.5 mg/mL corticosterone or 1.0 mg/mL corticosterone, respectively, consistent with previous studies (Breuner et al. 1998). Concentrations of these magnitudes elevate circulating corticosterone upon ingestion in a physiologically relevant range, consistent with what is produced in response to a stressor, such as capture and handling by researchers (Breuner et al. 1998; Merrill et al. 2012; Henderson et al. 2014). Five freshly injected mealworms were provided each morning beginning 3.1 ± 0.3 days (mean ± SE) prior to clutch initiation, whether or not the mealworms from the previous day had been taken (any mealworms remaining from the previous day were necrotic and discarded). Although it was not possible to ensure that every female consumed all of the mealworms provided (the resident male took mealworms on occasion), we did confirm that females ate at least a subset of the mealworms provided using digital videos (Fig. 1) and direct observations with binoculars. We continued providing mealworms each morning until egg laying ceased. We could not validate the effect of our manipulation on corticosterone levels in maternal circulation, as capturing and sampling blood from females during or shortly after egg laying dramatically increases rates of nest abandonment. Nonetheless, the increases in yolk-corticosterone concentrations resulting from this protocol (see Results) are consistent with increases in concentrations relative to control eggs that have been obtained in other experimental studies (e.g., Love et al. 2005; Haussmann et al. 2012).
We collected eggs two, four, and six (if laid) from each nest on the morning each was laid and replaced them with dummy eggs, which females readily accepted and incubated. In the laboratory, we weighed (± 0.001 g) and measured the size of these eggs (length and breadth; ± 0.01 mm), before storing them at −20ºC. The concentration of yolk corticosterone in each egg was subsequently measured using competitive-binding radioimmunoassay (RIA) following a standardized protocol (Paitz et al. 2011; Bowers et al. 2015b). For the RIAs, we diluted yolk samples in 500 μL water and added radiolabeled (tritiated) steroid tracer to quantify recoveries of the extracted corticosterone following column chromatography. Yolk steroids were extracted twice with 3 mL of an ether extraction solvent (30% petroleum ether : 70% diethyl ether). The organic fraction containing the extracted steroids was dried, reconstituted in 90% ethanol, and stored at −20ºC overnight to precipitate neutral lipids. We then fractionated different steroids from yolk extracts using column chromatography; samples were applied directly to the column and eluted using hormone-specific ethyl acetate : isooctane ratios (corticosterone = 50%; fractions containing progesterone, testosterone, and dihydrotestosterone were fractionated separately and discarded). We dried the corticosterone elutes and measured concentrations using competitive-binding RIAs with tritiated steroid. Average recovery was 61% (N = 112 eggs). We conducted two RIAs, and obtained corticosterone concentrations for 112 egg yolks from 48 nests; we discarded one datum because its estimated concentration was 5.8 SD above the mean of the other 111 eggs. The intra-assay coefficients of variation for the two assays were 1.5% and 5.9%; the inter-assay coefficient of variation was 5.4%.
Eggs not collected for RIAs remained in the nest and were allowed to develop and hatch naturally. For the nestlings hatching from these eggs, we quantified their begging effort four days after hatching began using a small microphone placed inside the nestbox, just under the lid. The microphone was attached to a digital voice recorder outside the nest following Barnett et al. (2011), and we quantified begging vocalizations using Raven Pro 1.4 sound analysis software (Cornell Lab of Ornithology). We monitored growth of nestlings and status of nests, and, 11 days after hatching began, we weighed nestlings and measured the length of their tarsus prior to fledging, traits that are positively associated with recruitment and future reproductive success in the study population (Bowers et al. 2014b, 2015a). We subsequently visited nests daily to monitor fledging.
Data and analyses
We used SAS (version 9.4) for all analyses, all tests are two-tailed (α = 0.05), and we converted data to z-scores prior to analysis to obtain standardized parameter estimates, which provide a measure of effect size (Schielzeth 2010). We used Satterthwaite’s degrees-of-freedom approximation, which can result in non-integer denominator degrees of freedom. We had a priori expectations that traits and behaviors expressed in low- and high-corticosterone groups would differ from those of controls; thus, we conducted pre-planned comparisons as follow-up tests to contrast the low- and high-corticosterone groups (pooled) with the control group.
We first analyzed clutch size using a linear model (PROC GLM), with treatment as a main effect and clutch-initiation date as a covariate. We then analyzed egg mass using a linear mixed model with clutch identity as a random effect to account for the non-independence of eggs within clutches, and we included relative egg-laying order (egg number divided by clutch size) to test for a laying-order effect across clutches of different size. We then analyzed yolk mass as residuals from a yolk-mass × egg-mass linear regression (there is a linear relationship between these variables); thus, the residuals reflect the amount of yolk per unit egg size, where eggs with positive and negative values have increased and decreased yolk, respectively, relative to what would be expected from the overall mass and size of the egg. We then analyzed the concentration of yolk corticosterone (per unit mass of yolk) in relation to treatment and egg-laying order, as described above. We also tested for an effect of corticosterone supplementation during egg formation on maternal body condition (i.e., size-adjusted body mass) after egg production stopped and incubation commenced, shortly after the period of the manipulation. We then analyzed the length of the incubation period in relation to the corticosterone treatment (i.e., time from clutch completion to the day on which hatching began within a nest) using a proportional hazards regression (i.e., survival analysis; PROC PHREG in SAS) with clutch size and clutch-initiation date as covariates. We analyzed the time from hatching until fledging using a similar approach, with nests that failed prior to fledging as censored observations, and we included hatching date as a covariate in our analysis of fledging age. We analyzed nestling mass using linear mixed models with nest as a random effect, and we analyzed nestling begging vocalizations at the level of the nest using a linear model. We calculated effect sizes for the terms in our linear models as η2, which represents the proportion of total variation in a dependent variable explained by a predictor; η2 values of 0.02, 0.13, and 0.26 generally reflect small, medium, and large effects, respectively. We also calculated Cohen’s d as the standardized differences between means in our follow-up comparisons; conventionally, values of 0.2, 0.5, and 0.8 are thought to reflect small, medium, and large effects, respectively.
Results
Effects on maternal investment and pre-natal allocation
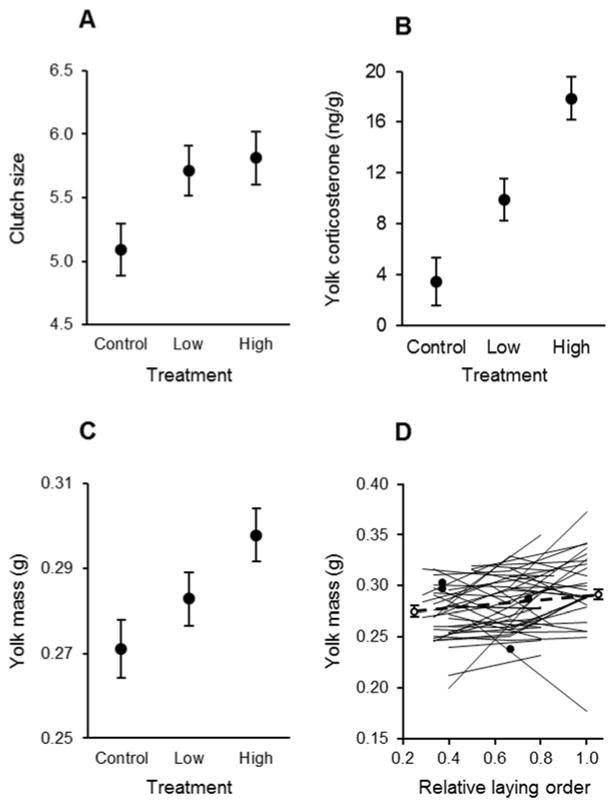
Females receiving supplemental corticosterone prior to and during egg laying produced more eggs per clutch than control females (Table 1A; follow-up comparison of control and experimental clutches: F1, 42 = 7.15, P = 0.011, Cohen’s d = 0.72; Fig. 2A). The corticosterone supplementation was also associated with an increase in the concentration of corticosterone within egg yolks (Table 1B), as females in both the low- and high-corticosterone groups produced eggs containing higher yolk-corticosterone concentrations than did controls (follow-up test: F1, 49 = 30.35, P < 0.001, Cohen’s d = 1.47; Fig. 2B). There was no effect of treatment on overall egg mass (Table 1C), but supplemental corticosterone increased yolk mass (Table 1D). For any given size of egg, corticosterone-supplemented females (low and high treatments pooled) produced relatively more yolk, on average, than did control females (follow-up test: F1, 45.9 = 8.57, P = 0.005, Cohen’s d = 0.70; Fig. 2C). There was also a modest, but significant increase in yolk mass from earlier- to later-laid eggs within clutches, regardless of their treatment (Table 1D; Fig. 2D). Supplemental corticosterone prior to and during egg laying had no effect on the body condition of females when we captured them after they completed egg laying (F2, 39 = 1.46, P = 0.245, η2 = 0.06), and there was no correlation between maternal body condition and the concentration of corticosterone in egg yolks (effect of maternal condition on yolk-corticosterone concentration: estimate ± SE = −1.28 ± 1.07, F1, 38.6 = 1.43, P = 0.239, η2 = 0.02).
Table 1.
Effects of corticosterone supplementation on components of maternal investment and allocation to offspring
| Estimate ± SE | F | df | P | η2 | |
|---|---|---|---|---|---|
|
|
|||||
| A. Clutch size | |||||
| Treatment | 3.61 | 2, 42 | 0.036 | 0.13 | |
| Controla | −0.69 ± 0.32 | ||||
| Higha | 0.11 ± 0.32 | ||||
| Clutch-initiation date | −0.37 ± 0.13 | 8.43 | 1, 42 | 0.006 | 0.15 |
| Intercept | 0.17 ± 0.22 | ||||
| B. Yolk-corticosterone concentration | |||||
| Treatment | 17.88 | 2, 46.2 | < 0.001 | 0.35 | |
| Controla | −0.85 ± 0.41 | ||||
| Higha | 0.07 ± 0.41 | ||||
| Relative egg-laying order | −0.04 ± 0.04 | 0.70 | 1, 62.0 | 0.405 | < 0.01 |
| Intercept | −0.07 ± 0.29 | ||||
| C. Egg mass | |||||
| Treatment | 0.23 | 2, 47.9 | 0.792 | < 0.01 | |
| Controla | 0.34 ± 0.49 | ||||
| Higha | −1.03 ± 0.49 | ||||
| Relative egg-laying order | 0.21 ± 0.05 | 20.94 | 1, 73.0 | < 0.001 | 0.04 |
| Intercept | 0.14 ± 0.35 | ||||
| D. Egg-yolk massb | |||||
| Treatment | 7.14 | 2, 47.4 | 0.002 | 0.16 | |
| Controla | −3.99 ± 0.66 | ||||
| Higha | −1.64 ± 0.66 | ||||
| Relative egg-laying order | 0.19 ± 0.07 | 7.66 | 1, 63.0 | 0.007 | 0.03 |
| Intercept | 1.48 ± 0.47 | ||||
relative to low corticosterone treatment,
corrected for total egg mass (i.e., residual egg-yolk mass)
Fig. 2.
(A–C) Clutch size, yolk-corticosterone concentration, and yolk mass in relation to treatment (least-squares means ± SE). (D) Yolk mass in relation to relative egg-laying order (egg number divided by clutch size); plotted are individual reaction norms for each female (filled dots represent a single egg from the clutch), and least-squares means ± SE are plotted on each side of the reaction norms for egg 2 and for last-laid and penultimate eggs.
Effects on nestling development
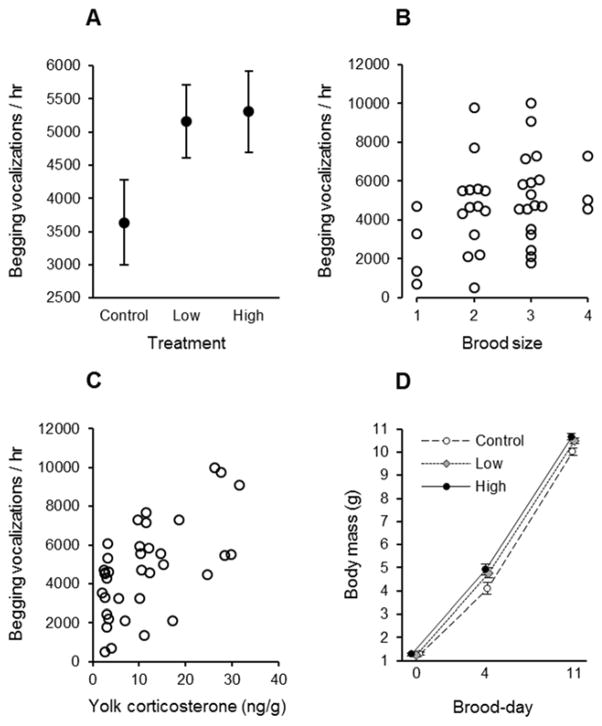
There was no effect of corticosterone supplementation on the length of the incubation or nestling stages (Table 2). There was also no effect of corticosterone supplementation on the body mass of recently hatched nestlings (Table 3A), but an effect on nestling body mass was apparent four days posthatching (Table 3B), with nestlings in both low- and high-corticosterone groups collectively having greater body mass than nestlings produced by control females (F1, 34.3 = 6.60, P = 0.015, Cohen’s d = 0.92). The frequency with which nestlings solicited food from parents at this age was also affected by maternal corticosterone treatment (Fig. 3A); although the main test of a treatment effect on begging was not statistically significant (Table 3C), our pre-planned comparisons revealed that nestlings produced by corticosterone-supplemented females begged for food at a higher rate than nestlings produced by control females (F1, 34 = 4.34, P = 0.045, Cohen’s d = 0.75; Fig. 3A). Begging rates also tended to vary positively with brood size, although this effect was marginally non-significant (Table 3C; Fig. 3B), and the concentration of corticosterone deposited in egg yolks positively predicted begging rates (r34 = 0.594, P < 0.001; Fig. 3C). By 11 days posthatching, experimental nestlings were in better body condition than control nestlings (Cohen’s d = 1.09; Table 3D; Fig. 3D).
Table 2.
Effects of maternal corticosterone supplementation on the duration of the incubation and nestling stages
| Estimate ± SE | χ2 | df | P | |
|---|---|---|---|---|
|
|
||||
| A. Incubation | ||||
| Treatment | 4.42 | 2 | 0.11 | |
| Controla | 0.11 ± 0.46 | |||
| Higha | −0.91 ± 0.48 | |||
| Clutch sizeb | 0.60 ± 0.34 | 3.23 | 1 | 0.072 |
| Clutch-initiation dateb | 0.06 ± 0.03 | 3.98 | 1 | 0.046 |
| B. Nestling | ||||
| Treatment | 3.95 | 2 | 0.139 | |
| Controla | 0.76 ± 0.49 | |||
| Higha | 0.89 ± 0.48 | |||
| Hatching dateb | −0.04 ± 0.02 | 2.66 | 1 | 0.103 |
parameter estimates are not standardized;
relative to low corticosterone treatment,
positive parameter estimates indicate a negative relationship between the independent variable and the time elapsed until hatching and fledging, and vice versa
Table 3.
Effects of maternal corticosterone supplementation during egg laying on nestling development. Brood-day 0 is the day hatching begins within a nest
| Estimate ± SE | F | df | P | η2 | |
|---|---|---|---|---|---|
|
|
|||||
| A. Body mass on brood-day 0 | |||||
| Treatment | 0.03 | 2, 33.2 | 0.972 | < 0.01 | |
| Controla | 0.06 ± 0.36 | ||||
| Higha | 0.08 ± 0.36 | ||||
| Hatching date | −0.01 ± 0.02 | 0.31 | 1, 34.3 | 0.583 | < 0.01 |
| Intercept | 1.92 ± 3.64 | ||||
| B. Body mass on brood-day 4 | |||||
| Treatment | 3.46 | 2, 34.1 | 0.043 | 0.09 | |
| Controla | −0.62 ± 0.31 | ||||
| Higha | 0.22 ± 0.31 | ||||
| Hatching date | −0.17 ± 0.13 | 1.81 | 1, 33.8 | 0.188 | 0.02 |
| Intercept | 0.10 ± 0.20 | ||||
| C. Begging rate on brood-day 4 | |||||
| Treatment | 2.17 | 2, 34.0 | 0.129 | 0.10 | |
| Controla | −0.64 ± 0.37 | ||||
| Higha | 0.07 ± 0.37 | ||||
| Brood size | 0.39 ± 0.19 | 3.99 | 1, 34.0 | 0.054 | 0.09 |
| Intercept | 0.19 ± 0.45 | ||||
| D. Body mass on brood-day 11 | |||||
| Treatment | 5.70 | 2, 35.6 | 0.007 | 0.13 | |
| Controla | −2.02 ± 0.46 | ||||
| Higha | −0.09 ± 0.63 | ||||
| Tarsus length | 0.53 ± 0.10 | 30.07 | 1, 53.0 | < 0.001 | 0.09 |
| Intercept | 0.74 ± 0.36 | ||||
relative to low corticosterone treatment
Fig. 3.
(A) Rate at which nestlings begged for food at the level of the nest by treatment (least-squares means ± SE). Variation in begging rate in relation to (B) brood size (overlapping data for broods of similar size are jittered) and (C) yolk-corticosterone concentrations (clutch means). (D) Body mass of nestlings in relation to treatment and brood-day (brood-day 0 is the day hatching begins within a nest); brood-day 4 represents the age at which growth is most rapid, and nestlings generally reach asymptotic body mass by brood-day 11, a few days prior to fledging (least-squares means ± SE).
Discussion
Females receiving supplemental corticosterone during the early stages of the nesting cycle produced more yolk per egg than controls. Moreover, the eggs produced by these females contained higher concentrations of corticosterone in the yolks of their eggs, and their offspring subsequently begged for food at a higher rate and were heavier than offspring produced by control females. Relative to control offspring, experimental offspring also attained greater body condition prior to fledging, a trait positively predictive of their recruitment and reproductive prospects in the study population (Bowers et al. 2014b, 2015a). Results of the current study and a recent one conducted in our study population that elevated in ovo corticosterone at laying (Strange 2015) are consistent with those of other studies reporting positive effects of glucocorticoids on offspring begging and development (e.g., Love and Williams 2008; Chin et al. 2009; Crino et al. 2011; Dantzer et al. 2013). Corticosterone supplementation also positively affected the number of eggs females produced and the amount of yolk per egg, but did not affect egg size. Although avian egg size is often not affected by food supplementation and resource availability (Christians 2002), clutch size and egg constituents, including yolk size, have often been found to be plastic and affected by resource availability and individual condition (Johnson and Barclay 1996; Nager et al. 1997, 2000; Rutkowska and Cichoń 2002; Williams and Miller 2003; Bowden et al. 2004; Ardia et al. 2006; Rollinson and Brooks 2008; Saino et al. 2010; Chin et al. 2012).
Results of our study and others suggest that glucocorticoids play a role in shaping allocation to eggs (Sinervo and DeNardo 1996; Giesing et al. 2011; Segers and Taborsky 2012; Kouwenberg et al. 2013; Khan et al. 2016). Given the link between glucocorticoids and metabolic rate (Burton et al. 2011) and in promoting foraging (Angelier et al. 2007; Crossin et al. 2012), circulating glucocorticoids may provide an important source of variation in maternal investment, particularly if it enhances the ability of females to acquire and assimilate food in the wild. For example, in a multi-year study, Hennin et al. (2015) recently obtained a large sample of female common eiders (Somateria mollissima) shortly before clutch initiation, and found a pronounced increase in baseline corticosterone leading up to egg laying, which was strongly positively associated with increases in body mass (see also Hennin et al. 2016), and with increases in very-low-density lipoprotein and vitellogenin, each of which is essential to mediating maternal investment in eggs (Hennin et al. 2015). Similarly, Sinervo and DeNardo (1996) experimentally increased corticosterone levels in female side-blotched lizards (Uta stansburiana) within physiological levels, which led to an increase in female body mass immediately prior to oviposition and to increased clutch and egg mass.
We recently found that the concentration of corticosterone in egg yolks produced by females in our study population positively predicted the rate at which they delivered food to unrelated foster offspring from hatching, and that offspring hatching from eggs with increased corticosterone attained enhanced body condition when reared by foster parents (Bowers et al. 2015b). In the current study, corticosterone-supplemented females also produced more yolk per egg in addition to the greater concentration of corticosterone within these eggs, a result consistent with that earlier study (Bowers et al. 2015b). Thus, it is unclear whether effects on posthatching development can be attributed to increased yolk and other nutrients, to increased corticosterone per se, or a combination of both. In other studies in which eggs were injected with corticosterone, this treatment induced posthatching effects on offspring development, albeit with mixed results (Rubolini et al. 2005; Saino et al. 2005; Love and Williams 2008; Chin et al. 2009; Strange 2015). Considering that we did not directly inject eggs in the current study, and that these eggs represent a complete “package” that females have been selected to produce, attempts to separate the effects of increased yolk allocation from those of increased corticosterone may be misplaced (see also Love et al. 2005). Indeed, because direct, exogenous manipulations to eggs cause embryos to develop under a hormonal milieu other than what the maternal phenotype was selected to produce, the biological relevance of hormonal injections into eggs has been questioned (Wagner and Williams 2007; Henriksen et al. 2011; Williams 2012). Notwithstanding effects that might be attributable to the type of manipulation (e.g., direct injection of eggs), effects of experimentally elevated in ovo corticosterone indeed appear to be context-dependent. Given that increased corticosterone increases metabolic rates in both embryos and postpartum animals (Burton et al. 2011), we propose that effects of in ovo corticosterone on offspring development may depend entirely on the level of available resources, whereby in ovo corticosterone enhances growth and development when resources are plentiful, but may be detrimental when resources are more strongly limiting. Therefore, variation in resource availability within the egg may mediate effects of in ovo corticosterone on offspring development and consequences for between-individual differences in fitness in the wild.
We did not detect a laying-order effect on egg-yolk corticosterone concentrations within clutches in this study or another one conducted on the same population over a two-year span (Bowers et al. 2015b; see also Navara et al. 2006 for a similar result in another species), contrary to results of studies that found an increase in corticosterone across the laying sequence in other species (e.g., Saino et al. 2005; Love et al. 2008, 2009; Larsen et al. 2015). Given the effects of yolk corticosterone that we observed on offspring begging, selection might be expected to favor increased allocation of this steroid in later-laid eggs within clutches, as detected in other species, to enhance the competitive vigor of younger nestlings, as they are often at a competitive disadvantage relative to older siblings and are more likely to starve when asynchronous hatching causes eggs of a clutch to hatch over several days (Mock and Parker 1997; Eising et al. 2001; Müller et al. 2007, 2010; Jeon 2008; Muller and Groothuis 2013). Such a strategy could improve the competitive vigor and potential survival of younger siblings when food is sufficiently abundant or, because increases in corticosterone within eggs elevate metabolic rate and offspring food demand, could also facilitate efficient brood reduction when resources are more strongly limiting (sensu Mock and Parker 1997; Love et al. 2008). However, results of recent studies from our population suggest that hatching patterns of eggs within nests, which are under maternal control, reflect distinct strategies for allocating offspring within broods, and that enhancing the competitive ability of younger siblings may actually reduce parental allocation and the fitness of older siblings within the brood (Bowers et al. 2011, 2016). Further studies assessing effects of yolk corticosterone on sibling rivalry in a comparative, life-history context may shed light on the adaptive significance of intra-clutch variation in corticosterone concentrations (see also Love et al. 2009).
Although rapid and dramatic increases in glucocorticoids can promote self-maintenance at a cost to investment in reproduction (Wingfield 2003; Romero et al. 2009; Lothery et al. 2014), subtle increases in glucocorticoid levels, below those induced by imminent threats, are predicted to facilitate, not inhibit, reproductive effort (Sapolsky et al. 2000; Bowers et al. 2015b). Indeed, there is accumulating evidence that variation in corticosterone can actually enhance parental care by mobilizing energetic reserves, thereby helping parents meet the demands of rearing altricial young (Love et al. 2004, 2014; Hau et al. 2010; Crossin et al. 2012, 2015; Ouyang et al. 2013; Sheriff and Love 2013). This is especially predicted for short-lived animals that have few reproductive opportunities in their lifetime (Sapolsky et al. 2000). For example, we previously showed that females facing an immune challenge increase allocation to offspring (Bowers et al. 2015b), as predicted if immunostimulation signals to an individual that its probability of future reproduction has been jeopardized (see also Sadd et al. 2006; Velando et al. 2006; Duffield et al. 2016). That work revealed that immune-stimulated females increased their provisioning of food to nestlings after hatching, an increase mediated by elevated maternal corticosterone (Bowers et al. 2015b). Given the association between yolk corticosterone and its effects on posthatching begging and growth in nestlings and on maternal provisioning, the maternal transfer of glucocorticoids to eggs may create a confluence of behavior between parent and offspring that enhances offspring growth. However, this may not be without costs, as embryonic development in a hormonal milieu characterized by high levels of glucocorticoids may accelerate aging (Haussmann et al. 2012). Whether effects of in ovo corticosterone on life histories influence between-individual differences in fitness in wild populations is unresolved.
In conclusion, we employed a non-invasive technique that elevated the maternal transfer of corticosterone to eggs. This treatment increased the number of eggs that females produced and the amount of yolk allocated to eggs. Offspring hatching from these eggs begged for food at a higher rate than control offspring four days posthatching, at an age when they were also heavier, and eventually attained increased prefledging body condition, a trait positively predictive of their probability of recruitment as breeding adults in the study population. These results indicate that in ovo corticosterone can enhance a suite of traits related to maternal investment and offspring development. Future work will shed light on the adaptive significance of maternal stress and glucocorticoid production as mediators of maternal effects.
Acknowledgments
We thank the 2015 Wren Crew for contributing to data collection and the ParkLands Foundation (Merwin Preserve), the Illinois Great Rivers Conference of the United Methodist Church, and the Sears and Butler families for the use of their properties. We also thank two anonymous reviewers for constructive comments that improved the manuscript. Financial support was provided by NIH grants R15HD076308-01 and R15ES023995; the School of Biological Sciences, Illinois State University; a postdoctoral research award from the American Ornithologists’ Union; a Research Internship in Science and Engineering (RISE) from the Deutscher Akademischer Austauschdienst to Raphael Ritter; and the Beta Lambda Chapter of the Phi Sigma Biological Sciences Honor Society. All activities complied with current laws of the United States and with Illinois State University Institutional Animal Care and Use Committee Protocol 04-2013, US Geological Survey banding permit 09211, and US Fish and Wildlife Service collecting permit MB692148-0.
References
- Almasi B, Rettenbacher S, Müller C, Brill S, Wagner H, Jenni L. Maternal corticosterone is transferred into the egg yolk. Gen Comp Endocr. 2012;178:139–144. doi: 10.1016/j.ygcen.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Angelier F, Shaffer SA, Weimerskirch H, Trouvé C, Chastel O. Corticosterone and foraging behavior in a pelagic seabird. Physiol Biochem Zool. 2007;80:283–292. doi: 10.1086/512585. [DOI] [PubMed] [Google Scholar]
- Ardia DR, Wasson MF, Winkler DW. Individual quality and food availability determine yolk and egg composition in tree swallows Tachycineta bicolor. J Avian Biol. 2006;37:252–259. [Google Scholar]
- Barnett CA, Clairardin SG, Thompson CF, Sakaluk SK. Turning a deaf ear: a test of the manipulating androgens hypothesis in house wrens. Anim Behav. 2011;81:113–120. [Google Scholar]
- Bowden RM, Harms HK, Paitz RT, Janzen FJ. Does optimal egg size vary with demographic stage because of a physiological constraint? Funct Ecol. 2004;18:522–529. [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Adaptive sex allocation in relation to hatching synchrony and offspring quality in house wrens. Am Nat. 2011;177:617–629. doi: 10.1086/659630. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Sibling cooperation influences the age of nest-leaving in an altricial bird. Am Nat. 2013;181:775–786. doi: 10.1086/670244. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Nietz D, Thompson CF, Sakaluk SK. Parental provisioning in house wrens: effects of varying brood size and consequences for offspring. Behav Ecol. 2014a;25:1485–1493. [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, Johnson LS, Thompson CF, Sakaluk SK. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology. 2014b;95:3027–3034. doi: 10.1890/14-0418.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. J Anim Ecol. 2015a;84:473–486. doi: 10.1111/1365-2656.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Sakaluk SK, Thompson CF. Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. Am Nat. 2015b;185:769–783. doi: 10.1086/681017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Within-female plasticity in sex allocation is associated with a behavioral polyphenism in house wrens. J Evol Biol. 2016;29:602–616. doi: 10.1111/jeb.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner CW. Maternal stress, glucocorticoids, and the maternal/fetal match hypothesis. Horm Behav. 2008;54:485–487. doi: 10.1016/j.yhbeh.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Greenberg AL, Wingfield JC. Noninvasive corticosterone treatment rapidly increases activity in Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii) Gen Comp Endocr. 1998;111:386–394. doi: 10.1006/gcen.1998.7128. [DOI] [PubMed] [Google Scholar]
- Burton T, Killen SS, Armstrong JD, Metcalfe NB. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc R Soc B. 2011;278:3465–3473. doi: 10.1098/rspb.2011.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin EH, Sharp CM, Burness G. Sex-biased resource allocation in ovo in a sexually size-dimorphic species. J Avian Biol. 2012;43:385–389. [Google Scholar]
- Chin EH, Love OP, Verspoor JJ, Williams TD, Rowley K, Burness G. Juveniles exposed to embryonic corticosterone have enhanced flight performance. Proc R Soc B. 2009;276:499–505. doi: 10.1098/rspb.2008.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians JK. Avian egg size: variation within species and inflexibility within individuals. Biol Rev. 2002;77:1–26. doi: 10.1017/s1464793101005784. [DOI] [PubMed] [Google Scholar]
- Crino OL, Klaassen Van Oorschot B, Johnson EE, Malisch JL, Breuner CW. Proximity to a high traffic road: glucocorticoid and life history consequences for nestling white-crowned sparrows. Gen Comp Endocr. 2011;173:323–332. doi: 10.1016/j.ygcen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Crossin GT, Trathan PN, Phillips RA, Gorman KB, Dawson A, Sakamoto KQ, Williams TD. Corticosterone predicts foraging behavior and parental care in macaroni penguins. Am Nat. 2012;180:E31–E41. doi: 10.1086/666001. [DOI] [PubMed] [Google Scholar]
- Crossin GT, Love OP, Cooke SK, Williams TD. Glucocorticoid manipulations in free-living animals: considerations of dose delivery, life-history context, and reproductive state. Funct Ecol. 2015;30:116–125. [Google Scholar]
- Dantzer B, Newman AEM, Boonstra R, Palme R, Boutin S, Humphries MM, McAdam AG. Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science. 2013;340:1215–1217. doi: 10.1126/science.1235765. [DOI] [PubMed] [Google Scholar]
- Duffield KR, Hunt J, Rapkin J, Sadd BM, Sakaluk SK. Terminal investment in the gustatory appeal of nuptial food gifts in crickets. J Evol Biol. 2015;28:1872–1881. doi: 10.1111/jeb.12703. [DOI] [PubMed] [Google Scholar]
- Eising CM, Eikenaar C, Schwabl H, Groothuis TGG. Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc R Soc Lond B. 2001;268:839–846. doi: 10.1098/rspb.2001.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau M, Ricklefs RE, Wikelski M, Lee KA, Brawn JD. Corticosterone, testosterone and life-history strategies of birds. Proc R Soc B. 2010;277:3203–3212. doi: 10.1098/rspb.2010.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc R Soc B. 2012;279:1447–1456. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LS, Wingfield JC. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen Comp Endocr. 2004;135:365–371. doi: 10.1016/j.ygcen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Henderson LJ, Evans NP, Heidinger BJ, Adams A, Arnold KE. Maternal condition but not corticosterone is linked to offspring sex ratio in a passerine bird. PLoS One. 2014;9:e110858. doi: 10.1371/journal.pone.0110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennin HL, Wells-Berlin AM, Love OP. Baseline glucocorticoids are drivers of body mass gain in a diving seabird. Ecol Evol. 2016;6:1702–1711. doi: 10.1002/ece3.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennin HL, Legagneux P, Bêty J, Williams TD, Gilchrist HG, Baker TM, Love OP. Pre-breeding energetic management in a mixed-strategy breeder. Oecologia. 2015;177:235–243. doi: 10.1007/s00442-014-3145-x. [DOI] [PubMed] [Google Scholar]
- Henriksen R, Rettenbacher S, Groothuis TGG. Prenatal stress in birds: pathways, effects, function and perspectives. Neurosci Biobehav Rev. 2011;35:1484–1501. doi: 10.1016/j.neubiorev.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Giesing ER, Suski CD, Warner RE, Bell AM. Female sticklebacks transfer information via eggs: effects of maternal experience with predators on offspring. Proc R Soc B. 2011;278:1753–1759. doi: 10.1098/rspb.2010.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano M, Groothuis TGG, Tschirren B. Interactions between prenatal maternal effects and posthatching conditions in a wild bird population. Behav Ecol. 2014;25:1459–1466. [Google Scholar]
- Jeon J. Evolution of parental favoritism among different-aged offspring. Behav Ecol. 2008;19:344–352. [Google Scholar]
- Johnson LS. House wren (Troglodytes aedon) In: Poole A, editor. The Birds of North America Online. 2. Cornell Lab of Ornithology and American Ornithologists’ Union; Ithaca, NY: 2014. [Google Scholar]
- Johnson LS, Barclay RMR. Effects of supplemental calcium on the reproductive output of a small passerine bird, the House Wren (Troglodytes aedon) Can J Zool. 1996;74:278–282. [Google Scholar]
- Jones BC, Smith AD, Bebus SE, Schoech SJ. Two seconds is all it takes: European starlings (Sturnus vulgaris) increase levels of circulating glucocorticoids after witnessing a brief raptor attack. Horm Behav. 2016;78:72–78. doi: 10.1016/j.yhbeh.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Khan N, Peters RA, Robert K. Compensating for a stressful start: maternal corticosterone, offspring survival, and size at fledging in the Zebra Finch (Taeniopygia guttata) Emu. 2016 doi: 10.1071/MU15095. [DOI] [Google Scholar]
- Kouwenberg AL, Hipfner JM, McKay DW, Storey AE. Corticosterone and stable isotopes in feathers predict egg size in Atlantic puffins Fratercula arctica. Ibis. 2013;155:413–418. [Google Scholar]
- Krist M. Egg size and offspring quality: a meta-analysis in birds. Biol Rev. 2011;86:692–716. doi: 10.1111/j.1469-185X.2010.00166.x. [DOI] [PubMed] [Google Scholar]
- Lambrechts MM, Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bańbura J, et al. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol. 2010;45:1–26. [Google Scholar]
- Larsen TR, Fairhurst GD, De Baere S, Croubels S, Müller W, De Neve L, Lens L. Novel insights into relationships between egg corticosterone and timing of breeding revealed by LC-MS/MS. J Avian Biol. 2015;46:643–647. [Google Scholar]
- Lothery CJ, Thompson CF, Lawler ML, Sakaluk SK. Food supplementation fails to reveal a trade-off between incubation and self-maintenance in female house wrens. PLoS One. 2014;9:e106260. doi: 10.1371/journal.pone.0106260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love OP, Williams TD. The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am Nat. 2008;172:E135–E149. doi: 10.1086/590959. [DOI] [PubMed] [Google Scholar]
- Love OP, Breuner CW, Vézina F, Williams TD. Mediation of a corticosterone-induced reproductive conflict. Horm Behav. 2004;46:59–65. doi: 10.1016/j.yhbeh.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Love OP, Chin EH, Wynne-Edwards KE, Williams TD. Stress hormones: a link between maternal condition and sex-biased reproductive investment. Am Nat. 2005;166:751–766. doi: 10.1086/497440. [DOI] [PubMed] [Google Scholar]
- Love OP, Wynne-Edwards KE, Bond L, Williams TD. Determinants of within- and among-clutch variation in yolk corticosterone in the European starling. Horm Behav. 2008;53:104–111. doi: 10.1016/j.yhbeh.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Love OP, Gilchrist HG, Bêty J, Wynne-Edwards KE, Berzins L, Williams TD. Using life-histories to predict and interpret variability in yolk hormones. Gen Comp Endocr. 2009;163:169–174. doi: 10.1016/j.ygcen.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Love OP, Williams TD. Manipulating developmental stress reveals sex-specific effects of egg size on offspring phenotype. J Evol Biol. 2011;24:1497–1504. doi: 10.1111/j.1420-9101.2011.02282.x. [DOI] [PubMed] [Google Scholar]
- Love OP, McGowan PO, Sheriff MJ. Maternal adversity and ecological stressors in natural populations: the role of stress axis programming in individuals, with implications for populations and communities. Funct Ecol. 2013;27:81–92. [Google Scholar]
- Love OP, Madliger CL, Bourgeon S, Semeniuk CAD, Williams TD. Evidence for baseline glucocorticoids as mediators of reproductive investment in a wild bird. Gen Comp Endocr. 2014;199:65–69. doi: 10.1016/j.ygcen.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Martin TE. Food as a limit on breeding birds: a life-history perspective. Ann Rev Ecol Syst. 1987;18:453–487. [Google Scholar]
- McCormick MI. Behaviorally induced maternal stress in fish influences progeny quality by a hormonal mechanism. Ecology. 1998;79:1873–1883. [Google Scholar]
- Merrill L, Angelier F, O’Loghlen AL, Rothstein SI, Wingfield JC. Sex-specific variation in brown-headed cowbird immunity following acute stress: a mechanistic approach. Oecologia. 2012;170:25–38. doi: 10.1007/s00442-012-2281-4. [DOI] [PubMed] [Google Scholar]
- Mock DW, Parker GA. The Evolution of Sibling Rivalry. Oxford University Press; Oxford: 1997. [Google Scholar]
- Mousseau TA, Fox CW. Maternal Effects as Adaptations. Oxford University Press; New York: 1998. [Google Scholar]
- Muller M, Groothuis TGG. Within-clutch variation in yolk testosterone as an adaptive maternal effect to modulate avian sibling competition: evidence from a comparative study. Am Nat. 2013;181:125–136. doi: 10.1086/668601. [DOI] [PubMed] [Google Scholar]
- Müller W, Lessells CM, Korsten P, von Engelhardt N. Manipulative signals of family conflict? On the function of maternal yolk hormones in birds. Am Nat. 2007;169:E84–E96. doi: 10.1086/511962. [DOI] [PubMed] [Google Scholar]
- Müller W, Boonen S, Groothuis TGG, Eens M. Maternal yolk testosterone in canary eggs: toward a better understanding of mechanisms and function. Behav Ecol. 2010;21:493–500. [Google Scholar]
- Nager RG, Ruegger C, van Noordwijk AJ. Nutrient or energy limitation on egg formation: a feeding experiment in great tits. J Anim Ecol. 1997;66:495–507. [Google Scholar]
- Nager RG, Monaghan P, Houston DC. Within-clutch trade-offs between the number and quality of eggs: experimental manipulations in gulls. Ecology. 2000;81:1339–1350. [Google Scholar]
- Navara KJ, Siefferman LM, Hill GE, Mendonça MT. Yolk androgens vary inversely to maternal androgens in eastern bluebirds: an experimental study. Funct Ecol. 2006;20:449–456. [Google Scholar]
- Nooker JK, Dunn PO, Whittingham LA. Effects of food abundance, weather, and female condition on reproduction in tree swallows (Tachycineta bicolor) Auk. 2005;122:1225–1238. [Google Scholar]
- Okuliarová M, Šárniková B, Rettenbacher S, Škrobánek P, Zeman M. Yolk testosterone and corticosterone in hierarchical follicles and laid eggs of Japanese quail exposed to long-term restraint stress. Gen Comp Endocr. 2010;165:91–96. doi: 10.1016/j.ygcen.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Ouyang JQ, Muturi M, Quetting M, Hau M. Small increases in corticosterone before the breeding season increase parental investment but not fitness in a wild passerine bird. Horm Behav. 2013;63:776–781. doi: 10.1016/j.yhbeh.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Paitz RT, Bowden RM, Casto JM. Embryonic modulation of maternal steroids in European starlings. Proc R Soc B. 2011;278:99–106. doi: 10.1098/rspb.2010.0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitk M, Tilgar V, Kilgas P, Mänd R. Acute stress affects the corticosterone level in bird eggs: a case study with great tits (Parus major) Horm Behav. 2012;62:475–479. doi: 10.1016/j.yhbeh.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Rivers JW, Liebl AL, Owen JC, Martin LB, Betts MG. Baseline corticosterone is positively related to juvenile survival in a migrant passerine bird. Funct Ecol. 2012;26:1127–1134. [Google Scholar]
- Rollinson N, Brooks RJ. Optimal offspring provisioning when egg size is “constrained”: a case study with the painted turtle Chrysemys picta. Oikos. 2008;117:144–151. [Google Scholar]
- Rollinson N, Hutchings JA. Environmental quality predicts optimal egg size in the wild. Am Nat. 2013;182:76–90. doi: 10.1086/670648. [DOI] [PubMed] [Google Scholar]
- Romero LM, Dickens MJ, Cyr NE. The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm Behav. 2009;55:375–389. doi: 10.1016/j.yhbeh.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Rubolini D, Romano M, Boncoraglio G, Ferrari RP, Martinelli R, Galeotti P, Fasola M, Saino N. Effects of elevated egg corticosterone levels on behavior, growth, and immunity of yellow-legged gull (Larus michahellis) chicks. Horm Behav. 2005;47:592–605. doi: 10.1016/j.yhbeh.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Ruffino L, Salo P, Koivisto E, Banks PB, Korpimäki E. Reproductive responses of birds to experimental food supplementation: a meta-analysis. Front Zool. 2014;11:80. doi: 10.1186/s12983-014-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowska J, Cichoń M. Maternal investment during egg laying and offspring sex: an experimental study of zebra finches. Anim Behav. 2002;64:817–822. [Google Scholar]
- Sadd B, Holman L, Armitage H, Lock F, Marland R, Siva-Jothy MT. Modulation of sexual signaling by immune challenged male mealworm beetles (Tenebrio molitor, L.): evidence for terminal investment and dishonesty. J Evol Biol. 2006;19:321–325. doi: 10.1111/j.1420-9101.2005.01062.x. [DOI] [PubMed] [Google Scholar]
- Saino N, Romano M, Ferrari RO, Martinelli R, Møller AP. Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J Exp Zool. 2005;303A:998–1006. doi: 10.1002/jez.a.224. [DOI] [PubMed] [Google Scholar]
- Saino N, Romano M, Caprioli M, Ambrosini R, Rubolini D, Fasola M. Sex allocation in yellow-legged gulls (Larus michahellis) depends on nutritional constraints on production of large last eggs. Proc R Soc B. 2010;277:1203–1208. doi: 10.1098/rspb.2009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol. 2010;1:103–113. [Google Scholar]
- Segers FHID, Taborsky B. Juvenile exposure to predator cues induces a larger egg size in fish. Proc R Soc B. 2012;279:1241–1248. doi: 10.1098/rspb.2011.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff MJ, Love OP. Determining the adaptive potential of maternal stress. Ecol Lett. 2013;16:271–280. doi: 10.1111/ele.12042. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Krebs CJ, Boonstra R. The ghosts of predators past: population cycles and the role of maternal programming under fluctuating predation risk. Ecology. 2010;91:2983–2994. doi: 10.1890/09-1108.1. [DOI] [PubMed] [Google Scholar]
- Sinervo B, DeNardo DF. Costs of reproduction in the wild: path analysis of natural selection and experimental tests of causation. Evolution. 1996;50:1299–1313. doi: 10.1111/j.1558-5646.1996.tb02370.x. [DOI] [PubMed] [Google Scholar]
- Smiseth PT, Scott MP, Andrews C. Hormonal regulation of offspring begging and mediation of parent-offspring conflict. Anim Behav. 2011;81:507–517. [Google Scholar]
- Strange MS. MS thesis. Illinois State University; 2015. Corticosterone in nestling house wrens: effects on fitness-related traits and the development of the stress response; p. 42. [Google Scholar]
- Velando A, Drummond H, Torres R. Senescent birds redouble reproductive effort when ill: confirmation of the terminal investment hypothesis. Proc R Soc B. 2006;273:1443–1448. doi: 10.1098/rspb.2006.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek MN, Jenkins BR, Safran RJ. Stress and success: individual differences in the glucocorticoid stress response predict behavior and reproductive success under high predation risk. Horm Behav. 2014;66:812–819. doi: 10.1016/j.yhbeh.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Wagner EC, Williams TD. Experimental (antiestrogen-mediated) reduction in egg size negatively affects offspring growth and survival. Physiol Biochem Zool. 2007;80:293–305. doi: 10.1086/512586. [DOI] [PubMed] [Google Scholar]
- Whittingham LA, Dunn PO, Lifjeld JT. Egg mass influences nestling quality in tree swallows, but there is no differential allocation in relation to laying order or sex. Condor. 2007;109:585–594. [Google Scholar]
- Williams TD. Physiological Adaptations for Breeding in Birds. Princeton University Press; Princeton: 2012. [Google Scholar]
- Williams TD, Miller M. Individual and resource-dependent variation in ability to lay supranormal clutches in response to egg removal. Auk. 2003;120:481–489. [Google Scholar]
- Wingfield JC. Control of behavioral strategies for capricious environments. Anim Behav. 2003;66:807–816. [Google Scholar]
- Wingfield JC, Maney DL, Breunner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. Ecological bases of hormone-behavior interactions: the “emergency life history stage. Am Zool. 1998;38:191–206. [Google Scholar]