Abstract
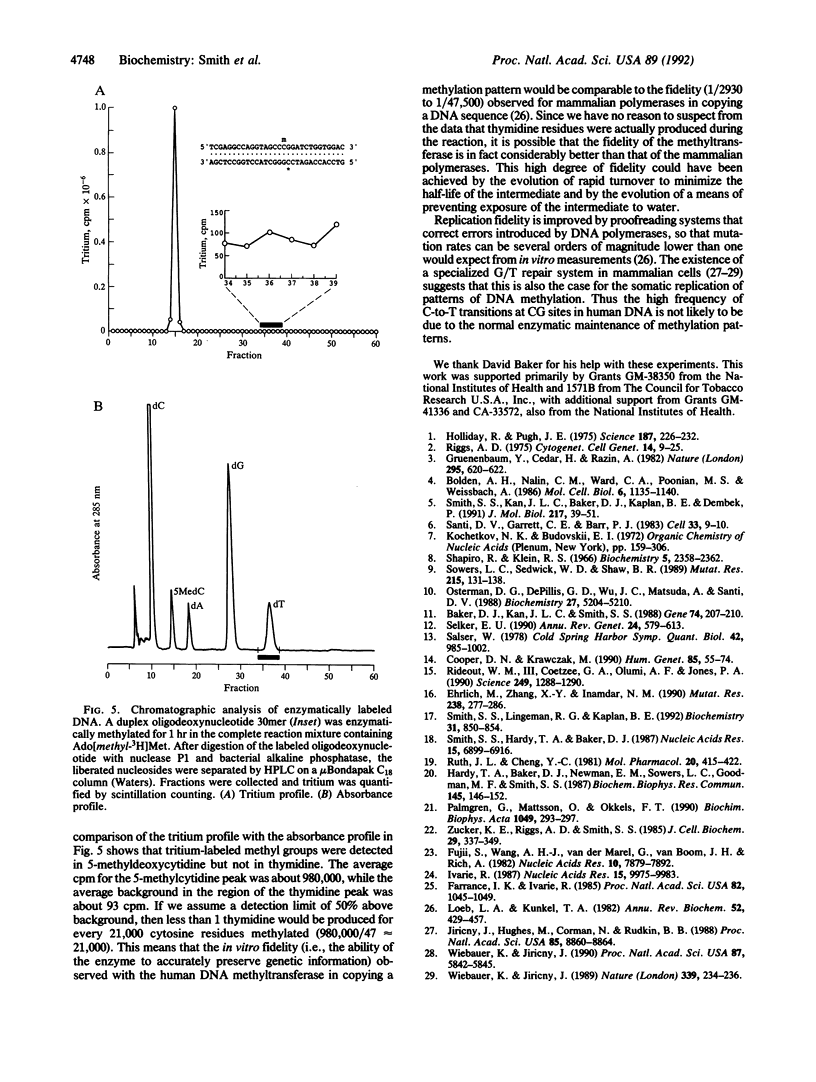
The properties of the methyl-directed DNA (cytosine-5-)-methyltransferase (EC 2.1.1.37) suggest that it is the enzyme that maintains patterns of methylation in the human genome. Proposals for the enzyme's mechanism of action suggest that 5-methyldeoxycytidine is produced from deoxycytidine via a dihydrocytosine intermediate. We have used an oligodeoxynucleotide containing 5-fluorodeoxycytidine as a suicide substrate to capture the enzyme and the dihydrocytosine intermediate. Gel retardation experiments demonstrate the formation of the expected covalent complex between duplex DNA containing 5-fluorodeoxycytidine and the human enzyme. Formation of the complex was dependent upon the presence of the methyl donor S-adenosylmethionine, suggesting that it comprises an enzyme-linked 5-substituted dihydrocytosine moiety in DNA. Dihydrocytosine derivatives are extremely labile toward hydrolytic deamination in aqueous solution. Because C-to-T transition mutations are especially prevalent at CG sites in human DNA, we have used high-performance liquid chromatography to search for thymidine that might be generated by hydrolysis during the methyl transfer reaction. Despite the potential for deamination inherent in the formation of the intermediate, the methyltransferase did not produce detectable amounts of thymidine. The data suggest that the ability of the human methyltransferase to preserve genetic information when copying a methylation pattern (i.e., its fidelity) is comparable to the ability of a mammalian DNA polymerase to preserve genetic information when copying a DNA sequence. Thus the high frequency of C-to-T transitions at CG sites in human DNA does not appear to be due to the normal enzymatic maintenance of methylation patterns.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker D. J., Kan J. L., Smith S. S. Recognition of structural perturbations in DNA by human DNA(cytosine-5)methyltransferase. Gene. 1988 Dec 25;74(1):207–210. doi: 10.1016/0378-1119(88)90288-0. [DOI] [PubMed] [Google Scholar]
- Bolden A. H., Nalin C. M., Ward C. A., Poonian M. S., Weissbach A. Primary DNA sequence determines sites of maintenance and de novo methylation by mammalian DNA methyltransferases. Mol Cell Biol. 1986 Apr;6(4):1135–1140. doi: 10.1128/mcb.6.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Krawczak M. The mutational spectrum of single base-pair substitutions causing human genetic disease: patterns and predictions. Hum Genet. 1990 Jun;85(1):55–74. doi: 10.1007/BF00276326. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Zhang X. Y., Inamdar N. M. Spontaneous deamination of cytosine and 5-methylcytosine residues in DNA and replacement of 5-methylcytosine residues with cytosine residues. Mutat Res. 1990 May;238(3):277–286. doi: 10.1016/0165-1110(90)90019-8. [DOI] [PubMed] [Google Scholar]
- Farrance I. K., Ivarie R. Ethylation of poly(dC-dG).poly(dC-dG) by ethyl methanesulfonate stimulates the activity of mammalian DNA methyltransferase in vitro. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1045–1049. doi: 10.1073/pnas.82.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Wang A. H., van der Marel G., van Boom J. H., Rich A. Molecular structure of (m5 dC-dG)3: the role of the methyl group on 5-methyl cytosine in stabilizing Z-DNA. Nucleic Acids Res. 1982 Dec 11;10(23):7879–7892. doi: 10.1093/nar/10.23.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y., Cedar H., Razin A. Substrate and sequence specificity of a eukaryotic DNA methylase. Nature. 1982 Feb 18;295(5850):620–622. doi: 10.1038/295620a0. [DOI] [PubMed] [Google Scholar]
- Hardy T. A., Baker D. J., Newman E. M., Sowers L. C., Goodman M. F., Smith S. S. Size of the directing moiety at carbon 5 of cytosine and the activity of human DNA(cytosine-5) methyltransferase. Biochem Biophys Res Commun. 1987 May 29;145(1):146–152. doi: 10.1016/0006-291x(87)91299-x. [DOI] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Ivarie R. Thymine methyls and DNA-protein interactions. Nucleic Acids Res. 1987 Dec 10;15(23):9975–9983. doi: 10.1093/nar/15.23.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiricny J., Hughes M., Corman N., Rudkin B. B. A human 200-kDa protein binds selectively to DNA fragments containing G.T mismatches. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8860–8864. doi: 10.1073/pnas.85.23.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Osterman D. G., DePillis G. D., Wu J. C., Matsuda A., Santi D. V. 5-Fluorocytosine in DNA is a mechanism-based inhibitor of HhaI methylase. Biochemistry. 1988 Jul 12;27(14):5204–5210. doi: 10.1021/bi00414a039. [DOI] [PubMed] [Google Scholar]
- Palmgren G., Mattsson O., Okkels F. T. Employment of hydrolytic enzymes in the study of the level of DNA methylation. Biochim Biophys Acta. 1990 Jul 30;1049(3):293–297. doi: 10.1016/0167-4781(90)90100-g. [DOI] [PubMed] [Google Scholar]
- Rideout W. M., 3rd, Coetzee G. A., Olumi A. F., Jones P. A. 5-Methylcytosine as an endogenous mutagen in the human LDL receptor and p53 genes. Science. 1990 Sep 14;249(4974):1288–1290. doi: 10.1126/science.1697983. [DOI] [PubMed] [Google Scholar]
- Riggs A. D. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Ruth J. L., Cheng Y. C. Nucleoside analogues with clinical potential in antivirus chemotherapy. The effect of several thymidine and 2'-deoxycytidine analogue 5'-triphosphates on purified human (alpha, beta) and herpes simplex virus (types 1, 2) DNA polymerases. Mol Pharmacol. 1981 Sep;20(2):415–422. [PubMed] [Google Scholar]
- Salser W. Globin mRNA sequences: analysis of base pairing and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):985–1002. doi: 10.1101/sqb.1978.042.01.099. [DOI] [PubMed] [Google Scholar]
- Santi D. V., Garrett C. E., Barr P. J. On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell. 1983 May;33(1):9–10. doi: 10.1016/0092-8674(83)90327-6. [DOI] [PubMed] [Google Scholar]
- Selker E. U. Premeiotic instability of repeated sequences in Neurospora crassa. Annu Rev Genet. 1990;24:579–613. doi: 10.1146/annurev.ge.24.120190.003051. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Klein R. S. The deamination of cytidine and cytosine by acidic buffer solutions. Mutagenic implications. Biochemistry. 1966 Jul;5(7):2358–2362. doi: 10.1021/bi00871a026. [DOI] [PubMed] [Google Scholar]
- Smith S. S., Hardy T. A., Baker D. J. Human DNA (cytosine-5)methyltransferase selectively methylates duplex DNA containing mispairs. Nucleic Acids Res. 1987 Sep 11;15(17):6899–6916. doi: 10.1093/nar/15.17.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. S., Kan J. L., Baker D. J., Kaplan B. E., Dembek P. Recognition of unusual DNA structures by human DNA (cytosine-5)methyltransferase. J Mol Biol. 1991 Jan 5;217(1):39–51. doi: 10.1016/0022-2836(91)90609-a. [DOI] [PubMed] [Google Scholar]
- Smith S. S., Lingeman R. G., Kaplan B. E. Recognition of foldback DNA by the human DNA (cytosine-5-)-methyltransferase. Biochemistry. 1992 Jan 28;31(3):850–854. doi: 10.1021/bi00118a030. [DOI] [PubMed] [Google Scholar]
- Sowers L. C., Sedwick W. D., Shaw B. R. Hydrolysis of N3-methyl-2'-deoxycytidine: model compound for reactivity of protonated cytosine residues in DNA. Mutat Res. 1989 Nov;215(1):131–138. doi: 10.1016/0027-5107(89)90225-x. [DOI] [PubMed] [Google Scholar]
- Wiebauer K., Jiricny J. In vitro correction of G.T mispairs to G.C pairs in nuclear extracts from human cells. Nature. 1989 May 18;339(6221):234–236. doi: 10.1038/339234a0. [DOI] [PubMed] [Google Scholar]
- Wiebauer K., Jiricny J. Mismatch-specific thymine DNA glycosylase and DNA polymerase beta mediate the correction of G.T mispairs in nuclear extracts from human cells. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5842–5845. doi: 10.1073/pnas.87.15.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker K. E., Riggs A. D., Smith S. S. Purification of human DNA (cytosine-5-)-methyltransferase. J Cell Biochem. 1985;29(4):337–349. doi: 10.1002/jcb.240290407. [DOI] [PubMed] [Google Scholar]






