Abstract
Purpose
In natural history studies, maintenance of higher levels of C-peptide secretion (a measure of endogenous insulin production) correlates with a lower incidence of major hypoglycemic events in patients with type 1 diabetes (T1D), but is unclear whether this will also be true for drug-induced C-peptide preservation.
Methods
We analyzed hypoglycemic events and glycemic control data from the T1DAL study, a trial of alefacept in new-onset T1D, which demonstrated significant C-peptide preservation at 1 and 2 years. We performed a post hoc analysis using mixed models of the relationship between the meal-stimulated 4-hour C-peptide area under the curve (4-hour AUC) and rates of major hypoglycemia, measures of glycemic control (HbA1c; average glucometer readings) and variability (glucometer SDs; highest and lowest readings), and an index of partial remission (insulin dose-adjusted HbA1c, IDAA1C).
Findings
Data from 49 participants (33 in the alefacept group, 16 in the placebo group) were analyzed at baseline and 12 and 24 months. We found that the 4-hour AUC at baseline and at 1 year was a significant predictor of the number of hypoglycemic events during the ensuing 12-month interval (p=0.030). There was a strong relationship between the 4-hour AUC and glucometer SDs (p<0.001), highest readings (p<0.001), and lowest readings (p=0.03), all measures of glycemic variability. There was a strong inverse correlation between the 4-hour AUC and two measures of glycemic control: HbA1c and average glucometer readings (both p<0.001). There was also a strong inverse correlation between the 4-hour AUC and IDAA1C values (p<0.001), as well as a strong correlation between IDAA1C values and glucometer SDs (p<0.001), suggesting that reduced glycemic variability is associated with a trend toward partial remission. For none of these analyses was there a significant difference between the alefacept and placebo groups.
Implications
Measures of glycemic variability and control, including rates of hypoglycemia, are significantly correlated with preservation of C-peptide regardless of whether this is achieved by immune intervention with alefacept or natural variability in patients with new-onset T1D. Thus, preservation of endogenous insulin production by an immunomodulatory drug may confer clinical benefits similar to those seen in patients with higher C-peptide secretion due to slow disease progression.
Keywords: New-onset T1D, islet function, glycemic control, alefacept, immune intervention, hypoglycemia
INTRODUCTION
Type 1 diabetes (T1D) is characterized by a progressive loss of β-cell function resulting in absolute insulin deficiency.1 Patients with T1D require lifelong insulin replacement therapy, which is lifesaving but heightens risks of major hypoglycemia and lessens but does not abolish serious complications, including death.2 Despite substantial technological advances over the past century, insulin replacement therapy remains imperfect and even with good glycemic control achievable with current regimens (glycated hemoglobin (HbA1c) levels <6.9%), mortality among patients with T1D is 2-fold or greater than matched controls.3 These increased mortality rates are observed across all age groups in T1D, including children and adolescents, in whom the cause of death most frequently relates to episodes of severe hyper- or hypoglycemia.3
Excessive glycemic variability in T1D also contributes to considerable morbidity. Hospitalization rates in younger patients with T1D are substantially higher than in the general population, most frequently because of inadequate glycemic control with life-threatening episodes of hyper- or hypoglycemia.4–7 T1D-induced glycemic variability, particularly repeated episodes of major hypoglycemia, has been linked to abnormalities in brain development, including cognitive dysfunction, which may extend into adulthood.8–11 Hypoglycemic episodes have been linked to cardiac abnormalities, including ventricular arrhythmias, which may be a contributing factor to the so-called “dead-in-bed” syndrome in younger T1D patients.12–14 Excess mortality from cardiovascular causes is associated with episodes of severe hypoglycemia in both T1D and type 2 diabetes and affects all age groups.3, 15, 16
The Diabetes Control and Complications Trial (DCCT) clearly demonstrated that maintenance of higher levels of C-peptide secretion, regardless of the insulin regimen, resulted in a lower frequency of retinopathy, nephropathy, and hypoglycemic events.17 The original analyses suggested that these clinical benefits were associated with C-peptide levels above a minimum threshold of 0.2 pmol/mL (“responders”), but more recent analyses indicate that reduced rates of hypoglycemia and microvascular complications are observed across a range of C-peptide values with no threshold or breakpoint,18 suggesting that even very low levels of preserved C-peptide secretion in T1D can confer clinical benefits.19
Data linking preservation of C-peptide with reduced rates of hypoglycemia in T1D are based on studies of the natural history of the disease and variability in rates of β-cell decline, which is influenced in part by the intensity of insulin therapy and glycemic targets and in part by the underlying variability in disease progression.17–21 The benefits of higher levels of C-peptide secretion are also seen after islet transplantation, with substantial reductions in severe hypoglycemia even when graft function is modest.22–24 In recent years, several immunomodulatory therapies have shown some success in preserving C-peptide secretion at 1 or 2 years after diagnosis in T1D.25–27 However, it has been unclear what effect preservation of C-peptide by an immune intervention will have on rates of hypoglycemia: on the one hand, preservation of islet function may decrease hypoglycemia by improving metabolic control and reducing exogenous insulin requirements; on the other hand, improved islet function may increase rates of hypoglycemia if insulin dose adjustments are not made commensurate with drug-induced changes in endogenous insulin secretion. The recent T1DAL trial (Inducing Remission in New-Onset T1D with Alefacept) demonstrated significant preservation of C-peptide and is the first immune intervention trial in T1D that clearly showed a significant reduction in rates of major hypoglycemia in the drug vs. placebo group.28, 29
In the current report we present the results of an extensive post hoc analysis of glycemic control data in the T1DAL trial, including rates of hypoglycemia, glycemic variability, and HbA1c, and their association with C-peptide preservation. We show that measures of glycemic control, including rates of hypoglycemia, are significantly correlated with preservation of C-peptide regardless of whether this was achieved by immune intervention with alefacept or natural variability.
PATIENTS AND METHODS
Study Design and Patient Population
The present study used data from the T1DAL trial, a phase 2, randomized, placebo-controlled, double-blind clinical trial of alefacept in patients with new-onset T1D, with a 9-month treatment period and 15 months of follow-up, conducted at 14 clinical centers in the United States.28, 29 Eligible participants were 12–35 years of age at time of screening; <100 days from diagnosis at the time of enrollment; positive for at least one diabetes-associated autoantibody (insulin, GAD-65, IA-2, ZnT8, or ICA); and had peak stimulated C-peptide of > 0.2 nmol/L during a mixed meal tolerance test (MMTT). Exclusion criteria included evidence of tuberculosis, hepatitis B or C, HIV, or active EBV or CMV infection; significant cardiac disease; conditions associated with immune dysfunction or hematologic dyscrasia (including malignancy, lymphopenia, thrombocytopenia, or anemia); liver or renal dysfunction; ongoing use of diabetes medications other than insulin; recent inoculation with a live vaccine; and lactating or pregnant females.
Eligible subjects were randomly assigned 2:1 to alefacept or placebo. All subjects and site personnel, including the independent diabetes educators, remained masked throughout the study. At outpatient visits, participants received 15 mg alefacept (Amevive®) or equivalent volume of saline (placebo) intramuscularly weekly for 12 weeks and, after a 12-week pause, 12 additional weekly doses of alefacept or placebo. Participants underwent a 4-hour MMTT at screening, 52 weeks, and 104 weeks, a 2-hour MMTT at 24 and 78 weeks, and intensive diabetes management.28, 29
The T1DAL study was conducted according to the Declaration of Helsinki and in accordance with good clinical practice guidelines, performed under an FDA investigational new drug application (IND 105,308), and approved by independent institutional review boards at each participating clinical center. All participants or parents provided written informed consent or assent (<18 years old). An independent data and safety monitoring board (DSMB) conducted regular safety reviews, and the sponsor’s medical monitor provided additional study oversight. AEs were recorded and reported according to the standards set forth in the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), Version 4.0 (May 28, 2009).
Assessments and Statistical Analyses
As part of their intensive diabetes management, all patients received identical glucometers and supplies (Bayer Contour, Bayer HealthCare LLC, Diabetes Care, Whippany, New Jersey, USA) as well as log books to record insulin use. Patients were instructed to measure blood glucose levels at least 4 times daily, record daily insulin use, and record hypoglycemic events. Clinical sites were trained in downloading and recording standardized glucometer data at each clinic visit. Standardized glucometer data included number of readings since last visit; number of readings ≤ 65 mg/dL; lowest reading; highest reading; standard deviation of readings; and average blood glucose across all readings. Major hypoglycemia events included episodes where glucose was < 55 mg/dL or clinical events involving seizure, loss of consciousness (coma), or requiring assistance from another individual. In addition, patients participated in up to three 4-hour MMTTs (screening, Months 52 and 104). The mean 4-hr C-peptide area under the curve (4-hour AUC) was computed as the total AUC divided by 240 minutes. At each of these visits, insulin use (units/kg) recorded for the prior 5 days and HbA1c levels were used to compute the insulin dose-adjusted HbA1c (IDAA1C). Models were fit using Proc Mixed in SAS ver. 9.3.
To account for multiple assessments per subject, mixed models were used to evaluate the relationship between 4-hour AUC and measures of glycemic control, which included outcomes derived from the glucometer data (i.e. standard deviation, lowest reading, highest reading, average reading), as well as HbA1c, and IDAA1C. For each measure of glycemic control, the mixed regression model included fixed effects for treatment as well as linear and quadratic terms for the 4-hour AUC, and a random subject-level intercept. Preliminary models included separate fixed-effect linear and quadratic slopes for each treatment, but linear and quadratic effects did not differ significantly between the two treatment arms for any outcome.
To evaluate the relationship between the rate of major hypoglycemic events and 4-hour AUC, the number of major hypoglycemic events for each subject were enumerated for 2 intervals: from screening up to the Week 52 visit, and between the Week 52 and Week 104 visits. To account for correlation between the intervals within subject, the relationship between the rate of hypoglycemic events and 4-hour AUC was evaluated using a repeated-measures generalized Gamma-Poisson mixture model. The log of the number of hypoglycemic events in each interval was modeled as a function of fixed effects for treatment, starting 4-hour AUC for the interval, and a random within-subject intercept. The log of number of days in the interval is the denominator. Preliminary models included terms for the change in the 4-hour AUC during the interval and an interaction term for treatment by starting 4-hour AUC. The error distribution is Poisson where the λ parameter follows a gamma distribution (i.e. generalized negative binomial). The model was fit using Proc GLIMMIX in SAS version 9.3. Missing data were not imputed, and no adjustments were made for multiple comparisons.
RESULTS
Patients
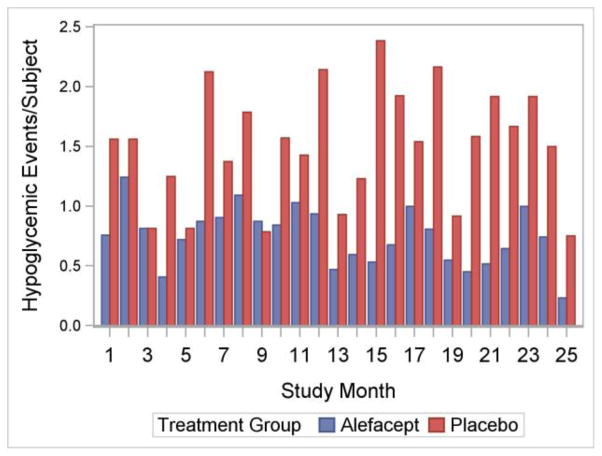
As reported previously,28, 29 of 73 individuals screened, 49 were enrolled in the trial, with 33 patients randomly assigned to receive alefacept and 16 to receive placebo. Demographic and baseline characteristics of the 49 participants enrolled were comparable between the alefacept and placebo groups. Alefacept-treated participants had significant preservation of endogenous insulin production at 12 and 24 months compared to placebo determined by the 4-hour AUC. Both groups achieved good glycemic control, with mean HbA1c levels at 24 months of ~7.4–7.5%. However, compared to the placebo group, participants who received alefacept had lower mean insulin requirements and had substantially less major hypoglycemia (blood glucose < 55 mg/dL).28, 29 Further analysis revealed that over the entire 2-year study period, rates of major hypoglycemia were substantially lower in the alefacept group (Figure 1).
Figure 1. Major Hypoglycemic Events over Time.
The height of each bar equals the total number of hypoglycemic events divided by the number of subject in the study at month x. Treatment groups are represented by different bars.
Relationship between Number of Hypoglycemic Events and C-Peptide Levels
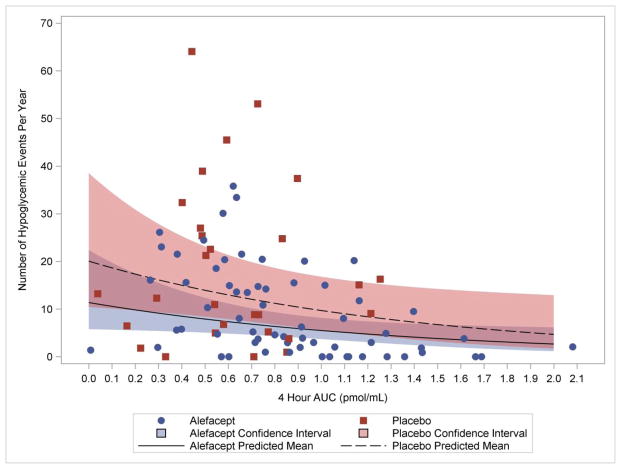
Figure 2 illustrates the relationship between hypoglycemic events during two intervals (screening to Week 52 and Week 52 to Week 104) and the 4-hour AUC at the start of the interval. Results from the Gamma-Poisson model indicate that the value of the 4-hour AUC at the start of the interval was a significant predictor of the number of hypoglycemic events that occurred during the interval (p=0.030), but, after accounting for the contribution of the 4-hour AUC, treatment was not a significant predictor of hypoglycemic events (0.072). A higher 4-hour AUC at the start of the interval was predictive of fewer hypoglycemic events during the interval in question. For example, the model-based parameter estimate for the starting AUC parameter equals −0.7271; thus, if the starting 4-hour AUC value falls from 0.65 pmol/mL to 0.45 pmol/mL, the expected rate of hypoglycemic events increases by 16% (i.e. {(0.45−0.65)×(−0.7271)}= 1.16). The relationship between the rate of hypoglycemic events and starting 4-hour AUC did not differ significantly by treatment group, and the change in AUC over the interval was not statistically significant (models not shown).
Figure 2. Major Hypoglycemic Events versus 4-hour AUC.
The lines are predicted Poisson means based on the parameter estimates (standard error) for the fitted Gauss-Poisson model: intercept −2.90 (0.324); alefacept −0.57 (0.308); starting AUC −0.73 (0.324), where the reference cell is placebo at AUC=0. P-values are <0.001, 0.07, and 0.03, respectively. The Pearson Chi-Square divided by the degrees of freedom = 0.44, suggesting good model fit. The analysis included 91 observations from 49 subjects. The 4-hour AUC represents the AUC at the start of the interval.
Relationship between Glycemic Variability and C-Peptide Levels
Determination of the standard deviations of the glucometer readings over time provides a useful surrogate for glycemic variability. Figure 3 illustrates the inverse correlation between glucometer SDs and C-peptide responses in both the alefacept and placebo groups. The mixed regression model indicated a strong relationship between 4-hour AUC and glucometer SDs; both the linear and quadratic terms for the 4-hour AUC were highly significant (p<0.001). The linear and quadratic effects did not differ significantly between the two treatment arms (model not shown), but, on average, standard deviations were significantly higher for the alefacept group (difference=17.4, p=0.02).
Figure 3. Standard Deviation of Glucometer Readings versus 4-hour AUC.
The lines are given by the parameter estimates (standard error) for the fitted model: intercept 98.8 (7.94); alefacept 17.4 (7.12); AUC −101.6 (13.71); AUC2 29.3 (5.95), where the reference cell is placebo at AUC=0. The shaded areas represent 95% confidence intervals. P-values are <0.001, 0.02, <0.001, and <0.001, respectively. The analysis included 111 observations from 49 subjects.
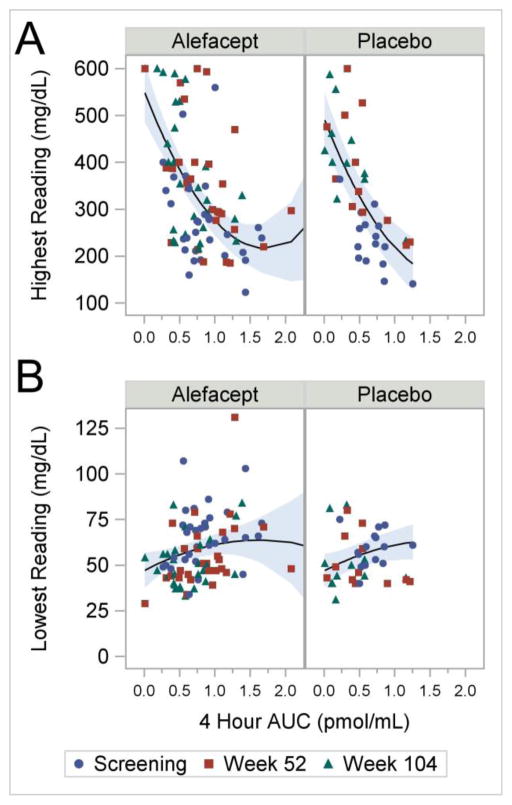
The validity of this association is supported by analyses of extreme glucometer readings (lowest and highest) versus the 4-hour AUC (Figure 4A and B). The highest and lowest blood glucose values are a reflection of glucose excursions and hence variability. Results for the highest glucometer reading are similar to those for the standard deviation (Figure 4A). Both the linear and quadratic terms for the 4-hour AUC were highly significant (both p<0.001). The linear and quadratic effects did not differ significantly between the two treatment arms (model not shown), but, on average, highest glucometer values were significantly higher for the alefacept group (difference=57.5, p=0.03). Figure 4B shows lowest glucometer readings decrease with decreasing 4 hour AUC. For this model, however, only the linear term was statistically significant (p=0.04); again, linear and quadratic effects did not differ significantly between the two treatment arms (model not shown). Thus, preservation of C-peptide, as assessed by the 4-hour AUC, correlates with a lower standard deviation of glucometer readings, and more stable extreme glucometer readings.
Figure 4.
(A) Highest Glucometer Readings versus 4-hour AUC. The lines are given by the parameter estimates (standard error) for the fitted model: intercept 493.5 (30.27); alefacept 57.5 (25.55); AUC −387.5 (58.08); AUC2 112.2 (26.12), where the reference cell is placebo at AUC=0. P-values are <0.001, 0.03, <0.001, and <0.001, respectively. (B) Lowest Glucometer Readings versus 4-hour AUC. The lines are given by the parameter estimates (standard error) for the fitted model: intercept 46.8 (4.56); alefacept 0.22 (4.02); AUC 20.4 (9.99); AUC2 −6.2 (5.43), where the reference cell is placebo at AUC=0. The shaded areas represent 95% confidence intervals. P-values are <0.001, 0.96, 0.04, and 0.26, respectively. The shaded areas represent 95% confidence intervals. Both analyses included 125 observations from 49 subjects.
Relationship between Glycemic Control and C-Peptide Levels
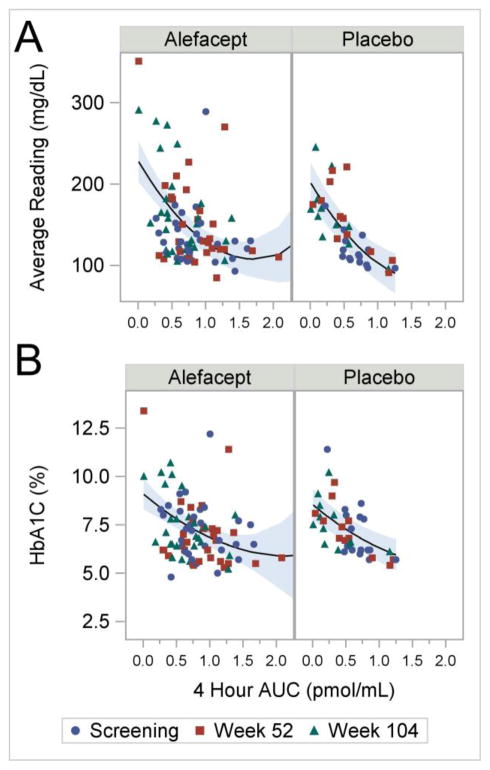
We analyzed two measures of glycemic control – average glucometer readings and glycated hemoglobin (HbA1c) – and plotted these variables as a function of the 4-hour AUC. In both cases we found a strong inverse correlation, which was similar in both the alefacept and placebo groups (Figure 5A and B). Higher 4-hour AUC values were associated with lower HbA1c values, including a highly significant linear effect (p<0.001) and a quadratic trend (p=0.10). Similar results are seen when analyzing the average glucometer readings; higher 4-hour AUC values were associated with lower average glucometer readings with highly significant linear and quadratic effects (both p<0.001). The linear and quadratic effects for the HbA1c and average glucometer readings did not differ significantly by treatment group (models not shown).
Figure 5.
(A) Average Glucometer Readings versus 4-hour AUC. The lines are given by the parameter estimates (standard error) for the fitted model: intercept 202.5 (12.78); alefacept 26.1 (11.92); AUC −140.8 (21.40); AUC2 41.0 (9.57), where the reference cell is placebo at AUC=0. The shaded areas represent 95% confidence intervals. P-values are <0.001, 0.03, <0.001, and <0.001, respectively. The analysis included 125 observations from 49 subjects. (B) HbA1c versus 4-hour AUC. The lines are given by the parameter estimates (standard error) for the fitted model: intercept 8.5 (0.42); alefacept 0.54 (0.41); AUC −2.9 (0.70); AUC2 0.6 (0.36), where the reference cell is placebo at AUC=0. The shaded areas represent 95% confidence intervals. P-values are <0.001, 0.19, 0.001, and 0.10, respectively. The analysis included 128 observations from 49 subjects.
Relationship between Index of Partial Remission and C-Peptide Levels
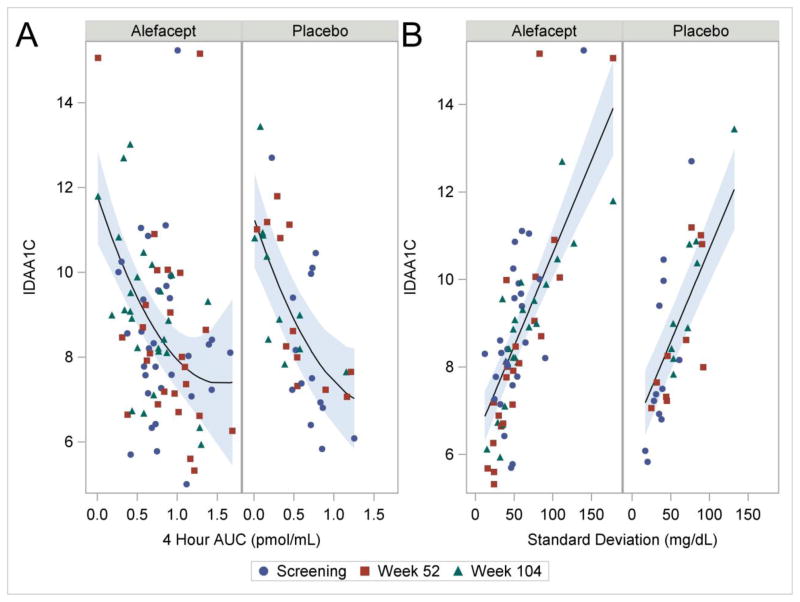
The IDAA1C has been proposed as an index of partial remission in T1D and is based on a simple formula that incorporates HbA1c and exogenous insulin dose. Using a threshold of ≤ 9 for the index, a correlation has been found with the partial remission (“honeymoon period”) that is frequently observed 3–6 months after diagnosis.30 Treating the IDAA1C as a continuous variable, we plotted this index against the 4-hour AUC and found a strong inverse correlation (Figure 6A). Higher 4-hour AUC values were associated with lower IDAA1C values, including significant linear and quadratic effects (p<0.001 and 0.04, respectively). The linear and quadratic effects did not differ significantly by treatment group (models not shown).
Figure 6.
(A) IDAA1C versus 4-hour AUC. The lines are given by the parameter estimates (standard error) for the fitted model: intercept 11.3 (0.57); alefacept 0.5 (0.54); AUC −5.7 (1.29); AUC2 1.8 (0.86), where the reference cell is placebo at AUC=0. The shaded areas represent 95% confidence intervals. P-values are <0.001, 0.35, <0.001, and 0.04, respectively. The analysis included 120 observations from 49 subjects. (B) IDAA1C versus Standard Deviation of Glucometer Readings. The lines are given by the parameter estimates (standard error) for the fitted model: intercept 6.5 (0.40); alefacept −0.1 (0.40); SD 0.042 (0.0041), where the reference cell is placebo at SD=0. P-values are <0.001, 0.79, <0.001, respectively. The analysis included 120 observations from 49 subjects.
To explore this further, we also plotted the IDAA1C versus the glucometer SDs and found a very strong correlation (Figure 6B), suggesting that reduced glycemic variability is closely associated with a trend toward partial remission. The linear trend is highly significant (p<0.001).
DISCUSSION
Preservation of residual islet function at the time of diagnosis is a major goal of intervention therapies in T1D.31 Even though the remaining β-cell mass at diagnosis may constitute only 20% or less of the original, pre-disease mass, accumulating evidence from the DCCT and other studies indicates that even modest amounts of endogenous insulin production substantially improve short- and long-term outcomes in these patients.17, 18, 20 It is known that the rate of decline of β-cell function after diagnosis in T1D is highly variable and many patients retain detectable insulin secretion (as measured by stimulated C-peptide production) for years or decades.19 The basis for this variability is not understood but is partly a function of age at disease onset as well as the intensity of diabetes management in the early period after diagnosis.32 Regardless of the mechanism of this variability, those with better preserved C-peptide secretion have lower rates of hypoglycemia and decreased microvascular complications.
However, until now it has been unclear whether C-peptide preservation with an immunomodulatory therapy would confer similar benefits. Therapies that have shown success in preserving C-peptide include drugs that target T cell frequency or function (anti-CD3 mAb, alefacept), costimulation blockade (CTLA4-Ig), inflammatory cytokines (anti-TNFα), and B cells (anti-CD20 mAb).31 Mechanistically these agents are very different and efficacy may result from alterations in the balance between regulatory and effector T cells, induction of hyporesponsiveness in autoreactive T cells, or general anti-inflammatory effects.31 One or more of these mechanisms could influence glucose metabolism – by affecting insulin sensitivity or glucose disposal rates – but in general this is not well understood. Components of both innate and adaptive immunity contribute to insulin resistance in diabetes and hence biologic agents may have complex effects on glycemic control and variability.33 For example, both TNFα and IL-6 influence insulin signaling,34, 35 but the potential consequences of this in the context of TNFα or IL-6 blockade in T1D are unknown. Lastly, while it is clear that poor glycemic control is related to the development of microvascular complications including retinopathy, this process also involves pro-inflammatory cytokines such as TNFα and IL-1β.36 Hence, treatment with an immunomodulatory drug may confer additional benefits on reducing such complications that extend beyond glycemic control.
The T1DAL trial is the first proof of concept that drug-induced preservation of islet function (as opposed to differences in natural rates of decline) can reduce hypoglycemia events in the context of intensive diabetes management.28, 29 Alefacept is an LFA3-Ig fusion protein that blocks CD2-mediated costimulation and depletes T cells that express high levels of CD2, principally memory and effector T cells, with preservation of regulatory T cells (Tregs).37 The primary analysis of the T1DAL clinical results indicated significant preservation of C-peptide responses at 1 and 2 years, and also showed significant reductions in exogenous insulin requirements and, remarkably, a 50% reduction in rates of major hypoglycemia in the treatment vs. placebo groups.28, 29
Here we show that rates of major hypoglycemia in the T1DAL trial are inversely correlated with endogenous insulin secretion, as measured by the meal-stimulated 4-hour C-peptide AUC. The 4-hour C-peptide AUC is a strong predictor of the number of hypoglycemic events for both study groups. The number of hypoglycemia events was also inversely correlated with the peak C-peptide levels during the 4-hour MMTT (results not shown). These results are consistent with the recent reanalysis of the DCCT data showing that there is a continuous relationship between rates of severe hypoglycemia and residual stimulated C-peptide levels.18 A similar conclusion was reached in a recent Danish study, which suggested that a level of meal-stimulated C-peptide of ≥ 0.04 nmol/L conferred beneficial effects on hypoglycemia and metabolic control in children with T1D of 3–6 years’ duration;38 the level of 0.04 nmol/L is considerably lower than the original responder threshold of 0.2 nmol/L identified by the DCCT.17 The value of higher levels of C-peptide secretion has also been borne out by the results of islet transplantation, which produces steep declines in the incidence of severe hypoglycemia, even at 5 years when significant loss of graft function has occurred, and positive C-peptide is strongly associated with all primary clinical outcomes.22–24 These results together with our analyses of the T1DAL data indicate that there is a continuous relationship between hypoglycemia rates and residual C-peptide secretion with no clear cut-point or threshold. The current data also suggest that preservation of C-peptide by an immune intervention achieves similar metabolic benefits as better preservation due to slow progression or islet transplantation.
Consistent with the correlation observed between hypoglycemia rates and C-peptide were correlations between measures of glycemic variability, glycemic control, and partial remission with residual β-cell function. Glycemic variability is an important problem in diabetes management in T1D and contributes to glycemic instability, suboptimal management, and episodes of hypoglycemia and ketoacidosis that require hospitalization. This is a particular problem in children and adolescents, in whom excess rates of hospitalization are approximately 5-fold those seen in the nondiabetic population and up to 40% of the excess hospitalization is due to hypoglycemia.4–7 We used the standard deviations (SDs) in the glucometer readings as a surrogate for glycemic variability and found an inverse correlation between SD values and the MMTT-stimulated 4-hour C-peptide AUC in both the alefacept and placebo groups. This was supported by a similar correlation between the highest glucometer readings and the 4-hour C-peptide AUC, and, albeit a weaker correlation, between the lowest glucometer readings and the 4-hour C-peptide AUC. The highest and the lowest glucometer readings in any given patient are a measure of the maximal glucose excursions and hence of glycemic variability. Related to glycemic variability are measures of glycemic control, of which the most widely used is glycated hemoglobin (HbA1c). HbA1c levels were inversely correlated with the 4-hour C-peptide AUC levels in both the alefacept and placebo groups.
Finally, we also explored the relationship between an index of partial remission and C-peptide responses. The IDAA1C combines HbA1c levels with exogenous insulin requirements using a simple formula and based on a threshold value of ≤ 9 has been proposed as a definition for the partial remission period (“honeymoon”) shortly after diagnosis in T1D.30 We explored this index as a surrogate for clinical response and found an inverse correlation between a continuous scale of IDAA1C values and the 4-hour C-peptide AUC in both the alefacept and placebo groups. We also found a very strong positive correlation between IDAA1C values and the SDs of glucometer readings, suggesting there is a relationship between clinical response and glycemic variability. This relationship strengthens the conclusion that preservation of C-peptide secretion improves clinical responses (as measured by an index combining HbA1c and insulin use) and this can be achieved by immunotherapy or by natural variation in disease progression.
Although the results of these analyses add important information to our understanding of the potential clinical benefits of immunomodulatory therapy in new-onset T1D, the findings are limited in several ways. First, this was a post hoc analysis of glycemia data collected from a comparatively small number of participants (n=49) in a proof-of-concept phase 2 clinical trial. Second, the follow-up period in this trial (2 years) was too short to assess longer-term durability of the effect on reduced hypoglycemia and improved glycemic control, or to assess effects on microvascular complications. Third, the results were derived from intervention with a specific immunomodulatory agent, alefacept, and the generalizability of the conclusions to agents with different mechanisms of action is unknown.
In previous immune intervention trials, rates of hypoglycemia have been difficult to quantify because many events are silent and there has not been a standardized approach to recording and collecting longitudinal blood glucose data in intervention trials. In T1DAL this was achieved by providing identical home glucometers to all randomized subjects, downloading glucometer data at every clinic visit, and recording standardized glucometer data. The next step in collecting this information and further enhancing the resolution of the data is continuous glucose monitoring (CGM) using implanted subcutaneous sensors that measure interstitial fluid (ISF) glucose. Although changes in ISF glucose levels lag behind changes in blood glucose,39 CGM has the advantage that it can provide a continuous readout over periods of days or weeks, thereby providing a more complete picture of the absolute number of hypoglycemic events during defined periods in a clinical trial.40 To date, CGM has been evaluated in patients with established T1D and is approved as an adjunct to blood glucose monitoring. Prospective studies are needed to establish the utility of CGM in the setting of investigational immune interventions in new-onset T1D.
Despite modern intensive diabetes management – including the use of insulin pumps, continuous glucose monitoring, sensor-augmented insulin pumps, or closed-loop pump-sensor systems (“artificial pancreas”) – normal or near-normal glycemic control (as measured by glycated hemoglobin (HbA1c) < 5.7%) cannot be achieved.41, 42 Recent developments in artificial pancreas technology have been encouraging, but in children and adolescents mean HbA1c values remain in the 7.6–7.9% range and rates of hypoglycemia are not improved compared to sensor-augmented pump therapy.43, 44 A recent analysis from the T1D Exchange indicated that most youth with T1D do not meet the HbA1c targets recommended by the American Diabetes Association (<7.5% for those between 13–20 years of age),45 and it is unclear whether artificial pancreas technology alone can substantially improve attainment of those targets. Moreover, it is sobering that even when HbA1c is lowered to <6.9%, patients with T1D, including children, still have a 2-fold greater mortality than their nondiabetic peers.3 Thus, it is likely that additional progress in further improvements in glycemic control will require sustained preservation or restoration of β-cell mass, either by immune-intervention or β-cell replacement therapies, or both.
An important consideration in T1D is safety. Alefacept therapy was well tolerated in the T1DAL trial: over the entirety of the study, the proportion of patients who had at least one adverse event (AE) was similar in the alefacept and placebo groups and there were no drug-related serious AEs.29 Four participants in the alefacept group had transient, asymptomatic declines in CD4 counts of <250 cells/μl. There were no deaths, opportunistic infections, or cytokine release syndrome in either group.29 Although the T1DAL trial was too small to detect uncommon AEs, alefacept has been widely used in psoriasis for over a decade with a strong safety record; based on a 2007 review of available safety data, alefacept does not increase susceptibility to infectious disease or malignancy.46 Maintenance of islet preservation over extended periods may require additional courses of treatment. In psoriasis, up to 9 courses of alefacept therapy have been given over a 5-year period with no evidence for increased toxicity with repeated exposure.47 Moreover, recent studies suggest it may be possible to lengthen the interval between doses of biologics in psoriasis48 and this could also be considered during the further development of alefacept for the treatment of T1D. These data suggest that the drug has a profile that would be acceptable for use as an adjunctive therapy in T1D, even in children.
In conclusion, in patients with new-onset T1D, treatment with the immune-modulating drug alefacept resulted in a substantial reduction in rates of major hypoglycemia, which correlated with the degree of C-peptide preservation. Levels of C-peptide secretion were also significantly correlated with measures of glycemic control, glycemic variability, and partial clinical remission. These analyses suggest that preservation of endogenous insulin production by an immunomodulatory drug confers clinical benefits similar to those seen in patients with higher C-peptide secretion due to slow disease progression or islet replacement. These results strengthen the conclusion that successful immune intervention with alefacept, which targets CD2+ memory and effector T cells, significantly enhances C-peptide secretion at 2 years and improves metabolic variables that are known correlates of long-term clinical outcomes.
Acknowledgments
This work was supported in part by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Number UM1AI109565. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. The T1DAL trial was conducted by the Immune Tolerance Network (ITN) and sponsored by NIAID under Award Numbers NO1-AI-15416 and UM1AI109565. Analyses were performed by Rho, the statistical and clinical coordinating center for the Immune Tolerance Network trials (HHSN272200800029C) and the NIH/NIAID/DAIT (1UM2AI117870). Additional funding was provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Astellas Pharma Global Development (Northbrook, IL, USA) provided alefacept (Amevive®) and gave input regarding dosage and safety, but had no direct involvement with study design, conduct, or management; data collection, analysis or interpretation; or manuscript preparation. The authors provided Astellas a copy of the original manuscript prior to submission. Bayer HealthCare LLC, Diabetes Care (Whippany, New Jersey, USA) provided blood glucose monitoring supplies through an Investigator Sponsored Research Grant. The authors provided Bayer a copy of the original manuscript prior to submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chiang JL, Kirkman MS, Laffel LM, Peters AL Type 1 Diabetes Sourcebook A. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37:2034–2054. doi: 10.2337/dc14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 4.Palta M, LeCaire T, Daniels K, Shen G, Allen C, D’Alessio D. Risk factors for hospitalization in a cohort with type 1 diabetes. Wisconsin Diabetes Registry. Am J Epidemiol. 1997;146:627–636. doi: 10.1093/oxfordjournals.aje.a009328. [DOI] [PubMed] [Google Scholar]
- 5.Icks A, Rosenbauer J, Haastert B, Giani G. Hospitalization among diabetic children and adolescents and non-diabetic control subjects: a prospective population-based study. Diabetologia. 2001;44(Suppl 3):B87–92. doi: 10.1007/pl00002960. [DOI] [PubMed] [Google Scholar]
- 6.Estrada CL, Danielson KK, Drum ML, Lipton RB. Hospitalization subsequent to diagnosis in young patients with diabetes in Chicago, Illinois. Pediatrics. 2009;124:926–934. doi: 10.1542/peds.2008-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayers A, Thayer D, Harvey JN, et al. Evidence for a persistent, major excess in all cause admissions to hospital in children with type-1 diabetes: results from a large Welsh national matched community cohort study. BMJ Open. 2015;5:e005644. doi: 10.1136/bmjopen-2014-005644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asvold BO, Sand T, Hestad K, Bjorgaas MR. Cognitive function in type 1 diabetic adults with early exposure to severe hypoglycemia: a 16-year follow-up study. Diabetes Care. 2010;33:1945–1947. doi: 10.2337/dc10-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorgaas MR. Cerebral effects of severe hypoglycemia in young people with type 1 diabetes. Pediatr Diabetes. 2012;13:100–107. doi: 10.1111/j.1399-5448.2011.00803.x. [DOI] [PubMed] [Google Scholar]
- 10.Mauras N, Mazaika P, Buckingham B, et al. Longitudinal assessment of neuroanatomical and cognitive differences in young children with type 1 diabetes: association with hyperglycemia. Diabetes. 2015;64:1770–1779. doi: 10.2337/db14-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin A, Northam EA, Werther GA, Cameron FJ. Risk factors for decline in IQ in youth with type 1 diabetes over the 12 years from diagnosis/illness onset. Diabetes Care. 2015;38:236–242. doi: 10.2337/dc14-1385. [DOI] [PubMed] [Google Scholar]
- 12.Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Characterizing sudden death and dead-in-bed syndrome in Type 1 diabetes: analysis from two childhood-onset Type 1 diabetes registries. Diabet Med. 2011;28:293–300. doi: 10.1111/j.1464-5491.2010.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark AL, Best CJ, Fisher SJ. Even silent hypoglycemia induces cardiac arrhythmias. Diabetes. 2014;63:1457–1459. doi: 10.2337/db14-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steineck I, Cederholm J, Eliasson B, et al. Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18,168 people with type 1 diabetes: observational study. BMJ. 2015;350:h3234. doi: 10.1136/bmj.h3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Origin Trial Investigators. Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur Heart J. 2013;34:3137–3144. doi: 10.1093/eurheartj/eht332. [DOI] [PubMed] [Google Scholar]
- 16.Lung TW, Petrie D, Herman WH, et al. Severe hypoglycemia and mortality after cardiovascular events for type 1 diabetic patients in Sweden. Diabetes Care. 2014;37:2974–2981. doi: 10.2337/dc14-0405. [DOI] [PubMed] [Google Scholar]
- 17.Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 18.Lachin JM, McGee P, Palmer JP DCCT/EDIC Research Group. Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes. 2014;63:739–748. doi: 10.2337/db13-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhtreiber WM, Washer SL, Hsu E, et al. Low levels of C-peptide have clinical significance for established type 1 diabetes. Diabet Med. 2015;32:1346–1353. doi: 10.1111/dme.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 21.Wherrett DK, Chiang JL, Delamater AM, et al. Defining pathways for development of disease-modifying therapies in children with type 1 diabetes: a consensus report. Diabetes Care. 2015;38:1975–1985. doi: 10.2337/dc15-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care. 2012;35:1436–1445. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vantyghem MC, Raverdy V, Balavoine AS, et al. Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (beta-score greater than 7) Is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (beta-score greater than 3) J Clin Endocrinol Metab. 2012;97:E2078–2083. doi: 10.1210/jc.2012-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lablanche S, Borot S, Wojtusciszyn A, et al. Five-year metabolic, functional, and safety results of patients with type 1 diabetes transplanted with allogenic islets within the Swiss-French GRAGIL Network. Diabetes Care. 2015;38:1714–1722. doi: 10.2337/dc15-0094. [DOI] [PubMed] [Google Scholar]
- 25.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herold KC, Gitelman SE, Ehlers MR, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766–3774. doi: 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rigby MR, DiMeglio LA, Rendell MS, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:284–294. doi: 10.1016/S2213-8587(13)70111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigby MR, Harris KM, Pinckney A, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015;125:3285–3296. doi: 10.1172/JCI81722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortensen HB, Hougaard P, Swift P, et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. 2009;32:1384–1390. doi: 10.2337/dc08-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehlers MR. Immune interventions to preserve β cell function in type 1 diabetes. Journal of Investigative Medicine. 2016;64:7–13. doi: 10.1097/JIM.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis AK, DuBose SN, Haller MJ, et al. Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care. 2015;38:476–481. doi: 10.2337/dc14-1952. [DOI] [PubMed] [Google Scholar]
- 33.Dai X, Zhan J, Demmy TA, et al. Monocytes play different roles in stimulating T cells in obese diabetic individuals. Int J Immunopathol Pharmacol. 2015;28:374–383. doi: 10.1177/0394632015598848. [DOI] [PubMed] [Google Scholar]
- 34.Schultz O, Oberhauser F, Saech J, et al. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One. 2010;5:e14328. doi: 10.1371/journal.pone.0014328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koulmanda M, Bhasin M, Awdeh Z, et al. The role of TNF-alpha in mice with type 1- and 2- diabetes. PLoS One. 2012;7:e33254. doi: 10.1371/journal.pone.0033254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bianchi E, Ripandelli G, Taurone S, et al. Age and diabetes related changes of the retinal capillaries: An ultrastructural and immunohistochemical study. Int J Immunopathol Pharmacol. 2016;29:40–53. doi: 10.1177/0394632015615592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis CN, Krueger GG Alefacept Clinical Study Group. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med. 2001;345:248–255. doi: 10.1056/NEJM200107263450403. [DOI] [PubMed] [Google Scholar]
- 38.Sorensen JS, Johannesen J, Pociot F, et al. Residual beta-cell function 3–6 years after onset of type 1 diabetes reduces risk of severe hypoglycemia in children and adolescents. Diabetes Care. 2013;36:3454–3459. doi: 10.2337/dc13-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freckmann G, Hagenlocher S, Baumstark A, et al. Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J Diabetes Sci Technol. 2007;1:695–703. doi: 10.1177/193229680700100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xing D, Kollman C, Beck RW, et al. Optimal sampling intervals to assess long-term glycemic control using continuous glucose monitoring. Diabetes Technol Ther. 2011;13:351–358. doi: 10.1089/dia.2010.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein BE, Klein R. Further insight on the limits of success of glycemic control in type 1 diabetes. Diabetes. 2015;64:341–343. doi: 10.2337/db14-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farrington C. The artificial pancreas: challenges and opportunities. Lancet Diabetes Endocrinol. 2015;3:937. doi: 10.1016/S2213-8587(15)00430-1. [DOI] [PubMed] [Google Scholar]
- 43.Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373:2129–2140. doi: 10.1056/NEJMoa1509351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tauschmann M, Allen JM, Wilinska ME, et al. Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2016 doi: 10.2337/dc15-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood JR, Miller KM, Maahs DM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36:2035–2037. doi: 10.2337/dc12-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheinfeld N. Alefacept: its safety profile, off-label uses, and potential as part of combination therapies for psoriasis. J Dermatolog Treat. 2007;18:197–208. doi: 10.1080/09546630701247955. [DOI] [PubMed] [Google Scholar]
- 47.Goffe B, Papp K, Gratton D, et al. An integrated analysis of thirteen trials summarizing the long-term safety of alefacept in psoriasis patients who have received up to nine courses of therapy. Clin Ther. 2005;27:1912–1921. doi: 10.1016/j.clinthera.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Lorenzin M, Ortolan A, de Hooge M, et al. Lengthening the time intervals between doses of biological agents in psoriatic arthritis patients: A single-center retrospective study. Int J Immunopathol Pharmacol. 2015;28:479–487. doi: 10.1177/0394632015599446. [DOI] [PubMed] [Google Scholar]