Abstract
Introduction and hypothesis
We present a technique for quantifying inter-individual variability in normal vaginal shape, axis, and dimension, and report findings in healthy women.
Methods
Eighty women (age: 28~70 years) with normal pelvic organ support underwent supine, multi-planar proton-density MRI. Vaginal width was assessed at five evenly-spaced locations, and vaginal axis, length, and surface area were quantified via ImageJ and MATLAB.
Results
The mid-sagittal plane angles, relative to the horizontal, of three vaginal axes were 90± 11, 72± 21, and 41± 22° (caudal to cranial, p < 0.001). The mean (± SD) vaginal widths were 17± 5, 24± 4, 30± 7, 41± 9, and 45± 12 mm at the five locations (caudal to cranial, p < 0.001). Mid-sagittal lengths for anterior and posterior vaginal walls were 63± 9 and 98 ± 18 mm respectively. The vaginal surface area was 72 ± 21 cm2 (range: 34 ~ 164 cm2). The coefficient of determination between any demographic variable and any vaginal dimension did not exceed 0.16.
Conclusions
Large variations in normal vaginal shape, axis, and dimensions were not explained by body size or other demographic variables. This variation has implications for reconstructive surgery, intravaginal and surgical product design, and vaginal drug delivery.
Keywords: Vagina, Vaginal axis, Dimension, Shape, Magnetic resonance imaging, Anatomy
Introduction
The goal of reconstructive surgery is to restore normal anatomy and function. However, there is a surprising dearth of quantitative data on normative vaginal size, shape, and geometry and their variations compared with data available for other parts of the body. These factors may affect the conduct and goals of pelvic floor reconstructive surgeries, design of surgical and intravaginal devices, and the delivery of intravaginal medications. It would therefore be helpful to know how much vaginal shape and/or dimensions vary and when they differ from normal.
Recently, magnetic resonance (MR) imaging has been used to study vaginal dimensions in healthy women using a noncontact measurement method. For example, Barnhart et al. used MR images to measure vaginal length and width in 28 women ranging from 18 to 39 years of age [1]. A second study demonstrates how MR images can be used non-invasively to analyze the structures comprising the female pelvic floor system in a way that allows its configuration relative to the bony pelvis to be established [2]. However, quantitative inter-individual differences in vaginal morphology remain poorly understood in women with different sizes and body types.
This study was therefore undertaken to address these knowledge gaps. We conducted a secondary analysis of MR image data gathered from a larger case–control study of pelvic organ prolapse, and developed a new technique for quantifying the vaginal shape and dimension. We used it to test the hypotheses that vaginal shape and dimension cannot be explained by any single demographic characteristic, and that the degree of variation will not be uniform along the vaginal length. The results of this study could help to enrich therapeutic targets for pelvic floor repair operations and improve the design of products related to drug and contraceptive delivery, and that of pessaries and other intravaginal devices.
Materials and methods
Magnetic resonance images of 80 women with normal pelvic organ support (controls) were sequentially selected from the most recent scans of a University of Michigan institutional review board-approved (IRB # 1999–0395) case–control study of pelvic organ prolapse. Women in the control group, defined as healthy women in this study, were recruited by a newspaper and radio advertisement for healthy volunteers and had to be asymptomatic for prolapse and have values for each of the POP-Q points that fall within the normal range, as determined by population-based studies of asymptomatic women [3]. None of the subjects had previously undergone hysterectomy or pelvic floor surgery.
As described in our previous work [4], each subject underwent supine multi-planar, two-dimensional, fast spin, proton density-weighted MR imaging both at rest and during maximal Valsalva using a 3 T superconducting magnet (Philips Medical Systems, Bothell, WA, USA) with version 2.5.1.0 software. At rest, 30 images were serially obtained in the axial, sagittal, and coronal planes using a 20 × 20 cm2 field of view, 4-mm slice thickness, and a 1-mm gap between slices.
Image analysis software (ImageJ v1.44) was used to visualize vaginal and cervical landmarks and contours on both axial and mid-sagittal scans and record vaginal topography. We first put markers on the MR images, with the locations of markers being then reviewed by the senior author.
The vaginal width was measured on axial scans (Fig. 1a–c). On the axial scan, and moving in a distal to proximal direction, we traced the vaginal wall on each scan, identifying the transition from the labia in the sagittal plane to the vagina lying in the frontal plane; we ended the tracing when we could no longer visualize either the anterior vaginal wall (AVW) or the posterior vaginal wall (PVW). The AVW and PVW were first traced together by considering that they were coapted; once they were at least 1 cm separate (on the axial scan), they were traced separately. This process was made easier by some of the women having had ultrasound gel inserted into the vagina. When viewing the MR images of the candidates, women with a large amount of gel inside (i.e., fully spread in the upper vagina), which may have distorted the vagina, were excluded from this study.
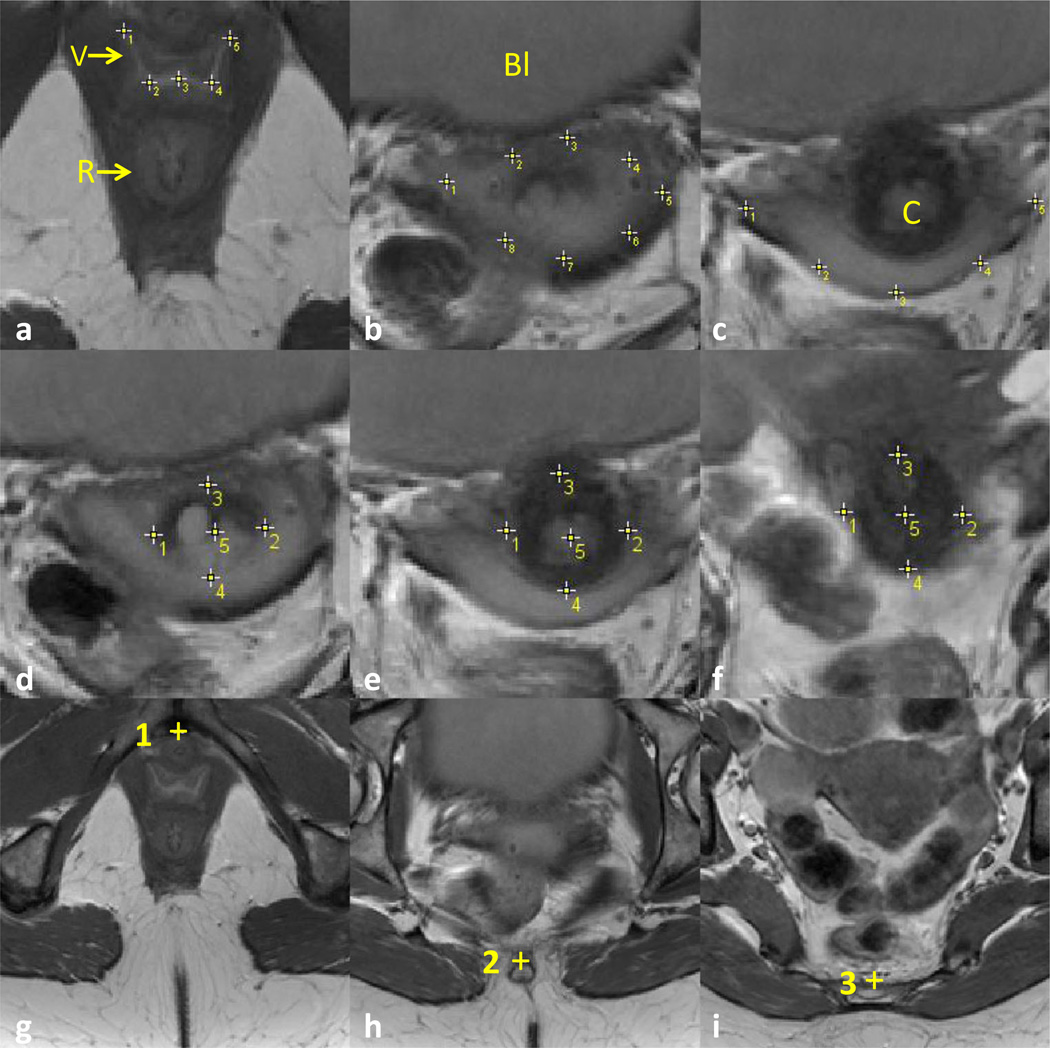
Fig. 1.
Axial measurements. a–c Vaginal axial measurement. Selected slices show typical vaginal axial measurements. Points 1 to 5 are used to identify the coapted anterior and posterior vaginal wall in a. Points 1 to 8 are used to identify the anterior vaginal wall (1 to 5) and posterior vaginal wall (1, 8, 7, 6, and 5) in b, with the largest separation between the anterior and posterior vaginal walls being at least 1 cm. When only the posterior vaginal wall was visible in c, points 1 to 5 were only used to identify the posterior vaginal wall. 1 is always the lateral-most point on the right and 5 is always the lateralmost point on the left. d–f Cervix measurement. Five points on axial slices were used to identify the cervix location. Points 1 and 2 were placed on the lateralmost margins on the right and left respectively. Likewise, points 3 and 4 were used to mark the anterior- and posteriormost margins. Point 5 marks the center of the cervix, with the first point 5 in d marking the location of the cervical os. g–i Bony pelvis mid-plane. The mid-sagittal plane of the pelvis was identified using three points placed on specific landmarks on different axial slices: point 1 at the center of pubic symphysis, point 2 at the center of the distal sacrum, and 3 on the mid-sacrum. V vagina, R rectum, BI bladder (DeLancey©)
The cervix was also traced on axial slices (Fig. 1d–f) to identify the cervical width and the location of the cervical os. To identify the symmetry of the vaginal wall and cervix, a mid-sagittal reference plane was passed through the bony pelvis using three points (Fig. 1g–i): one point at the middle of the pubic symphysis and two points centered on the distal and middle regions of the sacrum. To compare the regional differences in vaginal width among different women, the vaginal width measurement result was resampled at five evenly-spaced locations from the hymen to the most cranial image in which the vaginal lumen is visible (Fig. 2).
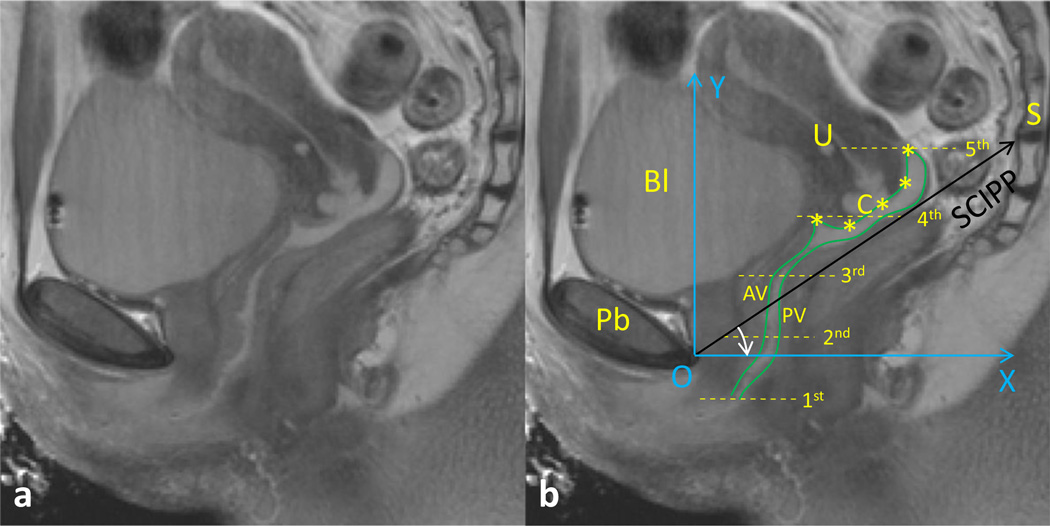
Fig. 2.
Vaginal analysis method. a and b mid-sagittal MR image seen from left without and with labels. b Vaginal width normalization and sagittal analysis system. To compare the transverse vaginal width at different locations along the vaginal canal among individuals, the width was resampled at five locations: from the level of the hymen to the top of the vagina. The anterior vaginal wall and posterior vaginal wall are identified with green lines (in reality, sampling points were placed along the lines using ImageJ to obtain the coordinates); the cervix was identified from the anterior fornix to the posterior fornix using five points, with the third point placed at the cervical os. To compare vaginal wall size and shape across subjects, a local coordinate system (XOY, in blue) was created to quantify the morphology. Axis OX was created by rotating the sacrococcygeal–inferior pubic point (SCIPP) line by 34° clockwise. Pb pubic bone, U uterus, C cervix, AV anterior vagina, PV posterior vagina, BI bladder, S sacrum (DeLancey©). The identified five points of cervix were represented by asterisks
Vaginal wall length and shape were measured in the mid-sagittal plane (Fig. 2). First in this plane, the AVW, PVW, and cervix were each identified. Next, the AVW was then traced from the introitus to the anterior fornix and the PVW from the introitus to the posterior fornix. Five points were then used to identify the cervix location and diameter, with the first point at the anterior fornix, the third point at the cervical os or external os, and the fifth point at the posterior fornix. The anterior fornix length was measured using a curved line through points 1, 2, and 3, and the posterior fornix length was similarly measured using points 3, 4 and 5.
To analyze the variation in vaginal wall shape relative to the pelvic bones among different women, a local coordinate system was created using bony landmarks (XOY, in blue), using the Pelvic Inclination Correction System (PICS) [5]. This system was set up by rotating the OX axis 34° clockwise from the sacrococcygeal-inferior pubic point (SCIPP) line. PICS produces an axis system aligned to the body axis (y axis) along which gravity acts, thereby providing the same coordinate system in which to compare each subject.
We also analyzed the vaginal axis angle in a left view of the mid-sagittal plane based on the PICS coordinates. After obtaining all 80 middle sagittal vaginal profiles, we viewed the profiles to find the common patterns of the vaginal axis. To measure the vaginal axis, the anterior vaginal wall between the introitus and the cervix was then divided into two regions based on the characteristics of these profiles: the distal half of the AVW length (lower vaginal region), and the proximal half of the AVW length (middle vaginal region). Each of the three regions was then fitted with a straight line. The cervical portion axis (upper vaginal region) was analyzed by connecting the anterior and posterior fornices. The angle of each axis from the horizontal PICS line was then calculated.
To measure the vaginal wall surface area, we reconstructed the AVW and PVW based on the coordinates of points traced in each axial plane (Fig. 1), fitting separate curves to the AVW and PVW. For the axial plane we next resampled each curve into ten equal regions. The AVW and PVW surfaces were then constructed by assembling separate small triangles from three points on adjacent curves for each surface. The surface area was then calculated by summing the areas of the small triangles.
A numerical computation program (MATLAB™) was used for data analysis, including transforming MRI coordinates into local coordinates and analyzing vaginal size, shape, axes orientations and surface area. Vaginal width was measured both as a frontal plane diameter, but also along the curve of the AVW in addition to the PVW in selected transverse plane sections. These were then defined as the “vaginal diameter” and the AVW or PVW as the “curved width”, with the sum of the last two constituting the “vaginal perimeter.”
Pearson’s correlation was calculated between each vaginal dimension and the main demographic variables such as height, age, weight, BMI, and parity. Two-sided independent t tests and one-way ANOVA tests were used with a significance level at p < 0.05.
Results
For the subjects’ characteristics, median parity was 2, mean ± standard deviation (SD) age was 45.5 ± 8.7, height was 163.3 ± 7.9 cm, weight was 73.4 ± 15.4 kg, BMI was 27.6 ± 5.5 kg/m2, and race was 91 % Caucasian. Mean ± SD POP-Q values were: Aa, −1.4 ± 1.0; Ba, −1.4 ± 1.0; C, −6.7 ±1.2; D, −9.3 ± 1.3; Ap, −1.6 ± 0.9; Bp, −1.5 ± 1.0. No subjects in this group had undergone a hysterectomy. Among the 80 subjects, only 2 were above 60 years of age; 1 was 67 years and another was 70 years. There were 4 nulliparas among the 80 women.
Measures of the distribution in vaginal dimensions are provided in Table 1, while descriptive statistics for vaginal width and symmetry are shown in Fig. 3.
Table 1.
Vaginal dimensions
| Parameters | Mean | SD | Minimum | Percentiles | Maximum | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | |||||
| AVW length | 63 | 9 | 44 | 48 | 56 | 63 | 69 | 77 | 84 |
| PVW length | 98 | 18 | 51 | 71 | 86 | 97 | 109 | 131 | 144 |
| Anterior fornix length | 18 | 7 | 8 | 9 | 13 | 17 | 22 | 31 | 35 |
| Posterior fornix length | 21 | 5 | 11 | 13 | 17 | 21 | 25 | 31 | 34 |
| Cervix curved length | 40 | 10 | 21 | 25 | 32 | 40 | 45 | 58 | 60 |
| Cervix transverse width | 25 | 5 | 18 | 19 | 22 | 24 | 27 | 35 | 43 |
| V 1st diameter | 17 | 5 | 9 | 11 | 13 | 16 | 19 | 27 | 31 |
| V 2nd diameter | 24 | 4 | 16 | 18 | 21 | 23 | 26 | 31 | 33 |
| V 3rd diameter | 30 | 7 | 15 | 21 | 26 | 29 | 34 | 43 | 56 |
| V 4th diameter | 41 | 9 | 17 | 28 | 35 | 40 | 47 | 58 | 69 |
| V 5th diameter | 45 | 12 | 24 | 29 | 35 | 43 | 52 | 64 | 81 |
| V 1st perimeter | 38 | 12 | 18 | 23 | 28 | 36 | 46 | 57 | 94 |
| V 2nd perimeter | 64 | 11 | 42 | 49 | 56 | 62 | 70 | 80 | 118 |
| V 3rd perimeter | 71 | 15 | 45 | 51 | 63 | 68 | 78 | 98 | 124 |
| V 4th perimeter | 93 | 25 | 55 | 59 | 75 | 88 | 109 | 137 | 209 |
| V 5th perimeter | 101 | 28 | 51 | 63 | 76 | 95 | 121 | 162 | 187 |
| V lower axis (°) | 90 | 11 | 60 | 73 | 82 | 89 | 99 | 107 | 121 |
| V middle axis (°) | 72 | 21 | 40 | 45 | 55 | 67 | 86 | 114 | 126 |
| V upper axis (°) | 41 | 22 | −26 | 10 | 28 | 41 | 56 | 76 | 88 |
| AVW surface area (cm2) | 35 | 10 | 17 | 20 | 30 | 35 | 40 | 48 | 82 |
| PVW surface area (cm2) | 37 | 11 | 17 | 22 | 30 | 35 | 42 | 57 | 88 |
| V surface area (cm2) | 72 | 21 | 34 | 43 | 60 | 70 | 81 | 106 | 164 |
Unless otherwise specified, all dimensions are in millimeters
V vagina, AVW anterior vaginal wall, PVW posterior vaginal wall
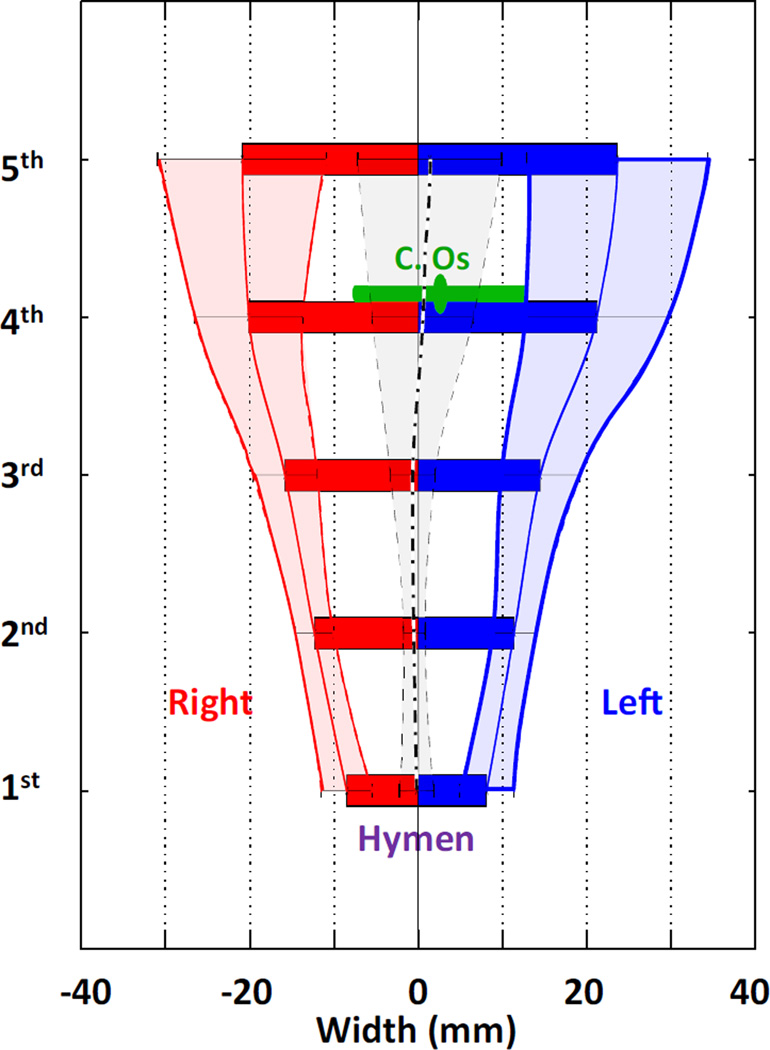
Fig. 3.
Vaginal axial dimension and symmetry analysis. Vaginal width (diameter) and symmetry information are shown in a bar plot at five equidistant locations. The first location denotes the vaginal width at the hymen, whereas the fifth location denotes the proximalmost vagina, with the other three locations being equally spaced between them. Vaginal width mean (middle solid line of dashed area) and standard deviation (curved outlines of dashed area) on both the left- and the right-hand sides of the bony middle plane are also shown. Zero on the x axis denotes the pelvic mid-line. The black lines show the average location (thick dash) and standard deviation (light dash) of the mid-vaginal point. Average location and standard deviation of the cervical os are shown in green (DeLancey©)
The most variable dimension was found to be vaginal width, for which the coefficient of variation reached nearly 30 % at the cranial end of the vagina. More specifically, the mean (± SD) vaginal widths at the five different levels in the frontal plane were, from cranial to caudal, 45 ± 12, 41 ± 9, 30 ± 7, 24 ± 4, and 17 ± 5 mm. These mean vaginal widths differed significantly from each other (p < 0.001). On average, the vagina was essentially bilaterally symmetrical relative to the mid-sagittal plane of the pelvis, but again more variation was found cranially than caudally.
The average location of the cervical os was at 83 % of the vaginal length and about 2 mm to the left of the mid-sagittal plane of the bony pelvis (47 cases on the left and 33 cases on right). The absolute value of the location of the cervical os was 8 ± 6 mm lateral to the mid-sagittal plane of the bony pelvis.
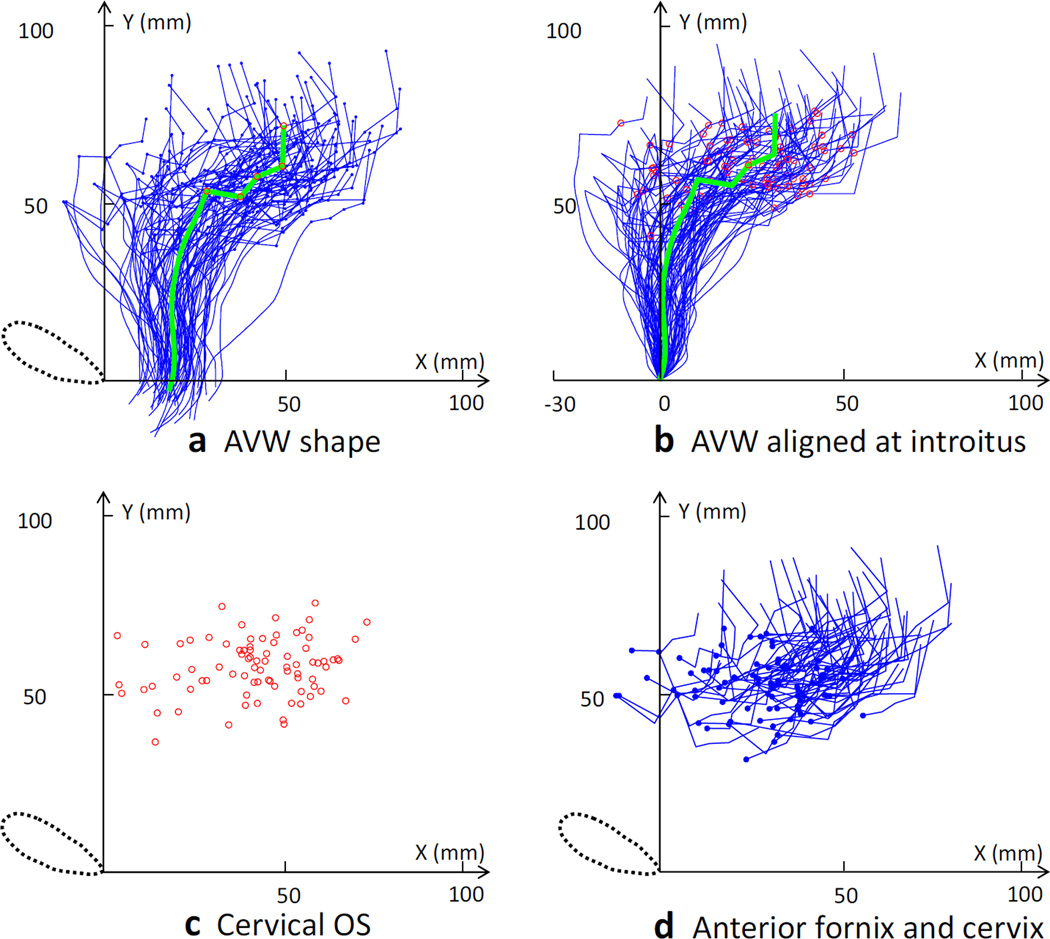
The results of the vaginal wall shape analysis based on the PICS coordinate system are shown in Fig. 4. Again, there was more variation in vaginal length and shape cranially than caudally. For example, the AVW length was 63 ± 9 mm (range: 44~84 mm), the cervix was 40 ± 10 mm (range: 21~60 mm) and the PVW (not shown) was 98 ± 18 mm (range: 51~144 mm). The average shape looks much like the sagittal profile of a curved spoon. The AVW portion can be viewed as two straight lines and the cervix portion as a flattened “W” shape. The average location of the introitus in terms of the x, y coordinates was 17 mm, −3 mm in the local coordinate system, or about 17 mm from the local origin at the pubic symphysis. The x, y coordinates (in millimeters) for the average location of the cervix relative to the inferior pubic point were as follows: anterior fornix (27, 53), anterior lip (37, 51), cervical os (42, 57), posterior lip (49, 60), and posterior fornix (49, 71). When the distal end of each vagina was aligned at the introitus (Fig. 4b), we found that there was less variation caudally than cranially. The location of the cervix in the PICS system varied more in the anterior–posterior direction than it did in the inferior–superior direction.
Fig. 4.
Mid-sagittal analysis of vaginal and cervix geometry. a Mid-sagittal shape and mean shape of the anterior vaginal wall and cervix are shown in terms of the local Pelvic Inclination Correction System (PICS) coordinates. Blue lines show results for the 80 subjects, with the small blue dots showing the location of the cervix. The green line shows the average shape with large red dots showing the location of the cervix. b Data for the vaginal shape when aligned at the introitus. Mid-sagittal shape and mean shape for the anterior vaginal wall and cervix are shown aligned at the average location of the introitus, with the blue lines representing data from the 80 subjects and the green line being the average shape, with red dots showing the location of the cervical os location. c The locations of the cervical os in 80 subjects are shown in terms of the local PICS coordinates. d Anterior fornix locations (blue dot) are shown with the cervix lines of the 80 subjects in terms of the local PICS coordinates (DeLancey©)
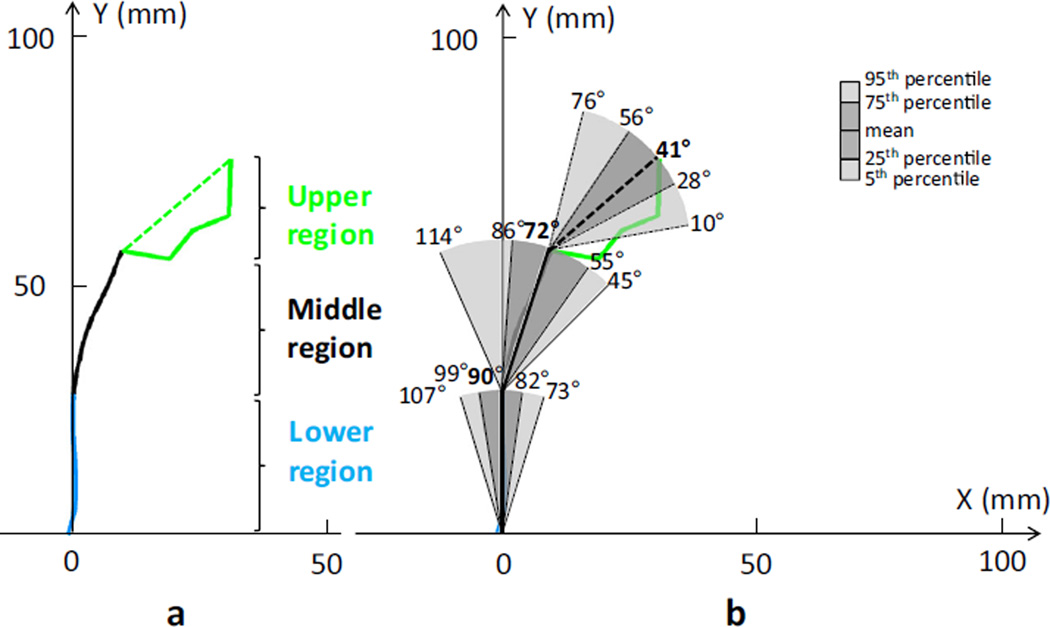
Three major vaginal axes were discernible along the vagina subtending, from caudal to cranial, angles of 90± 11° (range: 60~121), 72 ± 21° (range: 40~126), and 41 ± 22° (range: −26~88; Fig. 5), these being significantly different from one another (p < 0.001). The angle between the upper portion axis of the vagina surrounding the cervix and the middle region was 149 ± 34°, between the upper region axis and the lower region it was 132 ± 25°, and between the middle and the lower region axis it was 163 ± 22°.
Fig. 5.
Vaginal axis results. a Mean mid-sagittal shapes of the anterior vaginal wall and cervix are shown in terms of the local PICS coordinates. Three contiguous vaginal axes were defined as follows: the lower region (blue), the middle region (black), and the upper region with a line showing the cervical axis (green dashed line) connecting the anterior and posterior margins. b Variation in vaginal axis orientations is shown by the 5th, 25th, mean, 75th, and 95th percentile axis angles (DeLancey©)
The surface area of the vaginal walls was 72 ± 21 cm2 (range: 34~164 cm2). It varied markedly among the subjects (see Supplemental Material to view a video of the vaginal wall surface view of each subject). Some 75% of the subjects had a surface area of 60 cm2 or above, and 25 % of the subjects had a surface area of 81 cm2 or above. A surface area of 106 cm2 or above lay within the upper 5th percentile, whereas 43 cm2 or less lay within the lower 5th percentile. The smallest and largest surface areas were both from multiparous subjects. The surface area of 4 nulliparous subjects was 54± 11 cm2 (range: 44~65 cm2) compared with 73 ± 21 cm2 for parous women (p = 0.08).
Correlation coefficients were determined for each vaginal dimension and the following demographic parameters: height, age, weight, BMI, and parity (Appendix). None of the correlation values between dimension data and demographic parameters exceeded 0.4. Height was only significantly correlated with anterior fornix length, cervix curved length, and VW 5th diameter, but had a positive correlation value less than 0.3. Age exhibited a larger correlation (range: −0.4~0.4) than height, weight, BMI or parity. Age was negatively correlated with vaginal length and vaginal lower axis angle, but was positively correlated with vaginal width. Weight was only significantly positively correlated with the curved length of the cervix, VW 1st and 2nd perimeters, and the angle of the upper axis, and had a correlation coefficient less than 0.3. Likewise, BMI correlated with the angle of the vaginal middle and upper axes (correlation coefficient less than 0.3) and parity had no significant correlation.
Discussion
Large variations in vaginal dimension and shape were found in these 80 healthy women. For example, the minimum and maximum values for vaginal length and surface area exhibited 3- and 5-fold differences respectively. The standard deviations for the upper vaginal width and upper vaginal axis angle varied 2-fold from the lower vaginal width and lower vaginal axis angle. However, it is notable that no single demographic characteristic could explain more than 16 % of the variation in vaginal dimensions. There was more variation in vaginal dimensions and shape cranially than caudally. A larger sample might make these differences statistically significant, yet the fact would remain that only a small percentage of the variation in vaginal shape and size could be predicted by demographic characteristics. These findings define the normal ranges for a variety of vaginal characteristics against which treatments can be assessed. In addition, they provide the ranges of expected dimensions that need to be considered in treatment or device planning.
Let us first examine our results from a perspective of the functional anatomy of the pelvic floor. The upper vagina was wider than the lower vagina and exhibited a larger variation in transverse dimensions and sagittal distribution. Although this is generally known, the magnitude of these differences has to our knowledge not previously been quantified. These differences reflect the different natures of the vagina at these levels [6, 7]. The lower vaginal walls are spatially constrained by the levator ani muscles, perineal membrane and pubic bones, thereby reducing the distal spatial variability in the “high pressure” zone [8]. That is because in this region the vagina is surrounded by the “U-shaped” levator ani at the back and sides of the vagina, whose baseline tone closes the hiatus and supports the pelvic floor organs [9]. More proximally, the vagina is suspended by the level I apical supports, including the cardinal ligament and uterosacral ligaments [6, 10, 11]. These relatively long connections, with their curved trajectories like slack ropes, allow considerable variability in the location of the vagina [10–12]. In this way, the upper vagina and uterus tend to move relatively less encumbered by spatial constraints, thereby resulting in a much larger variation in spatial shape and location [13].
Our findings can be viewed within the context of earlier reports. When reporting the vaginal wall length, gynecology textbooks usually report single values for AVW length as 7 or 7.5 cm and PVW length as 9 cm [14, 15]. Although our average AVW (63 ± 9 mm, range: 44~84 mm) and PVW (98 ± 18 mm, range: 51~144 mm) length values are close to the textbook values, they also demonstrate a remarkably large variability across subjects, which is consistent with non-MRI based vaginal length assessment during physical examination [16]. Our results also corroborate those of Barnhart et al. [1], who found that vaginal width was largest in the cranial portion of the vagina, and decreased as it passed through the pelvic diaphragm, to be smallest at the introitus.
The vaginal axis has been considered an important parameter for pelvic organ support; restoring vaginal depth and axis has been deemed an important goal of surgical management [17]. Funt’s study of vaginograms in supine women revealed a 130° angle between the “upper and lower” vagina in a sample of 20 primarily nulliparous young women [18]. Our study extends these observations in a larger sample and adds further detail concerning the axis relative to the horizontal not addressed in the earlier study. We found that there were three major vaginal axes with angles of 90 ± 11° for the lower vagina, 72 ± 21° for the middle vagina, and 41 ± 22° for the upper vagina (cervical portion) with an angle between the upper and middle vagina of 149°. These quantitative results may help in considering suspension surgeries by allowing the normal axis and angles to be known, so that, as imaging postoperative patients comes into use, there will be normal values for reference. In this, however, the remarkable variation in normal must not be overlooked.
Vaginal dimensions are a primary determinant of vaginal surface area, which is an important factor in designing intravaginal and surgical products, and in vaginal drug delivery such as antimicrobials, spermicidal agents, and hormonal medications because it affects the area available for the distribution of and exposure to the drug [19]. The five-fold variation seen in the vaginal surface area (34~164 cm2) in the present study was nearly three times larger than that found in an earlier study (66~107 cm2) [19]. We believe that this is due to the larger age range in our sample because it included post-menopausal women as well. This variability has important implications for intravaginal drug delivery.
Although we have shown that there is a large variation in vaginal dimensions and shape, we still found only weak correlations between the vaginal dimension and demographic characteristics. This is consistent with other physical examination-based vaginal length quantifications [16] and may be informative in considering the role of stature in the design of vaginal products. It was true that body size (such as height) and age correlated better with vaginal dimension than weight, BMI, and parity, but body size explained less than 9 % (r2 of 0.3 = 0.09) and age explained less than 16 % of the variation in any vaginal parameter. Thus, vaginal dimensions cannot reliably be estimated from height.
There are many interesting questions to ask using the methods developed in this paper. For example, one might determine the relative contribution of factors such as nulliparity versus multiparity, sexual activity, marriage status, delivery mode, gestational status, race difference, and menopausal status to vaginal dimensions and shape, but those analyses would be most properly conducted in a population-based study.
Several factors should be considered when interpreting the present results. Our study has a moderate sample size with women selected as having normal support. The MR images were obtained in the supine position, which may systematically affect vaginal shape with regard to the standing posture. As we stated in Materials and methods, the MR images have 5-mm intervals between slices, and this may generate partial volume errors. To help minimize measurement errors, we chose to measure the vaginal width on the axial scans, and to measure the vaginal length on the sagittal scans. At the current resolution, this technique is not able to evaluate the effect of rugal folds on the vaginal surface area; thus, estimates from this paper should be interpreted with this limitation in mind. Finally, it is a limitation that we used gel in the vagina to help with visualization when in some normal women, it filled the upper vagina to such an extent that it slightly changed the contour. However, we tried to counter this by spreading the ultrasound gel evenly inside the vagina.
This study is a first step toward analyzing vaginal size, shape, and axis in healthy women. It quantifies vaginal dimensions based on axial and sagittal 2-D images using 3-D coordinates provided by a common coordinate system. The results were normalized to compare different subjects. In the future, one might study the vaginal shape in a 3-D space and divide the results statistically into characteristic shapes to better understand the role of vaginal morphology as it relates to women’s health.
Supplementary Material
Acknowledgments
We gratefully acknowledge support from the National Institute of Child Health and Human Development Grants R01 HD 038665, the Office for Research on Women’s Health SCOR on Sex and Gender Factors Affecting Women’s Health P50 HD 044406, the National Center for Advancing Translational Sciences 2UL1TR000433, and a Grant from the Swiss National Science Foundation.
Dr. Jiajia Luo doctoral studies were partially funded by American Medical Systems and Kimberly-Clark Corporation, and he has also received research support from Boston Scientific Corporation. The University of Michigan received research funding for partial salary support from Johnson & Johnson, American Medical Systems, Kimberly-Clark Corporation, Procter & Gamble, and Boston Scientific Corporation. They received an honorarium and travel reimbursement for giving an invited research seminar at Johnson & Johnson. Dr. Cornelia Betschart received research support from the Swiss National Science Foundation unrelated to the topic of this paper.
Appendix
Table 2.
Pearson’s correlations between vaginal dimensions and demographic variables along with p values
| Parameters | Height | Age | Weight | BMI | Parity | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Correlation | p | Correlation | p | Correlation | p | Correlation | p | Correlation | p | |
| AVW length | 0.112 | 0.322 | −0.165 | 0.144 | −0.118 | 0.297 | −0.160 | 0.156 | −0.012 | 0.918 |
| PVW length | 0.205 | 0.068 | −0.305** | 0.006 | 0.084 | 0.457 | −0.014 | 0.905 | 0.135 | 0.231 |
| Anterior fornix length | 0.231* | 0.039 | −0.338** | 0.002 | 0.182 | 0.106 | 0.070 | 0.536 | −0.021 | 0.852 |
| Positive fornix length | 0.100 | 0.378 | −0.269* | 0.016 | 0.188 | 0.095 | 0.144 | 0.203 | 0.067 | 0.552 |
| Cervix curved length | 0.224* | 0.046 | −0.396*** | 0.000 | 0.237* | 0.034 | 0.131 | 0.248 | 0.022 | 0.848 |
| Cervix transverse width | 0.141 | 0.213 | −0.053 | 0.641 | 0.196 | 0.081 | 0.142 | 0.209 | 0.171 | 0.130 |
| V 1st diameter | 0.135 | 0.233 | 0.192 | 0.088 | 0.105 | 0.354 | 0.055 | 0.627 | 0.095 | 0.403 |
| V 2nd diameter | −0.092 | 0.418 | 0.197 | 0.079 | 0.062 | 0.584 | 0.093 | 0.410 | 0.112 | 0.324 |
| V 3rd diameter | 0.150 | 0.184 | 0.366** | 0.001 | −0.082 | 0.468 | −0.190 | 0.092 | 0.174 | 0.124 |
| V 4th diameter | 0.119 | 0.294 | 0.354** | 0.001 | −0.053 | 0.637 | −0.144 | 0.201 | 0.144 | 0.202 |
| V 5th diameter | 0.224* | 0.046 | 0.221* | 0.049 | −0.086 | 0.449 | −0.208 | 0.064 | 0.053 | 0.643 |
| V 1st perimeter | 0.132 | 0.242 | 0.256* | 0.022 | 0.233* | 0.038 | 0.177 | 0.117 | 0.073 | 0.521 |
| V 2nd perimeter | 0.025 | 0.825 | 0.056 | 0.623 | 0.253* | 0.024 | 0.229* | 0.041 | 0.035 | 0.757 |
| V 3rd perimeter | 0.062 | 0.586 | 0.387*** | 0.000 | −0.030 | 0.795 | −0.087 | 0.443 | 0.188 | 0.096 |
| V 4th perimeter | 0.145 | 0.201 | 0.348** | 0.002 | 0.052 | 0.646 | −0.054 | 0.637 | 0.133 | 0.241 |
| V 5th perimeter | 0.165 | 0.144 | 0.172 | 0.127 | −0.093 | 0.413 | −0.197 | 0.080 | 0.048 | 0.674 |
| V lower axis | −0.095 | 0.402 | −0.250* | 0.026 | 0.017 | 0.880 | 0.084 | 0.459 | 0.015 | 0.891 |
| V middle axis | −0.144 | 0.201 | 0.143 | 0.207 | 0.165 | 0.143 | 0.262* | 0.019 | −0.085 | 0.454 |
| V upper axis | 0.052 | 0.646 | 0.094 | 0.405 | 0.297** | 0.007 | 0.281* | 0.012 | 0.186 | 0.099 |
| AVW surface area | 0.217 | 0.053 | 0.093 | 0.414 | 0.138 | 0.224 | 0.007 | 0.952 | 0.137 | 0.227 |
| PVW surface area | 0.168 | 0.136 | 0.057 | 0.616 | 0.065 | 0.565 | −0.045 | 0.694 | 0.163 | 0.149 |
| V surface area | 0.193 | 0.086 | 0.074 | 0.512 | 0.100 | 0.377 | −0.021 | 0.854 | 0.153 | 0.177 |
V vagina, AVW anterior vaginal wall, PVW posterior vaginal wall
Correlation is significant at the 0.05 level (two-tailed)
Correlation is significant at the 0.01 level (two-tailed)
Correlation is significant at the 0.001 level (two-tailed)
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00192-016-2949-0) contains supplementary material, which is available to authorized users
Compliance with ethical standards
Conflicts of interest Dr. Jiajia Luo does not have any conflicts of interest directly related to this study. Dr. John O.L. DeLancey and Dr. James A. Ashton-Miller do not have any conflicts of interest directly related to this study.
References
- 1.Barnhart K, Izquierdo A, Pretorius ES, Shera DM, Shabbout M, Shaunik A. Baseline dimensions of the human vagina. Hum Reprod. 2006;21(6):1618–1622. doi: 10.1093/humrep/del022. [DOI] [PubMed] [Google Scholar]
- 2.Nardos R, Thurmond AS, Worstell TR, Clark AL, Gregory WT. Reference lines in dynamic magnetic resonance imaging of the pelvic floor. Female Pelvic Med Reconstr Surg. 2010;16(4):242–245. doi: 10.1097/SPV.0b013e3181ec2070. [DOI] [PubMed] [Google Scholar]
- 3.Trowbridge ER, Fultz NH, Patel DA, DeLancey JO, Fenner DE. Distribution of pelvic organ support measures in a population-based sample of middle-aged, community-dwelling African American and white women in southeastern Michigan. Am J Obstet Gynecol. 2008;198(5):548.e1–548.e6. doi: 10.1016/j.ajog.2008.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo J, Larson KA, Fenner DE, Ashton-Miller JA, Delancey JO. Posterior vaginal prolapse shape and position changes at maximal Valsalva seen in 3-D MRI-based models. Int Urogynecol J. 2012;23(9):1301–1306. doi: 10.1007/s00192-012-1760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betschart C, Chen L, Ashton-Miller JA, Delancey JO. On pelvic reference lines and the MR evaluation of genital prolapse: a proposal for standardization using the pelvic inclination correction system. Int Urogynecol J. 2013;24(9):1421–1428. doi: 10.1007/s00192-013-2100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 Pt 1):1717–1724. doi: 10.1016/0002-9378(92)91562-o. discussion 1724–1718. [DOI] [PubMed] [Google Scholar]
- 7.Sze EH, Meranus J, Kohli N, Miklos JR, Karram MM. Vaginal configuration on MRI after abdominal sacrocolpopexy and sacrospinous ligament suspension. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(6):375–379. doi: 10.1007/s001920170016. discussion 379–380. [DOI] [PubMed] [Google Scholar]
- 8.Guaderrama NM, Nager CW, Liu J, Pretorius DH, Mittal RK. The vaginal pressure profile. Neurourol Urodyn. 2005;24(3):243–247. doi: 10.1002/nau.20112. [DOI] [PubMed] [Google Scholar]
- 9.Jung S, Pretorius DH, Padda BS, Weinstein MM, Nager CW, den Boer DJ, Mittal RK. Vaginal high-pressure zone assessed by dynamic 3-dimensional ultrasound images of the pelvic floor. Am J Obstet Gynecol. 2007;197(1):52.e1–52.e7. doi: 10.1016/j.ajog.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaisdell FE. The anatomy of the sacro-uterine ligaments. Anat Rec. 1917;12(1):1–42. [Google Scholar]
- 11.Campbell RM. The anatomy and histology of the sacrouterine ligaments. Am J Obstet Gynecol. 1950;59(1):1–12. doi: 10.1016/0002-9378(50)90334-6. [DOI] [PubMed] [Google Scholar]
- 12.Buller JL, Thompson JR, Cundiff GW, Krueger Sullivan L, Schon Ybarra MA, Bent AE. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97(6):873–879. doi: 10.1016/s0029-7844(01)01346-1. [DOI] [PubMed] [Google Scholar]
- 13.Paramore RH. The statics of the female pelvic viscera. Lewis, London: 1918. The uterus as a floating organ; p. 12. [Google Scholar]
- 14.Dutta DC, Konar H. Jaypee Brothers. New Delhi: Medical Publishers; 2014. DC Dutta’s textbook of gynecology. [Google Scholar]
- 15.Standring S, Gray H. Gray’s anatomy: the anatomical basis of clinical practice, 40th edn. Edinburgh: Churchill Livingstone/Elsevier; 2008. [Google Scholar]
- 16.Tan JS, Lukacz ES, Menefee SA, Luber KM, Albo ME, Nager CW. Determinants of vaginal length. Am J Obstet Gynecol. 2006;195(6):1846–1850. doi: 10.1016/j.ajog.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 17.Nichols DH, Milley PS, Randall CL. Significance of restoration of normal vaginal depth and axis. Obstet Gynecol. 1970;36(2):251–256. [PubMed] [Google Scholar]
- 18.Funt MI, Thompson JD, Birch H. Normal vaginal axis. South Med J. 1978;71(12):1534–1535. doi: 10.1097/00007611-197812000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Pendergrass PB, Belovicz MW, Reeves CA. Surface area of the human vagina as measured from vinyl polysiloxane casts. Gynecol Obstet Investig. 2003;55(2):110–113. doi: 10.1159/000070184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.