Abstract
At nerve terminals, endocytosis efficiently recycles vesicle membrane to maintain synaptic transmission under different levels of neuronal activity. Ca2+ and its downstream signal pathways are critical for the activity-dependent regulation of endocytosis. An activity- and Ca2+-dependent kinase, myosin light chain kinase (MLCK) has been reported to regulate vesicle mobilization, vesicle cycling and motility in different synapses, but whether it has a general contribution to regulation of endocytosis at nerve terminals remains unknown. We investigated this issue at rat hippocampal boutons by imaging vesicle endocytosis as the real-time retrieval of vesicular synaptophysin tagged with a pH-sensitive green fluorescence protein. We found that endocytosis induced by 200 action potentials (5 – 40 Hz) was slowed by acute inhibition of MLCK and downregulation of MLCK with RNA interference, while the total amount of vesicle exocytosis and somatic Ca2+ channel current did not change with MLCK downregulation. Acute inhibition of myosin II similarly impaired endocytosis. Furthermore, downregulation of MLCK prevented depolarization-induced phosphorylation of myosin light chain, an effect shared by blockers of Ca2+ channels and calmodulin. These results suggest that MLCK facilitates vesicle endocytosis through activity-dependent phosphorylation of myosin downstream of Ca2+/calmodulin, probably as a widely existing mechanism among synapses. Our study suggests that MLCK is an important activity-dependent regulator of vesicle recycling in hippocampal neurons, which are critical for learning and memory.
Keywords: endocytosis, vesicle cycling, hippocampus, myosin light chain kinase
Graphical abstract
In this issue: The kinetics of vesicle membrane endocytosis at nerve terminals has long been known to depend on activity and Ca2+. The present study provides evidence suggesting that myosin light chain kinase increases endocytosis efficiency at hippocampal neurons by mediating Ca2+/calmodulin-dependent phosphorylation of myosin. The authors propose that this signal cascade may serve as a common pathway contributing to the activity-dependent regulation of vesicle endocytosis at synapses.
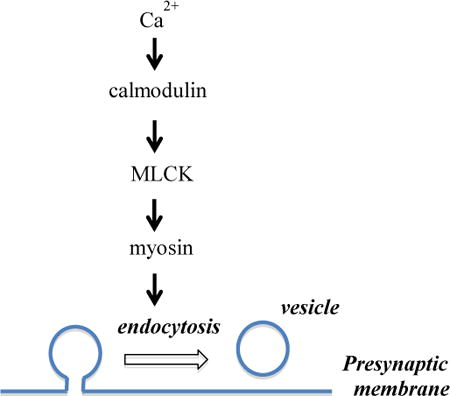
Introduction
Neurons communicate primarily by synaptic transmission at nerve terminals, where neuronal activity triggers vesicle exocytosis to release neurotransmitter molecules, followed by endocytosis to recycle vesicle membrane for future exocytosis. Efficiency of endocytosis depends on activity and Ca2+, which select different molecular machineries of endocytosis by activating Ca2+-dependent pathways. An activity- and Ca2+-dependent kinase, MLCK has been reported to regulate various cellular processes such as muscle contraction of smooth muscle cells, axonal growth in neurons, and membrane internalization after osmotic swelling in liver cells (Barfod et al., 2011). In synapses, MLCK and its typical downstream substrate myosin have long been known to regulate different aspects of neurotransmitter release (Mochida et al., 1994), including supply of fast releasing vesicles (Srinivasan et al., 2008, Lee et al., 2010, Gonzalez-Forero et al., 2012, Garcia-Morales et al., 2015), refilling of readily releasable pool after tetanus stimulation (Lee et al., 2008), vesicle mobility (Jordan et al., 2005, Seabrooke et al., 2010, Peng et al., 2012) and vesicle mobilization (Ryan, 1999, Polo-Parada et al., 2005, Seabrooke and Stewart, 2011). We recently suggested that MLCK accelerates vesicle endocytosis at the calyx of Held terminals (Yue and Xu, 2014). However, whether MLCK has a general contribution to the activity-dependent regulation of endocytosis in nerve terminals remains obscure. As one of the largest nerve terminals in the mammalian central nervous system, the calyx of Held can maintain synaptic transmission under neuronal firing activity up to 1000 Hz, which serves its role in sound localization along the auditory pathway (Schneggenburger and Forsythe, 2006). In contrast, many synapses such as hippocampal boutons function under neuronal activity below 100 Hz, and are probably subject to very different activity-dependent regulation of endocytosis and vesicle supply. For example, increase of stimulation intensity saturates the endocytosis machinery and prolongs endocytosis kinetics at hippocampal boutons (Balaji et al., 2008), but accelerates endocytosis at the calyx of Held to form an additional pathway with rapid kinetics (Wu et al., 2005). Effects of MLCK inhibitors on the release probability, vesicle priming, and endocytosis at the calyx (Lee et al., 2008, Srinivasan et al., 2008, Lee et al., 2010, Yue and Xu, 2014) are not consistently observed at conventional synapses (Ryan, 1999, Tokuoka and Goda, 2006). Therefore, whether regulation of vesicle endocytosis by MLCK represents a common mechanism is an open question in synaptic physiology.
Furthermore, the possible signalling pathway for MLCK to regulate synaptic endocytosis has not been defined. Many of the cellular effects of MLCK result from its phosphorylation of myosin light chain (MLC) (Somlyo and Somlyo, 2003), but alternative downstream targets have also been reported. For example, MLCK negatively controls cell migration and protrusion in smooth muscular cells, which does not involve phosphorylation of myosin light chain (Chen et al., 2014). MLCK is also subject to regulation by different pathways, including Ca2+/calmodulin, membrane-derived bioactive phospholipids (Garcia-Morales et al., 2015), the neural cell adhesion molecule (Polo-Parada et al., 2005), protein kinase C (Maeno-Hikichi et al., 2011), Rho kinase (Gonzalez-Forero et al., 2012), and p21-activated kinases (Sanders et al., 1999). Inhibitors of calmodulin and MLCK both impair endocytosis at the calyx (Wu et al., 2009, Yao and Sakaba, 2012, Yue and Xu, 2014), implicating that MLCK could function downstream of calmodulin. However, calmodulin can also activate calcineurin, a Ca2+-dependent serine/threonine protein phosphatase indicated in synaptic endocytosis (Liu et al., 1994, Chan and Smith, 2001, Cheung and Cousin, 2013). Inhibition and genetic deletion of calcineurin subunits both result in endocytosis defects at the calyx and hippocampal boutons, leading to the suggestion that calcineurin is the downstream signal of endocytosis regulation by Ca2+/calmodulin (Wu et al., 2009, Sun et al., 2010). On the other hand, MLCK may be activated by a signal cascade other than calmodulin, for example, the lysophosphatidic acid/Gαi/o-protein/phospholipase C to regulate the number of docked vesicles at synaptic boutons (Garcia-Morales et al., 2015). Given the complexity of Ca2+-dependent regulation of endocytosis, further evidence is needed to establish a possible link between calmodulin and MLCK in regulating synaptic vesicle endocytosis.
To address the above issues, we investigated the involvement of MLCK in vesicle endocytosis at rat hippocampal boutons by imaging pHluorin-tagged synaptophysin expressed into these boutons. We found that both chemical inhibition of MLCK and downregulation of MLCK with short hairpin RNAs (shRNAs) impaired endocytosis following action potentials delivered at 5 – 40 Hz, while MLCK downregulation did not change Ca2+ channel current in the soma or vesicle exocytosis at boutons. Acute inhibition of myosin II similarly impaired endocytosis. Furthermore, MLCK downregulation selectively reduced the depolarization-evoked increase of MLC phosphorylation, an effect shared by block of Ca2+ channels or calmodulin. These results collectively suggest that MLCK at hippocampal boutons functions downstream of Ca2+/calmodulin to facilitate endocytosis through phosphorylation of MLC.
Methods
Primary culture of rat hippocampal neurons
Dissociated culture of rat hippocampal neurons was prepared in accordance with guidelines of The Institutional Animal Care and Use Committee, Augusta University. Briefly, hippocampi were dissected from acutely decapitated postnatal day 0 Sprague-Dawley rats, which were purchased from Harlan Laboratories and bred in house. Hippocampi were cut into pieces of ∼1 mm3 and transferred into trypsin XI from bovine pancreas (2 mg/ml, Sigma-Aldrich, MO, USA) to digest under 37 °C. After trypsin digestion for 7 min, tissues were triturated and digested further in a mixture of trypsin and deoxyribonuclease I (from bovine pancreas, 60 U/ml, Sigma-Aldrich) for 7 min. The dispersed cells were plated with an estimated density of 53,000 cells/cm2 on 12 mm, round glass coverslips coated with poly-D-lysine, and maintained in Neurobasal medium (Life Technologies, NY, USA) containing 2% B-27 (Life Technologies) and 0.5 mM GlutaMAX™ -I (Life Technologies).
Optical imaging of vesicle exocytosis, endocytosis and reacidification
On 9 – 10 d in vitro (DIV), cultures were transfected with cDNA construct of synaptophysin-pHluroin2X (SypHy, kind gift from Dr. Yongling Zhu in Northwestern University, Illinois, USA), which was premixed with calcium phosphate or Lipofectamine® 3000 (Life Technologies) and diluted into MEM (1.8 μg SypHy/ml). After transfection for 40 min at 37 °C, cells on coverslips were moved back into the culture medium and maintained in culture for 2 – 3 d before imaging in a stimulation chamber (RC-21BRFS chamber, Warner Instruments, CT, USA) at room temperature (22 – 24 °C). The bath solution during imaging contained (in mM): 150 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, 10 HEPES, 0.01 6-cyano-7-nitroqunioxaline-2,3-dione (CNQX), and 0.05 aminophosphonopentanoic acid (AP-5); pH 7.4. CNQX and AP-5 (Tocris Bioscience, MN, USA) were added to block postsynaptic ionotropic glutamate receptors and thus prevent network activity. Vesicle exocytosis and endocytosis were evoked by electrical stimulation with a train of 200 brief current pulses (1 ms, 50 mA; 5 – 40 Hz) passing two parallel platinum electrodes, which were separated by about 7 mm. The current pulses were generated from a pulse stimulator (SIU-102, Warner Instruments) controlled by an EPC10/2 patch-clamp amplifier to set the number and frequency of pulses through the software Patchmaster (HEKA, Germany). Images of SypHy were acquired at 1 or 2 Hz using an EMCCD camera (Orca Flash2.8) through a 40X, 0.80 numerical aperture water-immersion objective (Olympus, PA, USA). To avoid interference from potential lateral diffusion of pHluorin (Granseth et al., 2006), we measured the average fluorescence intensity within a square of 1.6 μm × 1.6 μm at each functional bouton. Fluorescence traces from all boutons in an experiment (Nbouton) were averaged to yield one trace. Data presented for each treatment are further averaged from such traces from 4 – 9 imaging experiments (Nexp). SypHy carries an intraluminal domain with pH-sensitive green fluorescence, which is quenched by the acidic lumen (pH 5.5) of vesicles, induced by exposure to the extracellular neutral pH after vesicle fusion, and quenched again by vesicle reacidification following endocytosis (Granseth et al., 2006, Zhu et al., 2009). Thus we evaluated effects on endocytosis by comparing the fluorescence decay after stimulation, on the basis that treatments did not affect vesicle reacidification. To measure the kinetics of vesicle reacidification, the chamber containing ∼350 μl solution was perfused at a velocity of 150 μl/s by an acidic bath solution during ∼30 – 60 s after stimulation with 20 Hz action potentials. The acidic solution was similar to the standard bath except that HEPES was substituted with 2-(N-Morpholino)ethanesulfonic acid hydrate (10 mM) and titrated with NaOH to pH 5.5. The fluorescence decay during the quench was analyzed to estimate the rate of vesicle reacidification (Atluri and Ryan, 2006, Granseth et al., 2006). For measurements of exocytosis (Fig.4A), boutons were stimulated in the presence of 100 nM folimycin, which eliminated interference of endocytosis by blocking reacidification of endocytosed vesicles, and exposed to 50 mM NH4Cl at the end of tests. The fraction of exocytosed vesicles was calculated by normalizing the amplitude of fluorescence increase evoked by action potentials to that evoked by NH4Cl, which collapses the pH gradient across vesicle membrane and activates fluorescence from all copies of SypHy. Chemicals were from Sigma-Aldrich unless otherwise mentioned.
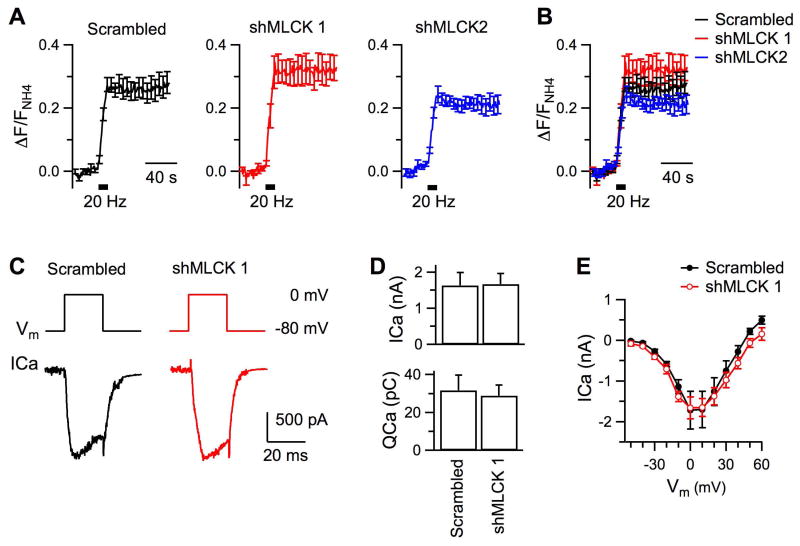
Figure 4. Downregulation of MLCK does not affect exocytosis or somatic Ca2+ channel current.
A, Averaged changes of SypHy fluorescence induced by 200 action potentials delivered at 20 Hz in the presence of 100 nM folimycin. The changes have been normalized to the peak increase of fluorescence in 50 mM NH4Cl in each experiment. Boutons had been transfected with scrambled shRNA (Nexp = 8), shMLCK 1 (Nexp = 8), or shMLCK 2 (Nexp = 7). The traces are superimposed in B for comparison. C, Sampled traces of somatic calcium channel current (ICa) induced by a 20 ms depolarization pulse from - 80 mV to 0 mV. D, Amplitude and total charge of ICa in hippocampal neurons transfected with either the scrambled shRNA (n = 7) or shMLCK 1 (n = 8). E, Current-voltage relationship of ICa from neurons transfected with either the scrambled shRNA (n = 7) or shMLCK 1 (n = 8).
Knockdown of endogenous MLCK and immunoblotting
For experiments on MLCK downregulation, we used two shRNA sequences (shMLCK 1: 5′-GCTAGATTTGACTGCAAGATT-3′; and shMLCK 2: 5′-ACTGTCCTCTATGGCAATGAT-3′) previously reported against rat MLCK (Leitman et al., 2011) and a scrambled shRNA sequence (scrambled: 5′-GCGAAAGATGATAAGCTAA-3′) as the control (Pasque et al., 2011). Oligos with these sequences were synthesized, annealed and ligated to pSuper (Oligoengine, Seattle, Washington, USA) at BglII/HindII restriction sites. Sequencing of the plasmids and their efficacy to ablate MLCK had been verified by Western blot. Specifically, we transfected neurons with shRNAs by electroporation with Neuron Nucleofector kit (Lonza, Basel, Switzerland), extracted proteins 4 d later and separated them on a 10% PAGE gel. We transferred the proteins onto a PVDF membrane (EMD Millipore, MA, USA), which was blocked with 5% non-fat milk for 1 h at room temperature and then incubated overnight with antibody against MLCK (Cat# ab76092 from Abcam, MA, USA) at 4 °C. The membrane was washed with Tris-buffered saline containing Tween 20, incubated with a horseradish peroxidase-conjugated secondary antibody for 2 h, and detected by Enhanced Chemiluminscent Kit (Thermo Scientific, USA) after washing with the Tris-buffered saline. The intensity of immunoblotting was normalized to that of tubulin. To examine effects of MLCK downregulation on endocytosis, hippocampal neurons were transfected on DIV 10 with 2 ml MEM solution that included 1.8 μg SypHy plasmid along with 2 μg of either a MLCK shRNA or the scrambled shRNA. Neurons were imaged 3 – 4 d after transfection.
Immunoblotting was similarly executed to quantify MLC and pMLC in hippocampal neurons, using antibodies against MLC II (Cat. ab48003 from Abcam) and MLC phosphorylated at Ser19 (Cat. #3671 from Cell Signaling, USA), respectively.
Patch-clamp recordings of hippocampal neurons
To evaluate possible changes in Ca2+ currents after MLCK knockdown, we measured the voltage-gated Ca2+ current (ICa) from soma of cultured hippocampal neurons by the standard whole-cell patch-clamp technique using an EPC-10/2 amplifier controlled by the Patchmaster program. Neurons were transfected with shMLCK 1 or the scrambled shRNA on DIV 10, and tested 3 – 4 d later. The bath solution contained (in mM): 120 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, 10 HEPES, 20 tetraethylammonium, 0.001 tetrodotoxin, 0.1 3,4-diaminopyridine, 0.01 CNQX, and 0.05 AP-5, pH 7.4. The pipette solution contained (in mM): 125 Cs-gluconate, 20 CsCl, 4 MgATP, 10 Na2-phosphocreatine, 0.3 GTP, 10 HEPES, 0.05 BAPTA, pH adjusted to 7.2 with CsOH. The series resistance (10 – 15 MΩ) was compensated by 65% with a lag of 10 μs. Recordings were made at room temperature (22-24 °C).
Data analysis
All the quantified results are presented as Mean ± SEM. The statistical test is Student's unpaired t-test for those in Fig.1 – 6, and two-way ANOVA followed by Newman–Keuls post hoc test in Fig.7, with the probability value p < 0.05 indicating a significant difference.
Figure 1. Chemical inhibition of MLCK slows down endocytosis.
A, Representative images showing SypHy fluorescence changes in response to stimulation with 200 action potentials of 20 Hz. Boutons had been incubated with 0.05% DMSO for 20 min at 37 °C before imaging. B, Averaged traces of SypHy fluorescence from boutons pretreated for 20 min with DMSO (Nexp = 7), wortmannin (Wort, 5 μM, Nexp = 7), or ML-7 (3.5 μM, Nexp = 5). The fluorescence change (ΔF) was normalized to the basal fluorescence level before stimulation (F0). Boutons were bathed in 2 mM Ca2+ for DMSO and wortmannin, and 5.5 mM Ca2+ for ML-7 which otherwise resulted in too small fluorescence increase in 2 mM Ca2+. C, The superimposed data are averaged from fluorescence traces that have been normalized to the peak ΔF induced by action potentials in each experiment. D, Comparison of endocytosis kinetics. Error bars in this and other figures are SEM. The symbols ** and * denote p < 0.01 and p < 0.05, respectively.
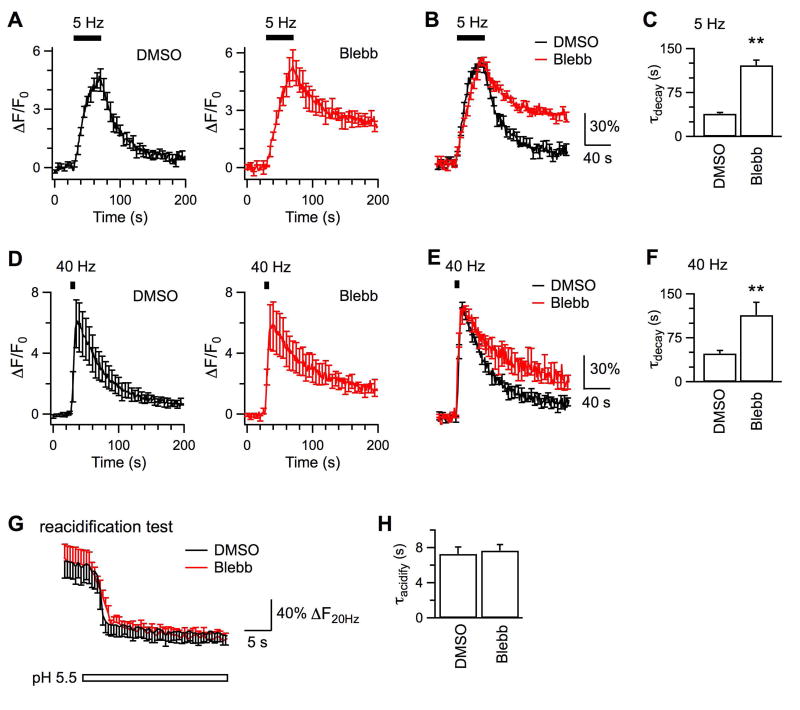
Figure 6. Acute inhibition of myosin II inhibits endocytosis induced by action potentials at 5 Hz and 40 Hz.
A, Averaged responses of SypHy fluorescence stimulated by 200 action potentials delivered at 5 Hz from boutons pre-treated with DMSO (0.01 - 0.05%, Nexp = 9) or blebbistatin (2.5 μM, Nexp = 5) for 20 min at 37 °C. B, The superimposed data are averaged from fluorescence traces that have been normalized to the peak ΔF induced by action potentials in each experiment. C, Comparison of endocytosis kinetics induced by action potentials of 5 Hz at boutons treated with DMSO and blebbistatin. D – F, Same as A – C except that boutons treated with DMSO (Nexp = 8) or blebbistatin (Nexp = 5) were stimulated by action potentials of 40 Hz. G, Averaged changes of SypHy fluorescence in tests to measure vesicle reacidification, same as in Fig.2B. Boutons had been treated with DMSO (Nexp = 5, from Fig.2B) or 2.5 μM blebbistatin (Nexp = 6). H, Time constants of vesicle reacidification from boutons treated with either DMSO or blebbistatin. Results in G and H suggest that blebbistatin does not affect vesicle reacidification.
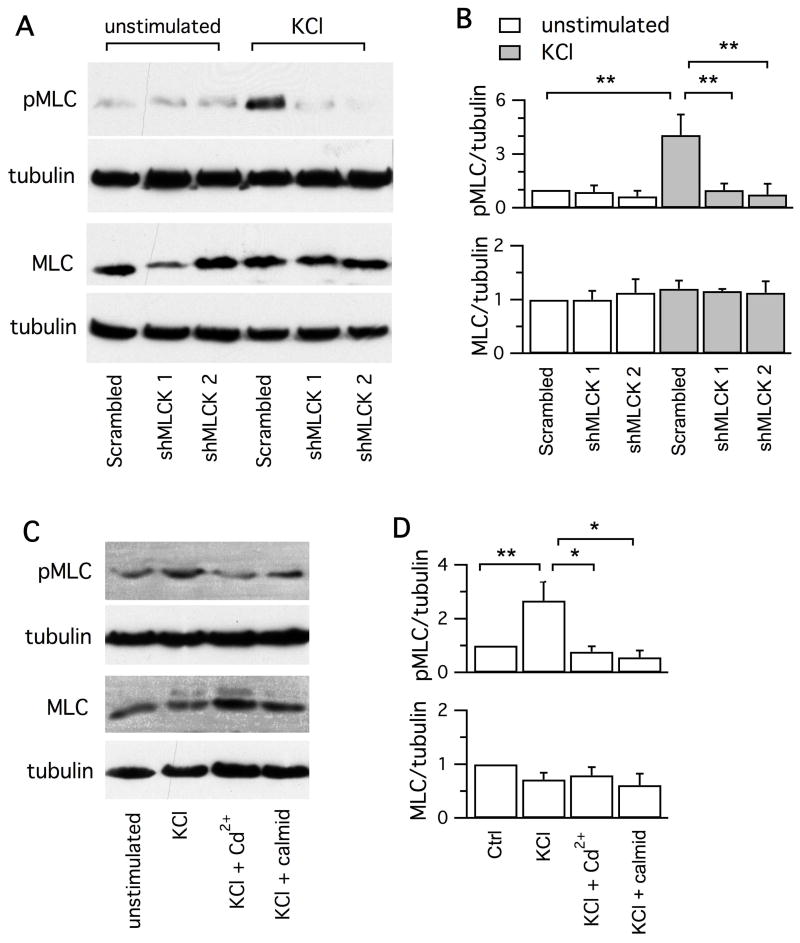
Figure 7. Activity-dependent phosphorylation of MLC is mediated by MLCK and Ca2+/calmodulin.
A, Immunoblots of MLC and pMLC from neuronal cultures with or without incubation with 55 mM KCl for 90 s. Neurons had been transfected for 3 d with the scrambled shRNA, shMLCK 1 and shMLCK 2, respectively. B, Downregulation of MLCK selectively reduced the KCl-evoked phosphorylation of MLC II (n = 4), but did not affect the total MLC (n = 3). For each treatment, the levels of pMLC and MLC in relative to tubulin are normalized to those acquired without KCl stimulation from neurons transfected with the scrambled shRNA. C, Immunoblots of MLC and pMLC from unstimulated neurons, neurons exposed for 90 s to 55 mM KCl, neurons exposed to 55 mM in the presence of CdCl2 (100 μM) or calmidazolium (100 μM). D, Inhibitors of Ca2+ current and calmodulin reduced phosphorylation of MLC (n = 4), without affecting the total MLC (n = 3). For each treatment, the levels of pMLC and MLC in relative to that of tubulin are normalized to the simultaneous measurements from neurons without KCl treatment.
Results
Inhibition or downregulation of MLCK impairs endocytosis induced by action potentials of 20 Hz
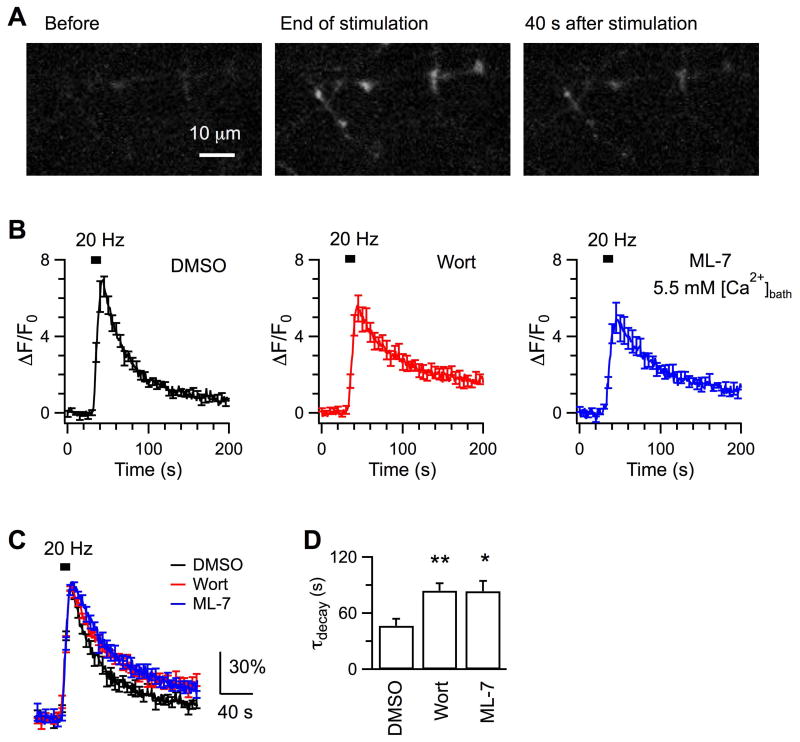
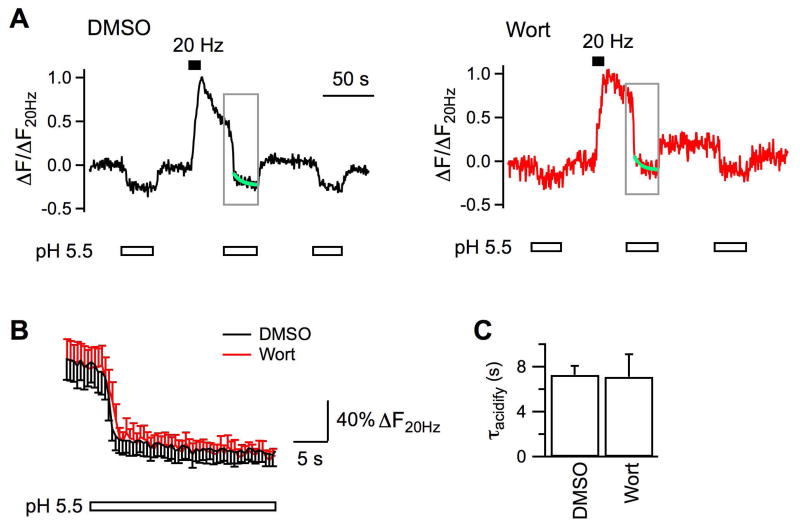
In this study endocytosis at hippocampal boutons was measured with SypHy, the exogenous synaptophysin tagged with a pH-sensitive green fluorescence protein at its intraluminal domain (Zhu et al., 2009). To test the possible involvement of MLCK in endocytosis, we first examined effects of two widely used and structurally unrelated inhibitors, wortmannin and ML-7 (Saitoh et al., 1987, Nakanishi et al., 1992). The fluorescence of axonal boutons was low at rest. When boutons were depolarized to fire action potentials by 200 current pulses delivered at 20 Hz, exocytosis inserted vesicular SypHy into plasma membrane, leading to >6 times of fluorescence increase in control boutons exposed to 0.05% DMSO (Fig.1A-C). After stimulation, the fluorescence started to decline, owing to intraluminal reacidification of vesicles regenerated from endocytosis (Sankaranarayanan and Ryan, 2000, Zhu et al., 2009). This fluorescence decay followed monoexponential kinetics with a time constant (τ) of 46.6 ± 7.5 s (Nexp = 6, Nbouton = 313; Fig.1C, D). In comparison, stimulation induced significantly slower fluorescence decay (τ = 83.9 ± 7.9 s, Nexp = 7, Nbouton = 266, p < 0.01) at boutons pre-incubated with 5 μM wortmannin (Fig.1B – D). At boutons pre-incubated with 3.5 μM ML-7, action potentials induced a small fluorescence increase (not shown), which made it difficult to accurately measure the decay kinetics. When the bath Ca2+ was elevated to 5.5 mM, stimulation increased the fluorescence by ∼5 times at boutons pre-treated with ML-7 (Nexp = 5, Nbouton = 179, Fig.1), followed by slower recovery (τ = 83.4 ± 11.1 s, p = 0.02). Because the fluorescence decay depends on both endocytosis and reacidification of endocytosed vesicles, we tested whether inhibition of MLCK affects vesicle reacidification using a previously reported method (Atluri and Ryan, 2006, Granseth et al., 2006). Specifically, when surface SypHy fluorescence was quenched by perfusion of a pH 5.5 solution during 30 – 60 s after stimulation, the decay kinetics of the remaining fluorescence reflected the kinetics of vesicle reacidification. Reacidification at boutons pre-treated with wortmannin had a time constant of 7.1 ± 2.0 s (Nexp = 6, Nbouton = 135; Fig.2), similar to that at DMSO-treated boutons (τ = 7.3 ± 0.8 s, Nexp = 5, Nbouton = 141). Our measured reacidification is >6 times as fast as the fluorescence decay, although a little slower than the reported 4 – 5 s in previous studies (Atluri and Ryan, 2006, Granseth et al., 2006), where solution exchange is more efficient with the acidic solution applied to boutons locally. This difference, however, should not affect our evaluation of MLCK inhibitors. To summarize, the slowing effect of wortmannin and ML-7 on the action potential-evoked fluorescence decay implicates that MLCK facilitates vesicle endocytosis at hippocampal boutons.
Figure 2. Chemical inhibition of MLCK does not affect vesicle reacidification.
A, Sample experiments on vesicle reacidification at boutons incubated with 0.05% DMSO (Nbouton = 34) or 5 μM wortmannin (Nbouton = 24) for 20 min at 37 °C before imaging. SypHy fluorescence, which was imaged every 500 ms, has been normalized to the amplitude of increase evoked by the 20 Hz stimulation (ΔF20Hz). Boutons were perfused constantly with either the standard solution of pH 7.4 or the solution of pH 5.5 before and after stimulation (started at 30 s, ∼110 s). By fitting the fluorescence decay during the quench (green line) between ∼35 – 60 s after the cessation of stimulation, we measured the time constant of vesicle reacidification as 7.5 s for DMSO and 6.4 s for wortmannin, respectively. B, Averaged changes of SypHy fluorescence to pH 5.5 in the time window indicated by gray frames in A. Boutons were pre-incubated with DMSO (Nexp = 5) or wortmannin (Nexp = 7). The similar fluorescence decay after rapid quenching of surface fluorescence suggests that wortmannin does not alter vesicle reacidification. C, Averaged time constants of vesicle reacidification from boutons incubated with DMSO and wortmannin.
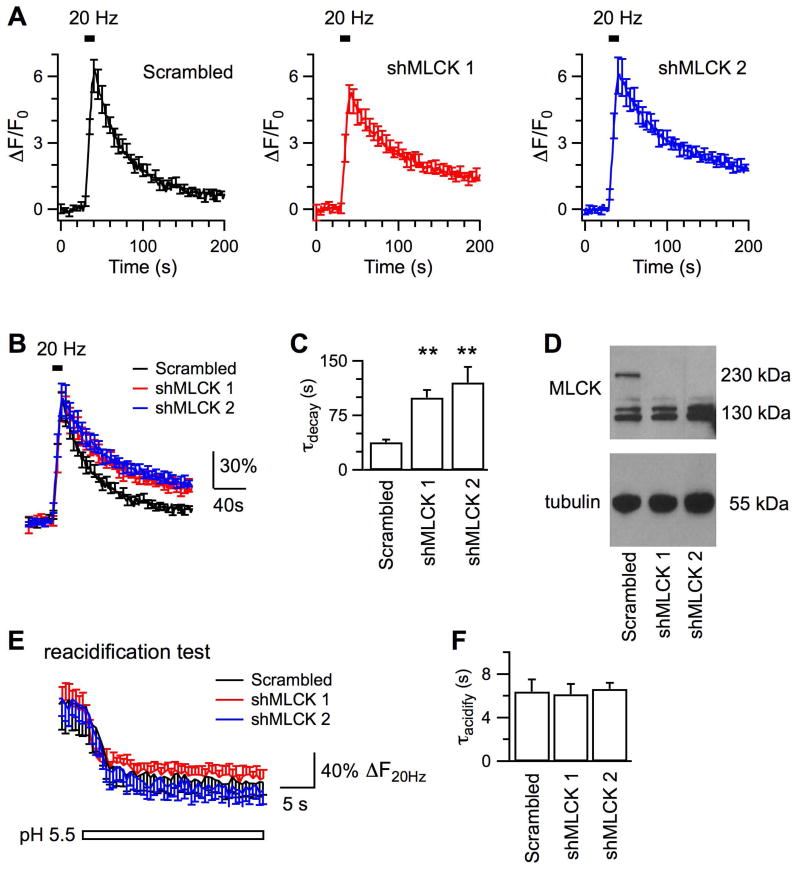
Considering that inhibitors of MLCK may cause off-target effects on action potential firings and voltage-gated Ca2+ channels (Tokimasa et al., 1995, Richards et al., 2004, Tokuoka and Goda, 2006), we next studied effects of genetic downregulation of endogenous MLCK. Two shRNAs targeting different regions of rat MLCK cDNA (Leitman et al., 2011), shMLCK 1 and shMLCK 2, were selected to transfect hippocampal neurons in culture. As confirmed by immunoblotting, transfection with either shMLCK led to significant decrease of endogenous MLCK of 230 kDa in hippocampal neurons, but not that of 130 kDa corresponding to the muscular MLCK isoform (Fig.3D). Transfection with the scrambled shRNA did not affect the level of MLCK, and was used as the control for shMLCKs.
Figure 3. Genetic downregulation of endogenous MLCK impairs endocytosis induced by action potentials at 20 Hz.
A, Averaged changes of SypHy fluorescence induced by 200 action potentials delivered at 20 Hz from hippocampal boutons transfected with the scrambled shRNA (Scrambled, Nexp = 9), shMLCK 1 (Nexp = 6) and shMLCK 2 (Nexp = 8), respectively. B, Averaged fluorescence traces after normalization to the peak ΔF induced by action potentials. C, Comparison of endocytosis kinetics. D, Immunoblotting results showing that transfection with shMLCK 1 or shMLCK 2, but not the scrambled shRNA, decreased the level of endogenous MLCK in hippocampal neurons. E, Averaged changes of SypHy fluorescence in tests to measure vesicle reacidification, same as in Fig.2B. Boutons had been transfected with the scrambled shRNA (Nexp = 5), shMLCK 1 (Nexp = 6) and shMLCK 2 (Nexp = 8), respectively. The similar decay kinetics of the stimulated fluorescence in pH 5.5 suggests that down-regulation of MLCK does not affect vesicle reacidification. F, Time constants of vesicle reacidification from boutons transfected with either the scrambled shRNA or a shMLCK to down-regulate the endogenous MLCK.
At boutons transfected with the scrambled shRNA, stimulation with action potentials at 20 Hz led to ∼6-fold fluorescence increase over the basal level (Fig.3A), followed by a monoexponential decay with τ of 37.8 ± 3.9 s (Nexp = 9, Nbouton = 290). Transfection with shMLCK 1 or shMLCK 2 prolonged τ of the fluorescence decay to 99.6 ± 10.2 s (p < 0.01, Nexp = 8, Nbouton = 351) or 120.5 ± 21.4 s (p < 0.01, Nexp = 8, Nbouton = 266), respectively. The slowing of fluorescence decay was caused by inhibition of endocytosis, because vesicle reacidification was similar between boutons transfected with scrambled shRNA (τ = 6.4 ± 1.1 s, Nexp = 6, Nbouton = 155, Fig.3E, F) and those transfected with either shMLCK 1 (τ = 6.1 ± 0.9 s, Nexp = 6, Nbouton = 228) or shMLCK 2 (τ = 6.6 ± 0.6 s, Nexp = 6, Nbouton = 109). Collectively, our data indicate that downregulation of endogenous MLCK led to impairment of endocytosis. Thus, effects of MLCK inhibitors and MLCK knockdown consistently suggest that MLCK positively regulates vesicle endocytosis at hippocampal boutons. Except for ML-7, other treatments did not drastically affect the fluorescence increase evoked by action potentials. Because the amplitude of fluorescence increase also depends on the expression levels of SypHy at boutons, we next examined more closely whether downregulation of MLCK affects exocytosis.
Downregulation of MLCK does not affect somatic Ca2+ current or exocytosis
Voltage-dependent Ca2+ influx and exocytosis are known to influence the kinetics of endocytosis at hippocampal boutons (Sankaranarayanan and Ryan, 2000, Balaji et al., 2008). ML-7 has been found to decrease exocytosis in hippocampal neurons by inhibiting voltage-dependent Ca2+ influx (Tokuoka and Goda, 2006). Therefore, we examined whether downregulation of MLCK with shRNAs affects exocytosis and Ca2+ influx.
First, we stimulated exocytosis and endocytosis with action potentials of 20 Hz from boutons bathed in 100 nM folimycin, a blocker of vesicular pH pumps, and applied 50 mM NH4Cl at the end of experiments. Endocytosis would uptake folimycin into the lumen of regenerated vesicles, which prevented lumen reacidification and quenching of SypHy fluorescence. The fluorescence increase measured in folimycin was thus not affected by concomitant endocytosis, and reflected the cumulative exocytosis. As shown in Fig. 4A – B, stimulation led to a steady state fluorescence rise at boutons transfected with the scrambled shRNA, which reached 0.28 ± 0.03 (Nexp = 8, Nbouton = 188) of the NH4Cl evoked fluorescence increase. This suggests that 28% of vesicles had undergone exocytosis. A similar amount of fluorescence increase was detected at boutons transfected with either shMLCK 1 (0.32 ± 0.05, p = 0.29, Nexp = 8, Nbouton = 203) or shMLCK 2 (0.23 ± 0.03, p = 0.2, Nexp = 7, Nexp = 177). Thus, downregulation of MLCK did not affect exocytosis evoked by the 20 Hz action potentials. In addition, transfection with shMLCKs did not change the rising course of fluorescence during or after the stimulation, ruling out the possibility that the fluorescence decay after stimulation was slowed down because of enhancement of asynchronous exocytosis.
Next, we measured somatic Ca2+ channel current from neurons co-transfected with shMLCK 1, or the scrambled shRNA, and a plasmid of green fluorescence protein (GFP) to indicate successful transfection. In neurons transfected with the scrambled shRNA (n = 7), depolarization from -80 mV to 0 mV for 20 ms induced an inward Ca2+ current (ICa) of 1.6 ± 0.4 nA in amplitude (Fig.4C, D) and 31.8 ± 8.1 pC in total charge (Fig.4E). The same depolarization evoked similar Ca2+ current in neurons transfected with shMLCK 1, which was 1.7 ± 0.3 nA (n = 8, p = 0.94) and 28.6 ± 5.6 pC (p = 0.77). Downregulation of MLCK did not affect activation or inactivation of Ca2+ channels either, because transfection with shMLCK1 had no effect on current-voltage curve of ICa (Fig.4E). Therefore, MLCK regulates endocytosis independent of exocytosis or Ca2+ channel current.
Downregulation of MLCK impairs endocytosis induced by action potentials of both 5 Hz and 40 Hz
At hippocampal synapses, stimulation of 10 – 20 Hz action potentials has been a widely used paradigm to induce endocytosis (Sankaranarayanan et al., 2003, Granseth et al., 2006, Sun et al., 2010, Zhang et al., 2013). A new study discovers that stimulation at 5 Hz mainly induces clathrin-mediated endocytosis at hippocampal boutons while stimulation at 40 Hz induces clathrin-independent endocytosis (Kononenko et al., 2014). To determine whether MLCK selectively targets clathrin-mediated or clathrin-independent endocytosis, we next studied effects of MLCK knockdown on endocytosis following stimulation of either 5 Hz or 40 Hz.
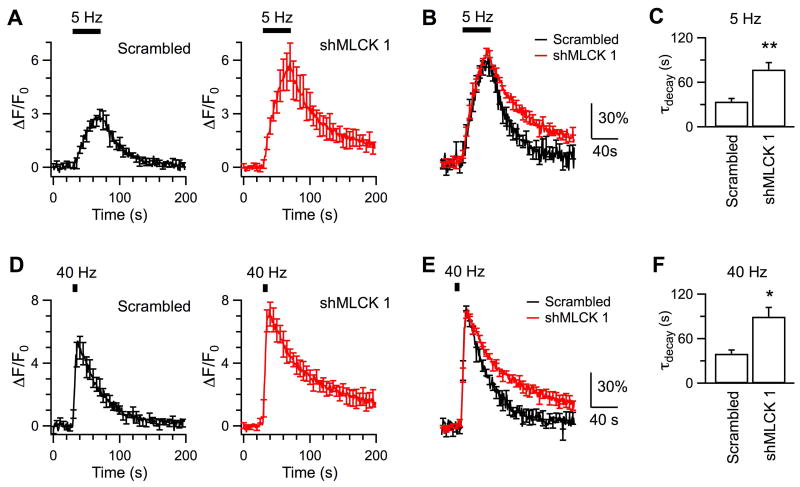
In response to 200 action potentials delivered at 5 Hz, SypHy fluorescence increased to ∼3-fold over the baseline at boutons transfected with the scrambled shRNA, and then decayed by monoexponential kinetics (τ = 34.1 ± 4.1 s, Nexp = 6, Nbouton = 217; Fig.5A-C). The fluorescence decay was slower at boutons transfected with shMLCK 1 (τ = 77.2 ± 9.3 s, Nexp = 5, Nbouton = 179, p < 0.01), suggestive of impaired endocytosis after downregulation of MLCK. Similar effect occurred for stimulation with 200 action potentials at 40 Hz (Fig. 5D-F). Specifically, the fluorescence decayed at τ of 39.9 ± 5 s at boutons transfected with the scrambled shRNA (Nexp = 6, Nbouton = 337) and 89.7 ± 12.3 s (p = 0.02) at boutons transfected with shMLCK 1 (Nexp = 4, Nbouton = 167). Taken together, downregulation of MLCK consistently slowed endocytosis induced by stimulation of 5 – 40 Hz, suggesting that endogenous MLCK modulates both clathrin-mediated endocytosis and clathrin-independent endocytosis. Action potentials of 5 Hz appeared to evoke larger fluorescence increase at boutons transfected with shMLCK 1, because impairment of endocytosis led to more retention of SypHy at surface. Transfection with shMLCK 1 did not change the fluorescence peak following stimulation of 40 Hz, because endocytosis has little impact on fluorescence accumulation during high frequency stimulation. Our observations are consistent with literature (Kononenko et al., 2014).
Figure 5. Downregulation of endogenous MLCK inhibits endocytosis evoked by action potentials at both 5 Hz and 40 Hz.
A, Averaged traces of SypHy fluorescence changes induced by 200 action potentials delivered at 5 Hz from boutons transfected with the scrambled shRNA (Nexp = 6) or shMLCK 1 (Nexp = 5). B, The superimposed data are averaged from fluorescence traces that have been normalized to the peak ΔF induced by action potentials in each experiment. C, Comparison of endocytosis induced by action potentials of 5 Hz at boutons transfected with shRNA and shMLCK 1. D – F, Same as A – C except that the boutons transfected with the scrambled shRNA (Nexp = 6) or shMLCK 1 (Nexp = 4) were stimulated by 200 action potentials delivered at 40 Hz.
MLCK regulates endocytosis by functioning between Ca2+/calmodulin and myosin
Both calmodulin and myosin have been shown to regulate vesicle endocytosis at hippocampal boutons, but without referring to a role of MLCK (Sun et al., 2010, Chandrasekar et al., 2013). In many cellular functions, MLCK acts downstream of Ca2+/calmodulin and activates the functions of myosin by phosphorylation of its regulatory light chain. But alternative signal cascades have been reported (Polo-Parada et al., 2005, Gonzalez-Forero et al., 2012, Garcia-Morales et al., 2015). For example, the lysophosphatidic acid/Gαi/o-protein/phospholipase C activates MLCK to reduce vesicle docking at synaptic terminals of motoneurons (Garcia-Morales et al., 2015), and MLCK regulates membrane tension of smooth muscle cells without involving phosphorylation of myosin (Chen et al., 2014). To test whether MLCK regulates endocytosis via interaction with Ca2+/calmodulin and myosin, we executed the following two sets of experiments.
First, we investigated whether myosin regulates clathrin-mediated endocytosis and clathrin-independent endocytosis like MLCK (Fig.5). At boutons pre-treated with DMSO, action potentials of 5 Hz induced >4 times increase of SypHy fluorescence, which was followed by monoexponential decay at τ of 38.6 ± 2.2 s (Nexp = 9, Nbouton = 274). Pre-treatment of boutons with blebbistatin (2.5 μM, 20 min) significantly prolonged the fluorescence decay following the 5 Hz stimulation (τ = 121.8 ± 8.7 s, Nexp = 5, Nbouton = 208, p < 0.01; Fig.6A-C). Similar effects occurred for stimulation with action potentials at 40 Hz, which induced a monoexponential fluorescence decay with τ of 47.9 ± 5.1 s at boutons pre-treated with DMSO (Nexp = 8, Nbouton = 463) and with τ of 113.8 ± 21.6 s at boutons pre-treated with blebbistatin (Nexp = 5, Nbouton = 187, Fig.6D-F). Since blebbistatin did not change the kinetics of vesicle reacidification (τ = 7.6 ± 0.6 s, Nexp = 6, Nbouton = 197; Fig.6G, H) when compared to DMSO (τ = 7.3 ± 0.8 s, Fig.2), the slower fluorescence decay after blebbistatin treatment should result from impairment of endocytosis. Pre-treatment with 50 μM blebbistatin inhibited endocytosis more potently, resulting in continuous accumulation of surface fluorescence during the observation. Previously photo toxicity has been reported for 20 min incubation with blebbistatin at 50 μM but not 2.5 μM (Kolega, 2004). Therefore, we only presented results from boutons pre-treated with 2.5 μM blebbistatin. Our data suggest that like MLCK, myosin regulates both the clathrin-mediated endocytosis and the clathrin-independent endocytosis.
Second, we performed immunoblotting to determine whether Ca2+, calmodulin and MLCK regulate phosphorylation of MLC (Somlyo and Somlyo, 2003). The level of phosphorylated MLC (pMLC), measured by the antibody against MLC phosphorylated at serine residue 19, was low at rest. It increased by ∼2-fold after exposure to 55 mM KCl for 90 s (Fig.7). Transfection by the scrambled shRNA had similar results. Interestingly, transfection with shMLCK 1 or shMLCK 2 did not affect the basal level of pMLC, but prevented the increase of pMLC induced by KCl exposure. These data suggest that MLCK mediates the activity-dependent phosphorylation of MLC, which is consistent with most of the literature. The KCl-evoked increase of pMLC was also reduced by block of Ca2+ channel current with 100 μM CdCl2 and by inhibition of calmodulin with 100 μM calmidazolium (Fig.7C, D). These results suggest that Ca2+/calmodulin regulates the MLCK-mediated phosphorylation of MLC in hippocampal neurons.
Discussion
Our main conclusion of this study is that MLCK facilitates vesicle endocytosis at the small hippocampal boutons by functioning between Ca2+/calmodulin and myosin. We have shown that both acute inhibition and downregulation of endogenous MLCK at cultured hippocampal boutons impaired endocytosis following the action potential-induced exocytosis (Fig.1, 3, 5). Downregulation of MLCK did not decrease the amount of exocytosis measured as SypHy fluorescence increase (Fig.4A, B) or the somatic Ca2+ currents (Fig.4C – E). Consistent with previous measurements of FM1-43 destaining and excitatory postsynaptic potentials (Tokuoka and Goda, 2006), ML-7 was found to reduce exocytosis at boutons bathed in 2 mM Ca2+, probably through off-target inhibition of Ca2+ current previously reported for hippocampal neurons (Tokuoka and Goda, 2006) (but see (Peng et al., 2012)). Our observation that downregulation of MLCK did not significantly affect the cumulated exocytosis induced by 200 action potential at 20 Hz agrees with a previous pharmacological study (Tokuoka and Goda, 2006). However, we cannot exclude a regulatory role of MLCK in exocytosis. As inferred from postsynaptic current recordings at different synapses, MLCK inhibits initial vesicle release (Srinivasan et al., 2008, Lee et al., 2010, Gonzalez-Forero et al., 2012, Garcia-Morales et al., 2015) and promotes replenishment of fast vesicles (Lee et al., 2008). If these dual functions occur at hippocampal boutons, reduction of MLCK activity is expected to first facilitate and then inhibit the increase of SypHy fluorescence triggered by 200 action potentials, which may deplete the recycling pool. As our SypHy imaging is limited in temporal and quantitative resolution, we could not accurately dissect such effects. Similarly, our recent capacitance measurements failed to reveal effects of MLCK inhibitors on the cumulative exocytosis at the calyx (Yue and Xu, 2014), where the inhibitors have been reported to enhance the initial release (Srinivasan et al., 2008) and reduce vesicle replenishment (Lee et al., 2008). With SypHy imaging, current results more convincingly indicated involvement of MLCK in endocytosis, which has much slower kinetics than exocytosis.
Knockdown of MLCK impaired endocytosis following 200 action potentials delivered at 5 – 40 Hz (Fig.5), suggesting that MLCK regulates endocytosis across a wide range of activity levels. As reported, stimulation with 200 action potentials at 5 Hz induces AP-2 dependent, clathrin-mediated endocytosis at hippocampal boutons while stimulation at 40 Hz induces clathrin-independent endocytosis, that depends on dynamin1/3 and endophilin and probably generates endosome-like vacuoles (Kononenko et al., 2014). Therefore, we suggest that MLCK modulates both clathrin-mediated endocytosis and clathrin-independent endocytosis. Consistent with this suggestion, blockers of MLCK inhibit the slow clathrin-mediated endocytosis and the rapid, putatively clathrin-independent endocytosis evoked by different stimulation paradigms from the calyx of Held (Yue and Xu, 2014). In non-neuronal cells, MLCK has also been reported to regulate endocytosis of very different mechanisms, such as caveolar endocytosis in intestinal epithelial (Schwarz et al., 2007, Marchiando et al., 2010), phagocytosis in polymorphonuclear leukocytes (Mansfield et al., 2000), and receptor-mediated phagocytosis and macropinocytosis in macrophages (Araki et al., 2003). It is likely that MLCK targets a universal mechanism in these different pathways of endocytosis.
In smooth muscle cells, MLCK regulates membrane tension to control cell migration and protrusion, which does not involve MLC phosphorylation (Chen et al., 2014). Our observations suggest that MLCK regulates synaptic vesicle endocytosis by phosphorylation of MLC. First, downregulation of endogenous MLCK did not affect the basal phosphorylation of MLC in cultured neurons, but selectively prevented the depolarization induced increase of pMLC (Fig.7). Because myosin phosphorylation leads to its activation, this result suggests that MLCK is important for activity-dependent functions of myosin in hippocampal neurons. Second, like downregulation of MLCK, acute inhibition of nonmuscle myosin II impaired endocytosis induced by action potentials at both 5 Hz and 40 Hz (Fig.6), indicating that myosin II and MLCK regulate endocytosis regardless of its clathrin-dependence. A recent study concludes that myosin II mainly regulates clathrin-mediated compensatory endocytosis at hippocampal boutons, based on inhibitory effects of blebbistatin and genetic knockout of myosin II on uptake of FM1-43 and horseradish peroxidase following prolonged potassium depolarization (Chandrasekar et al., 2013). This work thus extends the previous conclusion by suggesting that myosin plays a role in both clathrin-mediated endocytosis and clathrin-independent bulk endocytosis. Consistently, a number of studies have suggested that myosin II participates in a variety of endocytosis pathways, such as clathrin-mediated endocytosis in neurons (Chandrasekar et al., 2013, Yue and Xu, 2014), bulk endocytosis at endocrine cells and immune cells (Idrissi et al., 2012, Chandrasekar et al., 2014, Flores et al., 2014, Gormal et al., 2015, Kokotos and Low, 2015), and phagocytosis as well as caveolar endocytosis at other non-neuronal cells (Mansfield et al., 2000, Araki et al., 2003, Schwarz et al., 2007, Marchiando et al., 2010). The motor protein myosin II can function along with cortical actin to generate membrane tension needed for membrane bending (Idrissi et al., 2012) and/or fission (Flores et al., 2014, Gormal et al., 2015, Kokotos and Low, 2015). As an early step of bulk endocytosis, the acto-myosin II interaction constricts the neck of budding endosomes in chromaffin cells (Gormal et al., 2015). Capacitance measurements have revealed that both myosin II and actin regulate the fission pore kinetics of secretory cells (Yao et al., 2013, Flores et al., 2014). However, it should be noted that inhibition of actin polymerization, for example by latrunculin, generates relatively weak defects in endocytosis at mammalian central synapses. At the calyx terminals, latrunculin inhibits endocytosis less efficaciously (Yue and Xu, 2015) than blebbistatin (Yue and Xu, 2014). At hippocampal boutons, we observed strong inhibition of blebbistatin on endocytosis induced by 5 Hz and 40 Hz action potentials (Fig. 6), while latrunculin impairs the clathrin-independent endocytosis following the 40 Hz stimulation (Kononenko et al., 2014) and the ultrafast clathrin-independent endocytosis in physiological temperature (Watanabe et al., 2014), but not endocytosis induced by action potentials of lower frequency (Sankaranarayanan et al., 2003). In light of these studies, we speculate that MLCK/myosin may regulate clathrin-independent endocytosis through acto-myosin interaction. How MLCK/myosin modulates clathrin-mediated endocytosis remains an open question.
We found that the depolarization-induced phosphorylation of MLC was prevented by knockdown of MLCK (Fig.7A, B), block of Ca2+ channel current and inhibition of calmodulin (Fig.7C, D), respectively. These observations suggest that MLCK functions downstream of Ca2+/calmodulin to mediate the phosphorylation of MLC. Calmodulin has been indicated in regulating different forms of endocytosis at synapses (Wu et al., 2009, Sun et al., 2010, Yamashita et al., 2010) and adrenal medullary chromaffin cells (Artalejo et al., 1996). At hippocampal boutons, downregulation or inhibition of calmodulin results in slower retrieval of vesicular synaptobrevin following action potentials of 10 Hz (Sun et al., 2010). Inhibitors of calmodulin impair both clathrin-mediated endocytosis and clathrin-independent endocytosis at the calyx (Wu et al., 2009). Taken together, we conclude that MLCK contributes to the regulation of endocytosis by Ca2+/calmodulin. Since downregulation of MLCK did not affect the basal amount of pMLC (Fig.7), we cannot exclude the possibility that activity of MLCK and/or myosin is simultaneously regulated by other factors, for example, membrane-derived bioactive phospholipids (Garcia-Morales et al., 2015), the neural cell adhesion molecule (Polo-Parada et al., 2005), protein kinase C (Maeno-Hikichi et al., 2011), Rho/Rho-kinase (Garcia et al., 1999, Gonzalez-Forero et al., 2012) and p21-activated kinases (Sanders et al., 1999). Whether modulation of MLCK/myosin through alternative mechanisms contributes to synaptic endocytosis remains interesting to explore.
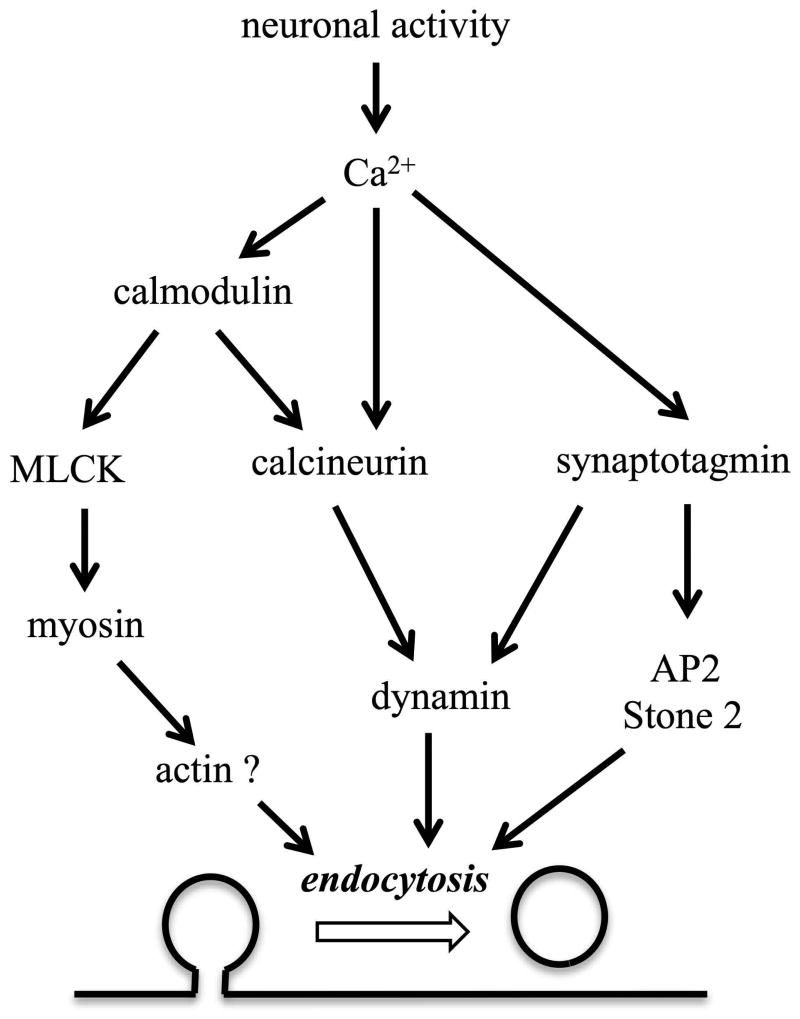
In addition to calmodulin, synaptotagmin and calcineurin are indicated as Ca2+ binding proteins to regulate synaptic endocytosis (Liu et al., 1994, Yao et al., 2012a, Cheung and Cousin, 2013, Wu et al., 2014)(Fig.8). Synaptotagmin can directly interact with the pleckstrin homology of dynamin 1 and regulate the fission pore kinetics in adrenal chromaffin cells (Yao et al., 2012b, McAdam et al., 2015). Synaptotagmin can also bind with the accessory proteins of endocytosis, such as AP2 and Stone 2 (Fergestad and Broadie, 2001). Calcineurin is a Ca2+-dependent protein serine/threonine phosphatase that may dephosphorylate dynamin to activate its function in detaching the membrane invagination from the plasma membrane (Liu et al., 1994, Clayton et al., 2007, Xue et al., 2011). While calmodulin may activate calcineurin, the current study suggests that MLCK mediated Ca2+/calmodulin dependent regulation of endocytosis by phosphorylation of myosin (Fig.8). It is possible that synaptotagmin, calcineurin, and calmodulin all regulate membrane scission by interacting with dynamin and myosin, respectively. The co-existence of multiple Ca2+ sensors and/or effectors reflects the complexity of activity-dependent regulation of endocytosis, a process of multiple steps that involve many different lipid and protein molecules (Saheki and De Camilli, 2012). Indeed, endocytosis is slower under prolonged mild Ca2+ elevation (von Gersdorff and Matthews, 1994, Wu and Wu, 2014) or following Ca2+ influx generated by a single action potential (Leitz and Kavalali, 2011, Armbruster et al., 2013), but significantly accelerates in response to strong Ca2+ signal following bursts of action potentials or a train of prolonged depolarization pulses (Wu et al., 2005, Hosoi et al., 2009, Wu and Wu, 2009, Yamashita et al., 2010, Armbruster et al., 2013). Such a complex regulation of vesicle endocytosis by different Ca2+ signals may be achieved through multiple distinct Ca2+-dependent pathways, including the previously suggested calmodulin/calcineurin pathway (Sun et al., 2010) and the currently proposed calmodulin/MLCK/myosin pathway (Fig.8). To summarize, we suggest that MLCK links Ca2+/calmodulin and myosin to facilitate clathrin-mediated and clathrin-independent endocytosis at conventional synapses, serving as a less-recognized mechanism of activity-dependent regulation of synaptic vesicle endocytosis. Our study will also contribute to better understanding of synaptic physiology of hippocampal neurons, which play a critical role in learning and memory.
Figure 8.
Synaptotagmin, calmodulin and calcineurin are the Ca2+ sensors indicated in synaptic vesicle endocytosis of different test systems. Synaptotagmin can control endocytosis through interaction with two clathrin adaptors, stoning and AP-2. Calcineurin may function directly or downstream of calmodulin to regulate membrane fission through dephosphorylation of dynamin. The current study presents evidence that MLCK can be an alternate pathway for Ca2+/calmodulin to regulate endocytosis through its phosphorylation of myosin, a motor molecule involved in vesicle endocytosis likely through actomyosin interaction.
Acknowledgments
The authors appreciate Drs. Tao Sun, Zhen Zhang and Ping-Yue Pan for technical advice on pHluorin imaging experiments, and Dr. Darrell Brann for insightful comments on the manuscript.
Funding: This work has been supported by 1R01NS082759 from National Institute of Neurological Disorders and Stroke, and the start-up fund from Georgia Regents University to JX.
Abbreviations
- MLCK
myosin light chain kinase
- pMLC
phosphorylated myosin light chain
- shRNA
short hairpin RNA
- shMLCK
short hairpin RNA for MLCK
- SypHy
synaptophysin with intraluminal domain tagged with pH-sensitive green fluorescence protein
Footnotes
Author contributions: All authors participated in research design, data collection and data analysis. J.X. wrote the paper with intellectual contribution from other authors.
ARRIVE guidelines have been followed: Yes. All experiments were conducted in compliance with the ARRIVE guidelines.
Conflicts of interest: none. The authors have no conflict of interest to declare.
Conflicts and Interest Disclosure: The authors do not have any conflicts of interest.
References
- Araki N, Hatae T, Furukawa A, Swanson JA. Phosphoinositide-3-kinase-independent contractile activities associated with Fcgamma-receptor-mediated phagocytosis and macropinocytosis in macrophages. J Cell Sci. 2003;116:247–257. doi: 10.1242/jcs.00235. [DOI] [PubMed] [Google Scholar]
- Armbruster M, Messa M, Ferguson SM, De Camilli P, Ryan TA. Dynamin phosphorylation controls optimization of endocytosis for brief action potential bursts. Elife. 2013;2:e00845. doi: 10.7554/eLife.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Elhamdani A, Palfrey HC. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. doi: 10.1016/s0896-6273(00)80036-7. [DOI] [PubMed] [Google Scholar]
- Atluri PP, Ryan TA. The kinetics of synaptic vesicle reacidification at hippocampal nerve terminals. J Neurosci. 2006;26:2313–2320. doi: 10.1523/JNEUROSCI.4425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji J, Armbruster M, Ryan TA. Calcium control of endocytic capacity at a CNS synapse. J Neurosci. 2008;28:6742–6749. doi: 10.1523/JNEUROSCI.1082-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfod ET, Moore AL, Van de Graaf BG, Lidofsky SD. Myosin light chain kinase and Src control membrane dynamics in volume recovery from cell swelling. Mol Biol Cell. 2011;22:634–650. doi: 10.1091/mbc.E10-06-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SA, Smith C. Physiological stimuli evoke two forms of endocytosis in bovine chromaffin cells. J Physiol. 2001;537:871–885. doi: 10.1111/j.1469-7793.2001.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar I, Goeckeler ZM, Turney SG, Wang P, Wysolmerski RB, Adelstein RS, Bridgman PC. Nonmuscle myosin II is a critical regulator of clathrin-mediated endocytosis. Traffic. 2014;15:418–432. doi: 10.1111/tra.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar I, Huettner JE, Turney SG, Bridgman PC. Myosin II Regulates Activity Dependent Compensatory Endocytosis at Central Synapses. J Neurosci. 2013;33:16131–16145. doi: 10.1523/JNEUROSCI.2229-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Tao T, Wen C, He WQ, Qiao YN, Gao YQ, Chen X, Wang P, Chen CP, Zhao W, Chen HQ, Ye AP, Peng YJ, Zhu MS. Myosin light chain kinase (MLCK) regulates cell migration in a myosin regulatory light chain phosphorylation-independent mechanism. J Biol Chem. 2014;289:28478–28488. doi: 10.1074/jbc.M114.567446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung G, Cousin MA. Synaptic vesicle generation from activity-dependent bulk endosomes requires calcium and calcineurin. J Neurosci. 2013;33:3370–3379. doi: 10.1523/JNEUROSCI.4697-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EL, Evans GJ, Cousin MA. Activity-dependent control of bulk endocytosis by protein dephosphorylation in central nerve terminals. J Physiol. 2007;585:687–691. doi: 10.1113/jphysiol.2007.137539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergestad T, Broadie K. Interaction of stoned and synaptotagmin in synaptic vesicle endocytosis. J Neurosci. 2001;21:1218–1227. doi: 10.1523/JNEUROSCI.21-04-01218.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores JA, Balseiro-Gomez S, Cabeza JM, Acosta J, Ramirez-Ponce P, Ales E. A new role for myosin II in vesicle fission. PLoS One. 2014;9:e100757. doi: 10.1371/journal.pone.0100757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, Verin AD, Schaphorst K, Siddiqui R, Patterson CE, Csortos C, Natarajan V. Regulation of endothelial cell myosin light chain kinase by Rho, cortactin, and p60(src) Am J Physiol. 1999;276:L989–998. doi: 10.1152/ajplung.1999.276.6.L989. [DOI] [PubMed] [Google Scholar]
- Garcia-Morales V, Montero F, Gonzalez-Forero D, Rodriguez-Bey G, Gomez-Perez L, Medialdea-Wandossell MJ, Dominguez-Vias G, Garcia-Verdugo JM, Moreno-Lopez B. Membrane-derived phospholipids control synaptic neurotransmission and plasticity. PLoS Biol. 2015;13:e1002153. doi: 10.1371/journal.pbio.1002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Forero D, Montero F, Garcia-Morales V, Dominguez G, Gomez-Perez L, Garcia-Verdugo JM, Moreno-Lopez B. Endogenous Rho-kinase signaling maintains synaptic strength by stabilizing the size of the readily releasable pool of synaptic vesicles. J Neurosci. 2012;32:68–84. doi: 10.1523/JNEUROSCI.3215-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormal RS, Nguyen TH, Martin S, Papadopulos A, Meunier FA. An Acto-Myosin II Constricting Ring Initiates the Fission of Activity-Dependent Bulk Endosomes in Neurosecretory Cells. J Neurosci. 2015;35:1380–1389. doi: 10.1523/JNEUROSCI.3228-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Hosoi N, Holt M, Sakaba T. Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron. 2009;63:216–229. doi: 10.1016/j.neuron.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Idrissi FZ, Blasco A, Espinal A, Geli MI. Ultrastructural dynamics of proteins involved in endocytic budding. Proc Natl Acad Sci U S A. 2012;109:E2587–2594. doi: 10.1073/pnas.1202789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R, Lemke EA, Klingauf J. Visualization of synaptic vesicle movement in intact synaptic boutons using fluorescence fluctuation spectroscopy. Biophys J. 2005;89:2091–2102. doi: 10.1529/biophysj.105.061663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokotos AC, Low DW. Myosin II and Dynamin Control Actin Rings to Mediate Fission during Activity-Dependent Bulk Endocytosis. J Neurosci. 2015;35:8687–8688. doi: 10.1523/JNEUROSCI.1172-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolega J. Phototoxicity and photoinactivation of blebbistatin in UV and visible light. Biochem Biophys Res Commun. 2004;320:1020–1025. doi: 10.1016/j.bbrc.2004.06.045. [DOI] [PubMed] [Google Scholar]
- Kononenko NL, Puchkov D, Classen GA, Walter AM, Pechstein A, Sawade L, Kaempf N, Trimbuch T, Lorenz D, Rosenmund C, Maritzen T, Haucke V. Clathrin/AP-2 mediate synaptic vesicle reformation from endosome-like vacuoles but are not essential for membrane retrieval at central synapses. Neuron. 2014;82:981–988. doi: 10.1016/j.neuron.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Lee JS, Ho WK, Lee SH. Post-tetanic increase in the fast-releasing synaptic vesicle pool at the expense of the slowly releasing pool. J Gen Physiol. 2010;136:259–272. doi: 10.1085/jgp.201010437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kim MH, Ho WK, Lee SH. Presynaptic release probability and readily releasable pool size are regulated by two independent mechanisms during posttetanic potentiation at the calyx of Held synapse. J Neurosci. 2008;28:7945–7953. doi: 10.1523/JNEUROSCI.2165-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman EM, Tewari A, Horn M, Urbanski M, Damanakis E, Einheber S, Salzer JL, de Lanerolle P, Melendez-Vasquez CV. MLCK regulates Schwann cell cytoskeletal organization, differentiation and myelination. J Cell Sci. 2011;124:3784–3796. doi: 10.1242/jcs.080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitz J, Kavalali ET. Ca(2)(+) influx slows single synaptic vesicle endocytosis. J Neurosci. 2011;31:16318–16326. doi: 10.1523/JNEUROSCI.3358-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Sim AT, Robinson PJ. Calcineurin inhibition of dynamin I GTPase activity coupled to nerve terminal depolarization. Science. 1994;265:970–973. doi: 10.1126/science.8052858. [DOI] [PubMed] [Google Scholar]
- Maeno-Hikichi Y, Polo-Parada L, Kastanenka KV, Landmesser LT. Frequency-dependent modes of synaptic vesicle endocytosis and exocytosis at adult mouse neuromuscular junctions. J Neurosci. 2011;31:1093–1105. doi: 10.1523/JNEUROSCI.2800-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield PJ, Shayman JA, Boxer LA. Regulation of polymorphonuclear leukocyte phagocytosis by myosin light chain kinase after activation of mitogen-activated protein kinase. Blood. 2000;95:2407–2412. [PubMed] [Google Scholar]
- Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, Turner JR. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam RL, Varga KT, Jiang Z, Young FB, Blandford V, McPherson PS, Gong LW, Sossin WS. The juxtamembrane region of synaptotagmin 1 interacts with dynamin 1 and regulates vesicle fission during compensatory endocytosis in endocrine cells. J Cell Sci. 2015 doi: 10.1242/jcs.161505. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Kakita S, Takahashi I, Kawahara K, Tsukuda E, Sano T, Yamada K, Yoshida M, Kase H, Matsuda Y, et al. Wortmannin, a microbial product inhibitor of myosin light chain kinase. J Biol Chem. 1992;267:2157–2163. [PubMed] [Google Scholar]
- Pasque V, Gillich A, Garrett N, Gurdon JB. Histone variant macroH2A confers resistance to nuclear reprogramming. EMBO J. 2011;30:2373–2387. doi: 10.1038/emboj.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng A, Rotman Z, Deng PY, Klyachko VA. Differential motion dynamics of synaptic vesicles undergoing spontaneous and activity-evoked endocytosis. Neuron. 2012;73:1108–1115. doi: 10.1016/j.neuron.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Polo-Parada L, Plattner F, Bose C, Landmesser LT. NCAM 180 acting via a conserved C-terminal domain and MLCK is essential for effective transmission with repetitive stimulation. Neuron. 2005;46:917–931. doi: 10.1016/j.neuron.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Richards DA, Rizzoli SO, Betz WJ. Effects of wortmannin and latrunculin A on slow endocytosis at the frog neuromuscular junction. J Physiol. 2004;557:77–91. doi: 10.1113/jphysiol.2004.062158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TA. Inhibitors of myosin light chain kinase block synaptic vesicle pool mobilization during action potential firing. J Neurosci. 1999;19:1317–1323. doi: 10.1523/JNEUROSCI.19-04-01317.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki Y, De Camilli P. Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol. 2012;4:a005645. doi: 10.1101/cshperspect.a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Ishikawa T, Matsushima S, Naka M, Hidaka H. Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem. 1987;262:7796–7801. [PubMed] [Google Scholar]
- Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Atluri PP, Ryan TA. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat Neurosci. 2003;6:127–135. doi: 10.1038/nn1002. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Forsythe ID. The calyx of Held. Cell Tissue Res. 2006;326:311–337. doi: 10.1007/s00441-006-0272-7. [DOI] [PubMed] [Google Scholar]
- Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, Fu YX, Turner JR. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383–2394. doi: 10.1053/j.gastro.2007.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrooke S, Qiu X, Stewart BA. Nonmuscle Myosin II helps regulate synaptic vesicle mobility at the Drosophila neuromuscular junction. BMC Neurosci. 2010;11:37. doi: 10.1186/1471-2202-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrooke S, Stewart BA. Synaptic transmission and plasticity are modulated by nonmuscle myosin II at the neuromuscular junction of Drosophila. J Neurophysiol. 2011;105:1966–1976. doi: 10.1152/jn.00718.2010. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Srinivasan G, Kim JH, von Gersdorff H. The pool of fast releasing vesicles is augmented by myosin light chain kinase inhibition at the calyx of Held synapse. J Neurophysiol. 2008;99:1810–1824. doi: 10.1152/jn.00949.2007. [DOI] [PubMed] [Google Scholar]
- Sun T, Wu XS, Xu J, McNeil BD, Pang ZP, Yang W, Bai L, Qadri S, Molkentin JD, Yue DT, Wu LG. The role of calcium/calmodulin-activated calcineurin in rapid and slow endocytosis at central synapses. J Neurosci. 2010;30:11838–11847. doi: 10.1523/JNEUROSCI.1481-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T, Ito M, Simmons MA, Schneider CR, Tanaka T, Nakano T, Akasu T. Inhibition by wortmannin of M-current in bullfrog sympathetic neurones. Br J Pharmacol. 1995;114:489–495. doi: 10.1111/j.1476-5381.1995.tb13253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuoka H, Goda Y. Myosin light chain kinase is not a regulator of synaptic vesicle trafficking during repetitive exocytosis in cultured hippocampal neurons. J Neurosci. 2006;26:11606–11614. doi: 10.1523/JNEUROSCI.3400-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994;370:652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Trimbuch T, Camacho-Perez M, Rost BR, Brokowski B, Sohl-Kielczynski B, Felies A, Davis MW, Rosenmund C, Jorgensen EM. Clathrin regenerates synaptic vesicles from endosomes. Nature. 2014;515:228–233. doi: 10.1038/nature13846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Xu J, Wu XS, Wu LG. Activity-dependent acceleration of endocytosis at a central synapse. J Neurosci. 2005;25:11676–11683. doi: 10.1523/JNEUROSCI.2972-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, Adachi R, Bai L, Wu LG. Ca(2+) and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat Neurosci. 2009;12:1003–1010. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Wu LG. Rapid endocytosis does not recycle vesicles within the readily releasable pool. J Neurosci. 2009;29:11038–11042. doi: 10.1523/JNEUROSCI.2367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Wu LG. The yin and yang of calcium effects on synaptic vesicle endocytosis. J Neurosci. 2014;34:2652–2659. doi: 10.1523/JNEUROSCI.3582-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Zhang Z, Zhao WD, Wang D, Luo F, Wu LG. Calcineurin is universally involved in vesicle endocytosis at neuronal and nonneuronal secretory cells. Cell Rep. 2014;7:982–988. doi: 10.1016/j.celrep.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Graham ME, Novelle AE, Sue N, Gray N, McNiven MA, Smillie KJ, Cousin MA, Robinson PJ. Calcineurin selectively docks with the dynamin Ixb splice variant to regulate activity-dependent bulk endocytosis. J Biol Chem. 2011;286:30295–30303. doi: 10.1074/jbc.M111.273110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Eguchi K, Saitoh N, von Gersdorff H, Takahashi T. Developmental shift to a mechanism of synaptic vesicle endocytosis requiring nanodomain Ca2+ Nat Neurosci. 2010;13:838–844. doi: 10.1038/nn.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Kwon SE, Gaffaney JD, Dunning FM, Chapman ER. Uncoupling the roles of synaptotagmin I during endo- and exocytosis of synaptic vesicles. Nat Neurosci. 2012a;15:243–249. doi: 10.1038/nn.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Sakaba T. Activity-dependent modulation of endocytosis by calmodulin at a large central synapse. Proc Natl Acad Sci U S A. 2012;109:291–296. doi: 10.1073/pnas.1100608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao LH, Rao Y, Bang C, Kurilova S, Varga K, Wang CY, Weller BD, Cho W, Cheng J, Gong LW. Actin Polymerization Does Not Provide Direct Mechanical Forces for Vesicle Fission during Clathrin-Mediated Endocytosis. J Neurosci. 2013;33:15793–15798. doi: 10.1523/JNEUROSCI.2171-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao LH, Rao Y, Varga K, Wang CY, Xiao P, Lindau M, Gong LW. Synaptotagmin 1 is necessary for the Ca2+ dependence of clathrin-mediated endocytosis. J Neurosci. 2012b;32:3778–3785. doi: 10.1523/JNEUROSCI.3540-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue HY, Xu J. Myosin light chain kinase accelerates vesicle endocytosis at the calyx of Held synapse. J Neurosci. 2014;34:295–304. doi: 10.1523/JNEUROSCI.3744-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue HY, Xu J. Cholesterol regulates multiple forms of vesicle endocytosis at a mammalian central synapse. J Neurochem. 2015;134:247–260. doi: 10.1111/jnc.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wang D, Sun T, Xu J, Chiang HC, Shin W, Wu LG. The SNARE proteins SNAP25 and synaptobrevin are involved in endocytosis at hippocampal synapses. J Neurosci. 2013;33:9169–9175. doi: 10.1523/JNEUROSCI.0301-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Xu J, Heinemann SF. Two pathways of synaptic vesicle retrieval revealed by single-vesicle imaging. Neuron. 2009;61:397–411. doi: 10.1016/j.neuron.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]