Abstract
Although aromatase is expressed in both male and female brains, its functional significance in females remains poorly understood. In female quail, sexual receptivity is activated by estrogens. However it is not known whether sexual motivation is similarly estrogen-dependent and whether estrogens locally produced in the brain contribute to these behavioral responses. Four main experiments were designed to address these questions. In experiment 1 chronic treatment of females with the anti-estrogen tamoxifen decreased their receptivity, confirming that this response is under the control of estrogens. In experiment 2 chronic treatment with tamoxifen significantly decreased sexual motivation as treated females no longer approached a sexual partner. In experiment 3 (a) ovariectomy (OVX) induced a significant decrease of time spent near the male and a significantly decreased receptivity compared to gonadally intact females, (b) treatment with testosterone (OVX+T) partially restored these responses and (c) this effect of T was prevented when estradiol synthesis was inhibited by the potent aromatase inhibitor Vorozole (OVX+T+VOR). Serum estradiol concentration was significantly higher in OVX+T than in OVX or OVX+T+VOR females. Together these data demonstrate that treatment of OVX females with T increases sexual motivation and that these effects are mediated at least in part by non-gonadal aromatization of the androgen. Finally, assays of aromatase activity on brain and peripheral tissues (Experiment 4) strongly suggest that brain aromatization contributes to behavioral effects observed here following T treatment but alternative sources of estrogens (e.g. liver) should also be considered.
Keywords: Japanese quail, female sexual behavior, aromatase activity, Vorozole, Tamoxifen, approach behavior, partner preference, estrogens, receptivity
Introduction
The function of aromatase in the brain has been a significant topic stimulating extensive work in the field of neuroendocrinology. This enzyme, which converts androgens into estrogens, is present in female ovaries but also in the brain of most/all vertebrate species (for example, (Callard et al., 1978)) including humans of both sexes (Azcoitia et al., 2011; Stoffel-Wagner et al., 1999). The central production of estrogens is involved in the control/modulation of multiple physiological or behavioral processes including memory, pain processing, neural plasticity and regeneration or tumor development (Balthazart and Ball, 2013; Garcia-Segura, 2009). In particular, central aromatization plays a limiting role in the long-term activation by testosterone of male reproductive behavior in birds (Balthazart et al., 2004) and in rodents (Hull and Rodriguez-Manzo, 2009). The long-term control of aggression (Trainor et al., 2006; Ubuka and Tsutsui, 2014; Unger et al., 2015) and parental behavior (Trainor and Marler, 2002) have also been shown to require a local production of estrogens.
Beside these long-term effects on behavior and physiology, brain aromatase is also involved in the short-term control of different behaviors. Estrogens rapidly control male sexual behavior in several species (Cornil et al., 2006a; Cornil et al., 2006b; Cross and Roselli, 1999; Taziaux et al., 2007). A recent study (Coturnix japonica) demonstrated that the specific blockade of central aromatization in quail decreases appetitive sexual behavior within 15 min without affecting consummatory sexual behavior (Seredynski et al., 2013). Other behaviors also seem to be modulated by acute changes in estrogen bioavailability, presumably at the brain level. Song preference and discrimination are rapidly regulated by the local concentrations of estrogens in the brain of male zebra finch (Taeniopygia guttata) (Remage-Healey et al., 2010; Tremere and Pinaud, 2011). Rapid effects of estradiol on aggression have been identified in male mice (Trainor et al., 2008) and effects of aromatase inhibitors have been observed on aggression 24h after the treatment in non-breeding male song sparrows, Melospiza melodia (Soma et al., 2000). In parallel, aromatase activity (AA) in the preoptic area of male quail is decreased within minutes following sexual or visual interaction with the female (Cornil et al., 2005; de Bournonville et al., 2013). Interestingly, changes in AA and/or estradiol concentrations have been identified in different brain regions of males a few minutes after stress (Dickens et al., 2011; Dickens et al., 2014), aggressive interactions (Charlier et al., 2011) or hearing song (Remage-Healey et al., 2008). Together these results strongly suggest that central aromatization of testosterone controls both the motivational and performance aspects of male sexual behavior and that these controls operate in different time-scales, short- and long-term (Cornil et al., 2015). Aromatase is thus a key-enzyme in the short- and long-term control of different behaviors, at least in males.
Most of the studies cited above have been performed in males. However, the female brain also contains a system of cells expressing aromatase and although the activity of this enzyme is slightly lower than in males it is still quite significant (Balthazart, 1997; Roselli et al., 1996; Schumacher and Balthazart, 1986). Moreover, the expression and activity of this enzyme in the female brain is regulated in the long-term by steroids as it is in males (reviewed in (Balthazart et al., 2008; Roselli et al., 1996)). Studies conducted in birds showed that in both sexes brain AA is also rapidly regulated in response to environmental stimulation (Dickens et al., 2011) or cellular neurochemical events (Konkle and Balthazart, 2011). Elevation of local estradiol concentration has also been identified in the caudomedial nidopallium of female zebra finches during the presentation of male song (Remage-Healey et al., 2011). Interestingly, individual variations in AA correlate with the estradiol content in the female preoptic area (Dickens et al., 2014). Together, these observations suggest that the presence of aromatase in the female brain is not simply a non-functional evolutionary vestige. The behavioral function of aromatase in the female brain is however poorly understood. This is probably due to the fact that the female ovaries express a high level of aromatase and consequently produce high concentrations of circulating estrogens suggesting that an alternative source would be redundant. However the observations that brain aromatase is active and acutely regulated raise the question of the function of an additional source of estrogens in the brain of females.
In mammals, female sexual receptivity and motivation are partly controlled by estrogens acting mostly in the ventromedial hypothalamus (reviewed in (Pfaus, 2015)). Non-ovarian testosterone aromatization, and more specifically aromatization in the medial preoptic area and in the ventromedial hypothalamus, is also involved in the regulation of female sexual behavior in musk shrews, a species that engages in sexual behavior before significant ovarian steroid hormone secretion occurs (Rissman et al., 1990; Rissman et al., 1996; Veney and Rissman, 2000). These data suggest that brain aromatization can also play a role in the control of behavior in females, a suggestion clearly reinforced by the recent finding that selective ablation of aromatase neurons of the medial amygdala significantly decreases maternal aggression in female mice (Unger et al., 2015).
The avian brain expresses a much higher level of AA than the rodent brain (Callard et al., 1978; Steimer and Hutchison, 1990), making birds a very interesting model in this field of research. In quail as in rodents, estrogens control female sexual receptivity. A treatment with exogenous estradiol was shown to increase receptivity in females with inactive ovaries (Adkins and Adler, 1972; Noble, 1973). Furthermore, sexual receptivity was restored in ovariectomized females in a dose-dependent manner by injections of estradiol benzoate (Delville and Balthazart, 1987). Finally, a decrease in sexual receptivity was observed in gonadally intact females after a chronic treatment with the anti-estrogens tamoxifen (TAM) (Delville and Balthazart, 1987) or nitromiphene citrate (CI-628) (Adkins and Nock, 1976). Whether sexual motivation is similarly affected by these treatments and whether estrogens affecting sexual receptivity and potentially sexual motivation derive from central aromatization of an androgen remain however unknown.
The main goal of the present study was to define whether estradiol derived from systemic or central aromatization is critical for the activation of all aspects of female sexual behavior in quail (i.e. sexual motivation as well as copulatory performance). Since estrogens derived from central aromatization were shown to differentially affect sexual motivation and sexual performance in males of this species (Seredynski et al., 2013), we decided to analyze both aspects of behavior in females and for this purpose we developed two new tests of sexual motivation in female quail. Four main experiments were then performed. Experiment 1 determined whether sexual receptivity and brain AA are affected by chronic treatment with the estrogen receptor antagonist, TAM. Experiment 2 tested whether estradiol differentially controls female sexual receptivity and motivation. Experiment 3 tested whether non-ovarian testosterone aromatization is involved in the control of both aspects of female sexual behavior. Finally experiment 4 compared aromatase activity in the brain and in peripheral tissues to provide information on the source of non-gonadal estrogens implicated in behavior control.
Materials and Methods
Subjects
Female Japanese quail (Coturnix Japonica) derived from our breeding colony in the laboratory at the University of Liege (Belgium) were employed as subjects in these studies. Sexually mature males obtained from the same colony served as stimuli during the behavioral tests. All subjects were raised in groups until adulthood (approximately 8 weeks) and were housed individually from this time until the end of the experiment. Subjects were maintained on a long day photoperiod (16h light and 8h dark) and provided with food and water ad libitum. All experimental procedures were in agreement with Belgian laws on the “Protection and Welfare of animals” and on the “Protection of experimental animals” and were approved by the Ethics Committee for the Use of Animals at the University of Liege (Protocol #1235).
Behavioral tests
Three different behavioral tests were used alone or in combination in these experiments.
Sexual receptivity test
In experiment 1, sexual receptivity was assessed by placing the female in an arena (60 cm × 40 cm × 50 cm) containing a freely moving male for 5 min. The female responds to male approaches by different behaviors that were quantified (see (Delville and Balthazart, 1987; Noble, 1972; Noble, 1973) for details). All analyses focused on the percentage of male approaches that were followed by crouches (the ultimate receptive posture) or by avoids once the female was within reach of the male.
Approach test
A short corridor (100 cm × 30 cm × 40 cm) was divided into three virtual compartments of equal size (33.3 cm long each) allowing the experimenter to quantify the time spent by an animal in each compartment. The female was placed in one end of this corridor and was allowed to freely move during 10 min. A tethered male was confined in the other end of the corridor preventing him from pursuing the female but allowing him to mount the female when she approached. The side of the corridor where the stimulus male was confined was counterbalanced between sessions. The time spent by the female in the compartment of the male was considered as a measure of sexual motivation. Sexual receptivity was also assessed during this test. Indeed, once the female approached the male, he usually tried to mount her. Female responses to male mounts were quantified as described above (see Sexual receptivity test).
Partner preference test
In this procedure, the experimental female was offered the choice between a stimulus female and a stimulus male tethered at the two ends of a short (100 cm × 30 cm × 40 cm for experiment 2) or long corridor (230 cm × 22 cm × 22 cm for experiment 3) divided into three virtual compartments of equal size (33.3 or 76.6 cm long). The time spent by the experimental female in each of these compartments was quantified. Male and female sides were counterbalanced between sessions. At the beginning of the test, the experimental female was placed in the central compartment of the arena and was then allowed to move freely in the whole corridor during 10 min. As in the approach test, the time spent by the female in the compartment of the male was considered as a measure of sexual motivation and once in the male compartment, her responses to male approaches were quantified to assess receptivity.
Experimental protocols
Prior to each experiment, stimulus males were pre-tested for copulatory behavior until they had gained sexual experience and were habituated to the tether in the test arena.
Experiment 1 evaluated the role of estrogens on sexual receptivity and brain AA. Gonadally intact adult females were chronically injected for 9 days with TAM (n=7) or with the control vehicle (n=6). They were tested twice for sexual receptivity during a baseline period (day −3 and day 0) and every other day during the treatment period. Females were 8 weeks old at the beginning of the experiment (day −3). On day 9, their brains were collected via rapid decapitation. These were quickly dissected out of the skull (see below for details), fresh frozen and stored at −80°C until assayed for AA.
Experiment 2 studied the role of estrogens on sexual receptivity and motivation. Gonadally intact adult females that were regularly laying eggs (8 weeks post-hatch) were first given sexual experience in three sessions of 5 min in the sexual receptivity test in order to habituate them to the handling and test conditions. Each female was then tested for sexual motivation and sexual receptivity using the approach test (five times) and the partner preference test (once) before the beginning of treatments. They were then chronically treated with TAM (n=7) or its vehicle (n=9) for 15 days. They were tested four times in the approach test and once in the partner preference test during the treatment period, and again four times in the approach test and once in the partner preference test during the recovery period (14 days) after cessation of the injections. At least 24h of rest were given between each test.
Experiment 3 tested whether non-ovarian aromatization is needed for testosterone to activate sexual receptivity and motivation. To this end, 26 females were ovariectomized while 12 females were sham-operated around 3 weeks of age. When birds were 12 weeks old, they were habituated to the test conditions and to approach a tethered male during three approach tests. They were then tested for sexual receptivity and motivation using the partner preference test (long corridor) for one session before the beginning of endocrine treatments. Ovariectomized females were then divided into three groups that received either an empty Silastic™ Implant (OVX, n=8), an implant filled with testosterone (OVX+T, n=9) or an implant filled with testosterone and two daily injections of the aromatase inhibitor Vorozole™ (OVX+T+VOR, n=9; see below). The other groups were injected in parallel with the Vorozole™ solvent, propylene glycol (see below). They were tested in the partner preference test once a week for 4 weeks. Birds were then killed by rapid decapitation and trunk blood was collected. Blood samples were centrifuged at 9000 g for 10 min and sera were frozen at −80°C until assayed for estradiol.
Experiment 4 was designed to compare the production of estradiol in the brain and the periphery. To this end, 11 females were ovariectomized while 6 females were Sham-operated. Half of ovariectomized females received an empty implant (OVX, n=5) while others received an implant filled with testosterone (see Experiment 3 for characteristics) (OVX+T, n=6). Sham females also received an empty implant (SHAM, n=6). At 10 weeks of age, i.e. 3 weeks after placement of the implants, the experiment was terminated and different tissue samples were collected. First, blood from the jugular vein (i.e. blood coming from the brain) was collected using a heparinized syringe. Second, the female was killed by rapid decapitation and the trunk blood (i.e. blood coming from the periphery) was collected in a heparinized Eppendorf tube. Blood samples were processed as in the previous experiment until assayed for estradiol and testosterone. The following organs were collected and frozen at −80°C until assayed for AA : the entire brain, portions of the pectoral muscle and of the thigh muscle, abdominal fat, heart, kidney, adrenals, small intestine, oviduct, and the ovarian follicle 3 of SHAM females (the 3rd one in the laying sequence, which is particularly rich in aromatase; (Rodriguez-Maldonado et al., 1996)) that was drained of its yolk.
Hormonal treatment
Females from experiments 1 and 2 were chronically treated with the antiestrogen Tamoxifen (TAM; Sigma Aldrich) or the vehicle solution (propylene glycol/saline, 4:1). They were injected twice a day in the pectoral muscles at a dose of 1mg/animal. This dose had been shown to significantly decrease receptivity in intact female quail (Delville and Balthazart, 1987).
In experiment 3 and 4, some ovariectomized females (OVX+T) received a 20 mm long Silastic™ implant (o.d.= 2.41 mm; i.d.= 1.57 mm) filled with crystalline testosterone (Fluka analytical, Sigma Aldrich) while other ovariectomized females (OVX) and SHAM-operated females (SHAM) received an empty implant of the same size. From the 18 testosterone treated-females of experiment 3, one half were chronically treated with the potent aromatase inhibitor Vorozole™ (graciously provided by Dr. R. DeCoster, Janssen Research Foundation, Beerse, Belgium; OVX+T+VOR) and the other half with the vehicle solution (OVX+T; propylene glycol/saline, 4:1). Females from all other groups (SHAM and OVX groups) were also injected twice daily with the vehicle solution. Vorozole™ injections (1mg/kg) were performed in the pectoral muscles twice a day. This dose was chosen based on studies in male quail demonstrating a strong inhibition of consummatory sexual behavior and a decrease of appetitive sexual behavior following such a treatment (Seredynski et al., 2013; Taziaux et al., 2004).
Morphological and physiological measures
Several morphological or physiological measures were collected every day (experiment 1), every four days (experiment 2), every week (experiment 3) or just before the sacrifice (experiment 4). First, the average number of eggs laid each day by each female was recorded for all experiments. Second, the cloacal diameter was measured with a caliper in females from experiments using estrogenic manipulations (experiment 1 and 2) to obtain an index of the circulating concentration of estradiol for each female (Noble, 1973). Third, the length and width of the cloacal gland were measured with a caliper in females from experiments using androgenic manipulations (experiment 3 and 4). The cloacal gland area (mm2) was defined by multiplying these two measures. This gland is an androgen-sensitive structure (Sachs, 1967) and thus provides a sensitive index of the testosterone plasma concentration (Delville and Balthazart, 1987; Follett and Maung, 1978). Finally, at autopsy, the completeness of ovariectomy was checked and females with gonadal regrowth were removed from the final analysis (7 out of 45 females were removed in experiment 3 and, 4 out of 21 females were removed in experiment 4). The presence of an implant (filled with testosterone or empty) was also successfully confirmed at autopsy.
Brain microdissection
In experiment 1, AA was quantified in the rostral and in the caudal parts of the preoptic-hypothalamic area (HPOA). Immediately after decapitation, the brain was removed from the skull and the entire HPOA block was collected by two coronal cuts at the level of the tractus septopallio-mesencephalicus (rostral edge of the preoptic area) and of the oculomotor nerves (caudal edge of hypothalamus), two parasagittal cuts placed approximately 2 mm lateral to the brain midline and one horizontal cut approximately 2 mm above the floor of the brain (Cornil et al., 2005). This HPOA block was cut in the middle of the antero-posterior axis in two equal parts. Based on the distribution of aromatase-immunoreactive cells and aromatase activity in the quail brain (Foidart et al., 1995; Cornil et al., 2011), the rostral part of this block (rostral HPOA) contained three large groups of aromatase-expressing cells namely the medial preoptic nucleus (POM), the bed nucleus of the stria terminalis (BNST) and the rostral ventromedial nucleus of the hypothalamus (VMN), while the caudal portion of the HPOA contained the population of aromatase cells belonging to the caudal VMN and the tuberal hypothalamus. Each dissection was made in less than 2 min to process a brain and samples were kept on ice to avoid any loss of enzymatic activity. These two brain parts were frozen on dry ice immediately after their dissection and were stored at −80°C until used for AA assay. In experiment 4, the entire brain was collected in less than 1 min on the day of sacrifice and was immediately frozen on dry ice and kept at −80°C. The microdissection of the HPOA block was performed in following days, on dry ice to avoid any loss of enzymatic activity. The left telencephalon was also microdissected and frozen at −80°C until assayed for AA.
AA assay
Every sample (rostral and caudal HPOA in experiment 1; HPOA, telencephalon, pectoral and thigh muscle, adipose tissue, heart, kidney, adrenals, small intestine, oviduct, and follicle 3 in experiment 4) were homogenized in 1 mL of ice-cold TEK buffer (150 mM KCl, 1 mM Na-EDTA, and 10 mM Tris—HCl; pH 7.2) and frozen at −80°C until assayed. AA was quantified by measuring the production of tritiated water associated with the conversion of [1β-3H]-androstenedione into estrone (Baillien and Balthazart, 1997; Roselli and Resko, 1991) as previously described and validated for quail brain (Baillien and Balthazart, 1997). Briefly, the enzymatic reaction was initiated by incubating the sample in the presence of 3H-androstenedione (final concentration 25 nM, specific activity 20.7 Ci/mmol for assays of experiment 1, 24.0 Ci/mmol for assays of experiment 4; Perkin-Elmer), NADPH (4.8 nM) and TEK buffer at 37°C for 15 min. This reaction was stopped by adding 2% charcoal in 10% trichloroacetic acid. 3H-Water was filtered through Dowex cation exchange columns and quantified by adding 10 mL of Optiphase Highsafe (Perkin Elmer) and counting for 3 min on a Wallac Winspectral 1414 Liquid Scintillation Counter. In addition, an extra tube was assayed for each sample in the presence of an excess (40 mM, final concentration) of the potent and specific aromatase inhibitor, Vorozole. This value was subtracted from the average value obtained from the duplicates of the experimental sample as a blank to determine the final specific activity. Blank values averaged between 92 and 147 CPM depending on the tissue, which is considerably lower than the experimental values obtained for HPOA and ovarian follicles (900 and 10000 CPM respectively). AA values were corrected for quenching, recovery, blank values and percentage of tritium in β-position in the substrate. All results from experiment 1 were expressed as pmol of aromatization product per hour for the total HPOA block. These samples were processed in one assay and the intra-assay variation coefficient was 9.56 ± 5.9. Results from experiment 4 were expressed in two different ways: as (1) fmol of aromatization product per hour and per mg of fresh weight and (2) pmol per hour for the entire organ (measured directly or estimated from data in the literature). AA in all tissues was determined during a total of 6 assays, with each assay containing all samples for 2 tissues. Internal standards were added in each assay to provide an inter-assay variation coefficient of 17.69 ± 5.4 and an intra-assay variation coefficient of 5.29 ± 3.2.
Estradiol extraction from serum and radioimmunoassay (RIA)
Sera from blood samples collected in experiment 3 and 4 were extracted using a solid phase extraction protocol previously used for songbirds (Newman et al., 2008) and subsequently described and validated for quail (Dickens et al., 2014). Briefly, this method uses uncapped C18 columns (Sep-Pak C18, 1cm3, 100 mg sorbent) set into a vacuum manifold. The difference between air pressure from inside and outside the manifold forces the liquid through the columns. Immediately after priming with 1 mL of ethanol, columns were equilibrated with 2 × 1 mL of deionized water, followed by sample loading (40 μl of serum previously diluted in 2 mL of water) and then a wash step (2 × 1 mL of deionized water). Finally, steroids were eluted with 1 mL of 90% HPLC-grade methanol and eluates were dried under a steady stream of nitrogen at 37°C. Dried samples were immediately reconstituted into 650 μl in phosphate-buffered saline containing 0.1% of gelatin and 0.7% of ethanol and were assayed for E2 the day following the extraction. Recovery was evaluated independently by adding a known amount of tritiated E2 (10.000 DPM) to four serum samples when the sample was diluted into water before being loaded on columns. The average recovery was 26.53% ± 0.83% and of 24.47 ± 0.41% respectively for samples from experiment 3 and 4. These specific values were used to calculate the final concentrations for all experimental serum samples.
Reconstituted serum samples were assayed for estradiol using a commercially available 125I-E2 radioimmunoassay kit (DSL-4800, Ultra-sensitive Estradiol RIA, Beckman Coulter). All samples (n = 34) were run in duplicates in one unique assay to avoid inter-assay variation. The intra-assay coefficient of variation was 1.67% with a standard deviation of 1.5%. The detection limit of the assay was 0.078 pg/tube. The procedure of this radioimmunoassay was previously described for quail serum samples (Dickens et al., 2014). Briefly, 300 μl of reconstituted sample were incubated at room temperature during 4 hours with 100 μl of anti-estradiol antiserum (diluted 1:3.5) then 100 μl of 125I-E2 (diluted 1:3) was added and incubated at 4°C for 24 h. Following this incubation, 500 μl of precipitating reagent were added and tubes were incubated for 20 min at room temperature and then centrifuged at 1500 g for 20 min. Finally, supernatants were decanted and pellets were counted. Gamma counts were compared to a standard curve established with known E2 quantities to calculate a final concentration per milliliter of serum. Validations of this assay for female quail serum samples were previously performed in our laboratory by controlling the accuracy of measurement of a known added amount of pure steroid (spiking) and measuring the same sample at different dilutions (parallelism) (Dickens et al., 2014). E2 concentration values found in female plasma from experiment 3 and 4 were consistent with previous published values (i.e. about 200 pg/ml in intact females) (Dickens et al., 2014).
Testosterone extraction from serum and enzyme immunoassay (EIA)
Carotid and jugular sera collected in experiment 4 were extracted using a liquid phase extraction protocol and assayed using enzyme immunoassay kits (Cayman Chemical Company, Ann. Arbor, MI) as previously described and validated for female quail serum (Dickens et al., 2011; Dickens et al., 2014). Briefly, 50μl of serum was extracted from its protein components using dichloromethane and dried at 37°C under a stream of nitrogen gas. Samples were reconstituted in 500 μl of EIA buffer supplied with the kit. Samples were assayed in duplicate randomly assigned to one of the two plates, which were both run in the same assay. No sample had concentrations below the detection threshold (0,049 pg/tube). The averaged inter- and intra-assay variation coefficients were respectively 0.43% ± 0.21 and 4.22% ± 3.86. Again, testosterone concentration values found in female plasma were consistent with previous published values (i.e. approximately 1.0 ng/ml in intact females) (Dickens et al., 2011; Dickens et al., 2014).
Statistical analysis
All data were analyzed with Statistica 10.0 (Statsoft Inc.). Behavioral and physiological data were analyzed by parametric statistics (two-way analyses of variance, ANOVAs) when normality conditions were fulfilled or when the number of comparisons within an analysis exceeded 12. Non-parametric tests with Bonferroni correction were used in the other cases. When the ANOVAs detected a significant interaction, post-hoc tests were used to compare all experimental groups at each time points. The choice of the post-hoc test was made based on the number of comparisons within the analysis (Newman-Keuls post-hoc test for less than five comparisons or HSD Tukey post-tests for six or more comparisons). When data were not normally distributed, Mann-Whitney U test were used to analyze the difference between independent groups (or Kruskal-Wallis followed by Mann-Whitney U if more than 2 groups were compared). Friedman and Wilcoxon tests were used to assess the evolution of a response over time. The alpha threshold was divided by the total number of comparisons (Bonferroni correction) to avoid type I error.
AA data in the two experimental groups of experiment 1 were analyzed by separate t-tests for the two hypothalamic sub-regions. Plasma concentrations of estradiol from experiment 3 were transformed with square root function to reach the normality condition and analyzed with a one-way ANOVA followed by a Newman-Keuls post-hoc test.
Given the small sample size, data from experiment 4 (physiological measurements, estradiol, testosterone and AA) were analyzed with non-parametric tests. Kruskal-Wallis followed by Mann-Whitney U tests were used to assess differences between the three groups. For the concentration of steroids in the blood, Friedman and/or Wilcoxon paired-test were also used to assess differences between jugular and peripheral bloods.
The following statistics were calculated depending on the statistical test to estimate the effect size of significant differences: Cohen d was calculated following ANOVAs and t tests, Cohen r was used following non-parametric Mann-Whitney U or Wilcoxon tests, while Cramer V was used after Fisher exact probability test (Fritz et al., 2012). Unfortunately, there, to our knowledge, is no direct way to calculate the effect size of Kruskal Wallis or Friedman tests.
Results
Experiment 1: Tamoxifen decreases female sexual receptivity and inhibits AA in the rostral hypothalamic-preoptic area
Morphological and physiological measures
The number of eggs laid per female per day was significantly different between groups during treatment (UN1=6,N2=7 = 0, p = 0.002; effect size r = 0.861) but not before (UN1=6,N2=7 = 16, p = 0.477; Table 1A). Fisher exact probability test on data collected after eight days of treatment also showed that the number of laying females was significantly different between groups (p < 0.001, none of the TAM females laid at that time, all VEH females did).
Table 1. Morphological and physiological measurements during hormonal manipulations.
Morphological and physiological measures in females treated with Tamoxifen (TAM) or vehicle (VEH) in experiments 1 and 2. The average egg laying was observed for the following time periods: before, during (Days/rows annotated with the mention “TRT”) and after (only in exp. 2) the treatment. The cloacal opening was measured on a day by day basis (Exp. 1) or every four days (Exp. 2: Days 18–34) during these time periods. TAM inhibited egg laying (A, C) and decreased cloacal diameter in both experiments (B, D).
| Exp. 1 | VEH | TAM | Treatment effect |
|---|---|---|---|
| (A) Egg laying | |||
| Before | 1.1 ± 0.07 | 1.0 ± 0.10 | NS |
| During TRT | 0.9 ± 0.03 | 0.09 ± 0.02 # | ** |
| (B) Cloacal opening | |||
| Day −3 | 12.2 ± 0.7 | 12.9 ± 0.8 | NS |
| Day 0 | 13.1 ± 0.5 | 12.0 ± 0.4 | NS |
| Day 1 TRT | 12.1 ± 0.8 | 11.3 ± 0.4 | NS |
| Day 2 TRT | 12.2 ± 0.5 | 10.0 ± 0.2 # | NS |
| Day 3 TRT | 12.6 ± 0.8 | 9.3 ± 0.5 ### | * |
| Day 4 TRT | 12.5 ± 1.0 | 9.1 ± 0.2 ### | * |
| Day 5 TRT | 11.9 ± 0.8 | 8.4 ± 0.4 ### | * |
| Day 6 TRT | 12.7 ± 0.7 | 8.2 ± 0.2 ### | *** |
| Day 7 TRT | 12.6 ± 1.0 | 8.0 ± 0.3 ### | *** |
| Day 8 TRT | 12.3 ± 0.5 | 7.8 ± 0.3 ### | *** |
|
| |||
| Exp. 2 | VEH | TAM | Treatment effect |
|
| |||
| (C) Egg laying | |||
| Before | 0.6 ± 0.07 | 0.7 ± 0.09 | NS |
| During TRT | 0.8 ± 0.05 | 0.05 ± 0.02 ### | *** |
| After | 0.7 ± 0.06 | 0.3 ± 0.07 ## | (*) |
| (D) Cloacal opening | |||
| Day −10 | 10.6 ± 0.3 | 10.6 ± 0.7 | NS |
| Day −6 | 10.6 ± 0.4 | 10.3 ± 0.6 | NS |
| Day −2 | 9.6 ± 0.5 | 9.9 ± 0.5 | NS |
| Day 2 TRT | 9.8 ± 0.3 | 7.8 ± 0.4 # | (*) |
| Day 6 TRT | 10.3 ± 0.4 | 7.3 ± 0.3 ### | *** |
| Day 10 TRT | 9.3 ± 0.3 | 7.4 ± 0.3 ### | NS |
| Day 14 TRT | 10.7 ± 0.2 | 7.0 ± 0.4 ### | *** |
| Day 18 | 9.3 ± 0.6 | 7.7 ± 0.6 ## | NS |
| Day 22 | 10.7 ± 0.4 | 10.2 ± 0.4 | NS |
| Day 26 | 10.7 ± 0.1 | 10.3 ± 0.5 | NS |
| Day 30 | 10.2 ± 0.3 | 9.9 ± 0.2 | NS |
| Day 34 | 10.1 ± 0.2 | 9.6 ± 0.5 | NS |
p < 0.1,
p < 0.05,
p < 0.01,
p < 0.001 compared to VEH.
p < 0.05,
p < 0.05,
p < 0.001 compared to corresponding group during before period (Exp. 1 : Day 0; Exp.2 : Day−2). p values are adapted with the Bonferroni correction when appropriate.
As expected, the estradiol-dependent cloacal opening was decreased by TAM (Table 1B). The ANOVA revealed significant group (F1,11 = 18.76, p = 0.001; effect size d = 2.62) and time effects (F9,99 = 10.81, p < 0.001; effect size d = 2.478) as well as a significant interaction between these factors (F9,99 = 10.30, p < 0.001; effect size d = 5.20). Post hoc analyses of this interaction showed that groups did not differ (p ≥ 0.997) before the beginning of the treatment (days −3 to day 0) and this measure remained stable in control animals during the whole experiment (p > 0.873 compared to day 0). In the TAM group however cloacal opening significantly decreased compared to day 0 starting on day 2 and this effect significantly differed across groups starting on day 3 (see Table 1B for details; effect size d > 1.892).
Sexual receptivity
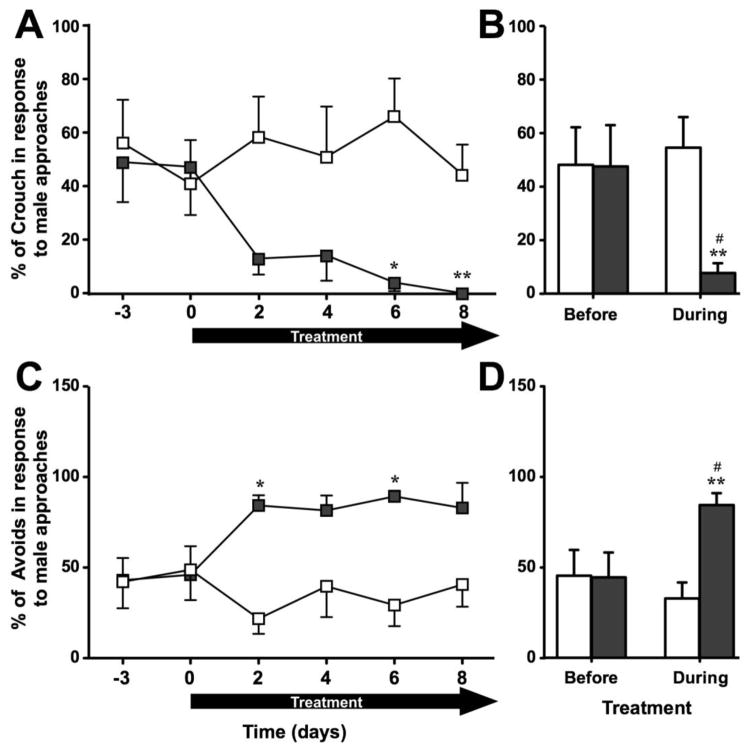
TAM induced a significant decrease in female sexual behavior as indicated by an increase in the number of avoiding behaviors and a decrease in the number of crouching behaviors in response to male approaches (Fig. 1A–D). Compared to other behaviors, the percentage of crouching displayed by the female over the total number approaches by the male did not change over time in controls (X2n=6 = 0.76, p = 0.979) but decreased significantly in TAM females (X2n=7 = 15.41, p = 0.009). Group comparisons showed a significantly decreased percentage of crouches in TAM females compared to controls starting on day 6 (see Fig. 1A for details; effect size r > 0.754). Moreover, when pooling the data across days before and during the treatment, TAM females showed a significantly decreased percentage of crouches compared to controls and compared to their baseline before the treatment (see Fig. 1B for detail; effect size r > 0.753).
Fig. 1.
Sexual receptivity quantified by the sexual receptivity test in females treated with Tamoxifen (TAM - dark grey) or vehicle (VEH - white) as observed on a day by day basis before and during treatment (left panels) or on average before and during treatment (right panels). Compared to controls, females treated with TAM showed fewer crouching responses (A–B, expressed as a percentage of the number of approaches by the male) and more avoidances of the male (C–D, expressed as a percentage of the number of approaches by the male). * p < 0.05, ** p < 0.01 compared to VEH. # p < 0.05 compared to corresponding group before the treatment. p values are adapted with the Bonferroni correction when appropriate.
Changes in the opposite direction were observed when considering the percentage of avoidances displayed by these females. The percentage of avoidances significantly varied over time in TAM but not in control females (X2n=7 = 19.54, p = 0.001; X2n=6 = 2.37, p = 0.796 respectively) and group differences were observed on days 2 and 6 (see Fig. 2C for details; effect size r > 0.774). Moreover, when pooling the data across days before and during the treatment, TAM females showed a significantly increased percentage of avoidances compared to controls and compared to their baseline before treatment (see Fig. 1D for details; effect size r > 0.733).
Fig. 2.
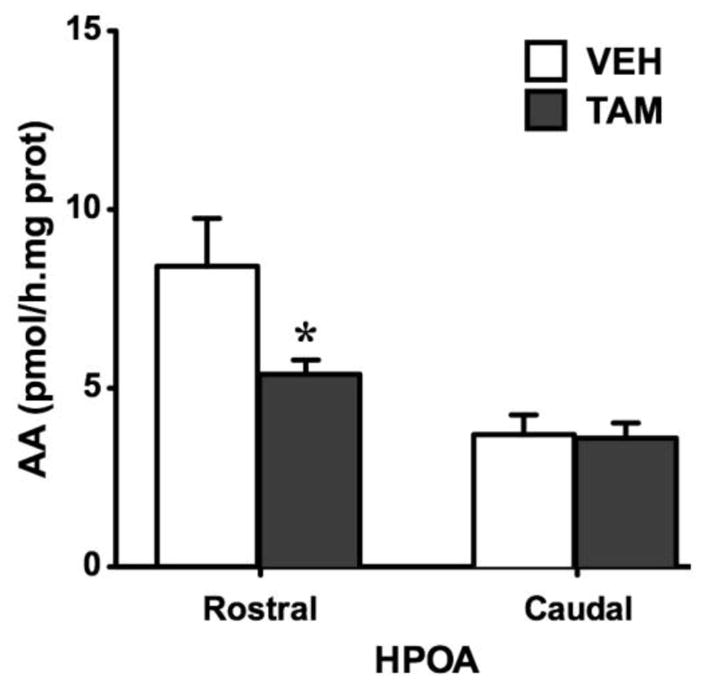
Aromatase activity (AA) in the rostral and the caudal parts of the hypothalamic-preoptic area (HPOA) of females treated with Tamoxifen (TAM - dark grey) or vehicle (VEH - white). TAM induced a significant inhibition of AA in the rostral HPOA. * p < 0.05 compared to VEH.
In conclusion, compared to control females, females treated with TAM showed less crouching responses and avoided the male more, which is indicative of a reduced receptivity.
Aromatase activity
As illustrated in figure 2, AA was significantly decreased by TAM in the rostral HPOA (t10 = 2.52, p = 0.030; effect size d = 1.594) that contains key nuclei implicated in the control of male and female sexual behavior in particular the VMN of the hypothalamus where estrogen action has been shown to induce female receptivity in ring doves, Streptopelia risoria (Gibson and Cheng, 1979). No group difference was observed in the caudal HPOA (t9 = 0.15, p = 0.887).
Experiment 2: Tamoxifen decreases female sexual motivation and receptivity
Morphological and physiological measures
We confirmed results from experiment 1 concerning cloacal diameter and egg laying (Table 1C–D). The analysis of variance of the number of egg layed per bird per day revealed a significant group (F1,14 = 16.47, p = 0.001; effect size d = 2.197) and time effect (F2,28 = 16.01, p < 0.001; effect size d = 1.647) and a significant interaction (F2,28 = 34.59, p < 0.001; effect size d = 5.28). Post hoc analyses indicated that the number of eggs laid per day remained constant in control animals over time (0.372 ≤ p ≤ 0.991) and was not different between groups before treatment (p = 0.936). By contrast, egg laying was decreased in the TAM group during and after treatment compared to baseline and differences between groups were also significant during these two periods (See Table 1C for details; effect size d > 3.745).
The analysis of cloacal opening data showed a significant difference between groups (F1,14 = 12.03, p = 0.004; effect size d = 1.172), an effect of time (F11,154 = 12.05, p < 0.001; effect size d = 2.126) and an interaction (F11,154 = 6.69, p < 0.001; effect size d = 3.79). Post hoc analyses of the interaction indicated, as expected, that if cloacal diameter in control females remained constant over time (Day −2 compared to other days: 0.827 ≤ p ≤ 0.999), TAM females showed a significant decrease in their cloacal diameter from day 2 to day 18 and group differences were observed on day 6 and 14 (See Table 1D for details; effect size d > 1.459).
Sexual motivation
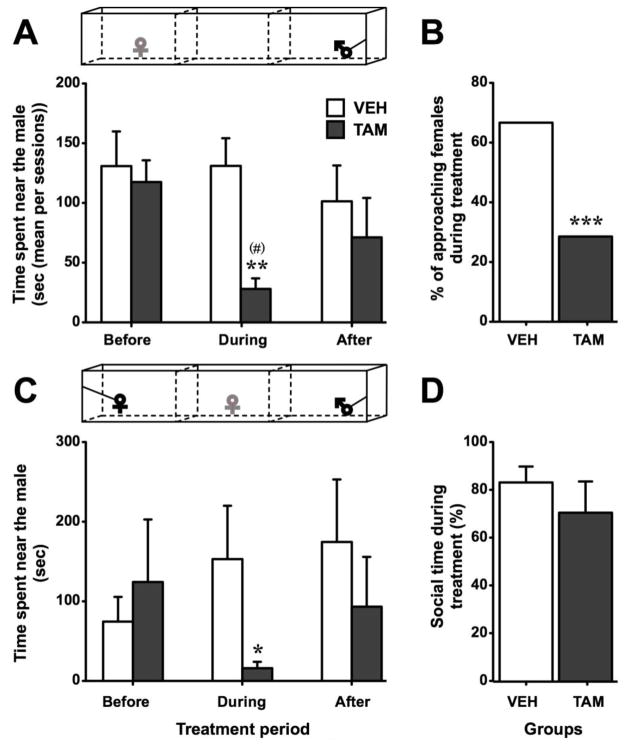
The time spent by the female near the male in the approach test, analyzed by Friedman test, did not change over time in control females (X2n=9 = 1.56, p = 0.459) while it significantly fluctuated in TAM females (X2n=7 = 6.00, p = 0.050). Indeed, in TAM females, this response tended to decrease during the treatment period compared to before. Group comparisons showed a significantly shorter time spent near the male by TAM females compared to controls specifically during treatment (see Fig. 3A for details; effect size r = 0.715). Accordingly, Fisher exact probability test demonstrated that the percentage of females approaching the male was lower in TAM than in control groups in the approach test session performed 12 days after the beginning of the treatment (p < 0.001; Fig. 3B).
Fig. 3.
Sexual motivation quantified in females treated with Tamoxifen (TAM - dark grey) or vehicle (VEH - white) before, during and after the treatment in the approach test (A, mean of all sessions per period) and in the partner preference test (C, one session per period). Females treated with TAM spent less time near the male compared to controls. During the treatment, the percentage of females approaching the male was lower in TAM treated-females compared to control (B). In the partner preference test, the total time spent near a congener (time near female + time near male) during the treatment did not differ between groups (D). * p < 0.05, ** p < 0.01, *** p < 0.001 compared to VEH. (#) p < 0.1 compared to corresponding group during “before” period. p values are adapted with the Bonferroni correction when appropriate.
Similar results were observed in the partner preference test. Indeed, even if the time spent near the male did not fluctuate significantly over time periods (X2n=9 = 1.75, p = 0.417 for VEH; X2n=7 = 2.77, p = 0.250 for TAM), group comparison showed a significant decrease in the time spent near the male by TAM females compared to controls during the treatment period (See Fig. 3C for details; effect size r = 0.493). The social time, i.e. the percentage of time spent by the female near a congener regardless of its sex, did not differ between groups (UN1=9,N2=7 = 26, p = 0.597; Fig. 3D).
In conclusion, in both behavioral tests, the time spent near a sexual partner was noticeably decreased by TAM. This effect was specific to the drug treatment and females resumed spending a larger amount of time near the male when treatment was discontinued.
Sexual receptivity
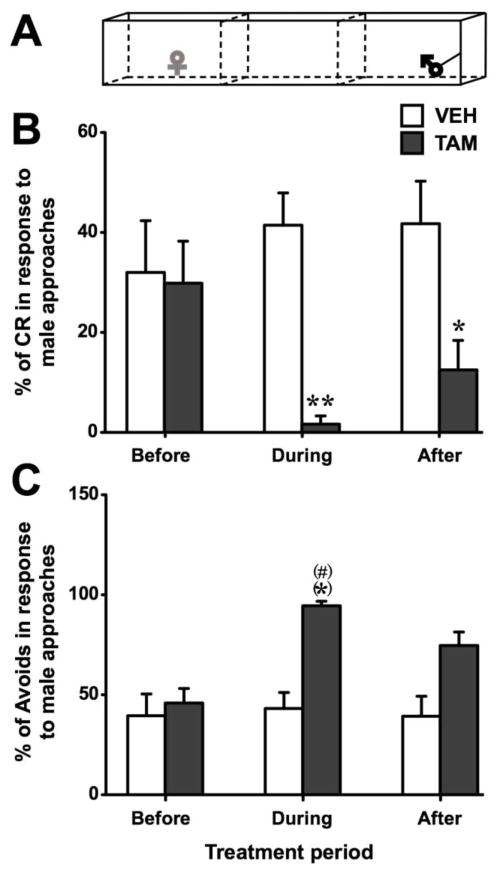
In the approach test (Fig. 4A), receptive and non-receptive behaviors were analyzed among the females that approached the male using Mann-Whitney tests. Although they did not differ from control females before treatment (p = 0.916), TAM females showed a significant decrease in the percentage of crouches displayed during the treatment (p = 0.005; effect size r = 0.708; Fig. 4B). This behavior did not fully recover levels similar to controls after the treatment had ceased (p = 0.029; effect size r = 0.546).
Fig. 4.
Sexual receptivity quantified in females that approached the male during the period when they were treated with Tamoxifen (TAM - dark grey) or vehicle (VEH - white). Data are shown separately for the periods before, during and after the treatment. During the approach test (A – experimental female in grey) when compared to controls females treated with TAM showed fewer crouching responses (B, expressed as a percentage of the number of approaches of the male) and more male avoidance (C, expressed as a percentage of the number of approaches of the male). (*) p < 0.1, * p < 0.05, ** p < 0.01 compared to VEH. (#) p < 0.1 compared to corresponding group during “before” period. p values are adapted with the Bonferroni correction when appropriate.
The ANOVA of the percentage of avoidances revealed a significant effect of group (F1,11 = 6.00, p = 0.032; effect size d = 1.600) and of time (F2,22 = 4.42, p = 0.024) while the interaction was at the threshold of significance (F2,22 = 3.34, p = 0.054; effect size d = 1.193). The suggested interaction presumably resulted from an increased percentage of avoidances in the TAM group during the treatment compared to baseline and compared to controls (p = 0.031 and p = 0.032 respectively; Fig. 4C; effect size d > 1.94).
To summarize, this experiment confirmed the results obtained in experiment 1 showing a decreased sexual receptivity during TAM-treatment as evidenced by an increase in the frequency of avoidances of the male along with a parallel decrease in the frequency of the typical receptive crouch response. In addition, TAM-treatment reduced time spent near a sexual partner in two different tests suggesting that chronic estrogen receptor blockade not only alters sexual receptivity but also motivation.
Experiment 3: Non-ovarian testosterone aromatization contributes to female sexual motivation
Physiological measures
SHAM females regularly laid eggs with an average of 0.84 egg per day. Obviously, no egg was laid by OVX females (data not shown). The cloacal gland area was, as expected, strongly affected by T (Table 2A). The ANOVA showed a main effect of group (F3,34 = 133.7, p < 0.001; effect size d = 2.892), a main effect of time (F9,340 = 68.91, p < 0.001; effect size d = 3.272) and a significant interaction (F27,340 = 19.45, p < 0.001; effect size d = 6.485). Post hoc analyses indicated that the cloacal gland size of SHAM females as well as OVX females remained constant over time (p ≥ 0.589, for day −5 compared to all following days) while OVX+T and OVX+T+VOR females showed an increased cloacal gland size compared to before the treatment (day −5) that was significant starting on day 9. Differences between groups were in agreement with these results: before treatment (from day −40 to day −5), all OVX females (regardless of the treatment) had similar sizes of cloacal gland while after day 9, OVX+T and OVX+T+VOR had a larger cloacal gland compared to OVX females (See Table 2A for details; effect size d > 2.646).
Table 2. Changes in cloacal gland area during hormonal manipulations.
Cloacal gland area of intact females (SHAM), ovariectomized (OVX), ovariectomized females treated with testosterone (OVX+T) or ovariectomized females treated with testosterone and Vorozole (OVX+T+VOR, only in exp. 3) measured before and during (Days/rows annotated with the mention “TRT”) treatment (exp. 3) or just before the sacrifice (exp. 4). Testosterone induced an increase of the size of cloacal gland in both experiments.
| Exp. 3 | SHAM | OVX | OVX+T | OVX+T+VOR |
|---|---|---|---|---|
| Day −40 | 74.6 ± 5.0 *** | 29.6 ± 2.5 | 28.7 ± 2.1 | 29.5 ± 1.8 |
| Day −33 | 84.0 ± 6.5 *** | 33.7 ± 5.3 | 36.0 ± 5.0 | 30.0 ± 3.2 |
| Day −26 | 78.7 ± 4.7 *** | 34.1 ± 4.7 | 41.6 ± 6.9 | 32.3 ± 3.7 |
| Day −19 | 79.3 ± 5.3 *** | 31.5 ± 5.4 | 38.3 ± 8.8 | 30.5 ± 2.8 |
| Day −12 | 83.2 ± 5.8 *** | 31.8 ± 4.4 | 29.3 ± 2.9 | 26.6 ± 1.4 |
| Day −5 | 83.2 ± 5.8 *** | 31.8 ± 4.4 | 29.3 ± 2.9 | 26.6 ± 1.4 |
| Day 2 TRT | 88.5 ± 6.5 *** | 28.5 ± 3.1 | 45.9 ± 2.7 | 59.9 ± 4.3 |
| Day 9 TRT | 94.9 ± 7.1 *** | 29.6 ± 2.9 | 120.2 ± 10.0 *** | 124.5 ± 8.7 *** |
| Day 16 TRT | 102.5 ± 5.8 *** | 26.8 ± 1.9 | 142.2 ± 10.0 *** | 149.6 ± 12.3 *** |
| Day 23 TRT | 93.5 ± 9.3 *** | 23.7 ± 3.0 | 137.2 ± 12. 4 *** | 147.2 ± 10.8 *** |
|
| ||||
| Exp. 4 | SHAM | OVX | OVX+T | |
|
| ||||
| Day 21 TRT | 100.0 ± 9.3 * | 30.3 ± 3.1 | 156.2 ± 13.6 * | |
p < 0.05,
p < 0.001 compared to OVX.
Sexual motivation
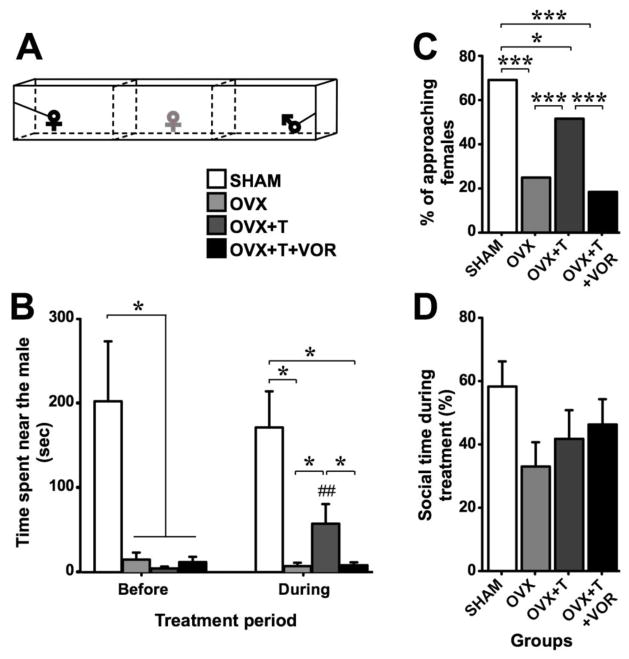
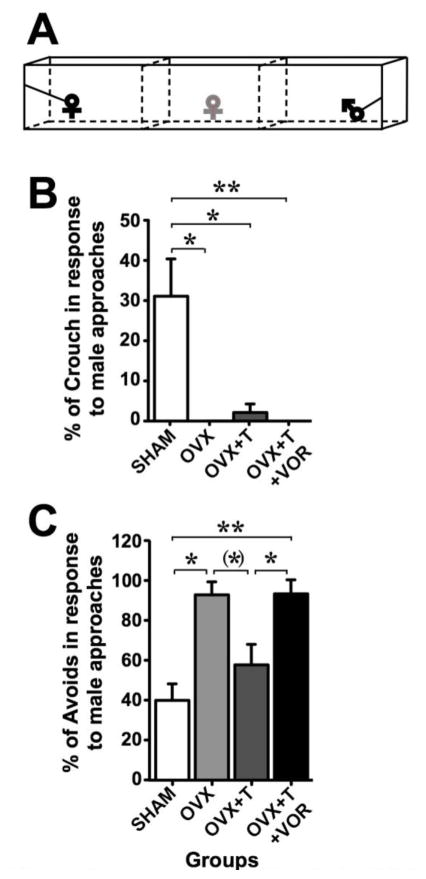
In the partner preference test (Fig. 5A), all ovariectomized females spent less time near the male than SHAM females before the treatment had started (UN1=12,N2=26 = 80, p = 0.011; effect size r = 0.411). Compared to the first session, the time spent by OVX+T females near the male was significantly (or tended to be) increased on the third (day 20, p = 0.043), fourth (day 27, p = 0.079) and fifth session (day 34, p = 0.046) but not on the second session (day 13, p = 0.345), as shown by Wilcoxon paired comparisons (Data not shown). Thus, we analyzed the average of time spent by the female near the male during the third to fifth partner preference test sessions (“During” values; Fig. 5B). During the treatment, SHAM females continued to spend more time near the male than OVX and OVX+T+VOR females (UN1=12,N2=8 = 10, p = 0.023, effect size r = 0.648 and UN1=12,N2=9 = 11, p = 0.015, effect size r = 0.660 respectively) but the difference between SHAM and OVX+T females disappeared (UN1=12,N2=9 = 28, p = 0.420). Moreover, OVX+T females spent significantly more time near the male than OVX and OVX+T+VOR females (UN1=9,N2=8 = 6, p = 0.027, effect size r = 0.689 and UN1=9,N2=9 = 7, p = 0.021, effect size r = 0.687 respectively). Confirming these results, the percentage of approaching females was different between groups (X23 = 70.09, p < 0.001). Specifically, Fisher exact probability test demonstrated that the percentage of approaching SHAM females was higher than in other groups (p ≤ 0.013) and the percentage of approaching OVX+T females was higher than in OVX and OVX+T+VOR (p < 0.001) (Fig. 5C). In contrast, the total time spent near the male or the female did not differ between groups (F3,34 = 1.70, p = 0.186; Fig. 5D).
Fig. 5.
Sexual motivation quantified in intact females (SHAM - white), ovariectomized females (OVX - light grey), ovariectomized females treated with testosterone (OVX+T - dark grey) or ovariectomized females treated with testosterone and vorozole (OVX+T+VOR - black). In the partner preference test (A – experimental female in grey), OVX+T females spent more time near the male compared to OVX and OVX+T+VOR females during the treatment period (B – right part) while there was no difference between these groups before the treatment (B – left part). The percentage of females approaching the male was higher in OVX+T females compared to OVX and OVX+T+VOR females (C) while the total social time did not differ between these two groups (D). * p < 0.05, *** p < 0.001. # p < 0.05 compared to corresponding group during the “before” period. p values are adapted with the Bonferroni correction when appropriate.
In conclusion, ovariectomy induced a significant decrease in time spent in close proximity to the male. Treatment with testosterone partially restored this response, an effect that was prevented when estradiol synthesis was inhibited by vorozole.
Sexual receptivity
The comparison between groups of the percentage of crouches in females who approached the male (other females were excluded from the analysis) in the partner preference test (Fig. 6A) showed that the endocrine treatments did not seem to influence this response since no difference was observed between OVX, OVX+T and OVX+T+VOR females (U ≥ 18, p ≥ 0.350). These three groups however showed a decreased percentage of crouches compared to SHAM females (See Fig. 6B for details; effect size r > 0.686). In contrast, the percentage of avoidances was higher in OVX and OVX+T+VOR females compared to SHAM females while OVX+T females did not differ from SHAM females (See Fig. 6C for details; effect size r > 0.658). Moreover, OVX+T females showed a decreased percentage of avoidances than OVX+T+VOR females (See figure 6 for details; effect size r = 0.709).
Fig. 6.
Sexual receptivity quantified in females that approached the male in the four treatment groups, i.e. intact females (SHAM - white), ovariectomized females (OVX - light grey), ovariectomized females treated with testosterone (OVX+T - dark grey) or ovariectomized females treated with testosterone and Vorozole (OVX+T+VOR - black). In the partner preference test (A – experimental female in grey), the mean of crouching responses (expressed as a percentage of male approaches) over sessions performed during the treatment was not influenced by endocrine treatment (B). The mean of male avoidance (expressed as a percentage of male approaches) over sessions performed during the treatment was lower in OVX+T females compared to OVX+T+VOR females (C). (*) p < 0.1, * p < 0.05, ** p < 0.01. p values are adapted with the Bonferroni correction when appropriate.
Circulating estradiol level
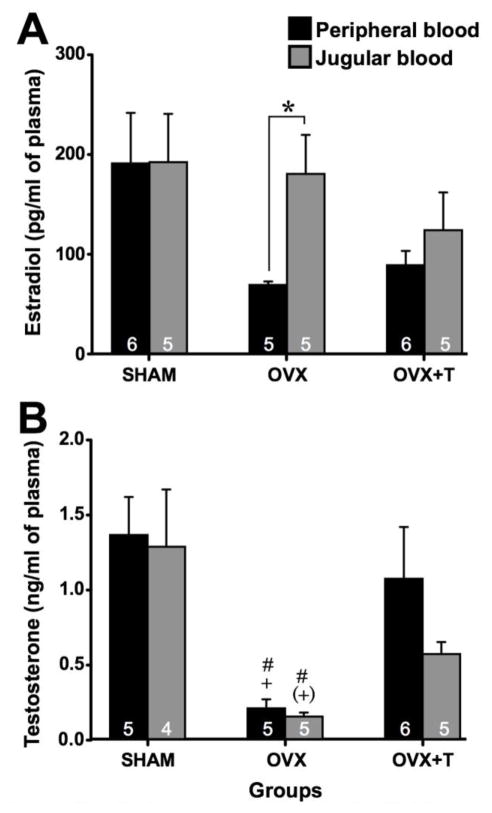
Circulating estradiol concentration significantly differed between groups (F3,27 = 3.27, p < 0.001; effect size d = 3.655). Post hoc analysis revealed that serum estradiol was significantly higher in SHAM females compared to all other groups while sera from OVX+T females contained significantly more estradiol than sera from OVX and OVX+T+VOR females (see Fig. 7 for details; effect size d > 1.03).
Fig. 7.
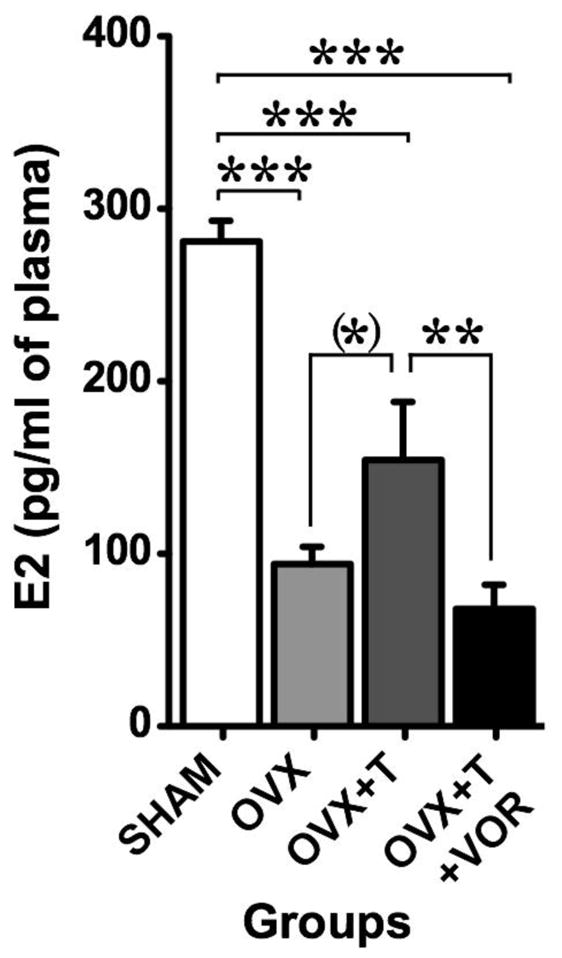
Circulating estradiol concentrations measured in intact females (SHAM - white), ovariectomized females (OVX - light grey), ovariectomized females treated with testosterone (OVX+T - dark grey) or ovariectomized females treated with testosterone and Vorozole (OVX+T+VOR - black). Testosterone induced a small increase of E2 concentration in the blood compared to females without testosterone and this effect was blocked by Vorozole. (*) p < 0.1, ** p < 0.01, *** p < 0.001. p values are adapted with the Bonferroni correction when appropriate.
In summary, this experiment showed that chronic depletion in estrogens results in a reduction of the time spent in contact with a male sexual partner and of crouch behavior but in an increase in avoiding behavior in females approaching their partner. Testosterone treatment increased the time spent in contact with males and restored normal avoiding frequencies but not crouching in approaching females suggesting that non-ovarian estrogens might play a role in sexual motivation but not performance. These behavioral effects were associated with a significant increase in E2 concentration in the blood of OVX+T females compared to OVX females and this increase was blocked by the concomitant aromatase inhibition. This result obviously raised the question of the site of aromatization producing these estrogens.
Experiment 4: Peripheral vs. central testosterone aromatization
Physiological measures
Mann-Witney U tests corrected for the number of comparisons revealed that OVX females had a smaller cloacal gland compared to SHAM females (U = 0, p = 0.024; effect size r = 0.826), both of these groups having a smaller gland than OVX+T females (U ≤ 2, p ≤ 0.038; effect size r > 0.74; Table 2B).
Brain contribution to estradiol and testosterone circulating concentrations
Estradiol and testosterone concentrations were measured in the trunk blood (coming from the periphery) and jugular blood (coming from the brain) of SHAM, OVX and OVX+T females (Fig. 8). For estradiol concentration, Kruskal-Wallis ANOVAs revealed no statistical difference between groups for the jugular samples (H = 2.02; p = 0.364) while a difference between groups was observed for the samples of peripheral blood (H = 5.96; p = 0.050). Estradiol level was significantly higher in jugular compared to peripheral blood samples in OVX females while no difference between blood samples was found in other groups (Wilcoxon tests; see figure 8A for details).
Fig. 8.
Peripheral (black) and Jugular (Grey) steroids concentrations measured in intact females (SHAM), ovariectomized females (OVX), ovariectomized females treated with testosterone (OVX+T). Estradiol concentration was higher in the jugular compared to peripheral blood in OVX females (A). Testosterone was lower in both blood samples of OVX females compared to other groups (B). Sample size is noted with white numbers in the bottom of histogram bars. * p < 0.05, # p < 0.05 vs. SHAM, (+) p < 0.1 vs. OVX+T, + p < 0.05 vs. OVX+T. p values are adapted with the Bonferroni correction when appropriate.
Testosterone concentrations were significantly different between groups both in peripheral (H = 16; p = 0.007) and in jugular blood samples (H = 14; p = 0.005; Fig. 8B). Post hoc Mann-Whitney U tests revealed that peripheral testosterone was lower in OVX females compared to SHAM and OVX+T females, with no difference between these two last groups (see figure for details; effect size r > 0.743). Similarly, jugular testosterone tended to be lower in OVX compared to SHAM females, was lower in OVX compared to OVX+T females and did not differ between SHAM and OVX+T females (see figure for details; effect size r > 0.776). No difference between peripheral vs. jugular blood samples was observed in any of the groups (p ≥ 0.225).
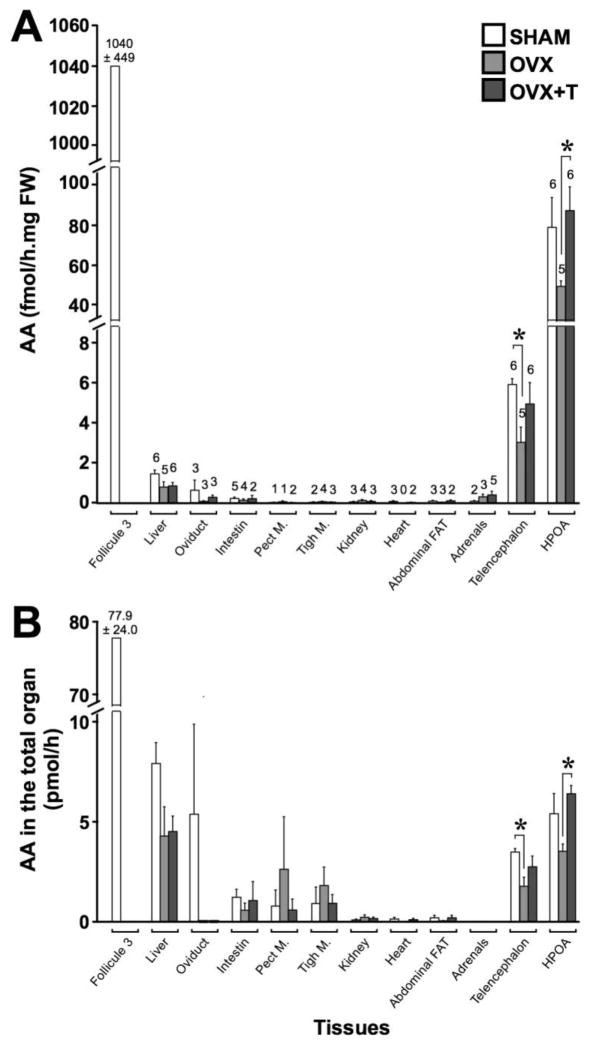
Peripheral vs. brain aromatase activity (AA)
AA was assayed in tissue homogenates including HPOA, telencephalon, pectoral and thigh muscle, adipose tissue, heart, kidney, adrenals, small intestine, and oviduct in SHAM, OVX and OVX+T females and expressed as fmol per mg of fresh weight (Fig. 9A) or pmol in the entire organ (Fig. 9B). The enzymatic activity in ovarian follicle 3, obviously measured only in SHAM females, is plotted on the figure allowing the direct comparison with the different tissues but was not included in the statistical analyses.
Fig. 9.
Aromatase activity assayed in different peripheral and brain tissues of intact females (SHAM - white), ovariectomized females (OVX – light grey), ovariectomized females treated with testosterone (OVX+T – dark grey). AA was expressed by mg of fresh weight in the three experimental groups (A) or as the total enzymatic activity of the organ in the three experimental groups (B). Numbers above histogram bars in (A) represent the number of samples in which AA was detectable on a total of 6 SHAM, 5 OVX and 6 OVX+T (only 5 for the oviduct because one sample was lost). * p < 0.05. p values are adapted with the Bonferroni correction when appropriate.
We first compared with a chi-square test the total number of animals in the pooled experimental groups (n=17) in which AA was detectable or not in all tissues. The number of detectable values (i.e. higher than the vorozole baseline value in the assay) for each tissue in each group is indicated at the top of the bars in Figure 9A. This test revealed a significant difference between tissues (X210 = 53.29; p < 0.001). Fisher exact tests were then used to compare tissues two-by-two (11 × 10/2 comparisons) and indicated that AA was detectable in more animals in the liver, the HPOA and the telencephalon than in all other tissues (p ≤ 0.018; effect size V > 0.493). AA in the intestine was also detectable in more animals compared to pectoral muscles (p = 0.037; effect size V = 0.469). All other comparisons were not significant (p ≥ 0.080).
AA was thus clearly detectable in the two brain samples and in the liver while being extremely low and often below the assay sensitivity in other tissues. The enzymatic activity in each tissue was then calculated for the entire organs (Fig. 9B) based on their weight measured during this experiment (follicle 3: 98.3 ± 14.4mg [means ± SEM]; adrenals: 15.6 ± 1.7mg; oviduct: 4.5 ± 1.0g; small intestine: 5.1 ± 0.2g; HPOA: 72.7 ± 2.9mg) or on estimations derived from the literature (liver: 5.4g; pectoral muscle: 53.8g; thigh muscle: 36.5g; kidney: 2.3g; heart: 2.2g; abdominal fat: 2.9g; telencephalon: 588mg). Interestingly, some organs (such as the liver) had low but detectable levels of enzymatic activity but due to their large size, the total AA in the entire organ was equivalent to the level observed in the brain.
Kruskal-Wallis ANOVAs of the data expressed per mg of fresh weight and for the entire organ revealed that endocrine manipulations significantly affected AA in both the HPOA and telencephalon (H ≥ 6.34; p ≤ 0.042). Subsequent Mann-Whitney post hoc tests showed that AA was lower in OVX compared to OVX+T females in HPOA (U = 1; p = 0.009; effect size r = 0.771) while in the telencephalon AA was lower in OVX compared to SHAM females (U = 0; p = 0.004; effect size r = 0.826; see Fig. 9A–B). No significant effect of manipulations was detected in the other tissues.
Discussion
This set of studies was designed to determine whether both motivational and performance aspects of female sexual behavior are controlled by estrogens in quail and whether non-gonadal (presumably central) aromatization plays a role in this process. The results confirm the estrogenic control of sexual receptivity (Adkins, 1973; Adkins and Adler, 1972; Adkins and Nock, 1976; Delville and Balthazart, 1987; Noble, 1972) and demonstrate that the motivation to approach a male sexual partner is also under the control of estrogens. Interestingly, although these two aspects (i.e., sexual motivation and receptivity) of female sexual behavior are inhibited by aromatase inhibition only the measure of sexual motivation appears to be enhanced by estrogens derived from testosterone aromatization taking place outside the ovary. Based on aromatase activity assays and measures of estrogen concentrations in jugular versus peripheral blood, it is hypothesized that these estrogens originate from the brain however the liver could also constitute a peripheral source of estrogens.
Physiological and morphological measures
Experiments 1 and 2 demonstrate that TAM decreases the cloacal diameter of females, a measure used as an external specific marker of the exposure to estrogenic stimulation (Ball and Balthazart, 2010). An increased cloacal opening following estradiol treatment in (functionally) ovariectomized females has been previously reported in quail (Delville and Balthazart, 1987; Noble, 1972; Noble, 1973) and the only published study using TAM in quail reported a slight decrease in the cloacal opening that did however not reach statistical significance for unknown reasons (Delville and Balthazart, 1987). A correlation has also been reported between the diameter of the cloacal opening and receptivity in female quail (Mills et al., 1997). The present data thus confirm the estrogenic dependency of this morphological trait and the functional removal of estrogenic stimulation after TAM treatment.
In addition, in experiments 3 and 4, ovariectomized females implanted with testosterone had a larger cloacal gland size than others. This observation is also in agreement with previous work showing that the size of this gland is androgen-dependent (Delville and Balthazart, 1987; Sachs, 1967) and such an increase has been previously reported in female quail implanted with testosterone (Schumacher and Balthazart, 1983).
Estrogens and sexual receptivity
In experiment 1, the chronic treatment with the anti-estrogen TAM increased avoiding behavior within two days while crouching responses were inhibited after a slightly longer latency. Results from experiment 2 confirmed this effect and also showed that these measures partially recovered when the TAM treatment was discontinued. Together these results corroborate previous work demonstrating that female quail receptivity is under the control of estrogens (Adkins, 1973; Adkins and Adler, 1972; Adkins and Nock, 1976; Delville and Balthazart, 1987; Noble, 1972). Accordingly, experiment 3 confirmed that ovariectomy decreases receptivity as shown previously after surgical or functional ovariectomy induced by exposure to short photoperiods (Adkins, 1973; Adkins and Adler, 1972; Noble, 1972).
Interestingly, treatment with exogenous testosterone of OVX females restored the percentage of avoiding behavior typical of intact females and this effect was blocked by a concomitant treatment with an aromatase inhibitor. However, testosterone could not restore crouching behavior as only one testosterone-treated female showed the typical receptive crouching behavior. This suggests that the crouching response is controlled by higher doses of estrogens or by ovarian estrogens whose effects cannot be mimicked by estrogens derived from non-ovarian aromatization. Alternatively behavior is partially activated by an ovarian product different from estradiol. This compound is however unlikely to be progesterone since females chronically treated with an anti-progestin did not decrease their receptivity and addition of progesterone to sub-optimal doses of estradiol benzoate did not clearly increase receptivity in OVX females (Delville and Balthazart, 1987).
The absence of crouching in T-treated OVX females might also relate to the need for a longer duration of estrogen action in order to activate this behavior. This is suggested by the observation in experiment 1 that TAM inhibited more rapidly avoiding behavior than crouching behavior. However, in previously published experiments sexual receptivity was fully restored in ovariectomized females to the level seen in gonadally intact females after 20–22 days of estradiol benzoate treatment (Delville and Balthazart, 1987). In experiment 3, females were exposed to testosterone for 34 days. It is possible that this duration was not long enough for the activation of crouching but it is more likely that non-gonadal aromatization did not produce enough estrogens to activate this specific response. Accordingly, circulating concentrations of estradiol in OVX+T females were not as high as in intact females both in the peripheral and in the jugular blood. The crouch response thus presumably involves motor circuits that are not specifically required for male avoidance and are activated only by higher doses of estrogens or exposure to estrogens for a longer time period.
Estrogens and sexual motivation
One main goal of the present experiments was to assess whether sexual motivation in female quail is also estrogen-dependent. In experiment 2, females treated with TAM almost completely stopped approaching the male and as a consequence spent less time near the male compared to the pre-treatment period and to control females during the treatment period. These observations suggest that estrogens are involved in the motivation to approach a sexual partner. Moreover, experiment 3 showed that non-ovarian testosterone aromatization specifically mediates this estrogen-dependent increase in sexual motivation as the decrease in the time spent near the male induced by ovariectomy was partially restored by testosterone and this effect was prevented when estradiol synthesis was blocked by Vorozole. These results confirm the reported decrease of sexually proceptive behaviors such as pecking and hopping/darting previously observed after a chronic treatment with TAM (Delville and Balthazart, 1987). Importantly, this regulation of approach behavior is specific to sexual motivation as it does not relate to general changes in social interest measured in both experiments by the time spent near a male or a female.
Together these results indicate that sexual motivation in female quail is, like in males, controlled by estrogens. The effects of estrogens on this aspect of sexual behavior seem to be even more prominent than the effects on the full sexual response expressed by crouching behavior suggesting a higher sensitivity to estrogenic activation. Future work should determine if as is the case in males (Seredynski et al., 2015; Seredynski et al., 2013), these effects are mediated by fast effects of estrogens initiated at the cell membrane or depend like consummatory sexual responses on slower genomic actions of the steroid.
Non-gonadal aromatization and female sexual behavior
Since the ovaries are a major source of estradiol, it is usually assumed that the activation of sexual interest and sexual receptivity is due to estrogens of ovarian origin. However, we showed here that T activates female sexual motivation to approach a male in the absence of ovaries and that this effect is blocked by an aromatase inhibitor. Another site of androgen aromatization is therefore obviously implicated.
The observation that blood estradiol concentrations were increased in OVX females treated with testosterone and that this effect was blocked by Vorozole (Experiment 3; Fig. 10) could indicate that either AA from peripheral organs is producing the estrogens implicated in the activation of female behavior or AA in the brain produces estradiol that leaks into the periphery. Experiment 4 revealed that, even if AA is very high in the brain, other organs in the periphery also contain an active aromatase displaying the same total level of activity when corrected for the entire size of the organ (Fig. 12B). It is known that in mammals, including humans, aromatase is expressed in peripheral organs/tissues such as bones, skin, adrenals and adipose tissue (Conley and Hinshelwood, 2001; Czajka-Oraniec and Simpson, 2010; Moreau et al., 2009; Simpson, 2004; Simpson et al., 2001). Aromatase expression and estrogen production have also been reported in the stomach of female rats (Kobayashi et al., 2013, 2015) as well as AA in the quail liver (Silverin et al., 2000). In zebra finches, one study compared AA in different tissues and concluded that the brain, besides the ovary, seems to be the only site of estrogen synthesis since AA was undetected in the adrenals, muscle, skin, liver, adipose tissue, kidney, and heart (Schlinger and Arnold, 1991). The technique used by this last study was however not the same as in the present experiment: they measured the various radioactive estrogenic products from tritiated androstenedione in the tissues whereas the present data are based on a tritiated water assay of AA. Catabolism of the estrogens produced in the tissues under study could therefore have limited their detection in zebra finches, a limitation that does not apply to the measure of tritiated water.
Even if AA has been detected in peripheral tissues such as the liver, we believe that aromatase in the HPOA is the non-gonadal source of estrogens that activated female sexual motivation and receptivity in OVX+T females for a variety of reasons. Firstly estrogens produced in the HPOA are at the site where they control female behavior whereas estrogens produced in the liver are exposed to the intense oxidative metabolism and conjugation present in the hepatic tissue (Barbier and Belanger, 2003; Mannisto and Kaakkola, 1999; Song, 2001; Song and Melner, 2000; Zhu and Conney, 1998) which result in a markedly reduced estrogenic activity. Secondly, AA in the HPOA is markedly regulated by sex steroids and by ovariectomy or testosterone treatment in particular. The changes in AA in HPOA in the OVX and OVX+T females thus correspond well with the fluctuations in sexual motivation and receptivity whereas AA is not affected by these endocrine manipulations in the liver. Thirdly experiment 1 showed that TAM injections decrease AA in the rostral HPOA in parallel with the decreased female sexual receptivity. These regulations of AA by ovariectomy or TAM treatment are fully consistent with previous work showing that both estradiol and testosterone up-regulate the quantity of aromatase mRNA (Harada et al., 1993), the number of aromatase-positive cells in the HPOA (Balthazart et al., 1996; Foidart et al., 1994), and the activity of the enzyme (Schumacher et al., 1987; Schumacher and Balthazart, 1986), with estrogens having a more prominent effect than non aromatizable androgens (reviewed in (Balthazart, 2003).
This focus on brain rather than peripheral aromatase in the control of female behavior is however challenged by one observation reported here: OVX females lost their sexual motivation and receptivity while they still had high concentrations of estradiol coming from the brain as measured in the jugular blood. This finding is fascinating in itself because it extends to a new species, the Japanese quail, the finding made earlier in zebra finches that the brain actually contributes to the systemic concentration of estrogens (Schlinger and Arnold, 1991, 1993). Furthermore, this leakage of brain estrogens is observed here in ovariectomized females instead of gonadally intact zebra finches which makes the finding even more unexpected. However this observation raises two major questions, namely a) what is the source of androgens that are aromatized in the OVX brain to produce the estrogens found in the jugular blood and b) why is sexual behavior of OVX females decreased while the brain is still releasing estrogens in the jugular blood in concentrations that are apparently not different from gonadally intact (SHAM) females.
Source of androgens being aromatized
Interestingly, the high estradiol concentration found in the jugular blood of OVX females was observed while testosterone concentration was markedly decreased in both the jugular and the peripheral blood. This result suggests that the substrate aromatized into estradiol is produced locally in the brain and rapidly transformed by aromatase into estradiol so that it does not appear in the jugular blood. All enzymes necessary for the synthesis of androgens from cholesterol are expressed and active in the avian diencephalon (London et al., 2003; London et al., 2006; Tsutsui, 2011; Tsutsui et al., 2006). Local concentrations in brain androgens can also change independently of systemic androgen concentrations in response to various stimuli in the male zebra finch telencephalon and diencephalon (Pradhan et al., 2010; Remage-Healey et al., 2008). It is therefore conceivable that the local production of testosterone increases in the female brain in response to ovariectomy and provides a substrate for aromatization. However, direct evidence for a local production of testosterone is still not available. Another aromatizable androgen such as dehydroepiandrosterone (DHEA) could also serve as a substrate that could explain the present observations. Indeed, in female birds, DHEA has been measured in the plasma (Landys et al., 2013; Prior et al., 2015; Shah et al., 2011; Soma et al., 2004) and in several brain regions (Shah et al., 2011). Interestingly, despite very low gonadal secretion plasma concentrations of DHEA in females outside the breeding season are maintained to the levels measured during the breeding season (Landys et al., 2013). Moreover, DHEA would circulate in higher concentrations than testosterone or even estradiol in seasonal or non-seasonal bird species (Hau et al., 2004; Prior et al., 2015). Therefore, the production of estradiol found here in the brain of ovariectomized females could be based on the metabolism of peripheral or brain DHEA. Such mechanism has been implicated in the control of aggressive behaviors (see (Soma et al., 2015) for a review) but further studies would be warranted to investigate its role in the regulation of sexual behavior.
Decrease in behavior while the brain output of estrogens is unaffected
One could also wonder why the estrogen-dependent female behavior was inhibited by ovariectomy when the brain output of estradiol was not decreased. One potential explanation could be that although brain production and concentration of estradiol decreases after ovariectomy, jugular E2 is not decreased because of a parallel decrease of estradiol use or catabolism. It has been shown that ovariectomy induces a down-regulation of estrogen receptors in the diencephalon, and this effect was reversed with estradiol treatment (Navarro et al., 2013). Moreover, ovariectomy down-regulates estrogen catabolism in rodent peripheral tissues (Ghraf et al., 1975a; Ghraf et al., 1975b) and the enzymes responsible for this catabolism are found in the periphery and in the brain of birds (Ambadkar and Kotak, 1978; Bhujle and Nadkarni, 1976; Matsunaga et al., 2002). A down-regulation of estrogen receptors available for interaction with E2 as well as estrogen catabolism could thus explain the effect of ovariectomy on sexual motivation and receptivity despite a leakage of estradiol from the brain.
Alternatively the estrogens found in the jugular blood might be produced in brain sites that are not implicated in behavior control and thus have no behavioral impact in the present context. Indeed, the amplitude of the regulation of AA by steroids in male quail is larger in the HPOA than in other brain sites (Balthazart et al., 1990) so that jugular estradiol in OVX females might come from sites outside the HPOA where AA is not decreased by gonadectomy.
Conclusions and perspectives
The present experiments demonstrate that estrogens control sexual motivation in female quail in addition to their control of sexual receptivity. Specifically in female quail, blocking estrogen receptors leads to an inhibition of the motivation to approach a male partner. They additionally show that a non-ovarian source of estrogens derived from testosterone aromatization can restore the motivation to approach a sexual partner in ovariectomized females and blocking this aromatization prevented this restoration. The crouching response was not affected as much by these endocrine manipulations suggesting that this behavior requires exposure to higher concentrations of estrogens possibly for a longer time period. The source of estrogens leading to these behavioral effects has not been unequivocally determined by the present studies; it is likely located in the HPOA but a contribution of aromatase located in peripheral tissues such as the liver cannot be ruled out at this stage.
The implication of central aromatization in the control of female behavior has been poorly documented. One set of studies showed however that female receptivity can be induced in female musk shrew by testosterone or estradiol implants in the ventromedial hypothalamus and the medial preoptic area (Veney and Rissman, 2000). The existence of a possible control of female behavior by non-gonadal, presumably central, aromatization could however be of major biomedical significance. Aromatase inhibitors are now commonly used in the treatment of breast cancer. In women like in other mammals, a large number of studies have shown improvements of sexual function following treatments with estrogenic compounds (Burger et al., 1987; Davis et al., 1995; Nathorst-Boos et al., 1993; Reed et al., 2014; Sherwin, 1991; Wiklund et al., 1993) or with a combined treatment by testosterone and estradiol (Burger et al., 1987; Davis et al., 1995; Sherwin et al., 1987; Shifren et al., 2000). These studies therefore suggest a role for aromatization in the control of sexual behavior in women. Accordingly treatments with aromatase inhibitors have been related to sexual dysfunction (Derzko et al., 2007). Other functions, such as cognition or neuroprotection, have also been documented to be controlled by brain-synthesized estrogens in humans (Boon et al., 2010; Garcia-Segura, 2008). If, as suggested by the present studies, aromatase inhibitors impair sexual motivation and receptivity (or other major functions) by acting at the brain level, their negative effects could then potentially be avoided if new inhibitors that do not cross the blood-brain barrier were developed. This possibility should certainly be entertained and investigated.
Highlights.
Blockade of estrogen action alters both female sexual motivation and receptivity
Ovariectomy alters both female sexual motivation and receptivity
Testosterone aromatization restores sexual motivation in ovariectomized females
Hormone assays indicate that non-ovarian estrogens most likely arise from the brain
Aspects of female sexual behavior likely rely on brain estrogen production
Acknowledgments
This research was supported by NIH/NIMH grant RO1 MH50388. CAC is F.R.S.-FNRS Research Associate. CdB was supported by a non-FRIA fellowship from the University of Liege. We thank M. Ceuleers for the AA assays in peripheral tissues and Dr. Julie Bakker for her constructive comments on a previous version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins EK. Functional castration of the female japanese quail. Physiol Behav. 1973;10:619–621. doi: 10.1016/0031-9384(73)90232-1. [DOI] [PubMed] [Google Scholar]
- Adkins EK, Adler NT. Hormonal control of behavior in the Japanese quail. J Comp Physiol Psychol. 1972;81:27–36. doi: 10.1037/h0033315. [DOI] [PubMed] [Google Scholar]
- Adkins EK, Nock BL. The effects of the antiestrogen CI-628 on sexual behavior activated by androgen and estrogen in quail. Horm Behav. 1976;7:417–429. doi: 10.1016/0018-506x(76)90013-1. [DOI] [PubMed] [Google Scholar]
- Ambadkar PM, Kotak VC. Histochemical observations on 3alpha- and 17beta- hydroxysteroid dehydrogenase in extra gonadal tissues of feral blue rock pigeon Columba livia (G.) Indian journal of experimental biology. 1978;16:298–301. [PubMed] [Google Scholar]
- Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–147. doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Baillien M, Balthazart J. A direct dopaminergic control of aromatase activity in the quail preoptic area. J Steroid Biochem Mol Biol. 1997;63:99–113. doi: 10.1016/s0960-0760(97)00080-0. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Japanese quail as a model system for studying the neuroendocrine control of reproductive and social behaviors. ILAR J. 2010;51:310–325. doi: 10.1093/ilar.51.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J. Steroid control and sexual differentiation of brain aromatase. J Steroid Biochem Mol Biol. 1997;61:323–339. [PubMed] [Google Scholar]
- Balthazart J. Multiple mechanisms control brain aromatase activity at the genomic and non-genomic level. The Journal of steroid biochemistry and molecular biology. 2003;86:367–379. doi: 10.1016/s0960-0760(03)00346-7. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Brain Aromatase, estrogens and behavior. Oxford University Press; New York: 2013. [Google Scholar]
- Balthazart J, Cornil CA, Charlier TD, Taziaux M, Ball GF. Estradiol, a key endocrine signal in the sexual differentiation and activation of reproductive behavior. Journal of Experimental Zoology. 2008;309A:1–23. doi: 10.1002/jez.464. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Schumacher M, Evrard L. Sex differences and steroid control of testosterone-metabolizing enzyme activity in the quail brain. J Neuroendocrinol. 1990;2:675–683. doi: 10.1111/j.1365-2826.1990.tb00464.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Tlemçani O, Harada N. Localization of testosterone-sensitive and sexually dimorphic aromatase-immunoreactive cells in the quail preoptic area. J Chem Neuroanat. 1996;11:147–171. doi: 10.1016/0891-0618(96)00149-4. [DOI] [PubMed] [Google Scholar]
- Barbier O, Belanger A. The cynomolgus monkey (Macaca fascicularis) is the best animal model for the study of steroid glucuronidation. Journal of Steroid Biochemistry and Molecular Biology. 2003;85:235–245. doi: 10.1016/s0960-0760(03)00235-8. [DOI] [PubMed] [Google Scholar]
- Bhujle BV, Nadkarni VB. Steroid dehydrogenases in the adrenal gland of four species of birds: a histochemical study. The Histochemical journal. 1976;8:591–596. doi: 10.1007/BF01003960. [DOI] [PubMed] [Google Scholar]
- Boon WC, Chow JD, Simpson ER. The multiple roles of estrogens and the enzyme aromatase. Prog Brain Res. 2010;181:209–232. doi: 10.1016/S0079-6123(08)81012-6. [DOI] [PubMed] [Google Scholar]
- Burger H, Hailes J, Nelson J, Menelaus M. Effect of combined implants of oestradiol and testosterone on libido in postmenopausal women. Br Med J (Clin Res Ed) 1987;294:936–937. doi: 10.1136/bmj.294.6577.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callard GV, Petro Z, Ryan KJ. Conversion of androgen to estrogen and other steroids in the vertebrate brain. Amer Zool. 1978;18:511–523. [Google Scholar]
- Charlier TD, Newman AE, Heimovics SA, Po KW, Saldanha CJ, Soma KK. Rapid effects of aggressive interactions on aromatase activity and oestradiol in discrete brain regions of wild male white-crowned sparrows. J Neuroendocrinol. 2011;23:742–753. doi: 10.1111/j.1365-2826.2011.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley A, Hinshelwood M. Mammalian aromatases. J Reprod Fertil. 2001;121:685–695. doi: 10.1530/rep.0.1210685. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. The dual action of estrogen hypothesis. Trends Neurosci. 2015;38:408–416. doi: 10.1016/j.tins.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006a;166:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology. 2005;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav. 2006b;49:45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross E, Roselli CE. 17beta-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- Czajka-Oraniec I, Simpson ER. Aromatase research and its clinical significance. Endokrynologia Polska. 2010;61:126–134. [PubMed] [Google Scholar]
- Davis SR, McCloud P, Strauss BJ, Burger H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas. 1995;21:227–236. doi: 10.1016/0378-5122(94)00898-h. [DOI] [PubMed] [Google Scholar]
- de Bournonville C, Dickens MJ, Ball GF, Balthazart J, Cornil CA. Dynamic changes in brain aromatase activity following sexual interactions in males: where, when and why? Psychoneuroendocrinology. 2013;38:789–799. doi: 10.1016/j.psyneuen.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville Y, Balthazart J. Hormonal control of female sexual behavior in the Japanese quail. Horm Behav. 1987;21:288–309. doi: 10.1016/0018-506x(87)90016-x. [DOI] [PubMed] [Google Scholar]
- Derzko C, Elliott S, Lam W. Management of sexual dysfunction in postmenopausal breast cancer patients taking adjuvant aromatase inhibitor therapy. Current oncology. 2007;14(Suppl 1):S20–40. doi: 10.3747/co.2007.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens MJ, Cornil CA, Balthazart J. Acute stress differentially affects aromatase activity in specific brain nuclei of adult male and female quail. Endocrinology. 2011;152:4242–4251. doi: 10.1210/en.2011-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens MJ, de Bournonville C, Balthazart J, Cornil CA. Relationships between rapid changes in local aromatase activity and estradiol concentrations in male and female quail brain. Hormones and behavior. 2014;65:154–164. doi: 10.1016/j.yhbeh.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foidart A, De Clerck A, Harada N, Balthazart J. Aromatase-immunoreactive cells in the quail brain: Effects of testosterone and sex dimorphism. PhysiolBehav. 1994;55:453–464. doi: 10.1016/0031-9384(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Follett BK, Maung SL. Rate of testicular maturation, in relation to gonadotrophin and testosterone levels, in quail exposed to various artificial photoperiods and to natural daylengths. J Endocrinol. 1978;78:267–280. doi: 10.1677/joe.0.0780267. [DOI] [PubMed] [Google Scholar]
- Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM. Aromatase in the brain: not just for reproduction anymore. J Neuroendocrinol. 2008;20:705–712. doi: 10.1111/j.1365-2826.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM. Hormones and brain plasticity. Oxford University Press; New York: 2009. [Google Scholar]
- Ghraf R, Lax ER, Hoff HG, Schriefers H. The role of the gonads and the hypophysis in the regulation of hydroxysteroid dehydrogenase activities in rat kidney. Hoppe-Seyler’s Zeitschrift fur physiologische Chemie. 1975a;356:135–142. doi: 10.1515/bchm2.1975.356.1.135. [DOI] [PubMed] [Google Scholar]
- Ghraf R, Lax ER, Schriefers H. The hypophysis in the regulation of androgen and oestrogen dependent enzyme activities of steroid hormone metabolism in rat liver cytosol. Hoppe-Seyler’s Zeitschrift fur physiologische Chemie. 1975b;356:127–134. doi: 10.1515/bchm2.1975.356.1.127. [DOI] [PubMed] [Google Scholar]
- Gibson MJ, Cheng MF. Neural mediation of estrogen-dependent courtship behavior in female ring doves. J Comp Physiol Psychol. 1979;93:855–867. [Google Scholar]
- Harada N, Abe-Dohmae S, Loeffen R, Foidart A, Balthazart J. Synergism between androgens and estrogens in the induction of aromatase and its messenger RNA in the brain. Brain Res. 1993;622:243–256. doi: 10.1016/0006-8993(93)90825-8. [DOI] [PubMed] [Google Scholar]
- Hau M, Stoddard ST, Soma KK. Territorial aggression and hormones during the non-breeding season in a tropical bird. Hormones and behavior. 2004;45:40–49. doi: 10.1016/j.yhbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Hull EM, Rodriguez-Manzo G. Male sexual behavior. In: Pfaff D, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; San Diego: 2009. pp. 5–65. [Google Scholar]
- Kobayashi H, Yoshida S, Sun YJ, Shirasawa N, Naito A. Gastric estradiol-17beta (E2) and liver ERalpha correlate with serum E2 in the cholestatic male rat. J Endocrinol. 2013;219:39–49. doi: 10.1530/JOE-13-0156. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Yoshida S, Sun YJ, Shirasawa N, Naito A. 17beta-Estradiol in the systemic circulation derives mainly from the parietal cells in cholestatic female rats. Journal of endocrinological investigation. 2015 doi: 10.1007/s40618-015-0374-8. [DOI] [PubMed] [Google Scholar]
- Konkle AT, Balthazart J. Sex differences in the rapid control of aromatase activity in the quail preoptic area. J Neuroendocrinol. 2011;23:424–434. doi: 10.1111/j.1365-2826.2011.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landys MM, Goymann W, Soma KK, Slagsvold T. Year-round territorial aggression is independent of plasma DHEA in the European nuthatch Sitta europaea. Hormones and behavior. 2013;63:166–172. doi: 10.1016/j.yhbeh.2012.10.002. [DOI] [PubMed] [Google Scholar]
- London SE, Boulter J, Schlinger BA. Cloning of the Zebra Finch androgen synthetic enzyme CYP17: a study of its neural expression throughout posthatch development. J Comp Neurol. 2003;467:496–508. doi: 10.1002/cne.10936. [DOI] [PubMed] [Google Scholar]
- London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. General and Comparative Endocrinology. 1999;51:593–628. [PubMed] [Google Scholar]
- Matsunaga M, Ukena K, Tsutsui K. Androgen biosynthesis in the quail brain. Brain Res. 2002;948:180–185. doi: 10.1016/s0006-8993(02)03147-5. [DOI] [PubMed] [Google Scholar]
- Mills AD, Crawford LL, Domjan M, Faure JM. The behavior of the Japanese or domestic quail Coturnix japonica. Neurosci Biobehav Rev. 1997;21:261–281. doi: 10.1016/s0149-7634(96)00028-0. [DOI] [PubMed] [Google Scholar]
- Moreau F, Mittre H, Benhaim A, Bois C, Bertherat J, Carreau S, Reznik Y. Aromatase expression in the normal human adult adrenal and in adrenocortical tumors: biochemical, immunohistochemical, and molecular studies. European journal of endocrinology / European Federation of Endocrine Societies. 2009;160:93–99. doi: 10.1530/EJE-08-0215. [DOI] [PubMed] [Google Scholar]
- Nathorst-Boos J, Wiklund I, Mattsson LA, Sandin K, von Schoultz B. Is sexual life influenced by transdermal estrogen therapy? A double blind placebo controlled study in postmenopausal women. Acta Obstet Gynecol Scand. 1993;72:656–660. doi: 10.3109/00016349309021160. [DOI] [PubMed] [Google Scholar]
- Navarro A, Del Valle E, Ordonez C, Martinez E, Perez C, Alonso A, Gonzalez C, Tolivia J. Aging and substitutive hormonal therapy influence in regional and subcellular distribution of ERalpha in female rat brain. Age. 2013;35:821–837. doi: 10.1007/s11357-012-9415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AE, Chin EH, Schmidt KL, Bond L, Wynne-Edwards KE, Soma KK. Analysis of steroids in songbird plasma and brain by coupling solid phase extraction to radioimmunoassay. Gen Comp Endocrinol. 2008;155:503–510. doi: 10.1016/j.ygcen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Noble R. The effects of estrogen and progesterone on copulation in female quail (Coturnix coturnix japonica) housed in continuous dark. Horm Behav. 1972;3:199–204. doi: 10.1016/0018-506x(72)90032-3. [DOI] [PubMed] [Google Scholar]
- Noble RF. Hormonal control of receptivity in female quail (coturnix corturnix japonica) Horm Behav. 1973;4:61–72. [Google Scholar]
- Pfaus JG, Jones SL, Flanagan-Cato LM, Blaustein JD. Female sexual behavior. In: Plant TM, Zeleznik AJ, Albertini DF, Goodman RL, Herbison AE, McCarthy MM, Muglia LJ, Richards DH, editors. Knobil and Neill’s Physiology of Reproduction. Elsevier; Amsterdam: 2015. pp. 2287–2370. [Google Scholar]
- Pradhan DS, Newman AE, Wacker DW, Wingfield JC, Schlinger BA, Soma KK. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm Behav. 2010;57:381–389. doi: 10.1016/j.yhbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior NH, Yap KN, Adomat HH, Mainwaring MC, Fokidis HB, Guns ES, Buchanan KL, Griffith SC, Soma KK. Sex steroid profiles and pair-maintenance behavior of captive wild-caught zebra finches (Taeniopygia guttata) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015 doi: 10.1007/s00359-015-1050-3. [DOI] [PubMed] [Google Scholar]
- Reed SD, Mitchell CM, Joffe H, Cohen L, Shifren JL, Newton KM, Freeman EW, Larson JC, Manson JE, LaCroix AZ, Guthrie KA. Sexual function in women on estradiol or venlafaxine for hot flushes: a randomized controlled trial. Obstet Gynecol. 2014;124:233–241. doi: 10.1097/AOG.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2011 doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF, Clendenon AL, Krohmer RW. Role of androgens in the regulation of sexual behavior in the female musk shrew. Neuroendocrinol. 1990;51:468–473. doi: 10.1159/000125376. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Harada N, Roselli CE. Effect of vorozole, an aromatase enzyme inhibitor, on sexual behavior, aromatase activity and neural immunoreactivity. J Neuroendocrinol. 1996;8:199–210. doi: 10.1046/j.1365-2826.1996.04505.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Maldonado E, Velazquez PN, Juarez-Oropeza MA, Pedernera E. Steroid metabolism in theca externa cells from preovulatory follicles of domestic hen (Gallus domesticus) Gen Comp Endocrinol. 1996;101:173–179. doi: 10.1006/gcen.1996.0019. [DOI] [PubMed] [Google Scholar]
- Roselli C, Resko JA. In vitro assay of aromatase activity in the central nervous system. In: Greenstein B, editor. Neuroendocrine Research methods. Harwood Academic Publishers; Switzerland: 1991. pp. 937–951. [Google Scholar]
- Roselli CE, Abdelgadir SE, Jorgensen E, Resko JA. Sex differences in androgen-regulated cytochrome p450 aromatase mRNA in the rat brain. Endocrine. 1996;5:59–65. doi: 10.1007/BF02738657. [DOI] [PubMed] [Google Scholar]
- Sachs BD. Photoperiodic control of the cloacal gland of the Japanese quail. Science. 1967;157:201–203. doi: 10.1126/science.157.3785.201. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP. Brain is the major site of estrogen synthesis in a male songbird. Proc Natl Acad Sci USA. 1991;88:4191–4194. doi: 10.1073/pnas.88.10.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP. Estrogen synthesis in vivo in the adult zebra finch: Additional evidence that circulating estrogens can originate in brain. Endocrinology. 1993;133:2610–2616. doi: 10.1210/endo.133.6.8243284. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Alexandre C, Balthazart J. Interactions of androgens and estrogens in the control of reproduction. Comptes Rendus de l’Academie des Sciences - Serie III. 1987;305:569–574. [PubMed] [Google Scholar]
- Schumacher M, Balthazart J. The effects of testosterone and its metabolites on sexual behavior and morphology in male and female Japanese quail. Physiol Behav. 1983;30:335–339. doi: 10.1016/0031-9384(83)90135-x. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain Res. 1986;370:285–293. doi: 10.1016/0006-8993(86)90483-x. [DOI] [PubMed] [Google Scholar]
- Seredynski AL, Balthazart J, Ball GF, Cornil CA. Estrogen receptor B activation rapidly modulates male sexual motivation through the transactivation of metabotropic glutamate receptor 1a. J Neurosci. 2015 doi: 10.1523/JNEUROSCI.2056-15.2015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seredynski AL, Balthazart J, Christophe VJ, Ball GF, Cornil CA. Neuroestrogens rapidly regulate sexual motivation but not performance. J Neurosci. 2013;33:164–174. doi: 10.1523/JNEUROSCI.2557-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AH, Chin EH, Schmidt KL, Soma KK. DHEA and estradiol levels in brain, gonads, adrenal glands, and plasma of developing male and female European starlings. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2011;197:949–958. doi: 10.1007/s00359-011-0655-4. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. J Clin Endocrinol Metab. 1991;72:336–343. doi: 10.1210/jcem-72-2-336. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Gelfand MM, Schucher R, Gabor J. Postmenopausal estrogen and androgen replacement and lipoprotein lipid concentrations. American journal of obstetrics and gynecology. 1987;156:414–419. doi: 10.1016/0002-9378(87)90295-x. [DOI] [PubMed] [Google Scholar]
- Shifren JL, Braunstein GD, Simon JA, Casson PR, Buster JE, Redmond GP, Burki RE, Ginsburg ES, Rosen RC, Leiblum SR, Caramelli KE, Mazer NA. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. The New England journal of medicine. 2000;343:682–688. doi: 10.1056/NEJM200009073431002. [DOI] [PubMed] [Google Scholar]
- Silverin B, Baillien M, Foidart A, Balthazart J. Distribution of aromatase activity in the brain and peripheral tissues of passerine and nonpasserine avian species. Gen Comp Endocrinol. 2000;117:34–53. doi: 10.1006/gcen.1999.7383. [DOI] [PubMed] [Google Scholar]
- Simpson ER. Aromatase: biologic relevance of tissue-specific expression. Seminars in reproductive medicine. 2004;22:11–23. doi: 10.1055/s-2004-823023. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Clyne C, Speed C, Rubin G, Bulun S. Tissue-specific estrogen biosynthesis and metabolism. Annals of the New York Academy of Sciences. 2001;949:58–67. doi: 10.1111/j.1749-6632.2001.tb04002.x. [DOI] [PubMed] [Google Scholar]
- Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepiandrosterone metabolism by 3beta-hydroxysteroid dehydrogenase/Delta5-Delta4 isomerase in adult zebra finch brain: sex difference and rapid effect of stress. Endocrinology. 2004;145:1668–1677. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- Soma KK, Rendon NM, Boonstra R, Albers HE, Demas GE. DHEA effects on brain and behavior: insights from comparative studies of aggression. The Journal of steroid biochemistry and molecular biology. 2015;145:261–272. doi: 10.1016/j.jsbmb.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Soma KK, Sullivan KA, Tramontin AD, Saldanha CJ, Schlinger BA, Wingfield JC. Acute and chronic effects of an aromatase inhibitor on territorial aggression in breeding and nonbreeding male song sparrows. J Comp Physiol [A] 2000;186:759–769. doi: 10.1007/s003590000129. [DOI] [PubMed] [Google Scholar]
- Song WC. Biochemistry and reproductive endocrinology of estrogen sulfotransferase. Annals of the New York Academy of Sciences. 2001;948:43–50. doi: 10.1111/j.1749-6632.2001.tb03985.x. [DOI] [PubMed] [Google Scholar]
- Song WC, Melner MH. Steroid transformation enzymes as critical regulators of steroid action in vivo. Endocrinology. 2000;141:1587–1589. doi: 10.1210/endo.141.5.7526. [DOI] [PubMed] [Google Scholar]
- Steimer T, Hutchison JB. Is androgen-dependent aromatase activity sexually differentiated in the rat and dove preoptic area? J Neurobiol. 1990;21:787–795. doi: 10.1002/neu.480210512. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Watzka M, Schramm J, Bidlingmaier F, Klingmuller D. Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J Steroid Biochem Mol Biol. 1999;70:237–241. doi: 10.1016/s0960-0760(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Taziaux M, Cornil CA, Balthazart J. Aromatase inhibition blocks the expression of sexually-motivated cloacal gland movements in male quail. Behav Processes. 2004;67:461–469. doi: 10.1016/j.beproc.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Taziaux M, Keller M, Bakker J, Balthazart J. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J Neurosci. 2007;27:6563–6572. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Finy MS, Nelson RJ. Rapid effects of estradiol on male aggression depend on photoperiod in reproductively non-responsive mice. Horm Behav. 2008;53:192–199. doi: 10.1016/j.yhbeh.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: how interactions between aromatase and the environment modulate aggression. Front Neuroendocrinol. 2006;27:170–179. doi: 10.1016/j.yfrne.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc Biol Sci. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K. Neurosteroid biosynthesis and function in the brain of domestic birds. Frontiers in endocrinology. 2011;2:37. doi: 10.3389/fendo.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Matsunaga M, Miyabara H, Ukena K. Neurosteroid biosynthesis in the quail brain: a review. Journal of experimental zoology Part A, Comparative experimental biology. 2006;305:733–742. doi: 10.1002/jez.a.302. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Tsutsui K. Gonadotropin-inhibitory hormone inhibits aggressive behavior of male quail by increasing neuroestrogen synthesis in the brain beyond its optimum concentration. Gen Comp Endocrinol. 2014;205:49–54. doi: 10.1016/j.ygcen.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Unger EK, Burke KJ, Jr, Yang CF, Bender KJ, Fuller PM, Shah NM. Medial Amygdalar Aromatase Neurons Regulate Aggression in Both Sexes. Cell reports. 2015 doi: 10.1016/j.celrep.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veney SL, Rissman EF. Steroid implants in the medial preoptic area or ventromedial nucleus of the hypothalamus activate female sexual behaviour in the musk shrew. J Neuroendocrinol. 2000;12:1124–1132. doi: 10.1046/j.1365-2826.2000.00567.x. [DOI] [PubMed] [Google Scholar]
- Wiklund I, Karlberg J, Mattsson LA. Quality of life of postmenopausal women on a regimen of transdermal estradiol therapy: a double-blind placebo-controlled study. American journal of obstetrics and gynecology. 1993;168:824–830. doi: 10.1016/s0002-9378(12)90828-5. [DOI] [PubMed] [Google Scholar]
- Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]