Abstract
The putative neuroprotective properties of various flavonoids have long been reported. Amongst this class of chemicals, quercetin, a major flavone/flavonol naturally occurring in plants, deserves focused attention because of the myriad of beneficial effects observed in various in vitro and in vivo models of central nervous system damage/degeneration. However, the mechanisms governing the beneficial outcomes mediated by quercetin remain to be elucidated. In an effort to define the underlying molecular mechanisms, our study employed human/rat neuroblastoma cell lines (SHSY5Y and B35 rat respectively) and E18 derived rat primary cortical neurons upon which the effects of various flavonoids were examined. Of note, increases in levels of global SUMOylation, a post-translational modification with the Small Ubiquitin-like MOdifier (SUMO) were pronounced. Quercetin treatment increased SUMOylation levels in both SHSY5Y cells and rat cortical neurons in a dose and time-dependent manner, possibly via the direct inactivation of certain SENPs (SUMO-specific isopeptidases). Of particular interest, cells treated with quercetin displayed increased tolerance to OGD (oxygen-glucose deprivation) exposure, an in vitro model of ischemia. SHSY5Y cells treated with quercetin also increased the expression of Nrf2 (via a decrease in the levels of Keap1), heme oxygenase-1 (HO-1), and nitric oxide synthase 1 (NOS1), which provide further protection from oxidative stress. In addition, the increased SUMOylation of HIF-1α was noted and deemed to be significant. We hypothesize that SUMOylated HIF-1α plays a fundamental role in the protection afforded and may underlie some of quercetin’s ability to protect cells from OGD-induced cell death, via an upregulation of HO-1 and NOS1, which ultimately leads to the induction of pro-life NOS1/PKG signaling.
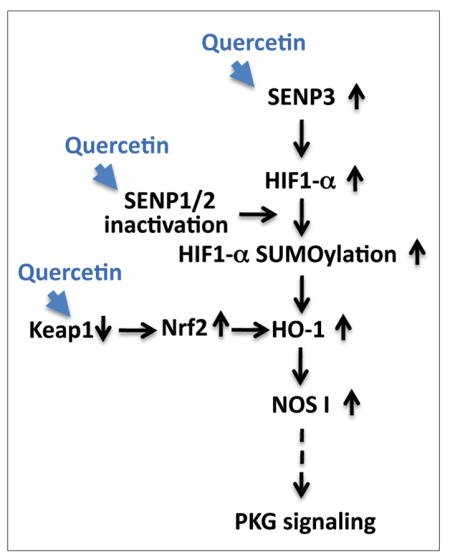
Putative pro-life pathway(s) induced by quercetin
Quercetin acts to increase survival in the face of ischemia via an increase of SENP3 expression, the possible inactivation of SENPs 1/2, and via a decrease in KEAP1 levels (thereby increasing Nrf2 stability). These changes may then lead to increase in HIF-1α SUMOylation and HO-1 activation, followed by an upregulation of NOS1/PKG signaling. Pathways altered via quercetin treatment within our experimental system are represented by blue arrowheads. Solid black arrows represent relationships that have been explored while a dotted arrow represents a relationship that has yet to be confirmed.
Introduction
Flavonoids, which are rich in tea, fruits, and vegetables, have been reported to exhibit a multifarious set of neuroprotective effects in conditions that include brain ischemia (reviewed in (Ossola et al. 2009, Bhullar & Rupasinghe 2013, Kawabata et al. 2015). While flavonoids are thought to work primarily as antioxidants, the wealth/diversity of these reported cellular actions cannot be explained solely via their antioxidant properties. As such, the manifold cellular effects of flavonoids in models of central nervous system (CNS) injury and degeneration prompted us to check whether they affect SUMOylation, a form of post-translational modification with the Small Ubiquitin-like MOdifer (SUMO) that appears to have wide-reaching influence in states of both cellular homeostasis and disease (reviewed in (Gill 2004)). As such, it is not surprising that post-translational modifications via SUMO have been demonstrated to be central in states of tolerance and act to preserve homeostasis under stress within ischemic networks (Tempe et al. 2008, Lee & Hallenbeck 2013, Bernstock et al. 2016).
Accordingly and through the use of the human/rat neuroblastoma cell lines (SHSY5Y and B35 respectively), and primary cortical neurons derived from rat embryos, we sought to examine the effects of various flavonoids on SUMOylation. It is interesting to note that most of the flavonoids studied did indeed show an ability to alter/increase the levels of global SUMOylation within these cells; however, among the compounds tested, quercetin, a flavone/flavonol, displayed the greatest capacity for increasing SUMOylation.
Quercetin’s biological repertoire includes the ability to function as an anti-oxidant, an anti-inflammatory, and an anti-viral agent, with some reports even suggesting the existence of an anti-cancer profile of activity (reviewed in (Kawabata et al. 2015)). Quercetin’s neuroprotective effects in the context of ischemia-induced brain damage have also been widely reported/examined (reviewed in (Ossola et al. 2009, Bhullar & Rupasinghe 2013)). While a large body of literature has clearly demonstrated the beneficial effects of quercetin in human health and models of disease, the precise molecular mechanisms governing the aforementioned remain to be elucidated.
Herein we show that quercetin treatments are capable of remarkable increases in the levels of global SUMOylation (in a dose/time-dependent manner) in both SHSY5Y cells and E18 rat cortical neurons and that this change may be mediated via the direct inactivation of certain SENPs (SUMO-specific isopeptidases) by quercetin. Critically, we found that the cells treated with quercetin displayed an increase in tolerance to OGD (oxygen-glucose deprivation) or OGD/ROG (restoration of oxygen and glucose) an in vitro model of ischemia and that the tolerance displayed was likely to be SUMO-dependent. Further, we show that SHSY5Y cells treated with quercetin increase the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) (possibility mediated via a decrease in Kelch-like ECH-associated protein 1 [Keap1]), heme oxygenase-1 (HO-1) and nitric oxide synthase 1 (NOS1); all of which are involved in antagonizing oxidative stress. In addition, definitive increases of the levels of SUMOylated hypoxia-inducible factor-1 alpha (HIF1-α) were noted. As such, we hypothesize that SUMOylated HIF1-α (induced via quercetin) plays a key role in protecting cells from OGD-induced cell death via an upregulation of HO-1 and NOS1, which leads to the induction of the pro-survival (NOS1/PKG) signaling pathway (Chan et al. 2011).
Materials and Methods
Flavonoids
All flavonoids were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA) and stock solutions were made in dimethyl sulfoxide (DMSO) at a concentration of 40 mM.
SHSY5Y and B35 cell culture
The human neuroblastoma cell line SHSY5Y (American Type Culture Collection, Manassas, VA, USA) and the B35 rat neuroblastoma cell lines stably expressing scrambled microRNA (cell line Y168) and SUMO 2/3 microRNA (cell line Y241) (generously provided by Drs. Wulf Pashen and Wei Yang) (Yang et al. 2009) ± transfected SUMO-1 shRNA #2 (Lee et al. 2009) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin (DMEM complete) in 5% CO2 at 37°C.
Isolation of E18 primary rat cortical neurons/neuronal culture
Timed pregnant Sprague-Dawley rats were purchased from Taconic (Taconic Biosciences, Hudson, NY, USA) and were used to prepare cortical neurons for culture. Briefly, the cortices were dissected from embryos (E18), dissociated with papain (Worthington Biochemicals, Lakewood, NJ, USA) and plated out at a density of 1,000,000 cells per well on poly-L-lysine-coated 6 well plates in Neurobasal/B27 media as has been previously described (Lee et al. 2009, Miyake et al. 2015). Cells were used after ~7 days in culture. All animal studies were performed in adherence to protocols approved by the NINDS Animal Care and Use Committee (ACUC).
Lentiviral transduction and plasmid transfection
SENP3 shRNA containing lentiviral particles (catalogue # iV022008) (Applied Biological Materials Inc, Richmond, Canada) were transduced into SHSY5Y cells at either a multiplicity of infection (MOI) of 0.5 or 1.0. Stable cells were then selected under the pressure of puromycin (2 μg/ml). The transient transfection of SHSY5Y cells with either flag or GFP-tagged SENP1 was done via electroporation with the Amaxa Nucleofactor™ (Lonza, Basel, Switzerland) as has been previously described (Lee et al. 2009).
Immunoprecipitation (IP) and Western blot analyses
Cell lysates for IP were made via the suspension of cells in IP buffer (50 mM HEPES buffer, pH7.4, 100 mM NaCl, 1.5 mM MgCl2, 1% NP40, 0.1% SDS) that also contained a protease inhibitor cocktail (Roche, Basal, Switzerland), 1 mM PMSF and 20 mM n-ethylmaleimide (NEM). This cell suspension was then incubated for 1 hr on ice and then centrifuged for 15 min at 20,000 x g. After pre-clearing with protein A/G plus agarose (Santa Cruz Biotechnology, Dallas, TX, USA), protein concentrations were measured via a Pierce BCA Protein Assay (Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of protein amongst the relevant samples were ultimately used for IP. After incubation with the primary antibody for 2 hr at 4 ºC, protein A/G plus agarose was added and incubated overnight at 4ºC. The washing and eluting of IP products that followed conformed entirely to the manufacturer’s (Santa Cruz) protocol. Total cell lysates for the Western blots from SHSY5Y, B35, or E18 primary cortical neurons were prepared as has been previously described (Lee et al. 2009, Lee et al. 2012). The antibodies used throughout the course of this study were as follows: rabbit polyclonal anti-SUMO-1 and anti-SUMO-2/3 antibodies (both developed in-house), anti-SAE1, anti-SAE2, and anti-Ubc9 antibodies were from Abcam (Cambridge, MA, USA), anti-SENP1, anti-SENP2, anti-SENP3, ant-HIF-1α, anti-HO-1, anti-NOS1, and anti-Nrf2 antibodies were from Santa Cruz Biotechnology, and the anti-β-actin antibody was from Sigma-Aldrich. Anti-SENP1 and anti-HIF-1α antibodies from Novus Biologicals (Littleton, CO, USA) were also used. Protein expression levels were determined via a densitometric analysis of the corresponding protein bands of interest using Image J (NIH, Bethesda, MD, USA). In order to measure SUMO-conjugation levels, regions corresponding to molecular weights above 100 kDa in each lane were cropped and the total intensity analyzed. All densities were normalized to the corresponding actin levels and expressed as the ratio to control (i.e. DMSO alone).
Oxygen/glucose deprivation (OGD), restoration of oxygen/glucose (ROG) and cell death assessments
OGD/ROG experiments on SHSY5Y, B35 and cortical neuronal cultures were performed as has been described previously (Lee et al. 2007, Lee et al. 2009, Lee et al. 2014). Briefly, after 16 hr (for SHSY5Y and B35) or 5 hr (for cortical neurons) of OGD with or without quercetin, the plates were removed from the hypoxia-chamber (Billups-Rothenberg, San Diego, CA, USA), and an equal volume of complete media (DMEM for SHSY5Y and B35, and Neurobasal/B27 for cortical neurons) containing 2x glucose (with or without quercetin) was added, and incubated for additional period of time (i.e. an ROG of 5 hr for SHSY5Y and B35, and 16 hr for cortical neurons). At the end of OGD, cell survival was measured via a premixed WST-1 cell proliferation reagent (Clontech Labratories, Mountain View, CA, USA) as per the manufacturer’s instructions and as has been described previously (Lee et al. 2012); cell viability is shown as the percentage of untreated (no OGD, no quercetin added) cells. Cell death after OGD/ROG was measured via LDH release according to the manufacturer’s instructions (Abcam); cell death is shown as the percentage of released LDH (i.e. of the total LDH present).
Assay for SENP activity
All AMC (7-amido-4-methylcoumarin)-conjugated substrates and recombinant SENP catalytic domains (CDs) were purchased from (Boston Biochem, Cambridge, MA, USA). Free AMC (control) was purchased from Sigma-Aldrich. Briefly, either SUMO-1-AMC (Cat# UL-551) or SUMO-2-AMC (Cat# UL-758) was used as a substrate at concentrations that ranged from 0.5~1.0 μM, in combination with either the His6-SENP1 CD (Cat# E-700) or His6-SENP2 CD (Cat# 710) as the enzymatic source at concentrations of between 0.5 ~1.0 nM. In order to achieve maximal activity, the CDs were preincubated in an activation buffer (50 mM Tris, pH 7.8, 10 mM DTT, 100 μg/ml ovalbumin) for 15 min at 25 °C. Reactions were initiated via the addition of the activated CD into a well (96 well format) containing one of the substrates (i.e. either SUMO-1-AMC or SUMO-2-AMC) in a reaction buffer consisting of 50 mM HEPES pH 8.0, 100 mM NaCl, 2 mM DTT, 0.1 mg/ml BSA, and the release of AMC, as measured via an increase in fluorescence, was monitored using Ex380 nm and Em460 nm wavelengths every 15 seconds. Reactions were carried out at 25 °C. Quercetin (1~10 μM) or other flavonoids (2 μM) were added either during the activation or reaction phase depending on the goals of the experiment (i.e. to examine effects on enzyme activation or activity). In order to examine the putative effects of quercetin on SENP activities in vivo, we treated SHSY5Y cells with quercetin at 0, 10 and 20 μM for 16 hr and measured SENP activities within the cellular extracts. Briefly, cell extracts were prepared by sonicating (3×10 sec pulses) the cells in lysis buffer (50 mM Tris HCl, pH 7.4, 5 mM MgCl2, 250 mM sucrose, 2 mM ATP, protease inhibitor cocktail), followed by centrifugation at 20,000 x g for 30 min. The protein concentrations of the supernatants of these extracts were measured via a BCA Protein Assay (Thermo Fisher Scientific). The same concentration of protein was then used to measure SENP activities via incubation with either SUMO-1-AMC or SUMO-2-AMC as the substrate.
Statistical analysis
To test for differences in treatments (i.e. flavonoids) vs control (i.e. DMSO), a one-way ANOVA, followed by Dunnett’s post-hoc test, was performed. Values of p ≤ 0.05 were deemed to be significant.
Results
Quercetin increases global SUMO-conjugation in SHSY5Y cells and E18 rat cortical neurons
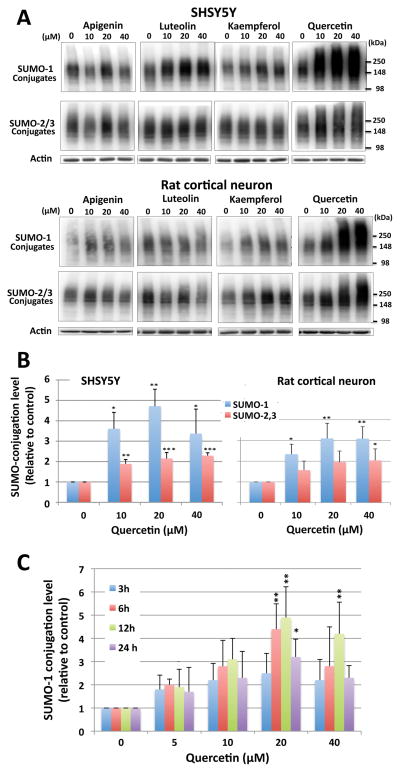
We examined levels of global SUMOylation in SHSY5Y cells and rat cortical neurons that were treated at several different concentrations and incubation times with the following flavonoids: apigenin, luteolin, kaempferol, epicatechin, quercetin, epigallocatechin gallate (EGCG), myricetin or naringenin. Several of these flavonoids were capable of increasing levels of global SUMOylation (Figure 1A, B and Supplemental Figure 1). Among the compounds tested quercetin displayed the greatest fold increases. Quercetin treatment increased SUMOylation levels in SHSY5Y cells and rat cortical neurons at doses ranging from 5–40 μM and at incubation times ranging from 3–24 hr (up to ~5-fold increase) (Figure 1C).
Figure 1. Quercetin treatment increases SUMO conjugation (both SUMO-1 and SUMO-2,3) in SHSY5Y cells and E18 rat cortical neurons.
(A) Representative immunoblots of SUMO-1 and SUMO-2,3 conjugates in SHSY5Y cells (upper panels) and rat cortical neurons (lower panels) that were treated with the flavonoids indicated at various concentrations (0, 10, 20, 40 μM) for 16 hr. (B) Quantitative analyses of the SUMO-conjugates in SHSY5Y and cortical neurons treated with quercetin at 10, 20 and 40 μM for 16 hr. The region corresponding to molecular weights > 100 kDa in each lane was cropped and the total intensity was analyzed. The densities were normalized to the corresponding actin levels and expressed as the ratio to control (no quercetin: DMSO alone). Data represent the mean ± standard deviation of at least three independent experiments. **p<0.01, *p<0.05 compared to the DMSO alone control via a one way ANOVA, followed by Dunnett’s post-hoc test. (C) Effects of dose and incubation time of quercetin on SUMO-1 conjugation levels in SHSY5Y cells. Cells were treated with various concentrations of quercetin (0, 5, 10, 20, 40 μM) for 3, 6, 12 and 24 hr and SUMO-1 conjugation levels were analyzed as per the aforesaid. Data represent the mean ± standard deviation of at least three independent experiments. **p<0.01, *p<0.05 compared to the DMSO alone control via a one way ANOVA, followed by Dunnett’s post-hoc test.
Putative mechanisms mediating quercetin induced increases in levels of global SUMOylation
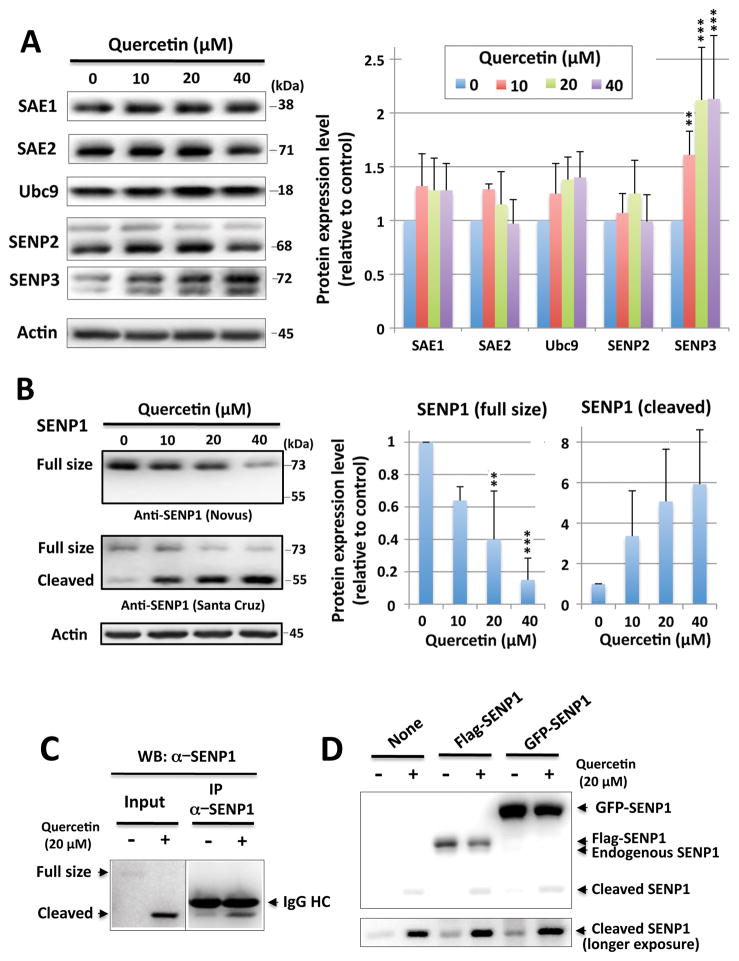
The conjugation/deconjugation of SUMO within the cell is a dynamic process and thus increased levels of SUMOylation may result from either an acceleration of the processes mediating conjugation and/or the inhibition of those processes governing deconjugation. We therefore checked the expression levels of proteins involved in both SUMO-conjugation (e.g. SAE1, SAE2, Ubc9) and deconjugation (SENPs). As shown in Figure 2A, the enzymes involved in SUMO conjugation (SAE1, SAE2 and Ubc9) displayed only a modest increase after quercetin treatment. Remarkably, the deconjugative enzymes, SENP2 and SENP3, were also shown to increase (i.e. especially SENP3), which seems to counter to the massive increases in levels of SUMOylation witnessed after treatment with quercetin; accordingly the significance of the increase in SENP3 expression via quercetin will be discussed later. In line with canonical enzymatic function, SENP1 protein levels were significantly decreased by quercetin treatments when the blots were probed with the anti-SENP1 antibody from Novus Biologicals (NB100-92101), which had been raised against the N-terminus of the SENP1 protein (amino acid [AA] 85~205) (Figure 2B). Interestingly, we also observed a smaller band (~55 kDa) that was consistently increased after quercetin treatment in a dose-dependent manner (Figure 2B) when we used an anti-SENP1 antibody from Santa Cruz Biotechnology (C12, cat# sc-271360), which had been raised against amino acids 361–425 (i.e. the middle part of the protein as SENP1 consists of 644 AA total), but not by anti-SENP1 antibody from Novus Biologicals which was raised against amino acids 85~205 (close to N-terminus). As shown in Figure 2B, the decreases in total SENP1 correlate well with increasing levels of the ~55 kDa band. Of note, the 55 kDa fragment could be pulled down with the former SENP1 antibody (Santa Cruz, C12) (Figure 2C). When N-terminus flag or GFP-tagged SENP1 were over-expressed and the cells treated with quercetin, the full length form of the overexpressed SENP1 also decreased (Figure 2D), yet the increased fragment’s size remained ~55 kDa. We therefore posit that the observed decrease in the levels of SENP1 (full size) was not the result of inhibited expression, but rather increased proteolysis (at around AA 150) mediated via quercetin and is therefore likely a mechanism related to the increased levels of SUMOylation demonstrated after treatments with quercetin.
Figure 2. Effects of quercetin on the expression of SUMOylation pathway related proteins.
(A) Representative immunoblots (left) and quantitative analyses (right) of the expression of SUMOylation pathway related proteins: levels of E1 (SAE1, SAE2), E2 (Ubc9) and the isopeptidases (SENP2, SENP3) in SHSY5Y cells that were incubated with quercetin at 0, 10, 20 and 40 μM for 16hr. The density of each band was normalized with the corresponding actin level and expressed as the ratio to control (no quercetin: DMSO alone). (B) Representative immunoblots (left) and quantitative analyses (right) of SENP1 level (full-size and cleaved form). Two SENP1 antibodies, one from Novus Biologicals (NBP1-89553), which was raised against AA 85–205, and one from Santa Cruz Biotechnology (sc-271360) which was raised against AA 361–425 were used. The density of each band was normalized with corresponding actin level and expressed as the ratio to control (no quercetin: DMSO alone). Data represent the mean ± standard deviation of at least three independent experiments. ***p<0.001, **p<0.01, *p<0.05 compared to the DMSO alone control via a one way ANOVA, followed by Dunnett’s post-hoc test. (C) Cell lysates from SHSY5Y cells, which were treated with or without quercetin (20 μM) overnight, were immunoprecipitated with an anti-SENP1 antibody from Santa Cruz. (D) SHSY5Y cells were transfected with either flag-SENP1 or GFP-SENP1, treated with or without quercetin (20 μM) overnight, and the various SENP1 expression levels were examined by the Western blot with anti-SENP1 antibody from Santa Cruz.
Quercetin inhibits SENP1 and SENP2 activity
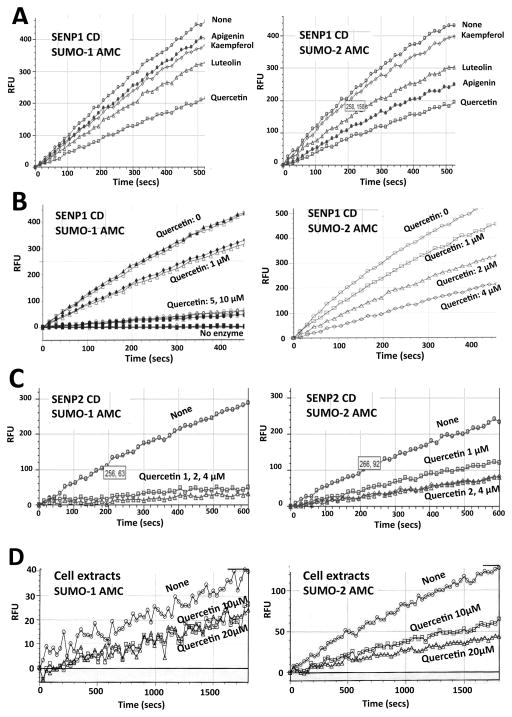
Beyond the putative induction of SENP1 degradation we wondered if quercetin could directly inhibit SENP activation and/or activity. The effect of quercetin on SENP1 and/or SENP2 activation or activity was examined in a cell-free system using SENP1 or SENP2 CDs as an enzymatic source and SUMO-1-AMC or SUMO-2-AMC compounds as reaction substrates. In order to achieve maximum activity, the enzyme CDs were pre-incubated in an activation buffer. Reactions were initiated via the addition of the activated enzyme CDs into wells containing the substrate of interest (i.e. either SUMO-1-AMC or SUMO-2-AMC) and the release of AMC via an increasing fluorescence signal was monitored. When quercetin was added to the reaction step, there was very modest reduction in SENP1 activity (data not shown), yet the activity was greatly reduced when quercetin was added to the CDs during the activation step. All of the data shown in Figure 3 is therefore derived from those experiments in which the addition of flavonoids was performed during the activation step. As shown in Figure 3A, most flavonoids examined (at 2μM) inhibit SENP1 CD activation and amongst these quercetin was the most potent inhibitor. SENP1 CD activation was inhibited by quercetin in a dose dependent manner for both SUMO-1 and SUMO-2, yet the effects were more pronounced for SUMO-1 (Figure 3B). Of note, SENP2 CD activation was inhibited in an even more profound manner by quercetin (Figure 3C). Pre-incubation of SENP2 CD with 1- 2μM quercetin completely inactivated SENP2 activity (when tested with either SUMO-1-AMC and SUMO-2-AMC as the substrate) (Figure 3C). Of note, we confirmed that there was no quenching activity of quercetin via the incubation of free AMC (~1 μM) with quercetin (up to 4 μM) and in so doing observed no reduction in fluorescence signal (data not shown); being that 2 μM quercetin was sufficient to cause inhibition within our cell free system we are confident in these findings. In order to examine the effects of quercetin on SENP activities in vitro, we treated SHSY5Y cells with quercetin at 0, 10 and 20 μM for 16 hr and then measured the SENP activities within the cell extracts. While higher concentrations (e.g. 20 μM) of quercetin were used to examine effects on SENP activities in vitro, they do not represent the final concentration of quercetin within the assay reaction mixtures and thus quenching at these elevated concentrations was not examined. As shown in Figure 3D, the SENPs activities within the cells were reduced by quercetin (10 and 20 μM) treatment (i.e. an ~50% reduction in SUMO-1-AMC cleavage and ~70% reduction in SUMO-2-AMC cleavage in the cells treated with 20 μM quercetin). It is prudent to note that the basal levels of SUMO-2 deconjugation were much higher than SUMO-1 deconjugation in the SHSY5Y cells; as such more than twice the amount of protein was used for the detection of SUMO-1 deconjugation. This is reasonable considering that SUMO-1 conjugates are deconjugated primarily by SENP1, whilst SUMO-2,3 conjugates are deconjugated by all of the SENPs including SENP1, 2, 3, 5, 6 and 7 (Hickey et al. 2012).
Figure 3. Quercetin is a potent inhibitor of SENP1 and SENP2 activity.
(A) Effects of various flavonoids on SENP1 activation. Apigenin, kaempferol, luteolin or quercetin was added at 2 μM (final concentration) during activation (pre-incubation with SENP1 catalytic domain CD) and SENP1 activity was measured by AMC release from the SUMO-1-AMC (left panel) or SUMO-2-AMC (right panel) substrate. AMC release was plotted every 15 seconds. (B) Quercetin was added at various concentrations to a pre-incubation mixture (i.e. activation stage) with the SENP1 CD, and SENP1 activity was measured by AMC release from the SUMO-1-AMC (left panel) or SUMO-2-AMC (right panel) substrate. AMC release was plotted every 15 seconds. (C) Quercetin was added at various concentrations to a pre-incubation mixture (i.e. activation stage) with the SENP2 CD, and SENP2 activity was measured by AMC release from the SUMO-1-AMC (left panel) or SUMO-2-AMC (right panel) substrate. AMC release was plotted every 15 seconds. (D) Effect of quercetin on SENP activities in vitro. SHSY5Y cells were treated with quercetin at 0, 10 and 20 μM for 16 hr. Equal amounts of protein (50 μg for SUMO-1-AMC cleavage assay, and 20 μg for SUMO-2-AMC cleavage assay) were used as the enzyme source to measure SENP activities within cell extracts. AMC release was plotted every 30 seconds.
Quercetin treatment protects both SHSY5Y cells and E18 rat cortical neurons from OGD and OGD/ROG-induced cell death
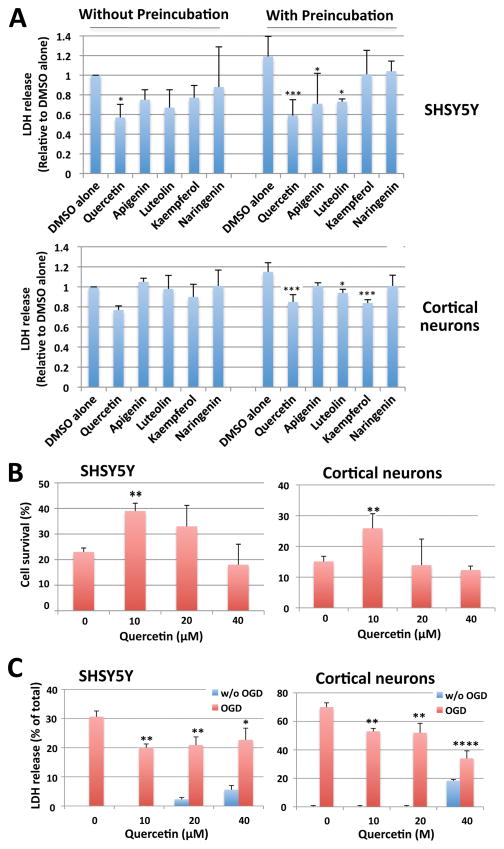
Flavonoids have been reported to display a litany of neuroprotective effects. As such we examined the protective effects of several flavonoids against OGD. SHSY5Y cells or rat cortical neurons were subjected to OGD for 16 hr (SHSY5Y) or 5 hr (cortical neurons) in the presence of the various flavonoids at a concentration of 10 μM. Further, some cells were also pre-incubated for 3 hr (cortical neurons) or 6 hr (SHSY5Y) with 10 μM of the same flavonoids used during OGD. Cell death was measured via LDH release and compared to DMSO alone. As shown in Figure 4A, the majority of flavonoids tested did display some level of protection (with the exception of naringenin) against OGD; again among the flavonoids quercetin provided the most protection. Of note, pretreatment with these flavonoids increased the trend noted previously and in so doing provided statistically significant levels of cytoprotection. We next examined the effect of dose on quercetin’s ability to induce neuroprotection. SHSY5Y cells and rat cortical neurons were subjected to OGD (16 hr for SHSY5Y, 5 hr for cortical neurons, respectively) in the presence of quercetin (0, 10, 20, 40 μM). Cell viability was measured immediately after OGD using a premixed WST-1 cell proliferation reagent. As shown in Figure 4B, without the addition of quercetin only 22% of SHSY5Y cells and less than 15% of the rat cortical neurons survived (i.e. as measured via intact/functional mitochondria). In the presence of quercetin (particularly 10 μM), the survival rate nearly doubled in both SHSY5Y cells and rat cortical neurons (Figure 4B). Some of the cell cultures that were subjected to OGD were subsequently returned to a media containing normal levels of oxygen and glucose (i.e. ROG) and incubated for an additional 5hr (for SHSY5Y) or 16 hr (for rat cortical neurons) with or without quercetin. Cell death after OGD/ROG was measured via LDH release and shown as the percentage of total LDH (Figure 4C). As shown in Figure 4C, OGD/ROG-induced death reached ~30% in SHSY5Y cells and ~70% in rat cortical neurons. The inclusion of 10~20μM quercetin during OGD/ROG significantly decreased cell death. Of note, incubating the cells with 40μM quercetin for ~24 hr did show signs of cellular toxicity (Figure 4C, blue column).
Figure 4. Effect of Quercetin and other flavonoids on OGD and OGD/ROG-induced cell death in SHSY5Y cells and rat cortical neurons.
(A) SHSY5Y cells or rat cortical neurons were subjected to OGD for 16 hr (SHSY5Y) or 5 hr (cortical neurons) in the presence of 10 μM of various flavonoids with or without pretreatment (10 μM for 6 hr for SHSY5Y and 3 hr for cortical neurons) as indicated. Cell death was measured via LDH release and is compared to DMSO alone. Upper panel: SHSY5Y cells; lower panel: cortical neurons. (B) SHSY5Y cells or rat cortical neurons were subjected to OGD for 16 hr (SHSY5Y) or 5 hr (cortical neurons) in the presence of various concentrations of quercetin (0, 10, 20, 40 μM). After OGD cell viability was measured using a premixed WST-1 cell proliferation reagent. Cell viability was shown as the percentage of untreated (no OGD, no quercetin added) cells. Left panel: SHSY5Y cells; Right panel: cortical neurons. (C) Cell death after OGD/ROG was measured via LDH release and is shown as the percentage of total LDH. Left panel: SHSY5Y; Right panel: cortical neurons. Data represent the mean ± standard deviation of at least three independent experiments. ***p<0.001, **p<0.01, *p<0.05 compared to the DMSO alone control via a one way ANOVA, followed by Dunnett’s post-hoc test.
The protection from OGD and OGD/ROG-induced cell death afforded by quercetin is SUMOylation dependent
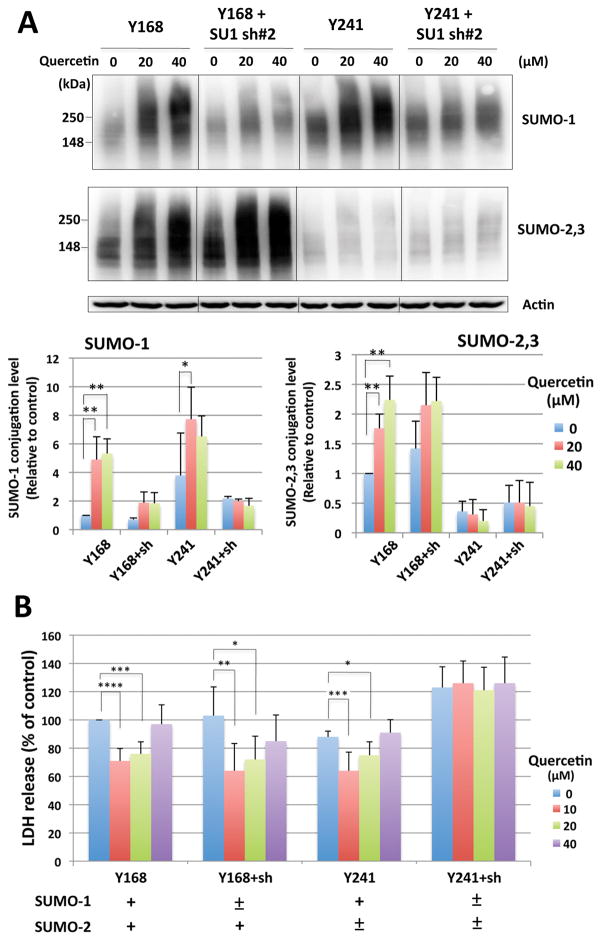
To determine if the cellular protection mediated by quercetin treatment during OGD or OGD/ROG was SUMOylation-dependent, we made use of rat B35 neuroblastoma cell lines stably expressing scrambled (control) microRNA (cell line Y168) (i.e. wild type for SUMO) and SUMO-2,3 microRNA which inhibits SUMO-2,3 expression (cell line Y241) (Yang et al. 2009) with or without the stable expression of SUMO-1 shRNA#2 which depletes (i.e. reduces) the levels of SUMO-1 (Lee et al. 2009). As shown in Figure 5A, these four engineered cell lines exhibited the expected levels of SUMOylation in response to quercetin: Y168 cells (wild type), quercetin induced a dose-dependent increase in the levels of both SUMO-1 and SUMO-2,3 conjugation; Y168 cells expressing SUMO-1 shRNA, showed reduced levels of SUMO-1 conjugation but an increased (compensatory) SUMO-2,3 conjugation and further increases were seen with quercetin treatment; Y241 (SUMO-2,3 depleted), displayed a minimal increase in SUMO-2,3 conjugation, but SUMO-1 conjugation levels were dramatically induced by quercetin; Y241 expressing SUMO-1 shRNA, displayed low levels of SUMO-1 and SUMO-2,3 conjugation with no significant responses to quercetin. These engineered cell lines were ultimately subjected to OGD/ROG (for 16hr/5hr respectively) in the absence or presence of quercetin at 10, 20 or 40 μM, and cell death measured via LDH release. As shown in Figure 5B, quercetin at a concentration of 10~20 μM displayed significant levels of protection from OGD/ROG-induced cell death in the cell lines capable of expressing either SUMO-1 or SUMO-2,3 yet no protection was found in those cells in which both SUMO-1 and -2,3 were depleted (i.e. Y241 + SUMO-1 shRNA). These results strongly suggest that the protection mediated by quercetin during OGD/ROG is at least in part SUMOylation-dependent.
Figure 5. Quercetin induced cellular protection against OGD/ROG is SUMOylation dependent.
Rat B35 neuroblastoma cell lines stably expressing miR negative (Y168), miR-negative with SUMO-1 shRNA#2, SUMO-2,3 microRNA (Y241) alone, and SUMO-2,3 miRNA with SUMO-1 shRNA#2 were incubated with quercetin at 20 and 40 μM for 16 hr, and the levels of SUMO-conjugation and cellular tolerance to OGD/ROG were examined. (A) Representative immunoblots (upper panel) and their quantitative analyses (lower panel). (B) Cell death after OGD/ROG (for 16hr/5hr respectively) was measured via LDH release and is shown as percentages of control (Y168 cells without quercetin, DMSO alone). Data represent the mean ± standard deviation of at least three independent experiments. ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05 compared to the DMSO alone control via a one way ANOVA, followed by Dunnett’s post-hoc test.
Putative molecular mechanism(s) underlying the protection induced by quercetin against OGD or OGD/ROG-induced cell death
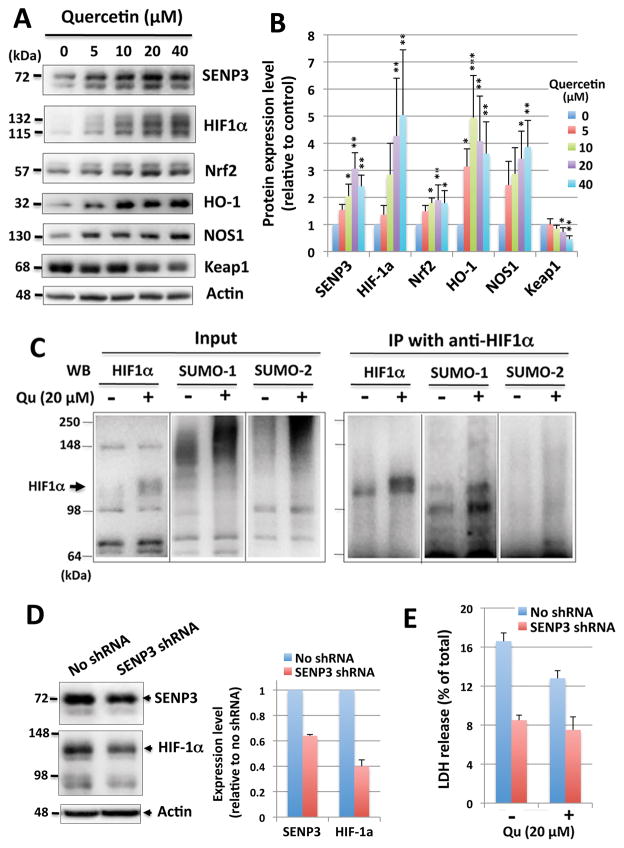
In an effort to understand how quercetin protects neuronal cells from OGD and/or OGD/ROG-induced cell death, we examined the effects of quercetin on the expression of several proteins, which are known to be involved in cellular responses to oxidative stress (e.g. Nrf2, Keap1, HIF-1α, NOS1, and HO-1). We found that for most of the proteins we examined, with the exception of Keap1, levels significantly increased after quercetin treatment in a dose-dependent manner (Figure 6A, B). Interestingly, Keap1 levels were decreased significantly via quercetin treatment (Figure 6A, B). Being that Keap1 is known to facilitate the ubiquitination and the subsequent proteolysis of Nrf2, we believe the decrease in Keap1 noted after quercetin treatment led to the increase in Nrf2 levels noted within our study (Figure 6A, B). We also noticed that the probing of HIF-1α yielded multiple bands, which were also increased after exposure to quercetin (Figure 6A, B). Such banding patterns are typical of SUMOylated proteins; being that quercetin treatment induced massive global protein SUMOylation, we regard it as highly likely that HIF-1α is one of the critical SUMO targets. We found these bands did indeed represent a SUMOylated form of HIF-1α via immunoprecipitaion/blotting. Lysates from SHSY5Y cells treated with either DMSO alone or quercetin at 20 μM for 16 hr were immunoprecipitated with anti-HIF1α followed by immunoblotting against anti-HIF1α, anti-SUMO-1 or anti-SUMO-2 antibodies. As shown in Figure 6C, the immunoprecipitated product did reacted with the anti-SUMO-1 antibody. Interestingly, our SUMO-2 antibody did not detect these forms thereby suggesting that HIF-1α is mainly SUMOylated via SUMO-1. In addition, it is prudent to mention the significant increases in the levels of SENP3 (Figure 2 and 6).
Figure 6. Differential expression of several proteins influenced by quercetin treatment.
SHSY5Y cells were treated with different concentrations of quercetin for 16 hr and protein expression levels in total cell lysates were analyzed. (A) Representative immunoblots for differentially expressed proteins. (B) Quantitative analyses of the protein expression levels. The density of each band was normalized to the corresponding actin level and expressed as the ratio to control (no quercetin: DMSO alone). Data represent the mean ± standard deviation of at least three independent experiments. ***p<0.001, **p<0.01, *p<0.05 compared to the DMSO alone control via a one way ANOVA, followed by Dunnett’s post-hoc test. (C) HIF-1α is SUMOylated after quercetin-treatment. Lysates from SHSY5Y cells treated either with DMSO alone or quercetin at 20 μM for 16 hr were immunoprecipitated with anti-HIF-1α antibody followed by immunoblotting using anti-HIF1α, anti-SUMO-1 or anti-SUMO-2,3 antibodies. Left panel shows input and right panel shows immunoprecipitated products. Arrow shows the predicted HIF-1α band. (D) SENP3 depletion (reduction) by SENP3 shRNA down regulates HIF-1α expression in SHSY5Y cells. SHSY5Y cells were infected with SENP3/shRNA lentiviral particles, and selected under the pressure of puromycin (2 μg/ml). After established stable lines, the SENP3 and HIF-1α protein levels in the total cell lysates were analyzed by the Western blot. Actin was used as a loading control. Left, immunoblots; right, quantitative analysis. (E) SENP3-depleted cells were more resistant to OGD and no additional protection by quercetin was afforded in these cells. SHSY5Y cells with or without SENP3 depletion were subjected to OGD with or without quercetin (20 μM) for 16 hr, and LDH levels (released and total) were measured. Released LDH were shown as percentage of the total LDH.
Discussion
Flavonoids are polyphenolic compounds that are present in plant-derived foods and as such occur ubiquitously throughout the human diet. The physiologic functions of flavonoids have been widely investigated since their bioactivities were first described more than 80 years ago (Kawabata et al. 2015). Of the flavonoids, quercetin is one of the most extensively studied to date owing to the myriad of beneficial biologic effects that have been reported in a range of in vitro and in vivo models of a number of diseases/disorders (e.g. anti-oxidative, anti-inflammatory, anticancer etc.) (reviewed in (Brito et al. 2015, Kawabata et al. 2015)). Of critical importance are the multitude of neuroprotective properties of quercetin that have been described and reviewed in detail by Ossola et al. (Ossola et al. 2009) and Kawabata et al. (Kawabata et al. 2015). While most of the work has focused on quercetin’s ability to act as an anti-oxidant and in so doing to relieve the oxidative stress caused by a vast array of neurotoxic insults (e.g. hydrogen peroxide (H2O2), linoleic acid hydroperoxide, tert-butyl-hydroperoxide or amyloid-β peptide [Aβ]) (Ossola et al. 2009, Kawabata et al. 2015), it has become clear that quercetin has a more complex repertoire of mechanisms of action than were initially anticipated/realized (Ossola et al. 2009). In line with this, quercetin has been reported to have many other profiles of activity which facilitate neuroprotection (e.g. an ability to reduce neuroinflammation (Chen et al. 2005, Sharma et al. 2007, Bureau et al. 2008), anti-amyloidogenic and fibril destabilization effects with regard to Aβ deposition (Ono et al. 2003, Jimenez-Aliaga et al. 2011, Ho et al. 2013), an ability to upregulate the AKT pathway (Lei et al. 2015), and/or to activate AMP-activated protein kinase (Lu et al. 2010, Chen et al. 2014, Leyton et al. 2015)).
Understanding that quercetin possesses such a vast array of putative neuroprotective mechanisms herein we sought to identify molecular targets and/or pathways capable of coordinating such a response. One candidate capable of coordinating the multimodal molecular mechanisms that underlie the vast cytoprotection induced by quercetin is that of global SUMOylation, a post-translational modification that has been shown to have a multiplicity of effects (reviewed in (Gill 2004, Hay 2005)). In line with such reasoning, it is prudent to note that we found that quercetin treatment led to a remarkable increase in global SUMOylation in both SHSY5Y cells and rat cortical neurons in a time/dose-dependent manner (Figure 1). SUMO, like ubiquitin, is synthesized as an inactive precursor that is subsequently processed by the SENPs to yield a mature di-glycine C-terminus. A single heterodimeric E1 activating enzyme (SAE1/SAE2) initiates conjugation via the adenylation of SUMO and this step is followed by the formation of a covalent thioester E1-SUMO intermediate. SUMO is then transferred to the catalytic cysteine of the sole E2 conjugase, Ubc9 (ubiquitin conjugase 9), which alone or in concert with an E3 (i.e. target specific) ligase catalyzes the formation of an isopeptide linkage between the C-terminal glycine residue of SUMO and the epsilon-amino group of the substrate’s target lysine residue. The conjugative process described above is regulated/opposed by the same SENP isopeptidases, which catalyze de-SUMOylation, thereby modulating steady-state levels of SUMO conjugates (reviewed in (Muller et al. 2001, Hay 2005)). Per the abovementioned, SUMO conjugation and deconjugation are dynamic processes within the cell, and thus increased SUMOylation occurs via an acceleration of the conjugative processes and/or via the inhibition of the deconjugative process. Interestingly, we found that quercetin was able to directly inhibit the catalytic domains of SENPs 1/2 and the SENP activity of cell lysates that had been treated with quercetin (Figure 3). Understanding that the concentrations of enzymes, which participate in the conjugation of SUMO (i.e. SAE1/SAE2 and Ubc9) were not significantly altered after quercetin treatment, we therefore posit that the major cause of the increases noted in global SUMOylation relate primarily to the inactivation and/or degradation of the SENPs by quercetin. Of note, quercetin’s ability to directly inhibit enzyme activities (other than SENPs) has been reported previously (i.e. as an inhibitor of PI3K (Matter et al. 1992) and MEK1 (Lee et al. 2008)). It is prudent to again note that we remain unsure as to whether SENP1 was inactivated directly by quercetin or via its presumed cleavage (i.e. possibly mediated by as of yet unknown quercetin-activated protease(s)) of SENP1 (Figure 2B). As such, future studies will be required to more definitely define the relationship between quercetin and the perturbation of SENP activity.
The neuroprotective effects of elevated SUMO-conjugation were first uncovered in 13-lined ground squirrels (Ictidomys tridecemlineatus) during hibernation torpor (Lee et al. 2007), later confirmed in cell culture systems (Lee et al. 2007, Lee et al. 2009, Datwyler et al. 2011, Cimarosti et al. 2012) and animal models (Yang et al. 2008, Lee et al. 2011, Cimarosti et al. 2012, Lee et al. 2014, Yang et al. 2014). In line with such work, herein we have also shown that quercetin-treated cells displayed an increase in tolerance to OGD or OGD/ROG-induced cell death (Figure 4) and that this tolerance is in fact SUMO-dependent (Figure 5). These results strongly suggest that quercetin-induced tolerance to stress (OGD or OGD/ROG in our experimental system) is at least in part mediated via increases in the levels of global SUMOylation.
While such increases in global SUMOylation are believed to be in part responsible for both natural and acquired tolerance to ischemic stress (reviewed in (Lee & Hallenbeck 2013)), the precise molecular mechanisms governing such ischemic protection remain unknown. Herein we have found that quercetin treatment increases the expression levels of the SUMOylated forms of hypoxia-inducible factor-1 alpha (HIF-1α) in a dose dependent manner (Figure 6). HIF-1, which consists of an oxygen-regulated α-subunit and a constitutively expressed β-subunit, serves as a master transcriptional regulator of gene expression in response to hypoxia (reviewed in (Semenza 2001, Sharp & Bernaudin 2004)). It is known that HIF-1α is among the many transcription factors that are SUMOylated (Hay 2005), and that hypoxia is capable of inducing HIF-1α SUMOylation (Bae et al. 2004, Shao et al. 2004, Berta et al. 2007, Carbia-Nagashima et al. 2007). It should be noted that controversy exists with regard to the underlying cellular mechanisms/outcomes of HIF-1α SUMOylation (i.e. some reports claim that HIF-1α SUMOylation stabilizes the HIF-1α and in so doing enhances its transcriptional activity, while others report that hypoxia-induced HIF-1α SUMOylation negatively regulates its stability and transactivation) (Bae et al. 2004, Carbia-Nagashima et al. 2007, Berta et al. 2007, Chen & Shen 2007, Cai et al. 2010, Kang et al. 2010, Nunez-O’Mara & Berra 2013). However it does seem that the effects of SUMOylation on HIF-1α vary from cell type to cell type (Dimova & Kietzmann 2010) with the SUMOylation patterns influenced by specific E3 ligases (Kang et al. 2010, Li et al. 2014). Interestingly, Chan et al. have reported differential functions of SUMOylated HIF-1α (i.e. with SUMOylated HIF-1α, but not free HIF-1α, contributing to the amelioration of brain stem cardiovascular regulatory failure) (Chan et al. 2011). The pro-life pathway put forth by Chan et al. centered on the fact that HIF-1α SUMOylation upregulated heme oxygenase-1 (HO-1) which was followed by nitric oxide synthase I (NOSI)/protein kinase G (PKG) signaling (Dai et al. 2010, Chan et al. 2011). The induction of such a pathway resonates with the findings ascertained in our experimental system as the expression levels of both HO-1 and NOS1 were increased by quercetin treatment in SHSY5Y cells (Figure 6).
As per the abovementioned, quercetin increased not only the levels of SUMOylated HIF-1α, but also the levels of the non-SUMOylated form (Figure 6). It is known that HIF-1α can be activated under normoxic conditions (e.g. during inflammation (Cramer et al. 2003, Jung et al. 2003), growth factor activation (Tacchini et al. 2001, Patten et al. 2010) and insulin administration (Zelzer et al. 1998, Carroll & Ashcroft 2006)) and that the production of reactive oxygen species (ROS) accompanies these events. Further, it is well known that ROS production increases during ischemia/reperfusion and that they are in fact required for HIF-1α activation (Chandel et al. 1998, Chandel & Budinger 2007, Jung et al. 2008). Of note, HIF-1α activation via ROS has been shown to be biphasic (i.e. with mild ROS activating, but high ROS inhibiting, HIF-1α transactivation) (Huang et al. 2009). Critically, Huang et al. found that the SUMO-2,3 specific protease, SENP3, is required for ROS-induced HIF-1α transactivation (i.e. via the SENP3-mediated deconjugation of SUMO-2,3 from p300, the co-regulator of HIF-1α) (Huang et al. 2009). Of note, the SENP3 protein was induced by mild oxidative stress yet its catalytic activity was inhibited by severe oxidative stress (Huang et al. 2009). This work was especially interesting to us given the fact that within our experimental system quercetin treatment significantly increased the levels of SENP3 expression (Figures 2 and 6). Despite this it is important to note that recent work has emerged to highlight the potential destructive role(s) of SENP3 during OGD/ROG. Guo et al. have reported that SENP3 is degraded during OGD via a pathway involving the unfolded protein response kinase, PERK and the lysosomal enzyme cathepsin B thereby preventing the de-SUMOylation of the GTPase Drp1, which plays a major role in regulating mitochondrial fission (Guo et al. 2013). In their hands depletion of SENP3 prolongs Drp1 SUMOylation, which suppresses Drp1-mediated cytochrome c release and caspase-mediated cell death (Guo et al. 2013). They went on to show that SENP3 levels do in fact recover after ROG and in so doing facilitate the removal of SUMO from Drp1, ultimately promoting mitochondria-induced apoptosis (Guo et al. 2013). In line with this eloquent study we found that SENP3 depletion was in fact cytoprotective (Figure 6E) yet in our system it appears as though the modulation of the cellular redox state via quercetin treatments (and the associated upregulation of SENP3) is critical in facilitating the activation and/or stability of HIF-1α and its associated axis (Figure 6D).
When we consider the protective mechanisms upregulated by quercetin, we would be remiss to ignore quercetin’s well-documented capacity to function as an antioxidant; with the understanding that ROS sit at the center of the pathobiology that unfolds as a result of OGD/ROG. Quercetin has been previously reported capable of inducing the expression of the nuclear factor erythroid 2-related factor 2 (Nrf2) (Ji et al. 2015), which is the redox-sensitive transcription factor that plays a predominant role in the cellular defense(s) against oxidative stress (reviewed in (Kaspar et al. 2009, Niture et al. 2010)). Accordingly, we found that Nrf2 expression was increased (most likely through reduction of Keap1). Briefly, the transcription factor Nrf2 and its negative regulator Keap1 control the expression of ~500 genes with a diverse set of cytoprotective functions that underlie responses to oxidative stress (reviewed in (Kensler et al. 2007)). Keap1, acts as substrate adaptor protein for Cullin3/Rbx1 ubiquitin ligase which under conditions of cellular homeostasis continuously targets Nrf2 for degradation (Kensler et al. 2007) (Baird et al. 2014). Being that Keap1 levels were reduced via exposure to quercetin, in our experimental system we believe that Nrf2 was able accumulate via this mechanism and in so doing go on to facilitate the transcription of its protective target genes. Of note, heme oxygenase-1 (HO-1) was also shown to be upregulated (Figure 6). HO-1 has been documented to protect against the cytotoxicity caused by oxidative stress and ultimately against apoptotic cell death (while concurrently modulating states of inflammation) (Aburaya et al. 2006, Paine et al. 2010, Bastianetto & Quirion 2010, Sakata et al. 2010), and a number of natural compounds (including quercetin), have been reported to induce HO-1 (reviewed in (Barbagallo et al. 2013)). As such, we postulate that Nrf2-mediated (Surh et al. 2009, Niture et al. 2010, Lee et al. 2012, Na & Surh 2014) and/or HIF-1α (SUMOylated HIF-1α)-mediated (Lee et al. 1997, Yang & Zou 2001, Dai et al. 2010, Chan et al. 2011) HO-1 induction is likely responsible for a significant portion of the quercetin induced tolerance to OGD/ROG witnessed in our experimental system.
In conclusion, we propose the following mechanism(s) of action underlying quercetin’s protection of cells from OGD/ROG and/or other potential stressors: (i) quercetin alters the level of oxidative stress which leads to an upregulation of SENP3 (ii) quercetin inhibits SENP1/2 activation which leads an upregulation of global SUMOylation (and modifications of proteins involved in the ischemic stress response e.g. HIF-1α) (iii) quercetin down-regulates Keap1 which stabilizes Nrf2 and its associated oxidative axis. All these effects ultimately converge and induce HO-1 upregulation and the enhancement of the pro-life signaling pathway, NOS1/PKG (Figure 7).
Figure 7. Putative pro-life pathway(s) induced by quercetin.
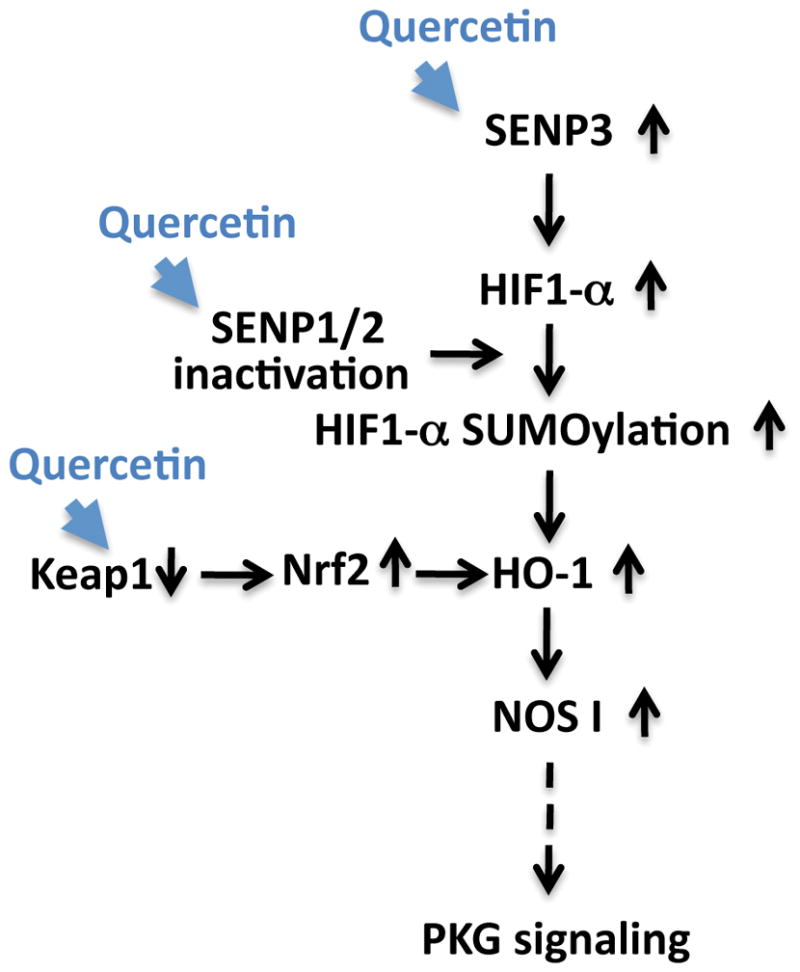
Quercetin acts to increase survival in the face of ischemia via an increase of SENP3 expression, the possible inactivation of SENPs 1/2, and via a decrease in KEAP1 levels (thereby increasing Nrf2 stability). These changes may then lead to increase in HIF-1α SUMOylation and HO-1 activation, followed by an upregulation of NOS1/PKG signaling. Pathways altered via quercetin treatment within our experimental system are represented by blue arrowheads. Solid black arrows represent relationships that have been explored while a dotted arrow represents a relationship that has yet to be confirmed.
Supplementary Material
Representative immunoblots of SUMO-1 and SUMO-2,3 conjugates in SHSY5Y cells (upper panels) and rat cortical neurons (lower panels) which were treated with the indicated flavonoids at various concentrations (0, 10, 20, 40 μM) for 16 hr.
Acknowledgments
We the authors would like to thank Professors Wulf Paschen and Wei Yang (Multidisciplinary Neuroprotection Laboratories, Duke University, Durham, NC, USA) for the provision of the B35 rat neuroblastoma cell lines utilized during the course of this study. This work was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NINDS/NIH).
Abbreviation
- SUMO
small ubiquitin-like modifier
- SENP
SUMO-specific isopeptidase
- OGD
oxygen and glucose deprivation
- ROG
restoration of oxygen and glucose
- Nrf2
nuclear factor erythroid 2-related factor 2
- Keap1
Kelch-like ECH-associated protein 1
- HO-1
heme oxygenase-1
- NOS1
nitric oxide synthase 1
- HIF-1α
hypoxia-inducible factor-1 alpha
- DMSO
dimethyl sulfoxide
- PKG
protein kinase G
- LDH
lactate dehydorogenase
- AMC
7-amido-4-methlcoumarin
- SAE1/SAE2
SUMO activating enzyme 1 and 2
- Ubc9
ubiquitin conjugating enzyme 9
Footnotes
Conflict of Interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Aburaya M, Tanaka K, Hoshino T, Tsutsumi S, Suzuki K, Makise M, Akagi R, Mizushima T. Heme oxygenase-1 protects gastric mucosal cells against non-steroidal anti-inflammatory drugs. The Journal of biological chemistry. 2006;281:33422–33432. doi: 10.1074/jbc.M602074200. [DOI] [PubMed] [Google Scholar]
- Bae SH, Jeong JW, Park JA, Kim SH, Bae MK, Choi SJ, Kim KW. Sumoylation increases HIF-1alpha stability and its transcriptional activity. Biochemical and biophysical research communications. 2004;324:394–400. doi: 10.1016/j.bbrc.2004.09.068. [DOI] [PubMed] [Google Scholar]
- Barbagallo I, Galvano F, Frigiola A, et al. Potential therapeutic effects of natural heme oxygenase-1 inducers in cardiovascular diseases. Antioxidants & redox signaling. 2013;18:507–521. doi: 10.1089/ars.2011.4360. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Quirion R. Heme oxygenase 1: another possible target to explain the neuroprotective action of resveratrol, a multifaceted nutrient-based molecule. Exp Neurol. 2010;225:237–239. doi: 10.1016/j.expneurol.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Bernstock JD, Lee YJ, Peruzzotti-Jametti L, et al. A novel quantitative high-throughput screen identifies drugs that both activate SUMO conjugation via the inhibition of microRNAs 182 and 183 and facilitate neuroprotection in a model of oxygen and glucose deprivation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36:426–441. doi: 10.1177/0271678X15609939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta MA, Mazure N, Hattab M, Pouyssegur J, Brahimi-Horn MC. SUMOylation of hypoxia-inducible factor-1alpha reduces its transcriptional activity. Biochemical and biophysical research communications. 2007;360:646–652. doi: 10.1016/j.bbrc.2007.06.103. [DOI] [PubMed] [Google Scholar]
- Bhullar KS, Rupasinghe HP. Polyphenols: multipotent therapeutic agents in neurodegenerative diseases. Oxidative medicine and cellular longevity. 2013;2013:891748. doi: 10.1155/2013/891748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito AF, Ribeiro M, Abrantes AM, Pires AS, Teixo RJ, Tralhao JG, Botelho MF. Quercetin in Cancer Treatment, Alone or in Combination with Conventional Therapeutics? Current medicinal chemistry. 2015;22:3025–3039. doi: 10.2174/0929867322666150812145435. [DOI] [PubMed] [Google Scholar]
- Bureau G, Longpre F, Martinoli MG. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J Neurosci Res. 2008;86:403–410. doi: 10.1002/jnr.21503. [DOI] [PubMed] [Google Scholar]
- Cai QL, Verma SC, Kumar P, Ma M, Robertson ES. Hypoxia Inactivates the VHL Tumor Suppressor through PIASy-Mediated SUMO Modification. Plos One. 2010;5 doi: 10.1371/journal.pone.0009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbia-Nagashima A, Gerez J, Perez-Castro C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F, Arzt E. RSUME, a small RWD-containing protein, enhances SUMO conjugation and stabilizes HIF-1alpha during hypoxia. Cell. 2007;131:309–323. doi: 10.1016/j.cell.2007.07.044. [DOI] [PubMed] [Google Scholar]
- Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer research. 2006;66:6264–6270. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- Chan JY, Tsai CY, Wu CH, Li FC, Dai KY, Sun EY, Chan SH, Chang AY. Sumoylation of hypoxia-inducible factor-1alpha ameliorates failure of brain stem cardiovascular regulation in experimental brain death. PLoS One. 2011;6:e17375. doi: 10.1371/journal.pone.0017375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, Budinger GR. The cellular basis for diverse responses to oxygen. Free Radic Biol Med. 2007;42:165–174. doi: 10.1016/j.freeradbiomed.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BL, Wang LT, Huang KH, Wang CC, Chiang CK, Liu SH. Quercetin attenuates renal ischemia/reperfusion injury via an activation of AMP-activated protein kinase-regulated autophagy pathway. The Journal of nutritional biochemistry. 2014;25:1226–1234. doi: 10.1016/j.jnutbio.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Chen JC, Ho FM, Pei-Dawn Lee C, Chen CP, Jeng KC, Hsu HB, Lee ST, Wen Tung W, Lin WW. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. European journal of pharmacology. 2005;521:9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Chen M, Shen X. Nuclear actin and actin-related proteins in chromatin dynamics. Curr Opin Cell Biol. 2007;19:326–330. doi: 10.1016/j.ceb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Cimarosti H, Ashikaga E, Jaafari N, Dearden L, Rubin P, Wilkinson KA, Henley JM. Enhanced SUMOylation and SENP-1 protein levels following oxygen and glucose deprivation in neurones. J Cereb Blood Flow Metab. 2012;32:17–22. doi: 10.1038/jcbfm.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai KY, Chan SH, Chang AY. Heme oxygenase-1 plays a pro-life role in experimental brain stem death via nitric oxide synthase I/protein kinase G signaling at rostral ventrolateral medulla. Journal of biomedical science. 2010;17:72. doi: 10.1186/1423-0127-17-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datwyler AL, Lattig-Tunnemann G, Yang W, Paschen W, Lee SL, Dirnagl U, Endres M, Harms C. SUMO2/3 conjugation is an endogenous neuroprotective mechanism. J Cereb Blood Flow Metab. 2011;31:2152–2159. doi: 10.1038/jcbfm.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimova EY, Kietzmann T. Hypoxia-inducible factors: post-translational crosstalk of signaling pathways. Methods Mol Biol. 2010;647:215–236. doi: 10.1007/978-1-60761-738-9_13. [DOI] [PubMed] [Google Scholar]
- Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes & development. 2004;18:2046–2059. doi: 10.1101/gad.1214604. [DOI] [PubMed] [Google Scholar]
- Guo C, Hildick KL, Luo J, Dearden L, Wilkinson KA, Henley JM. SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes cell death following ischaemia. The EMBO journal. 2013;32:1514–1528. doi: 10.1038/emboj.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Hickey CM, Wilson NR, Hochstrasser M. Function and regulation of SUMO proteases. Nature reviews. Molecular cell biology. 2012;13:755–766. doi: 10.1038/nrm3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Ferruzzi MG, Janle EM, et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:769–781. doi: 10.1096/fj.12-212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Han Y, Wang Y, et al. SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. Embo J. 2009;28:2748–2762. doi: 10.1038/emboj.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji LL, Sheng YC, Zheng ZY, Shi L, Wang ZT. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic Biol Med. 2015;85:12–23. doi: 10.1016/j.freeradbiomed.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Jimenez-Aliaga K, Bermejo-Bescos P, Benedi J, Martin-Aragon S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life sciences. 2011;89:939–945. doi: 10.1016/j.lfs.2011.09.023. [DOI] [PubMed] [Google Scholar]
- Jung SN, Yang WK, Kim J, et al. Reactive oxygen species stabilize hypoxia-inducible factor-1 alpha protein and stimulate transcriptional activity via AMP-activated protein kinase in DU145 human prostate cancer cells. Carcinogenesis. 2008;29:713–721. doi: 10.1093/carcin/bgn032. [DOI] [PubMed] [Google Scholar]
- Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- Kang X, Li J, Zou Y, Yi J, Zhang H, Cao M, Yeh ET, Cheng J. PIASy stimulates HIF1alpha SUMOylation and negatively regulates HIF1alpha activity in response to hypoxia. Oncogene. 2010;29:5568–5578. doi: 10.1038/onc.2010.297. [DOI] [PubMed] [Google Scholar]
- Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata K, Mukai R, Ishisaka A. Quercetin and related polyphenols: new insights and implications for their bioactivity and bioavailability. Food & function. 2015;6:1399–1417. doi: 10.1039/c4fo01178c. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annual review of pharmacology and toxicology. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kang NJ, Heo YS, et al. Raf and MEK protein kinases are direct molecular targets for the chemopreventive effect of quercetin, a major flavonol in red wine. Cancer research. 2008;68:946–955. doi: 10.1158/0008-5472.CAN-07-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 1997;272:5375–5381. [PubMed] [Google Scholar]
- Lee Y-j, Mou Y, Klimanis D, Bernstock JD, Hallenbeck JM. Global SUMOylation is a molecular mechanism underlying hypothermia-induced ischemic tolerance. Frontiers in cellular neuroscience. 2014;8 doi: 10.3389/fncel.2014.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Castri P, Bembry J, Maric D, Auh S, Hallenbeck JM. SUMOylation participates in induction of ischemic tolerance. Journal of neurochemistry. 2009;109:257–267. doi: 10.1111/j.1471-4159.2009.05957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Hallenbeck JM. SUMO and ischemic tolerance. Neuromolecular medicine. 2013;15:771–781. doi: 10.1007/s12017-013-8239-9. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Johnson KR, Hallenbeck JM. Global rotein conjugation by Ubiquitin-Like-Modifiers during ischemic stress is regulated by microRNAs and confers robust tolerance to ischemia. PLoS One. 2012;7:e47787. doi: 10.1371/journal.pone.0047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Miyake S, Wakita H, McMullen DC, Azuma Y, Auh S, Hallenbeck JM. Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells. J Cereb Blood Flow Metab. 2007;27:950–962. doi: 10.1038/sj.jcbfm.9600395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Mou Y, Maric D, Klimanis D, Auh S, Hallenbeck JM. Elevated global SUMOylation in Ubc9 transgenic mice protects their brains against focal cerebral ischemic damage. PLoS One. 2011;6:e25852. doi: 10.1371/journal.pone.0025852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Chao H, Zhang Z, Lv J, Li S, Wei H, Xue R, Li F, Li Z. Neuroprotective effects of quercetin in a mouse model of brain ischemic/reperfusion injury via anti-apoptotic mechanisms based on the Akt pathway. Molecular medicine reports. 2015;12:3688–3696. doi: 10.3892/mmr.2015.3857. [DOI] [PubMed] [Google Scholar]
- Leyton L, Hott M, Acuna F, Caroca J, Nunez M, Martin C, Zambrano A, Concha MI, Otth C. Nutraceutical activators of AMPK/Sirt1 axis inhibit viral production and protect neurons from neurodegenerative events triggered during HSV-1 infection. Virus Res. 2015;205:63–72. doi: 10.1016/j.virusres.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Li J, Xu Y, Long XD, et al. Cbx4 governs HIF-1alpha to potentiate angiogenesis of hepatocellular carcinoma by its SUMO E3 ligase activity. Cancer cell. 2014;25:118–131. doi: 10.1016/j.ccr.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF, Shan Q, Zheng ZH, Liu CM, Wang YJ. Quercetin activates AMP-activated protein kinase by reducing PP2C expression protecting old mouse brain against high cholesterol-induced neurotoxicity. The Journal of pathology. 2010;222:199–212. doi: 10.1002/path.2754. [DOI] [PubMed] [Google Scholar]
- Matter WF, Brown RF, Vlahos CJ. The inhibition of phosphatidylinositol 3-kinase by quercetin and analogs. Biochem Biophys Res Commun. 1992;186:624–631. doi: 10.1016/0006-291x(92)90792-j. [DOI] [PubMed] [Google Scholar]
- Miyake SI, Wakita H, Bernstock JD, Castri P, Ruetzler C, Miyake J, Lee YJ, Hallenbeck JM. Hypophosphorylation of Ribosomal Protein S6 is a Molecular Mechanism Underlying Ischemic Tolerance Induced by either Hibernation or Preconditioning. Journal of neurochemistry. 2015 doi: 10.1111/jnc.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, Hoege C, Pyrowolakis G, Jentsch S. SUMO, ubiquitin’s mysterious cousin. Nat Rev Mol Cell Biol. 2001;2:202–210. doi: 10.1038/35056591. [DOI] [PubMed] [Google Scholar]
- Na HK, Surh YJ. Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic Biol Med. 2014;67:353–365. doi: 10.1016/j.freeradbiomed.2013.10.819. [DOI] [PubMed] [Google Scholar]
- Niture SK, Kaspar JW, Shen J, Jaiswal AK. Nrf2 signaling and cell survival. Toxicology and applied pharmacology. 2010;244:37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-O’Mara A, Berra E. Deciphering the emerging role of SUMO conjugation in the hypoxia-signaling cascade. Biol Chem. 2013;394:459–469. doi: 10.1515/hsz-2012-0319. [DOI] [PubMed] [Google Scholar]
- Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. J Neurochem. 2003;87:172–181. doi: 10.1046/j.1471-4159.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- Ossola B, Kaariainen TM, Mannisto PT. The multiple faces of quercetin in neuroprotection. Expert opinion on drug safety. 2009;8:397–409. doi: 10.1517/14740330903026944. [DOI] [PubMed] [Google Scholar]
- Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Patten DA, Lafleur VN, Robitaille GA, Chan DA, Giaccia AJ, Richard DE. Hypoxia-inducible factor-1 activation in nonhypoxic conditions: the essential role of mitochondrial-derived reactive oxygen species. Mol Biol Cell. 2010;21:3247–3257. doi: 10.1091/mbc.E10-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata Y, Zhuang H, Kwansa H, Koehler RC, Dore S. Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Experimental neurology. 2010;224:325–329. doi: 10.1016/j.expneurol.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- Shao R, Zhang FP, Tian F, Anders Friberg P, Wang X, Sjoland H, Billig H. Increase of SUMO-1 expression in response to hypoxia: direct interaction with HIF-1alpha in adult mouse brain and heart in vivo. FEBS letters. 2004;569:293–300. doi: 10.1016/j.febslet.2004.05.079. [DOI] [PubMed] [Google Scholar]
- Sharma V, Mishra M, Ghosh S, Tewari R, Basu A, Seth P, Sen E. Modulation of interleukin-1beta mediated inflammatory response in human astrocytes by flavonoids: implications in neuroprotection. Brain research bulletin. 2007;73:55–63. doi: 10.1016/j.brainresbull.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5:437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Kundu JK, Li MH, Na HK, Cha YN. Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch Pharm Res. 2009;32:1163–1176. doi: 10.1007/s12272-009-1807-8. [DOI] [PubMed] [Google Scholar]
- Tacchini L, Dansi P, Matteucci E, Desiderio MA. Hepatocyte growth factor signalling stimulates hypoxia inducible factor-1 (HIF-1) activity in HepG2 hepatoma cells. Carcinogenesis. 2001;22:1363–1371. doi: 10.1093/carcin/22.9.1363. [DOI] [PubMed] [Google Scholar]
- Tempe D, Piechaczyk M, Bossis G. SUMO under stress. Biochemical Society transactions. 2008;36:874–878. doi: 10.1042/BST0360874. [DOI] [PubMed] [Google Scholar]
- Yang W, Ma Q, Mackensen GB, Paschen W. Deep hypothermia markedly activates the small ubiquitin-like modifier conjugation pathway; implications for the fate of cells exposed to transient deep hypothermic cardiopulmonary bypass. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:886–890. doi: 10.1038/jcbfm.2009.16. [DOI] [PubMed] [Google Scholar]
- Yang W, Sheng H, Homi HM, Warner DS, Paschen W. Cerebral ischemia/stroke and small ubiquitin-like modifier (SUMO) conjugation--a new target for therapeutic intervention? Journal of neurochemistry. 2008;106:989–999. doi: 10.1111/j.1471-4159.2008.05404.x. [DOI] [PubMed] [Google Scholar]
- Yang W, Sheng H, Thompson JW, Zhao S, Wang L, Miao P, Liu X, Moseley MA, Paschen W. Small ubiquitin-like modifier 3-modified proteome regulated by brain ischemia in novel small ubiquitin-like modifier transgenic mice: putative protective proteins/pathways. Stroke; a journal of cerebral circulation. 2014;45:1115–1122. doi: 10.1161/STROKEAHA.113.004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Zou AP. Transcriptional regulation of heme oxygenases by HIF-1alpha in renal medullary interstitial cells. Am J Physiol Renal Physiol. 2001;281:F900–908. doi: 10.1152/ajprenal.2001.281.5.F900. [DOI] [PubMed] [Google Scholar]
- Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. Embo J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative immunoblots of SUMO-1 and SUMO-2,3 conjugates in SHSY5Y cells (upper panels) and rat cortical neurons (lower panels) which were treated with the indicated flavonoids at various concentrations (0, 10, 20, 40 μM) for 16 hr.