Abstract
Objective
To characterize a novel “worst” symptom visual analogue scale (WS-VAS) versus the traditional dyspnea visual analogue scale (DVAS) in an acute heart failure (AHF) trial.
Background
AHF trials assess symptom relief as a pivotal endpoint using dyspnea scores. However, many AHF patients’ worst presenting symptom (WS) may not be dyspnea. We hypothesized that a WS-VAS may reflect clinical improvement better than DVAS in AHF.
Methods
AHF patients (n=232) enrolled in the Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE-AHF) Trial indicated their WS at enrollment and completed DVAS and WS-VAS at enrollment (BL), 24, 48 and 72 hours.
Results
Dyspnea was the WS in 61%, body swelling in 29% and fatigue in 10% of patients. Clinical characteristics differed by WS. In all patients, DVAS scores were higher (less severe symptoms) than WS-VAS and the change in WS-VAS over 72 hours was greater than DVAS (P<0.001). Changes in DVAS were smaller in patients with body swelling and fatigue than in patients with dyspnea as their WS (p=0.002) whereas changes in the WS-VAS were similar regardless of patients’ WS. Neither score, nor its change was associated with available decongestion markers (change in N-terminal of the prohormone brain natriuretic peptide [NT-proBNP] or weight or cumulative 72 hour urine volume).
Discussion
Many AHF patients have symptoms other than dyspnea as their most bothersome symptom. The WS-VAS better reflects symptom improvement across the spectrum of AHF phenotypes. Symptom relief and decongestion were not correlated in this AHF study.
Keywords: Acute Heart Failure, Clinical Trials, Quality of Life
INTRODUCTION
Dyspnea relief is a primary goal of acute heart failure (AHF) therapy,(1) a regulatory benchmark for the approval of novel therapeutic agents and a common endpoint in AHF clinical trials.(2) However, a previous trial of advanced HF patients reported that only 52% of patients cite dyspnea as their most bothersome symptom with the remainder citing fatigue and body swelling instead.(3) In a contemporary AHF trial, we hypothesized that the change in an individual patient’s most bothersome symptom may be more closely linked to therapeutic responses than changes in dyspnea alone.
METHODS
Study Design
The Reliable Evaluation of Dyspnea in the Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE-AHF) study (RED-ROSE; ClinicalTrials.gov identifier: NCT01132846) was an ancillary ROSE-AHF study designed to assess novel symptom assessment tools in AHF. ROSE-AHF was performed within the National Heart Lung and Blood Institute (NHLBI)-sponsored Heart Failure Research Network (HFN). Its design and primary results have been published previously.(4) RED-ROSE was approved by the HFN data and safety monitoring board and by each site’s institutional review board. Participants provided written informed consent.
Symptom assessment tools
Trained study coordinators used standardized scripts to administer each symptom assessment tool.
Dyspnea visual analogue scale (DVAS)
Patients indicated how their breathing felt “right now” on an analogue scale from 0 (worst possible) to 100 (no breathlessness) throughout the study [baseline (randomization), 24, 48 and 72-hours].
Worst symptom VAS (WS-VAS)
At enrollment, patients indicated their most bothersome symptom (choice of fatigue, body swelling or dyspnea). Patients with swelling or fatigue as their WS completed an additional 100-mm VAS (0, worst; 100, none) for that specific WS throughout the study.
Global well-being VAS (GVAS)
We also administered a global well-being VAS (GVAS) throughout the study where patients ranked their global health status from 0 (worst) to 100 (best).
Decongestion markers
Clinical markers of decongestion included weight change, cumulative urine volume and percent change in NT-proBNP from randomization to 72 hours.
Statistical Analysis
Differences in baseline characteristics and symptom scores between the group with dyspnea as a WS and those with fatigue or those with swelling as their WS was assessed with Wilcoxon rank test for continuous variables or Likelihood Ratio Chi-square for discrete variables. Nonparametric repeated measures test (Friedman) was used to assess whether the DVAS or WS-VAS scores differed over time. The Kruskal-Wallis test was used to analyze for differences in the change in symptom scores across all 3 groups. Subsequently, each of the fatigue and swelling groups were compared to the dyspnea group using the Mann Whitney test. Spearman correlation coefficients were used to examine the correlation between scores. The relationship between changes in scores and markers of clinical decongestion was examined using general linear models adjusting for baseline values of scores and baseline congestion markers. No imputation or carry forward was used to account for missing data. All analyses were conducted with SAS statistical software, version 9.2 or JMP, version 9.
RESULTS
Baseline Characteristics
RED-ROSE commenced after ROSE-AHF had started and enrolled 232 of the 360 ROSE-AHF patients. Baseline characteristics of the RED-ROSE participants were similar to those of the ROSE cohort (Table 1).
Table 1.
Baseline characteristics of RED-ROSE cohort versus ROSE cohort
| Characteristic | RED-ROSE (N=232) |
ROSE (N=360) |
|---|---|---|
| Age, years | 69 (62–79) | 70 (62–79) |
| Male sex | 159 (69%) | 264 (73%) |
| White race | 166 (72%) | 272 (76%) |
| HF hospitalization | 143 (62%) | 240 (67%) |
| Ejection fraction, % | 35 (23–54) | 34 (21–53) |
| Ejection fraction ≥ 50% | 76 (33%) | 94 (26%) |
| Hypertension | 195 (84%) | 298 (83%) |
| Diabetes | 127 (55%) | 200 (56%) |
| Stroke | 21 (9%) | 31 (9%) |
| Atrial fibrillation | 135 (58%) | 215 (60%) |
| COPD | 59 (25%) | 95 (26%) |
| Medications | ||
| Loop diuretic | 215 (93%) | 340 (94%) |
| ACE or ARB | 112 (48%) | 179 (50%) |
| Hydralazine | 52 (22%) | 68 (19%) |
| Nitrates | 60 (26%) | 90 (25%) |
| Beta blocker | 196 (84%) | 300 (83%) |
| Aldosterone antagonist | 68 (29%) | 109 (30%) |
| Digoxin | 53 (23%) | 89 (25%) |
| Clinical Examination | ||
| Heart rate (bpm) | 74 (66–85) | 74 (65–85) |
| Systolic BP, mmHg | 116 (104–129) | 114 (103–127) |
| Body mass index, Kg/m2 | 31 (27–37) | 31 (27–37) |
| JVP ≥ 8 cm, | 212 (95%) | 327 (95%) |
| Rales | 122 (54%) | 197 (56%) |
| Edema ≥ 2+/4+ | 159 (69%) | 251 (70%) |
| Orthopnea | 193 (88%) | 307 (90%) |
| Laboratory data | ||
| Hemoglobin, g/dL | 11.5 (10.4–12.8) | 11.4 (10.3–12.7) |
| eGFR, mL/min/1.73m2 | 45 (33–56) | 42 (32–53) |
| NT Pro-BNP, pg/mL | 5055 (2358–10348) | 5323 (2420–10797) |
In RED-ROSE participants, 141 (61%) reported dyspnea, 24 (10%) fatigue and 67 (29%) body swelling as their WS at enrollment (Table 2). Compared to patients with dyspnea as their WS, those with fatigue were less likely to have been hospitalized for HF in the last year, be treated with renin-angiotensin system (RAS) antagonists, or report orthopnea but were more likely to be in atrial fibrillation. Compared to patients with dyspnea as their WS, those with body swelling were less likely to have been hospitalized for HF in the last year or have rales or orthopnea but were more likely to have severe edema and had worse renal function.
Table 2.
Baseline Characteristics of RED-ROSE cohort
| WORST REPORTED SYMPTOM | ||||
|---|---|---|---|---|
| Characteristic | All (N=232) |
Dyspnea (N=141) |
Fatigue (N=24) |
Body Swelling (N=67) |
| Age, years | 69 (62–79) | 68 (60–75) | 71 (62–82) | 72 (62–82) |
| Male sex | 159 (69%) | 96 (68%) | 17 (71%) | 46 (69%) |
| White race | 166 (72%) | 98 (70%) | 18 (75%) | 50 (75%) |
| HF hospitalization | 143 (62%) | 93 (67%) | 13 (54%)* | 37 (55%)* |
| Ejection fraction, % | 35 (23–54) | 33 (23–52) | 43 (18–55) | 38 (23–53) |
| Ejection fraction ≥ 50% | 76 (33%) | 42 (30%) | 10 (42%) | 24 (36%) |
| Hypertension | 195 (84%) | 120 (85%) | 19 (79%) | 56 (84%) |
| Diabetes | 127 (55%) | 80 (57%) | 14 (58%) | 33 (49%) |
| Stroke | 21 (9%) | 10 (7%) | 4 (17%) | 7 (10%) |
| Atrial fibrillation | 135 (58%) | 74 (52%) | 18 (75%)* | 43 (64%) |
| COPD | 59 (25%) | 41 (29%) | 3 (13%) | 15 (22%) |
| Medications | ||||
| Loop diuretic | 215 (93%) | 128 (91%) | 24 (100%) | 63 (94%) |
| ACE or ARB | 112 (48%) | 74 (52%) | 6 (25%)* | 32 (48%) |
| Hydralazine | 52 (22%) | 30 (21%) | 6 (25%) | 16 (24%) |
| Nitrates | 60 (26%) | 39 (28%) | 5 (21%) | 16 (24%) |
| Beta blocker | 196 (84%) | 120 (85%) | 22 (92%) | 54 (81%) |
| Aldosterone antagonist | 68 (29%) | 44 (31%) | 6 (25%) | 18 (27%) |
| Digoxin | 53 (23%) | 29 (21%) | 8 (33%) | 16 (24%) |
| Clinical Examination | ||||
| Heart rate (bpm) | 74 (66–85) | 76 (68–85) | 72 (66–81) | 71 (63–85) |
| Systolic BP, mmHg | 116 (104–129) | 117 (106–127) | 114 (106–124) | 114 (100–132) |
| Body mass index, Kg/m2 | 31 (27–37) | 31 (28–37) | 29 (25–37) | 31 (27–37) |
| JVP ≥ 8 cm, (n=224) | 212 (95%) | 125 (93%) | 24 (100%) | 63 (97%) |
| Rales (n=227) | 122 (54%) | 81 (59%) | 12 (52%) | 29 (46%)* |
| Edema ≥ 2+/4+ (n=229) | 159 (69%) | 89 (64%) | 18 (75%) | 52 (79%)* |
| Orthopnea, (n=219) | 193 (88%) | 126 (95%) | 20 (87%)* | 47 (75%)* |
| Laboratory data | ||||
| Hemoglobin, g/dL | 11.5 (10.4–12.8) | 11.8 (10.4–12.9) | 11.4 (10.7–12.6) | 11.2 (10.3–12.6) |
| eGFR, mL/min/1.73m2 | 45 (33–56) | 45 (35–57) | 48 (33–57) | 42 (30–51)* |
| NT Pro-BNP, pg/mL | 5055 (2358–10348) | 5109 (2371–9516) | 4269 (2223–12402) | 5268 (2905–11888) |
| Baseline Symptom Scores | ||||
| Dyspnea VAS | 62.5 (40.0–82.0) | 54.0 (35.0–75.0) | 77.0 (54.0–92.0)* | 74.5 (50.0–90.0)* |
| WS-VAS | 50.0 (25.0–71.0) | 54.0 (35.0–75.0) | 52.0 (47.0–80.0) | 32.5 (18.0–56.0)*† |
Data are number (%) or median (interquartile range).
p<0.05 vs dyspnea as worse symptom,
p <0.05 vs fatigue as worst symptom for “Baseline Symptom Scores” analysis only.
Abbreviations:
ACE, Angiotensin Converting Enzyme; ARB, Angiotensin Receptor Blocker; BP, Blood Pressure; COPD, Chronic Obstructive Pulmonary Disease; eGFR, Estimated Glomerular Filtration Rate; HF, Heart Failure; JVP, Jugular Venous Pressure; NT Pro-BNP, N-terminal pro-brain natriuretic peptide
Symptom scores at baseline
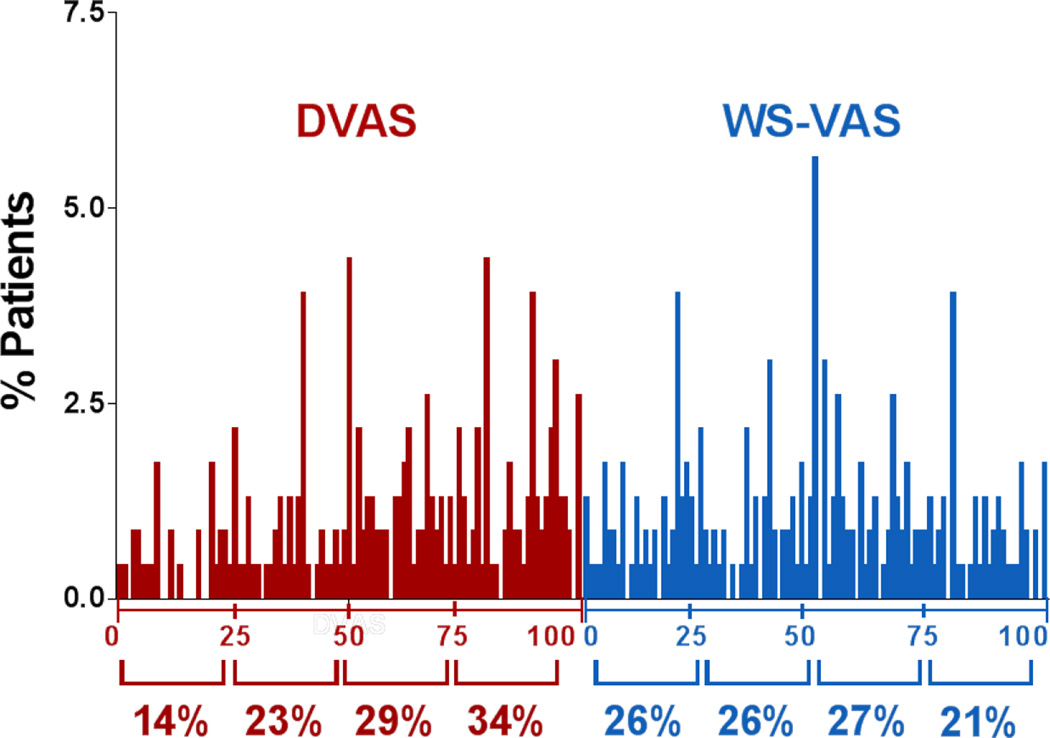
At enrollment, the distribution of DVAS scores was slightly skewed towards higher scores while patients were more evenly distributed across the range of possible WS-VAS scores (Figure 1). As compared to patients with dyspnea as their WS, the DVAS was higher (less dyspnea) in patients with fatigue or body swelling as their WS (Table 2). As compared to those with dyspnea or fatigue as their WS, the WS-VAS was lower (more severe) in those with body swelling as their WS (Table 2). The median WS-VAS score was significantly lower (worse) than the DVAS in patients with fatigue (52.0 vs 77.0, p=0.04) or body swelling (32.5 vs 74.5, p<0.001) as their WS (Table 2).
Figure 1. Distribution of symptom scores at enrollment.
The frequency distribution of the dyspnea visual analogue scale (DVAS: n= 230), or worst symptom visual analogue scale (WS-VAS; n=230) are shown per quartile of each scale. Patients were more evenly distributed across the range of possible WS-VAS scores compared to DVAS scores.
Change in symptom scores over time
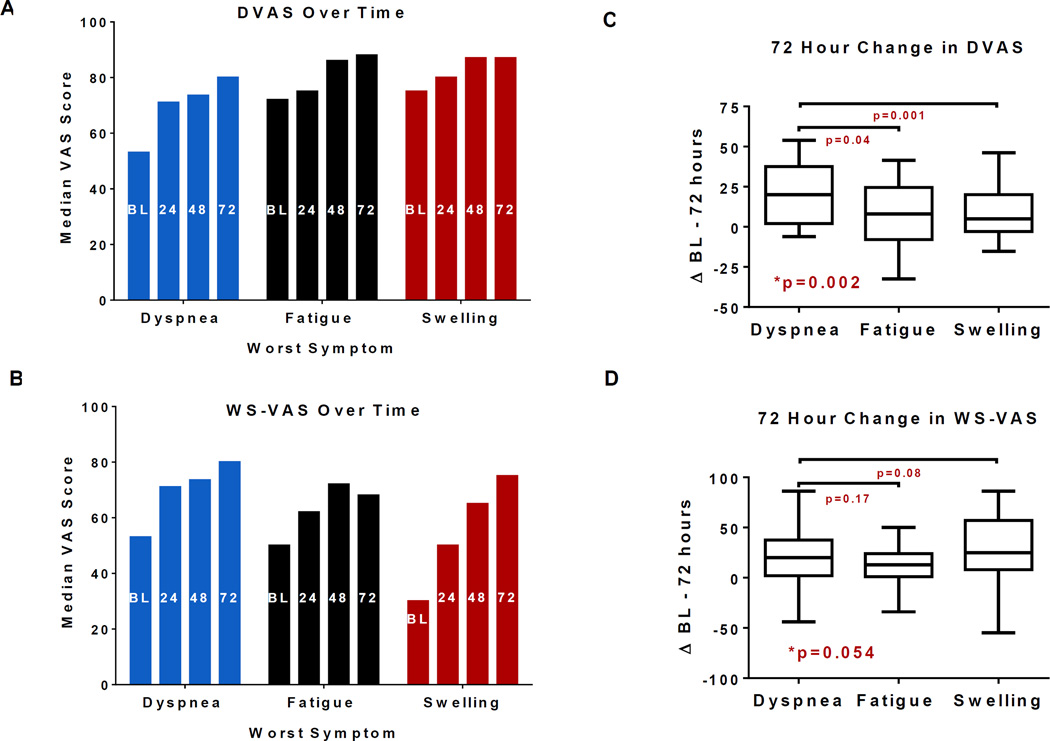
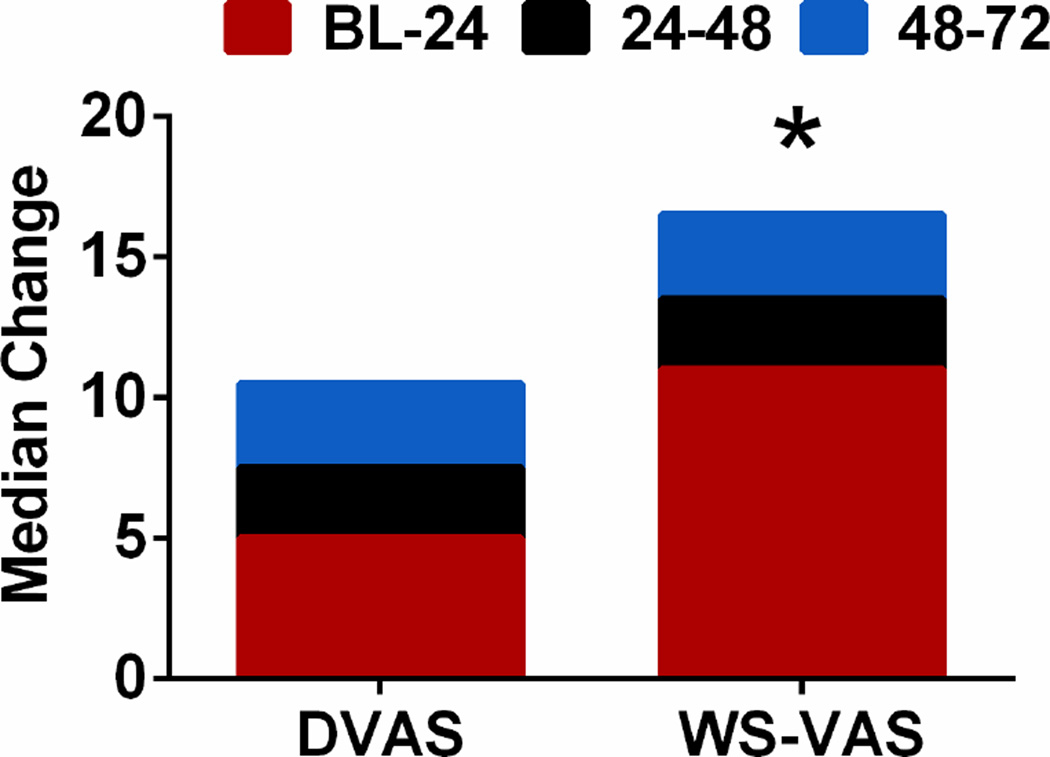
In patients with paired daily assessments (enrollment through 72 hours) of both symptom scores (n=206), both the DVAS and WS-VAS increased over time indicating symptom improvement (Figure 2 A–B). The change in DVAS from enrollment (BL) to 72 hours was lower in patients with body swelling and fatigue as their WS than in patients with dyspnea as their WS (Figure 2 C). The change in WS-VAS from BL to 72 hours was similar regardless of patients’ WS (Figure 2 D). Overall, the BL to 72 hour improvement in the WS-VAS [19 (4,41)] was greater than that of the DVAS [13 (0,31); p<0.001] with the majority of improvement in both scores occurring in the first 24 hours compared to subsequent days (Figure 3).
Figure 2. Symptom scores over time.
Median dyspnea visual analogue scale (DVAS: A), or worst symptom visual analogue scale (WS-VAS; B) at enrollment (BL) through 72 hours. Both scores increased over time within each WS group (p<0.0001 for all). Median (IQR) for changes in DVAS (C) or WS-VAS (D) from BL to 72 hours according to the self-identified worst symptom (dyspnea, fatigue or body swelling); *p: overall Kruskal-Wallis test. The p values for comparison between patients with fatigue or with body swelling versus those with dyspnea (Mann Whitney test) are shown.
Figure 3. Changes in symptom scores over time.
Median change in dyspnea visual analogue scale (DVAS), or worst symptom visual analogue scale (WS-VAS) from BL to 72hrs in 24 hour increments. * p<0.001 vs DVAS
Association of changes in symptom scores with decongestion markers
There were no clinically meaningful associations between changes in DVAS or WS-VAS from BL to 72 hours and extent of decongestion at 72 hours as the model R2 values were all quite low (< 0.05; Table 3).
Table 3.
Association between changes in symptom scores and extent of decongestion.
| Cumulative Urine Volume (BL to 72 Hours) |
Weight Change (BL to 72 Hours) |
Percent Change in NT-proBNP (BL to 72 Hours) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Model R2 | Parameter Estimate (CI) ml |
P value |
Model R2 | Parameter Estimate (CI) lbs |
P value |
Model R2 | Parameter Estimate (CI) % |
P value |
|
| Change DVAS (BL to 72 Hours) | 0.04 | 29 (9 – 50) | 0.005 | 0.02 | −0.05 (−0.10 – 0.004) | 0.07 | 0.01 | −0.23 (−0.50 − 0.04) | 0.10 |
| Change WS-VAS (BL to 72 Hours) | 0.02 | 17 (−3 – 36) | 0.09 | 0.02 | −0.04 (−0.09 – 0.01) | 0.13 | 0.02 | −0.35 (−0.70 − 0.00) | 0.05 |
Linear regression model adjusts for baseline symptom score value and when appropriate, for baseline value of the congestion marker (weight and NT-proBNP level).
Global well-being
The WS-VAS and GVAS (n=206 with both scores at BL and 72 hours) were moderately correlated at BL (r=0.47, p<0.0001) and 72 hours (r=0.69, p<0.0001). The change from BL to 72 hours in the GVAS (18 (3–38)) was similar to that in the WS-VAS (20 (4–41); p=0.20). As with the WS-VAS, changes in the GVAS were similar in patients with dyspnea (20 (6–40)) or other (13 (0–32), p=0.15) symptoms as their WS.
DISCUSSION
Dyspnea assessment tools in AHF
The DVAS is a widely used measure of dyspnea severity in AHF trials and may be more sensitive to modest changes in dyspnea severity than a Likert based score in AHF(5, 6). In RED-ROSE, on average, changes in DVAS scores (16.5 mm) over 72 hours were modest and consistent with other AHF studies assessing dyspnea relief at 6 hours(7), 96 hours(8) or 5 days(6) where changes in DVAS ranged from 14 to 28 mm. The relatively modest improvement in DVAS here and in other AHF studies, despite aggressive therapy, supports the need for alternate measures of symptom relief in AHF.
Symptom specific assessment tools in AHF
In RED-ROSE, 39% of patients identified a symptom other than dyspnea as their WS, similar to findings in the ESCAPE trial of hemodynamic guided therapy on outcomes in advanced HF patients, where half of patients reported fatigue, abdominal discomfort or body swelling rather than dyspnea as their WS. In ESCAPE, patients with WS other than dyspnea also had worse renal function and more physical signs of right sided failure as observed in RED-ROSE. Importantly, in ESCAPE, hemodynamic profiles (cardiac index, pulmonary capillary wedge pressure and right atrial pressure) were similar across WS groups(3). While invasive hemodynamics were not assessed in RED-ROSE, together, these studies suggest that the hemodynamic perturbations associated with AHF and targeted in AHF therapy are perceived differently by patients, potentially due to differences in the interplay of age, HF, comorbid conditions and affective conditions or simply differences in the perception or interpretation of physical limitations.(9, 10)
In RED-ROSE, the WS-VAS showed greater change over 72 hours than the DVAS and in contrast to the DVAS, changes were similar regardless of WS. Recognizing that distinct symptom clusters exist in AHF(10), some studies have utilized a global well-being scale to assess overall health status in AHF.(6, 11, 12) Here and in ESCAPE,(3, 12) changes in the GVAS correlated with changes in WS-VAS. These findings suggest that the WS-VAS or GVAS are more sensitive to symptom improvement than dyspnea focused symptom assessments across the spectrum of AHF phenotypes. Despite the correlation between the WS-VAS and GVAS, they may not provide similar information as GVAS may be more sensitive to affect and comorbid conditions whereas the WS-VAS assesses a single, HF related symptom which may be specifically impacted by AHF treatment.
Symptom relief and decongestion
In RED-ROSE, neither DVAS nor WS-VAS change at 72 hours were meaningfully correlated with changes in markers of decongestion. Our findings do not provide evidence that the use of the WS-VAS greatly strengthens the relationship between symptom relief and decongestion. Regardless of WS, the lack of correlation between extent of decongestion and symptom improvement may reflect the fact neither fluid output nor weight change were scaled to an assessment of the goal fluid or weight loss and thus, are only rough (but widely used) markers of decongestion. Further, symptom relief may require only a threshold degree of fluid loss as the greatest change in symptom severity occurred in the first 24 hours. Finally, symptom improvement may be affected by other therapeutic measures not impacting decongestion markers.
Limitations
Exclusion of AHF patients without significant renal dysfunction may have affected our findings. Patients were enrolled up to 24 hours after presentation and dyspnea may have been more severe prior to enrollment with change from presentation to enrollment not captured. Patients may have different symptoms that are most bothersome to them at different time points. Females were underrepresented in this cohort.
Conclusion
Approximately 40% of AHF patients have symptoms other than dyspnea as their most bothersome symptom. Worst symptom specific scores appear more sensitive than dyspnea specific scores to clinical improvement across the spectrum of AHF phenotypes. Symptom relief is poorly correlated to widely used markers of decongestion efficacy. Additional prospective studies are needed to support the use of the worse symptom score as a novel metric of symptom relief in AHF.
Acknowledgments
Funding Sources:
This work was supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) (coordinating center: U10 HL084904; regional clinical centers and Heart Failure Network Clinical Research Skills Development Cores : U01 HL084861, U10 HL110312, U109 HL110337, U01 HL084889, U01 HL084890, U01 HL084891, U10 HL110342, U10 HL110262, U01 HL084931, U10 HL110297, U10 HL110302, U10 HL110309, U10 HL110336, U10 HL110338). This work was also supported by the National Center for Advancing Translational Sciences (UL1TR000454, UL1TR000135, UL1RR025008, UL1TR000439) and the National Institute on Minority Health and Health Disparities (8 U54 MD007588).
Disclosures:
RJM receives research support from the NIH, Amgen, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Gilead, Novartis, Otsuka, and ResMed; honoraria from Thoratec; and has served on an advisory board for Luitpold Pharmaceuticals, Inc. AFH reports grants from Amgen, Bristol Myers Squibb, GlaxoSmithKline and Novartis, outside the submitted work. All others report no disclosures relevant to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B, Masoudi FA, Butler J, McBride PE, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Teerlink JR. Dyspnea as an end point in clinical trials of therapies for acute decompensated heart failure. Am Heart J. 2003;145(2 Suppl):S26–S33. doi: 10.1067/mhj.2003.151. [DOI] [PubMed] [Google Scholar]
- 3.Kato M, Stevenson LW, Palardy M, Campbell PM, May CW, Lakdawala NK, et al. The worst symptom as defined by patients during heart failure hospitalization: implications for response to therapy. J Card Fail. 2012;18(7):524–533. doi: 10.1016/j.cardfail.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen HH, AbouEzzeddine OF, Anstrom KJ, Givertz MM, Bart BA, Felker GM, et al. Targeting the kidney in acute heart failure: can old drugs provide new benefit? Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE AHF) trial. Circ Heart Fail. 2013;6(5):1087–1094. doi: 10.1161/CIRCHEARTFAILURE.113.000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen LA, Metra M, Milo-Cotter O, Filippatos G, Reisin LH, Bensimhon DR, et al. Improvements in signs and symptoms during hospitalization for acute heart failure follow different patterns and depend on the measurement scales used: an international, prospective registry to evaluate the evolution of measures of disease severity in acute heart failure (MEASURE-AHF) J Card Fail. 2008;14(9):777–784. doi: 10.1016/j.cardfail.2008.07.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381(9860):29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 7.Mebazaa A, Pang PS, Tavares M, Collins SP, Storrow AB, Laribi S, et al. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J. 2010;31(7):832–841. doi: 10.1093/eurheartj/ehp458. [DOI] [PubMed] [Google Scholar]
- 8.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'Connor CM, Bull DA, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367(24):2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjellstrom B, van der Wal MH. Old and new tools to assess dyspnea in the hospitalized patient. Curr Heart Fail Rep. 2013;10(3):204–211. doi: 10.1007/s11897-013-0141-0. [DOI] [PubMed] [Google Scholar]
- 10.Jurgens CY, Moser DK, Armola R, Carlson B, Sethares K, Riegel B. Symptom clusters of heart failure. Res Nurs Health. 2009;32(5):551–560. doi: 10.1002/nur.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294(13):1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]