Abstract
Addictions, including alcohol use disorders (AUDs), are characterized by the loss of control over drug seeking and consumption, but the neural circuits and signaling mechanisms responsible for the transition from controlled use to uncontrolled abuse remain incompletely understood. Prior studies have shown that ‘compulsive-like’ behaviors in rodents, e.g., persistent responding for ethanol (EtOH) despite punishment, are increased after chronic exposure to EtOH. The main goal of the current study was to assess the effects of chronic intermittent EtOH (CIE) exposure on multiple, putative measures of compulsive-like EtOH seeking in C57BL/6J mice. Mice were exposed to two or four weekly cycles of CIE and then, post-withdrawal, tested for progressive ratio responding for EtOH, sustained responding during signaled EtOH-unavailability and (footshock) punished-suppression of responding for EtOH. Results showed that mice exposed to CIE exhibited attenuated suppression of EtOH-seeking during punishment, as compared to air-exposed controls. By contrast, CIE exposure affected neither punished food reward-seeking behavior, nor other putative measures of compulsive-like EtOH-seeking. Ex vivo RT-PCR analysis of brain tissue found reduced sensitivity to punished EtOH-seeking after CIE exposure was accompanied by a significant increase in gene expression of the GluN1 and GluN2A subunits of the N-methyl-D-aspartate receptor (NMDAR), specifically in the medial orbitofrontal cortex (mOFC). Moreover, slice electrophysiological analysis revealed increased NMDAR-mediated currents in the mOFC after CIE exposure in test-naïve mice. Collectively, the current findings add to growing body of evidence demonstrating that chronic exposure to EtOH fosters resistance to punished EtOH-seeking in association with adaptations in cortical glutamatergic transmission.
Keywords: alcohol, CIE, NMDAR, mouse, orbitofrontal cortex, addiction
Introduction
A hallmark of drug addictions, including alcohol use disorders (AUDs), is the loss of control over the seeking and consuming of the substance. Over time, individuals spend increasing amounts of time using and seeking drugs until these behaviors become uncontrolled (DSM-5, 2013). Characterizing the neural circuits and signaling mechanisms responsible for the transition from controlled use to uncontrolled abuse is a major focus of current preclinical research (Everitt et al., 2008; Koob and Volkow, 2010; Vengeliene et al., 2009).
To this end, the field of addiction has made efforts to develop rodent models of compulsive drug-seeking that in various forms measure the persistence of drug-seeking, the motivation to obtain a drug, and continued drug-seeking in the face of adverse consequences (Belin-Rauscent et al., 2015; Deroche-Gamonet et al., 2004; Vanderschuren and Everitt, 2004). For example, prior studies have demonstrated that rats with extended (i.e., > 1 month) access to cocaine achieve higher breakpoints on a progressive ratio (PR) schedule of reinforcement, seek cocaine when it is unavailable, and continue to respond for the drug when such seeking behavior is punished by footshock (Belin et al., 2011; Deroche-Gamonet et al., 2004). A number of subsequent studies have used these and other behavioral procedures to investigate changes in neural circuits (Chen et al., 2013; Hollander et al., 2010; Jonkman et al., 2012; Xue et al., 2012) and synaptic functions (Cannella et al., 2013; Kasanetz et al., 2013) associated with compulsive cocaine-seeking.
There is growing interest in developing rodent models of compulsive-responding for other drugs of abuse (Wade et al., 2014), including alcohol (ethanol, EtOH) (Hopf and Lesscher, 2014; Leão et al., 2015; Vengeliene et al., 2009). Putative measures of compulsive-like EtOH intake have included sustained operant responding for EtOH during footshock or EtOH-unavailability (Radwanska and Kaczmarek, 2012; Seif et al., 2013) and continued EtOH consumption following adulteration with bitter-tasting quinine (Spanagel et al., 1996; Wolffgramm and Heyne, 1991). Interestingly, recent studies in rats and C57BL/6J mice have shown that the aversion to EtOH drinking caused by quinine-adulteration is absent after prolonged intermittent, or limited, access (IA) to EtOH (Hopf et al., 2010; Lesscher et al., 2010; Loi et al., 2010) or following chronic exposure to EtOH vapors (Vendruscolo et al., 2012). These observations suggest that compulsive-like EtOH consumption may emerge in parallel with the development of EtOH dependence. A few studies have provided initial insight into the potential molecular mechanisms underlying these changes. The effects of EtOH vapor on quinine-attenuated drinking are prevented by systemic glucocorticoid receptor blockade via mifepristone (Vendruscolo et al., 2012). Moreover, the effects of intermittent EtOH drinking on quinine-attenuated drinking are blocked by the partial N-methyl-D-aspartate receptor (NMDAR) agonist, D-serine, administered systemically or directly into the nucleus accumbens (NAc) core (Seif et al., 2015), while IA-induced resistance to footshock-suppression of EtOH responding are reversed by viral-knockdown of GluN2C-containing NMDAR in the NAc core (Seif et al., 2013).
The goal of the current study was to extend the existing literature by examining, in C57BL/6J mice, the effects of chronic EtOH exposure on measures of elevated or compulsive-like reward seeking in C57BL/6J mice: progressive ratio responding, sustained responding during signaled reward-unavailability, and punished-suppression of responding. We employed a chronic intermittent EtOH (CIE) vapor procedure previously shown to increase voluntary EtOH consumption (Becker and Lopez, 2004; DePoy et al., 2013; Gilpin et al., 2008; Griffin III et al., 2009b; Holmes et al., 2012; Lopez and Becker, 2005; O'Dell et al., 2004; Rimondini et al., 2002; Rimondini et al., 2003), enhance signs of withdrawal (Kliethermes, 2005; Metten et al., 2010; O'Dell et al., 2004; Roberts et al., 1996; Roberts et al., 2000), produce tolerance to EtOH intoxication (Daut et al., 2015), insensitivity to EtOH-devaluation (Lopez et al., 2014) and alter various cognitive behaviors (Badanich et al., 2011; DePoy et al., 2013; DePoy et al., 2015; Holmes et al., 2012). In order to explore neural correlates of CIE-induced changes in compulsive-like behavior, we assessed NMDAR gene expression in various brain regions and measured NMDAR-mediated synaptic currents in the medial orbitofrontal cortex (mOFC).
Materials and methods
Subjects
Adult male C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) at 7-9 weeks of age and housed in a temperature and humidity controlled vivarium under a 12 h light/dark cycle (lights on 0600 h). Testing began at least 1 week after acclimation to the animal facility. Test-naïve mice were used in each experiment. All procedures were approved by the NIAAA Animal Care and Use Committee and Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill, and followed the NIH guidelines outlined in ‘Using Animals in Intramural Research.’ The number of mice tested is indicated in the figure legends.
Behavioral training
Testing was conducted in a chamber measuring 21.6 × 17.8 × 12.7 cm (model ENV-307W, Med Associates, St. Albans, VT, USA) housed in a sound- and light-attenuating box. A receptacle for delivery of food pellets or liquid rewards, a house light, a tone generator, and 2 levers (left = active, right = inactive) were located on one wall of the chamber. To motivate performance, mice were food-restricted and maintained at 80-85% of their free-feeding body weight.
Mice were trained to press the active lever for reward on a fixed-ratio (FR) 1 (for 2 daily sessions) and then a FR3 schedule of reinforcement until criterion. Following each reward delivery, a 65 dB tone was played for 2 seconds. Responses on the inactive lever had no programmed consequences. Training sessions were 60 minutes in duration. A 20-minute reward-available period was followed by a 10-minute reward-unavailable period and this sequence was then repeated. Reward-available periods were signaled by continuous illumination of the reward receptacle.
One cohort of mice was trained and tested for responses to a 14 mg food pellet reward (F05684, BioServ, Frenchtown, NJ, USA). A separate cohort was trained and tested for responses to a liquid EtOH reward using a modified sucrose-fading procedure. Reward was 0.01 mL of a 10% sucrose solution during the 2 FR1 sessions and the first 2 FR3 sessions. EtOH was then introduced. The solution was 10% sucrose/10% EtOH for 3 FR3 sessions and then 5% sucrose/10% EtOH for 3 FR3 sessions. Thereafter, the solution was 10% EtOH for a minimum of 5 sessions until criterion of reliable responding on the active lever (<20% variability in responding on 3 successive sessions) was attained. Mice earned an average of 14 rewards, equal to a 0.5 g/kg dose of EtOH during the 60 minute session for a 22 g mouse. At the end of training, response rate on the final 3 test days was used to match mice into groups (air vs. CIE) with similar overall mean response rates.
CIE exposure
Mice were chronically exposed to EtOH after attaining operant training criterion and received weekly “reminder” operant sessions throughout exposure. For EtOH exposure, we employed a vapor inhalation procedure as previously described (Holmes et al., 2012; Lopez and Becker, 2005). Mice were removed from the home cage and housed in clean 60 × 36 × 60 cm mouse cages (PlasLabs, Lansing, MI, USA). Cages were placed in Plexiglas vapor chambers. Air was passed through an air stone submerged in 95% EtOH and mixed with fresh air to deliver 19–22 mg of EtOH/L of air at a rate of ∼10 L/minute. Blood EtOH levels were confirmed via weekly blood samples taken from ‘sentinel’ mice exposed to EtOH simultaneously with the test mice (operant: 150.1 ± 6.99 mg/dL, n=23; fear: 198 ± 16.24 mg/dL, n=12). Prior to entering the chambers, the CIE group received 71.6 mg/kg injections (i.p.) of the alcohol dehydrogenase inhibitor pyrazole (Sigma, St. Louis, MO, USA) combined with 1.5 g/kg of 20% EtOH (v/v), in a volume of 10 mL/kg. This served to initiate intoxication and stabilize blood EtOH levels. Chamber exposure lasted 16 hours/day (in at 1700 h, out at 0900 h), followed by an 8-h withdrawal. Intermittent exposure continued for 4 consecutive days (Monday through Thursday) and was followed by 3 days (80 hours) of withdrawal (Friday through Monday). This complete weekly cycle was repeated for a total of either two or four times. Air controls received a 68.1 mg/kg injection (i.p.) of pyrazole before placement in dedicated air chambers receiving vaporized air at the same exchange rate as the EtOH chambers.
Behavioral testing
Beginning 3 days after CIE, mice were assessed for responding for food or EtOH reward (according to reward type received during training) in each of 3 separate test conditions (for schematic, see Figure 1a).
Figure 1. Behavioral procedures. (a).

Mice were trained to lever-press for a 14 mg food pellet or a 0.01 mL 10% EtOH reward, on a FR3 schedule of reinforcement, and then given CIE exposure or air for 2 or 4 weekly cycles. A series of three behavioral tests of compulsive behavior followed. (b) Mice were tested for rewarded lever-pressing during ‘reward-available’ (signaled by illumination of the reward magazine) and ‘reward-unavailable’ periods. (c) Motivation for reward was assayed from lever-pressing breakpoints on a progressive ratio (PR) schedule. (d) Punished-suppression of reward-seeking was tested with a 0.4 mA (food-reward) or 0.6 mA (EtOH-reward) footshock.
Responding for unavailable reward
To measure the persistence of reward-seeking when reward was unavailable, mice were tested for lever-pressing (FR3 schedule) during signaled reward-available and reward-unavailable periods (Figure 1b). Testing was conducted over 5 sessions on consecutive days. Performance was calculated as the average of responding over the 5 sessions.
Progressive ratio responding for reward
On the next session (10 days post-CIE), motivation for reward was tested using a progressive ratio (PR) schedule, in which a progressively higher number of presses (range 1-737) were required to obtain a reward (Figure 1c). The session terminated when the mouse failed to earn a reward in a 60-minute period. The breakpoint was taken as the highest number of presses that resulted in an earned reward.
Responding for a punished reward
Following PR testing, responding on a FR3 schedule of reinforcement was reestablished for 1 session (pilot experiments showed 1 session was sufficient to reestablish response rates evident prior to PR testing). On the next session (12 days post-CIE), punished-suppression of lever-pressing was assessed during a 40-minute session (Figure 1d). Here, the first active lever press in a sequence signaled a pending punishment by illuminating the house light. The second active lever press produced a footshock via a metal grid connected to a shock generator (model ENV-416S, Med Associates, St. Albans, VT, USA). The third active lever press produced a reward. The signal-shock-reward sequence was then repeated. Because baseline response rates varied after CIE exposure, active lever pressing during the punished session was compared with active lever presses during the reward-available portion of the 5 post-CIE sessions described above (=baseline).
Pilot studies conducted in EtOH naïve mice showed that 0.4 mA footshock was sufficient to significantly suppress lever pressing in mice trained and tested for food reward (t12 = 12.83, P<.001). However, 0.4 mA was ineffective in mice trained and tested for EtOH reward (t6 = 0.59, P>.05), whereas 0.6 mA significantly suppressed lever pressing (t6 = 2.65, P<.05) (Table 1). Therefore, footshock intensity was set at 0.4 and 0.6 mA for food and EtOH reward experiments, respectively.
Table 1. Higher punishment required to suppress EtOH-seeking than food-seeking in CIE naïve mice.
A footshock level of 0.4 mA significantly suppressed food- but not EtOH-seeking. A footshock level of 0.6 mA significantly suppressed EtOH-seeking. n=12-15 per group. *P<.05 compared to pre-punished baseline.
| Reward | Baseline presses/min | Punished presses/min (0.4 mA) | Punished presses/min (0.6 mA) |
|---|---|---|---|
| Food | 7.09 ± 0.42 | 0.71 ± 0.17 * | not tested |
| EtOH | 0.91 ± 0.23 | 0.76 ± 0.17 | 0.35 ± 0.11 * |
Pavlovian fear conditioning
Three days after exposure to 4 weekly cycles of CIE, mice underwent cued Pavlovian fear conditioning as previously described (Fitzgerald et al., 2014). The conditioning box measured 27 × 27 × 11 cm and the metal-rod floor was cleaned with a 79.5% water/19.5% ethanol/1% vanilla-extract solution between mice. The conditioned stimulus (30-second, 80 dB, 3 kHz tone) was paired with the unconditioned stimulus (2-second, 0.6 mA scrambled foot shock) 3 times during the last 2 seconds of each tone presentation. Stimulus presentation was controlled by MED Associated VideoFreeze system (MED Associated, Burlington, VT, USA), which also automatically scored freezing behavior. 24 h after conditioning the mice were tested for retrieval of fear in a distinct context (27×27×11 cm chamber with a white smooth floor insert that was positioned over the grid floor and a white curved wall insert cleaned with a 1% acetic acid/99% water solution) housed in a different room from conditioning. Following a 180 s acclimation period, the conditioned stimulus was presented 5 times with an inter-stimulus interval of 5 s.
RT-PCR of NMDAR subunit gene expression
The day after the completion of behavioral testing (i.e., punished session), a subset of mice responding for EtOH reward were sacrificed via rapid cervical dislocation and decapitation to remove and freeze brains for NMDAR gene expression analysis. Tissue from the medial orbitofrontal cortex (mOFC), ventromedial prefrontal cortex (vmPFC), dorsolateral striatum (DLS), and basolateral amygdala (BLA) was dissected with a 1 mm-diameter micro-punch and stored in RNAlater solution (Ambion, Grand Island, NY, USA). Total RNA was isolated with RNeasy kit (Qiagen, Valencia, CA, USA). Reverse transcription was performed with 1 microgram of total RNA using the Iscript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) with C1000 thermal cycler (Bio-Rad). Expression of the Grin1 (GluN1 receptor), Grin2A (GluN2A receptor), and Grin2B (GluN2B receptor) genes was quantified with the Qiagen QuantiTect Primer Assays (cat# QT00159061, QT00093562, and QT00169281, respectively) and Power SYBR Green PCR master mix (Applied Biosystems, Grand Island, NY, USA) using a StepOnePlus™ Real-Time PCR instrument (Applied Biosystems). Gene expression was quantified by normalization with the Qiagen QuantiTect Primer Assay against mouse beta-Actin (cat# QT01136772), and calculated as proportions relative to values in the corresponding air control group. Given the absence of major differences in behavior or mRNA expression in mice exposed to 2 or 4 CIE cycles, data from both groups, and their respective air controls, were combined to increase the statistical power to detect effects.
Slice electrophysiology
Test-naive mice were exposed to 4 weekly cycles of CIE and, 12-13 days later (time point corresponding to post-CIE time point at which brain tissue was extracted for RT-PCR) deeply anesthetized with isoflurane and decapitated for recordings. Whole-cell voltage-clamp electrophysiological recordings were performed in mOFC from acutely-prepared coronal brain slices according to landmarks based on the Allen Mouse Brain Atlas (Allen Institute for Brain Science, 2014). Brains were rapidly removed and placed in ice-cold sucrose-artificial cerebrospinal fluid (aCSF) containing (in mM) 194 sucrose, 20 NaCl, 4.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3 and saturated with 95% O2/5% CO2. Slices were transferred to a submerged recording chamber (Warner Instruments, Hamden, CT, USA), where they were perfused with heated, oxygenated aCSF at a rate of approximately 2 ml/minute and allowed to equilibrate for 30 minutes before electrophysiological recordings.
Recording electrodes (3–5 MΩ) were pulled from thin-walled borosilicate glass capillaries with a Flaming-Brown Micropipette Puller (Sutter Instruments, Novato, CA, USA). Recordings were performed in pyramidal neurons of layer 2/3 of the mOFC. In experiments where stimulated release was examined, bi-polar tungsten stimulating electrodes were placed dorsal to the recording site. In experiments where the input-output response of NMDAR-mediated currents were examined, recordings were conducted with a cesium gluconate internal solution and picrotoxin and NBQX in the extracellular solution. Stimulation response curves were constructed by taking the peak amplitude at each voltage. Spontaneous excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs, respectively) were measured in voltage-clamp mode using electrodes filled with an intracellular recording solution containing (in mM): 135 Cs-methane sulfonate, 10 KCl, 10 HEPES, 1 MgCl2, 0.2 EGTA, 4 Mg-ATP, 0.3 GTP, 20 phosphocreatine. Lidocaine N-ethyl bromide (1 mg/ml) was included in the intracellular solution to block postsynaptic sodium currents. Neurons were held at -55 mV to isolate glutamatergic synaptic transmission and record spontaneous EPSCs (sEPSCs) or +10 mV to isolate GABAergic synaptic transmission and record spontaneous IPSCs (sIPSCs) within individual neurons. Electrophysiological recordings of synaptic transmission were used to determine PSC frequency and amplitude, as well as to calculate synaptic drive ratios for spontaneous and miniature currents using the following equation: (EPSC frequency × amplitude) / (IPSC frequency × amplitude).
Statistical analysis
Data were analyzed using t-tests or repeated-measures analysis of variance (ANOVA). The between-subjects factor was group (AIR versus CIE) and the within-subjects factor was either reward-availability period (available versus unavailable), punishment session (baseline versus punished), or stimulation intensity (2-20 V). ANOVA's were followed by Sidak's multiple comparison test, using GraphPad Prism 6.04 (GraphPad Software Inc, La Jolla, CA, USA). Data in figures are means ± SEM. The threshold for statistical significance was set at P<.05.
Results
Effects of CIE on responding for EtOH reward
Mice did not significantly change their lever press response rates for the EtOH reward on the FR3 schedule from the pre-CIE period to the post-CIE baseline period.
Reward available versus unavailable responding
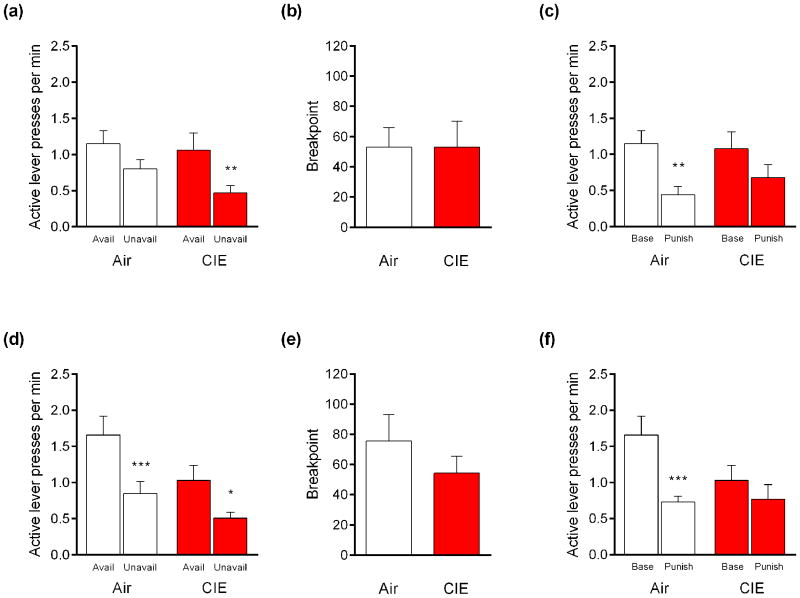
During available/unavailable testing, the active lever pressing response rate was significantly higher during reward-available than reward-unavailable periods in mice exposed to 2 cycles (main effect of period, F1,26 = 16.29, P<.001) and 4 cycles (main effect of period, F1,26 = 29.59, P<.01). Follow-up comparisons indicated that this effect held for mice exposed to 2 CIE-cycles (t26 = 3.62, P<.01), but not the respective air controls (t26 = 1.987, P>.05) (Figure 2a), and in mice exposed to 4 CIE-cycles (t26 = 3.00, P<.05) and their air controls (t26 = 4.70, P<.01) (Figure 2d). There was minimal responding on the inactive lever across experiments and these data are not shown. Post-CIE, the average dose of EtOH earned during these sessions was 0.49 g/kg for CIE-exposed mice and 0.52 g/kg for air controls.
Figure 2. CIE attenuates punished-suppression of EtOH-seeking. (a).
Mice exposed to 2 cycles of CIE made more rewarded lever-presses during available than unavailable periods. (b) Progressive ratio breakpoints were unaffected by exposure to 2 CIE cycles. (c) Punishment suppressed rewarded lever-pressing in air controls, but not mice exposed to 2 CIE cycles. (d) Mice exposed to 4 cycles of CIE or air made more rewarded lever-presses during available than unavailable periods. (e) Exposure to 4 CIE cycles did not affect progressive ratio breakpoints. (f) Punishment suppressed rewarded lever-pressing in air controls, but not mice exposed to 4 CIE cycles. n=12-15 per group. *P<.05, **P<.01, ***P<.001
PR responding
PR breakpoints did not differ between mice exposed to 2 CIE-cycles and their air controls (t-test: P>.05) (Figure 2b). There were also no group differences for mice exposed to 4 CIE-cycles (t-test: P>.05) (Figure 2e). The average dose of EtOH earned during this session was 0.38 g/kg and 0.40 g/kg for the CIE and air groups, respectively.
Punished responding
Overall, there was significantly lesser active lever pressing in the 2-cycle (main effect of session, F1,25 = 11.64, P<.01) and 4-cycle (main effect of session, F1,26 = 13.73, P<.01, session × group interaction, F1,26 = 4.36, P<.05) experiments. However, multi-test corrected follow-up comparisons indicated that mice exposed to 2 CIE-cycles did not significantly suppress responding during punishment relative to baseline (t25 = 1.61, P>.05), whereas their respective air controls did (t25 = 3.32, P<.01) (Figure 2c). Similarly, mice exposed to 4 CIE cycles did not significantly suppress responding during punishment relative to baseline (t26 = 1.14, P>.05), but their air controls did (t26 = 4.10, P<.001) (Figure 2f). The average dose of EtOH earned was 0.33 g/kg for CIE-exposed mice and 0.28 g/kg for air controls.
Effects of CIE on responding for food reward
Mice did not significantly change their lever press response rates for food reward on the FR3 schedule from the pre-CIE period to the post-CIE baseline period.
Reward available versus unavailable responding
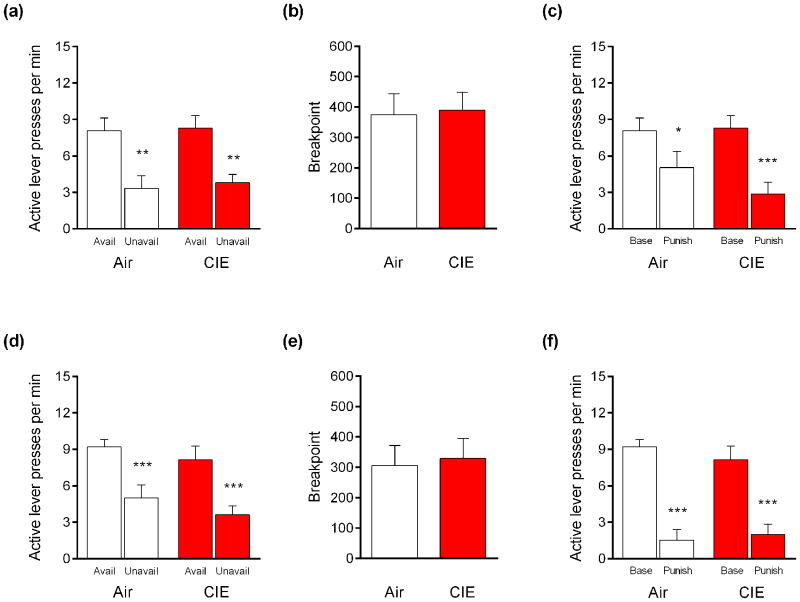
The rate of active lever pressing was significantly higher during reward-available than reward-unavailable periods in mice exposed to 2 cycles (main effect of period, F1,17 = 26.43, P<.01) and 4 cycles (main effect of period, F1,13 = 47.61, P<.01). Multi-test corrected follow-up comparisons indicated that this difference was evident in all groups: mice exposed to 2 CIE-cycles (t17 = 3.44, P<.01) and their air controls (t17 = 3.84, P<.01) (Figure 3a) and mice exposed to 4 CIE-cycles (t13 = 5.640, P<.01) and their air controls (t13 = 4.30, P<.001) (Figure 3d).
Figure 3. CIE does not decrease punished-suppression of food reward-seeking. (a).
Mice exposed to 2 cycles of CIE or air made more rewarded lever-presses during available than unavailable periods. (b) Progressive ratio breakpoints were unaffected by 2 cycles of CIE. (c) Punishment suppressed rewarded lever-pressing in air controls and mice exposed to 2 cycles of CIE. (d) Mice exposed to 4 cycles of CIE or air made more rewarded lever-presses during available than unavailable periods. (e) Exposure to 4 CIE cycles did not affect progressive ratio breakpoints. (f) Punishment suppressed rewarded lever-pressing in air controls and mice exposed to 4 cycles of CIE. n=6-10 per group. *P<.05, **P<.01, ***P<.001
PR responding
Breakpoints on the PR schedule did not differ between mice exposed to 2 CIE-cycles and their air controls (t-test: P>.05) (Figure 3b), or mice exposed to 4 CIE-cycles (t-test: P>.05) (Figure 3e).
Punished responding
Punishment significantly suppressed lever pressing in mice exposed to 2 cycles (main effect of session, F1,17 = 35.00, P<.01) and 4 cycles (main effect of session, F1,12 = 50.33, P<.01). Multi-test corrected follow-up comparisons confirmed this effect was apparent in mice exposed to 2 CIE-cycles (t17 = 5.42, P<.001) and their air controls (t17 = 3.05, P<.05) (Figure 3c), and in mice exposed to 4 CIE-cycles (t12 = 5.40, P<.01) and their air controls (t12 = 4.82, P<.01) (Figure 3f).
Effects of CIE on Pavlovian fear conditioning
There were no differences in freezing between CIE-exposed mice and air controls at any point during conditioning or retrieval (Table 2).
Table 2. CIE exposure did not affect Pavlovian fear conditioning.
Mice exposed to 4 cycles of CIE showed similar levels of freezing to air controls during cued fear conditioning or cue retrieval testing. n=11-13 per group. Data are expressed as average percent time freezing ± SEM.
| Conditioning | Retrieval Test | |||||
|---|---|---|---|---|---|---|
| Group | Baseline | Tone 1 | Tone 2 | Tone 3 | Baseline | Tones 1-5 |
| AIR | 5.45 ± 2.82 | 6.15 ± 2.67 | 20.00 ± 6.03 | 50.91 ± 5.63 | 3.39 ± 1.33 | 49.23 ± 5.07 |
| CIE | 6.15 ± 2.67 | 9.23 ± 4.87 | 21.54 ± 5.76 | 58.46 ± 6.59 | 0.27 ± 0.27 | 47.82 ± 4.23 |
Effects of CIE on NMDAR gene expression
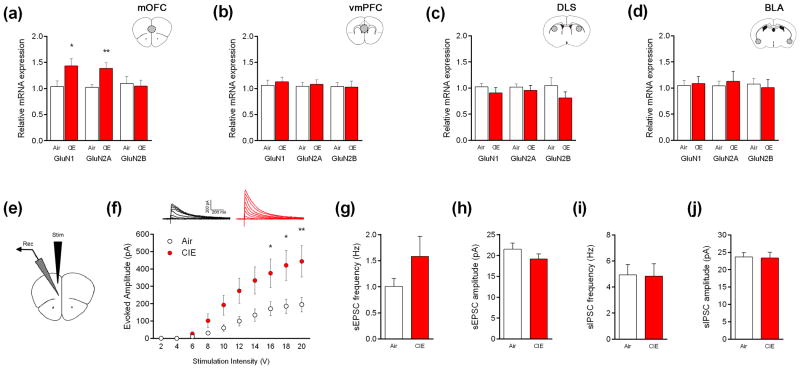
Mice exposed to 4-CIE cycles had significantly increased mRNA expression of GluN1 (t29 = 2.19, P<.05) and GluN2A (t29 = 2.95, P<.01), as compared to air controls, in the mOFC (Figure 4a), but not vmPFC, DLS or BLA (Figure 4b-d).
Figure 4. CIE exposure increases NMDA receptor mRNA expression and NMDAR-mediated synaptic currents in the mOFC.
Mice exposed to 4 cycles of CIE had higher GluN1 and GluN2A gene expression in the (a) medial orbitofrontal cortex (mOFC), not (b) ventromedial prefrontal cortex (vmPFC), (c) dorsolateral striatum (DLS) or (d) basolateral amygdala (BLA) (n=13-16). (e,f) Mice exposed to 4 cycles of CIE had higher evoked NMDAR-mediated currents in the mOFC. Neither sEPSC nor sIPSCs were affected by CIE exposure (n=13-16 neurons from n=8 mice). *P<.05, **P<.01 compared to air-exposed control.
Effects of CIE on mOFC synaptic transmission
NMDAR-mediated currents were significantly higher in mice exposed to 4 CIE-cycles, relative to air controls, in a stimulation current-dependent manner (main effect of group, F1,150 = 32.78, P<.01; main effect of stimulation, F9,150 = 12.95, P<.01, group × stimulation interaction, F9,150 = 1.97, P<.05) (Figure 4f). There were no differences between the CIE and air groups in the frequency or amplitude of either sEPSCs (Figure 4g,h) or sIPSC (Figure 4i,j) or in synaptic drive ratios for spontaneous and miniature currents (all t-tests: P>.05).
Discussion
The major finding of the current study was that mice chronically exposed to EtOH via vapor inhalation showed a loss of suppression of EtOH-seeking behavior in the face of punishment. This effect of CIE was behaviorally selective, in that CIE exposure neither attenuated punished food reward-seeking behavior, nor affected other putative measures of compulsive-like EtOH-seeking. We also found that reduced sensitivity to punished EtOH-seeking after CIE exposure was associated with a significant increase in NMDAR expression in the mOFC, and that CIE exposure produced an increase in NMDAR-mediated currents in the mOFC.
The suppression of EtOH seeking/consumption after associating EtOH with a ‘punisher,’ in the form of footshock, air puff, or quinine-adulteration, has previously been documented in rats (Marchant et al., 2013; Seif et al., 2013; Spanagel et al., 1996; Vendruscolo et al., 2012; Wolffgramm and Heyne, 1991) and mice (Lesscher et al., 2010; Radwanska and Kaczmarek, 2012). Interestingly, and in agreement with a recent study that tested C57BL/6 mice in ‘IntelliCages’ using an air puff punishment (Radwanska and Kaczmarek, 2012), we found that a higher footshock level was needed to suppress an EtOH-seeking response than a response for food reward in CIE-naïve mice. The marked suppression of food reward-seeking after CIE discounts possible loss of pain perception as an explanation of the attenuation of punished effects on EtOH seeking. This is substantiated by the finding that exposure to four cycles of CIE did not alter cued Pavlovian fear conditioning to the same footshock intensity used to punish EtOH-seeking.
Interestingly, despite the development of resistance to punishment after CIE exposure, we did not observe changes in baseline (FR3) responding for EtOH. This was noteworthy in light of prior studies that have found such increases (Chu et al., 2007; Lopez and Becker, 2014). One critical factor accounting for these differences may be that, unlike the current study, subjects in studies where increased self-administration was observed received exposure to CIE between test sessions. Indeed, we also did not observe changes in either PR breakpoint or response-persistence – two other purported measures of compulsive-like behavior (Deroche-Gamonet et al., 2004; Radwanska and Kaczmarek, 2012). The selectivity in this pattern of effects contrasts with previous studies showing that rats that developed resistance to quinine-suppressed EtOH-drinking after CIE also demonstrated a parallel increase in PR breakpoint (Vendruscolo et al., 2012). Along similar lines, chronic cocaine exposure has been shown to produce greater resistance to shock-punishment, increased PR breakpoint, and persistent responding for cocaine during periods of drug unavailability, in a subset of rats (Belin et al., 2011; Deroche-Gamonet et al., 2004; Jonkman et al., 2012). There could be species differences in the effects of chronic drug exposure that explain these apparent discrepancies, or they may be due to procedural variations between the studies. For example, the increased PR responding following CIE exposure reported by (Vendruscolo et al., 2012) was tested when rats were still under CIE exposure, whereas mice in the current study were assessed post-CIE. Thus, it may be that some EtOH phenotypes such as increased motivation for EtOH, as measured by PR responding, are transiently affected by CIE, while other behaviors, including resistance to punishment, are longer lasting.
It could also be the case that the brain mechanisms responsible for resistance to punishment are dissociable, and potentially more sensitive to CIE exposure, than those mediating other measures of compulsive behavior. In this regard, we have recently shown that mice with genetic deletion of the NMDAR subunit GluN2B in cortical principal neurons demonstrate a similar, selective phenotypic profile to CIE-exposed mice in the current study: i.e. resistance to punishment but no alterations in PR responding or responding during a period of reward unavailability (Radke et al., 2015). Clearly, the field remains at an early stage in defining the precise mechanisms underlying the development of compulsive-like behaviors.
We found that punished suppression of EtOH-seeking occurred after exposure to either two or four weekly cycles of CIE. It is worth pointing out that, although AIR and CIE groups were matched for pre-CIE response-rates in each experiment, mice exposed to 4 CIE cycles had a lower rate of post-CIE responding than the 4 AIR cycles group. This difference in post-CIE baseline was largely due to high responding in the 4 AIR cycles group post-CIE in this particular experiment. We do not, however, think that the comparably low baseline in the 4-cycle CIE group confounded the ability to observe a punishment-induced suppression, because we observed significant punishment-induced reductions from similar baselines in other groups – such as the AIR group in the 2-cycles experiment (Figure 2c). Still, it is possible that this baseline difference contributed to the robustness of the observed effects.
The finding of similar suppression of punished behavior after two or four cycles in the current study is in line with the earlier finding that C57BL/6J mice exhibited reduced sensitivity to quinine-adulteration after only two weeks of limited-access EtOH drinking (Lesscher et al., 2010). Together, these data indicate that EtOH-seeking in C57BL/6J mice may develop resistance to punishment with relatively limited EtOH experience – consistent with the characterization of this strain as high EtOH-preferring (Belknap et al., 1993). Indeed, prior work has demonstrated two CIE-cycles is sufficient to increase two-bottle EtOH drinking (DePoy et al., 2013; Griffin III et al., 2014; Griffin III et al., 2009a; Griffin III et al., 2009b; Lopez and Becker, 2005; Lopez et al., 2012) and tolerance to the discriminative stimulus properties of ethanol (Becker and Baros, 2006). Interestingly, however, not all behaviors in C57BL/6J mice are sensitive to this relatively limited EtOH exposure regimen. For example, extinction of a Pavlovian fear memory in C57BL/6J mice is impaired by four, but not two, CIE weekly cycles (Holmes et al., 2012), while eight cycles are required to produce lasting tolerance to EtOH intoxication (Daut et al., 2015). Taken together, these findings indicate that the specific constellation of EtOH-related alterations caused by CIE exposure is critically dependent on the chronicity of EtOH exposure.
The brain circuits and molecular mechanisms regulating EtOH-induced increases in compulsive-like behaviors are only beginning to be to uncovered. For instance, Vendruscolo et al. (Vendruscolo et al., 2012) recently demonstrated that inhibition of the glucocorticoid receptor by mifepristone injection effectively prevented CIE-induced insensitivity to quinine-adulteration of EtOH. Lateral hypothalamic projections to the ventral tegmental area have also been implicated in the control of compulsive sucrose consumption (Nieh et al., 2015). An elegant series of studies by Seif and colleagues using an intermittent EtOH drinking model has also shown that resistance to footshock-suppression of EtOH-seeking is reversed by nucleus accumbens core knockdown of GluN2C-containing NMDARs (Seif et al., 2013) or intra-accumbens injection of D-serine (Seif et al., 2015). Providing further evidence of a role for the NMDAR in compulsive-like behaviors in the current study, we found significant increases in the expression of the NMDAR subunits, GluN1 and GluN2A, but not GluN2B, in the punishment-resistant CIE-exposed mice. These effects were enduring, reflecting long-term neuroadaptations, given measurements were taken approximately two weeks after the end of CIE exposure. This is particularly notable given prior findings that elevated NMDAR synaptic currents persist for at least one week after CIE or other means of chronic EtOH exposure, while receptor expression changes, whether increases or decreases, are seen in some but not other studies (Follesa and Ticku, 1995; Holmes et al., 2012; Kalluri et al., 1998; Kim et al., 2014; Kroener et al., 2012; Nelson et al., 2005; Pian et al., 2010; Staples et al., 2015). While the current study only assessed one post-CIE time point, future studies correlating resistance to punishment with changes in NMDAR expression/function at multiple post-CIE time points would be of value.
Interestingly, the NMDAR gene expression changes currently observed after CIE were highly localized to the mOFC – with no alterations evident in the vmPFC, BLA or dorsal striatum. These changes in gene expression may also manifest as an increase in NMDAR function, as we observed that four cycles of CIE alone (i.e. in behaviorally-naïve mice) increased synaptic currents in the mOFC at the same time point at which NMDAR gene expression was increased. Upregulation of synaptic NMDAR expression and function are likely an adaptive response to long-term exposure to the inhibitory effects of EtOH. The association of altered physiology in the mOFC with compulsive EtOH-seeking is consistent with literature implicating cortical regions, including the OFC, in the control of behavioral flexibility and compulsive drug-seeking (Brigman et al., 2013; Chen et al., 2013; Everitt et al., 2008; Schoenbaum and Shaham, 2008). More generally, an augmentation of NMDAR transmission in response to CIE concurs with previous studies in rats and mice that have reported similar increases in the hippocampus (Nelson et al., 2005), vmPFC (Kroener et al., 2012), BLA (Läck et al., 2007), and cortical cultures (Hu and Ticku, 1997), one to three weeks following chronic EtOH. However, other studies have found a decrease in NMDAR synaptic transmission in regions including the vmPFC (Holmes et al., 2012), central amygdala (Roberto et al., 2004) and nucleus accumbens (Jeanes et al., 2011), during the withdrawal period or in the days soon after. These findings speak to the dynamic nature of NMDAR adaptations in the face of chronic EtOH, with potential implications for EtOH's effects on behavioral functions mediated by the receptor. Fully delineating these interrelationships awaits further study.
In summary, the current study shows that C57BL/6J mice exposed to chronic intermittent EtOH, via EtOH vapors, showed attenuated suppression of EtOH-seeking during punishment, a putative measure of ‘compulsive-like’ behaviors in rodent. Chronic EtOH did not affect punished food reward-seeking behavior or other measures of compulsive-like EtOH-seeking - progressive ratio and persistent responding for EtOH. Increased responding for EtOH under punishment was associated with an upregulation in NMDAR gene expression, while a similar regimen of CIE exposure in test-naïve mice increased NMDAR-mediated synaptic transmission in the mOFC, suggesting a potential molecular mechanism underlying this compulsive-like behavior. Taken together, these findings provide further evidence that chronic EtOH promotes resistance to punished EtOH-seeking in association with glutamatergic adaptations in specific cortical areas.
Acknowledgments
We are very grateful to Mr. Miguel Mendez for technical assistance and Dr. Jonathan Brigman for cartoons of behavioral procedures. Research supported by the NIAAA IRP (AR, NJ, AH), The Bowles Center for Alcohol Studies (TK), and NIH grants AA019454, AA020911, AA011605 (TK), AA022549 (EL), AA023559 (KP), AA023555 (ZM), AA007573 (JM).
Footnotes
Authors contribution: AR, NJ, AK, CM, ELG, KP, ZM, and JM performed experiments. AR and NJ analyzed data. AR, TK, and AH wrote the manuscript.
References
- Allen Institute for Brain Science. Allen Mouse Brain Connectivity Atlas [Internet] 2014 Available from: http://connectivity.brain-map.org/
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5 2013. [Google Scholar]
- Badanich KA, Becker HC, Woodward JJ. Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behavioral Neuroscience. 2011;125:879. doi: 10.1037/a0025922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Baros AM. Effect of duration and pattern of chronic ethanol exposure on tolerance to the discriminative stimulus effects of ethanol in C57BL/6J mice. Journal of Pharmacology and Experimental Therapeutics. 2006;319:871–878. doi: 10.1124/jpet.106.108795. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcoholism: Clinical and Experimental Research. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Belin-Rauscent A, Fouyssac M, Bonci A, Belin D. How preclinical models evolved to resemble the diagnostic criteria of drug addiction. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–579. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young E. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Jiang Z, Saksida LM, Jinde S, Pease M. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nature Neuroscience. 2013;16:1101–1110. doi: 10.1038/nn.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N, Halbout B, Uhrig S, Evrard L, Corsi M, Corti C, Deroche-Gamonet V, Hansson AC, Spanagel R. The mGluR2/3 agonist LY379268 induced anti-reinstatement effects in rats exhibiting addiction-like behavior. Neuropsychopharmacology. 2013;38:2048–2056. doi: 10.1038/npp.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau H-J, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF 1 receptor antagonist antalarmin and by CRF 1 receptor knockout. Pharmacology Biochemistry and Behavior. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut RA, Busch EF, Ihne J, Fisher D, Mishina M, Grant SG, Camp M, Holmes A. Tolerance to ethanol intoxication after chronic ethanol: role of GluN2A and PSD-95. Addiction Biology. 2015;20:259–262. doi: 10.1111/adb.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proceedings of the National Academy of Sciences. 2013;110:14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Wright T, Camp M, Crowley N, Noronha B, Lovinger D, Holmes A. Chronic alcohol alters rewarded behaviors and striatal plasticity. Addiction Biology. 2015;20:345–348. doi: 10.1111/adb.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P, Pinard C, Camp M, Feyder M, Sah A, Bergstrom H, Graybeal C, Liu Y, Schlüter O, Grant S. Durable fear memories require PSD-95. Molecular Psychiatry. 2014 doi: 10.1038/mp.2015.44. [DOI] [PubMed] [Google Scholar]
- Follesa P, Ticku MK. Chronic ethanol treatment differentially regulates NMDA receptor subunit mRNA expression in rat brain. Molecular Brain Research. 1995;29:99–106. doi: 10.1016/0169-328x(94)00235-7. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Current Protocols in Neuroscience. 2008;9(29):1–19. doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, III, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39:707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, III, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcoholism: Clinical and Experimental Research. 2009a;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, III, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology. 2009b;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Im H-I, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, Kenny PJ. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nature Neuroscience. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcoholism: Clinical and Experimental Research. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Lesscher HM. Rodent models for compulsive alcohol intake. Alcohol. 2014;48:253–264. doi: 10.1016/j.alcohol.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X-J, Ticku MK. Functional characterization of a kindling-like model of ethanol withdrawal in cortical cultured neurons after chronic intermittent ethanol exposure. Brain Research. 1997;767:228–234. doi: 10.1016/s0006-8993(97)00581-7. [DOI] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA. In vivo chronic intermittent ethanol exposure reverses the polarity of synaptic plasticity in the nucleus accumbens shell. Journal of Pharmacology and Experimental Therapeutics. 2011;336:155–164. doi: 10.1124/jpet.110.171009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Pelloux Y, Everitt BJ. Differential roles of the dorsolateral and midlateral striatum in punished cocaine seeking. The Journal of Neuroscience. 2012;32:4645–4650. doi: 10.1523/JNEUROSCI.0348-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri HS, Mehta AK, Ticku MK. Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Molecular Brain Research. 1998;58:221–224. doi: 10.1016/s0169-328x(98)00112-0. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest J, Berson N, Balado E, Fiancette J, Renault P, Piazza P, Manzoni O. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Molecular Psychiatry. 2013;18:729–737. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- Kim A, Zamora-Martinez ER, Edwards S, Mandyam CD. Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Structure and Function. 2014:1–16. doi: 10.1007/s00429-014-0755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neuroscience & Biobehavioral Reviews. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre-and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. Journal of Neurophysiology. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão RM, Cruz FC, Vendruscolo LF, de Guglielmo G, Logrip ML, Planeta CS, Hope BT, Koob GF, George O. Chronic nicotine activates stress/reward-related brain regions and facilitates the transition to compulsive alcohol drinking. The Journal of Neuroscience. 2015;35:6241–6253. doi: 10.1523/JNEUROSCI.3302-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher H, Van Kerkhof LW, Vanderschuren LJ. Inflexible and indifferent alcohol drinking in male mice. Alcoholism: Clinical and Experimental Research. 2010;34:1219–1225. doi: 10.1111/j.1530-0277.2010.01199.x. [DOI] [PubMed] [Google Scholar]
- Loi B, Lobina C, Maccioni P, Fantini N, Carai MA, Gessa GL, Colombo G. Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcoholism: Clinical and Experimental Research. 2010;34:2147–2154. doi: 10.1111/j.1530-0277.2010.01311.x. [DOI] [PubMed] [Google Scholar]
- Lopez M, Becker H, Chandler L. Repeated episodes of chronic intermittent ethanol promote insensitivity to devaluation of the reinforcing effect of ethanol. Alcohol. 2014;48:639–645. doi: 10.1016/j.alcohol.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Operant ethanol self-administration in ethanol dependent mice. Alcohol. 2014;48:295–299. doi: 10.1016/j.alcohol.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Griffin WC, Melendez RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure leads to the development of tolerance to aversive effects of ethanol in C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2012;36:1180–1187. doi: 10.1111/j.1530-0277.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Khuc TN, Pickens CL, Bonci A, Shaham Y. Context-induced relapse to alcohol seeking after punishment in a rat model. Biological Psychiatry. 2013;73:256–262. doi: 10.1016/j.biopsych.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Sorensen ML, Cameron AJ, Yu CH, Crabbe JC. Withdrawal severity after chronic intermittent ethanol in inbred mouse strains. Alcoholism: Clinical and Experimental Research. 2010;34:1552–1564. doi: 10.1111/j.1530-0277.2010.01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T, Ur C, Gruol D. Chronic intermittent ethanol exposure enhances NMDA-receptor-mediated synaptic responses and NMDA receptor expression in hippocampal CA1 region. Brain Research. 2005;1048:69–79. doi: 10.1016/j.brainres.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Leppla CA, Wichmann R, Neve R, Wildes CP, Tye KM. Decoding neural circuits that control compulsive sucrose seeking. Cell. 2015;160:528–541. doi: 10.1016/j.cell.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism: Clinical and Experimental Research. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Milner R, Ehlers CL. N-methyl-D-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neuroscience. 2010;170:645–654. doi: 10.1016/j.neuroscience.2010.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Nakazawa K, Holmes A. Cortical GluN2B deletion attenuates punished suppression of food reward-seeking. Psychopharmacology. 2015:1–9. doi: 10.1007/s00213-015-4033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska K, Kaczmarek L. Characterization of an alcohol addiction-prone phenotype in mice. Addiction Biology. 2012;17:601–612. doi: 10.1111/j.1369-1600.2011.00394.x. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. The FASEB Journal. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. Journal of Studies on Alcohol and Drugs. 2003;64:445. doi: 10.15288/jsa.2003.64.445. [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. The Journal of Neuroscience. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcoholism: Clinical and Experimental Research. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biological Psychiatry. 2008;63:256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang S-J, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nature Neuroscience. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Simms JA, Lei K, Wegner S, Bonci A, Messing RO, Hopf FW. D-Serine and D-Cycloserine Reduce Compulsive Alcohol Intake in Rats. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Hölter SM, Allingham K, Landgraf R, Zieglgänsberger W. Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. European Journal of Pharmacology. 1996;305:39–44. doi: 10.1016/0014-2999(96)00174-4. [DOI] [PubMed] [Google Scholar]
- Staples MC, Kim A, Mandyam CD. Dendritic remodeling of hippocampal neurons is associated with altered NMDA receptor expression in alcohol dependent rats. Molecular and Cellular Neuroscience. 2015;65:153–162. doi: 10.1016/j.mcn.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. The Journal of Neuroscience. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Celerier E, Chaskiel L, Penzo F, Spanagel R. Research focus on compulsive behavior in animals: Compulsive alcohol drinking in rodents. Addiction Biology. 2009;14:384–396. doi: 10.1111/j.1369-1600.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacology Biochemistry and Behavior. 1991;38:389–399. doi: 10.1016/0091-3057(91)90297-f. [DOI] [PubMed] [Google Scholar]
- Xue Y, Steketee JD, Sun W. Inactivation of the central nucleus of the amygdala reduces the effect of punishment on cocaine self-administration in rats. European Journal of Neuroscience. 2012;35:775–783. doi: 10.1111/j.1460-9568.2012.08000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]