Abstract
A prominent hypothesis holds that “sticky” attention early in life in children with autism spectrum disorder (ASD) limits their ability to explore and learn about the world. Under this hypothesis, the core clinical symptoms of ASD – restricted interests, repetitive behaviors and impaired social/communication abilities – could all result from impaired attentional disengagement during development. However, the existence of disengagement deficits in children with ASD is controversial, and a recent study found no deficit in five- to twelve-year-olds with ASD. Nonetheless, the possibility remains that disengagement is impaired earlier in development in children with ASD, altering their developmental trajectory even if the attentional deficit itself is remediated or compensated for by the time children with ASD reach school age. Here, we tested this possibility by characterizing attentional disengagement in a group of toddlers just diagnosed with ASD (age 21- to 37-months). We found strikingly similar performance between the ASD and age-matched typically developing (TD) toddlers, and no evidence of impaired attentional disengagement. These results show that even at a young age when the clinical symptoms of ASD are first emerging, disengagement abilities are intact. Sticky attention is not a fundamental characteristic of ASD, and probably does not play a causal role in its etiology.
Keywords: ASD, visual attention, eye tracking, saccade, reaction time, free viewing
Introduction
From the first moments of life, we explore the world with our eyes, finding important objects to look at and learn about (Goren, Sarty, & Wu, 1975; Johnson, Dziurawiec, Ellis, & Morton, 1991; Salapatek & Kessen, 1966). This ability to fluidly move attention through the visual environment helps bring our eyes to objects that may provide reward, or threaten harm, or present a mystery to be solved. What would the consequence be of an inability to move attention fluidly throughout the visual world? Having “sticky” attention (a difficulty disengaging gaze from one object in order to move to the next), would dramatically constrain what we look at and learn about, and could have diverse and far-reaching effects on cognitive abilities.
This fundamental link between attention and cognitive development has led many to ask whether impaired attention might be a root cause of autism spectrum disorder (ASD; Dawson et al., 2004; Keehn, Müller, & Townsend, 2013; Loveland & Landry, 1986; Maestro et al., 2002; Toth, Munson, Meltzoff, & Dawson, 2006). In particular, slower attentional disengagement is often implicated in the etiology of ASD because it could so neatly account for some of the core clinical symptoms. Children with ASD show restricted interests and repetitive behaviors (Szatmari et al., 2006; Turner, 1999), such as spinning the wheel on a toy car over and over, which could naturally arise from “sticky” attention. But disengagement impairments could also shape development in more subtle ways. If sticky attention in ASD comes at the cost of rich and varied experience with the visual and social world during early development, then it could ultimately cascade into a family of impairments, including the social and communication difficulties that are hallmarks of ASD (Zwaigenbaum et al., 2005). Thus, the attentional disengagement hypothesis features prominently in current theories of ASD (Keehn et al., 2013; Menon, 2011; Sacrey, Armstrong, Bryson, & Zwaigenbaum, 2014).
Despite the intuitive appeal of the disengagement hypothesis, the actual evidence for it is conflicting. A recent review (Sacrey et al., 2014) found that more than a quarter of published studies on attentional disengagement in ASD found no evidence of a deficit (and this is likely an underestimate, given the bias toward publication of positive findings in clinical research; Easterbrook, Gopalan, Berlin, & Matthews, 1991). Stimuli and procedures vary widely across these studies, and many existing studies have shortcomings in design or analysis that complicate the interpretation of their results, leaving no clear single reason for the discrepancies (see Discussion). In an effort to overcome these limitations, Fischer et al. (Fischer, Koldewyn, Jiang, & Kanwisher, 2014) recently tested attentional disengagement in a large group of five- to twelve-year-old children with ASD, and age- and IQ-matched typically developing controls. They analyzed gaze behavior during a free-viewing experiment using novel images on each trial, and found strikingly similar disengagement abilities in ASD and TD children; there was no hint of sticky attention in children with ASD.
The results of this previous study in 5- to 12-year-old children suggest that a general deficit in attentional disengagement is not a fundamental characteristic of ASD, even if it and other attentional impairments appear in some children with ASD under some conditions. An alternative possibility, however, is that attentional disengagement may be impaired in very young children with ASD, shaping early development, but children with ASD may “catch up” to typical children or be largely remediated by interventions by the time they reach school age. If so, then sticky attention could still factor importantly into the etiology of ASD, even if disengagement itself is not impaired later in life. While disengagement has been studied in young children with ASD with mixed results (Sacrey et al., 2014), the existing studies share many of the same limitations found in studies of disengagement in older children and adults with ASD (see Discussion). It remains unclear whether very young children with ASD exhibit sticky attention.
To test the hypothesis that disengagement deficits are present in very young children with ASD, we measured attentional disengagement abilities in a group of 21- to 37-month-old toddlers who had just been diagnosed with ASD, along with an age-matched group of TD controls. We employed a nearly identical experimental design to the one previously used with older children by Fischer et al. (Fischer et al., 2014), using precise eye tracking in a free-viewing paradigm and presenting novel stimuli on each trial.
Methods
Participants
Eighteen toddlers with ASD and twenty-six TD toddlers participated in the study (seven TD toddlers were excluded due to potential developmental concerns, and seven children with ASD and six TD children were run but excluded from further analysis because their eye tracking data were incomplete; see below). Toddlers with ASD were recruited through local early intervention agencies. The experiment was run at the beginning of the same visit in which the diagnostic testing was performed, and only toddlers who met criteria for ASD were included in subsequent analyses. Toddlers at risk for ASD who did not meet ASD criteria were excluded from the study.
TD toddlers were recruited from the Greater Boston area via mailings. The TD toddlers analyzed here were drawn from a larger initial pool, taking the largest subset that yielded a tight age match between groups (ASD mean age = 876±128 days; TD mean age = 859±133 days; p = .68 for the group difference).
Toddlers’ general development was assessed using the Mullen Scales of Early Learning Early Learning Composite score (ELC), which provides a general measure of cognitive ability and is computed on the basis of four scales: Visual Reception, Fine Motor, Expressive Language, and Receptive Language (Mullen, 1995). We verified that no TD participant had a history of neurological disorders, developmental issues, ASD behaviors, or siblings with developmental disorders.
The Autism Diagnostic Observation Schedule-2 (ADOS-2; Lord, Rutter, et al., 2012; Lord, Luyster, Gotham, & Guthrie, 2012) was administered to aid in assigning clinical diagnosis and to characterize the ASD sample. Table 1 shows a summary of subjects’ demographic information and assessment scores.
Table 1.
Summary of subjects’ demographic information and scores on standardized assessments.
| ASD | TD | |
|---|---|---|
| N | 18 | 26 |
| # Females | 2 | 15 |
| Total trials collected | 656 | 952 |
| (% trials retained) | 66.50% | 71.80% |
| Ethnicity: | # of children | # of children |
| Hispanic or Latino | 11 | 3 |
| Not Hispanic or Latino | 7 | 23 |
| Race: | # of children | # of children |
| Asian | 0 | 2 |
| Black/African American | 2 | 2 |
| White | 11 | 16 |
| other/multiple/no response | 5 | 6 |
| Mullen Scales: | mean ± SD | mean ± SD |
| VR | 35.7 ± 7.6 | 58.1 ± 10.3 |
| FM | 31.7 ± 9.5 | 46.9 ± 11.5 |
| RL | 28.4 ± 13.7 | 57.0 ± 13.8 |
| EL | 28.6 ± 9.4 | 54.7 ± 12.9 |
| ELC | 65.8 ± 14.0 | 112.3 ± 16.6 |
| ADOS-2: | mean ± SD | |
| SA | 14.4 ± 2.9 | |
| RRB | 5.9 ± 1.5 | |
| Total | 20.3 ± 3.5 | |
| CSS | 8.7 ± 1.2 | |
| BITSEA: | mean ± SD | |
| Competence Total Score | 12.6 ± 4.5 | |
| ASD Problem Score | 5.9 ± 3.3 | |
| ASD Competence Score | 9.2 ± 3.1 |
| RBS-R: | median | min | max |
|---|---|---|---|
| Stereotyped | 8 | 3 | 14 |
| Self-injurious | 1 | 0 | 9 |
| Compulsive | 5 | 1 | 11 |
| Ritualistic/Sameness | 7.5 | 0 | 34 |
| Restricted | 5 | 0 | 10 |
The Mullen scales collected were Visual Reception, (VR), Fine Motor (FM), Receptive Language (RL), and Expressive Language (EL). The Early Learning Composite (ELC) was computed from these four scales, and reflects general cognitive ability. Age-equivalent scores were used for analyses. To ensure that the TD sample was not developmentally delayed, we excluded any TD child with a Mullen ELC score of less than 85 (>1 SD below the mean based on Mullen norms; seven children). TD children in our sample scored significantly higher than children with ASD on the Mullen ELC (t = 9.1; p < 0.001). For ADOS-2 testing, fourteen children below 30 months old with language skills ranging from no verbal language to single words were administered the Toddler Module; 3 children who were nonverbal or using single words were administered Module 1 based on their age (31 months or over); and 1 child with phrase speech completed Module 2. ADOS-2 Total Social Affect (SA), Restricted and Repetitive Behavior (RRB), Overall Total (SA + RRB), and Calibrated Severity (CSS) scores were computed for each child in the ASD sample. Parents of children with ASD completed the Brief Infant Toddler Social Emotional Assessment (BITSEA)(Briggs-Gowan & Carter, 2002), a screener for social-emotional difficulties and competencies, and the Repetitive Behaviors Scale – Revised (RBS-R)(Bodfish, Symons, Parker, & Lewis, 2000), a questionnaire that characterizes restricted and repetitive behaviors in ASD.
Stimuli and Experimental Design
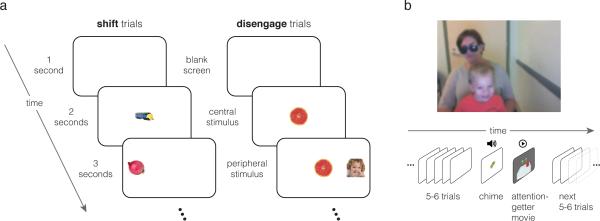
The experiment was a modified gap-overlap task (Reulen, 1984a, 1984b; Reuter-Lorenz, Hughes, & Fendrich, 1991; Saslow, 1967). Figure 1a shows the events within a trial. Each six-second trial consisted of a blank white screen for one second, followed by an image appearing in the center of the screen (approximately 7×7 degrees visual angle, depending on the exact viewing distance of the toddler). After two seconds, another image of the same size appeared on the left or right side of the screen (unpredictably, with equal probability), approximately 13 deg. from the center. This peripheral stimulus was intended to capture the infant's attention, eliciting a saccade away from the center of the screen. The peripheral stimulus remained onscreen for three seconds, until the end of the trial. Two trial types were randomly interleaved in the trial sequence: “shift” trials and “disengage” trials. On shift trials, the central image was present for two seconds, and disappeared at the moment the peripheral image appeared, an inter-stimulus interval (ISI) of zero (Fig. 1a). On disengage trials, the central stimulus remained onscreen for the remainder of the trial after it appeared; for the last three seconds of the trial both the central and the peripheral stimuli were onscreen simultaneously. Thus, on shift trials, attention was free to shift to the peripheral stimulus when it appeared since the central stimulus was no longer present, while on disengage trials, moving attention to the peripheral stimulus required first disengaging attention from the central stimulus, which remained onscreen. The images in the central and peripheral locations were either faces or objects (fruits, vegetables, or vehicles). Trials were counterbalanced such that all possible pairings of central and peripheral stimulus type (face or object) occurred equally often within the shift and disengage conditions. A run consisted of 32 trials.
Figure 1.
Experimental design. Panel a shows the event sequence within one example trial of each type. Each trial began with a blank screen, followed by the presentation of an image in the center of the screen. After 2 seconds, another image appeared in the periphery, which remained onscreen for 3 seconds, until the end of the trial. In shift trials, the central stimulus disappeared at the moment the peripheral stimulus appeared. In disengage trials, the central stimulus remained onscreen, requiring participants to disengage attention from the central image before shifting their eyes to the peripheral image. b) Toddlers viewed the stimuli while sitting on their caregiver's lap. Every 5-6 trials, an attention-getter played to maintain the toddler's focus on the screen. During the attention-getter, an image appeared in the center of the screen for two seconds as in a standard trial, followed by a chime sound to draw the toddler's interest to the screen if he or she had been looking away. The toddler then saw a six-second video clip of moving, colorful objects (such as billiard balls bouncing on a table). After the clip finished, the experiment continued with the presentation of shift and disengage trials.
Data Collection
Toddlers viewed the stimuli on a 17-inch LCD screen while seated on a caregiver's lap, approximately 55-65 cm from the screen (Fig. 1b). Caregivers wore blacked-out sunglasses or kept their eyes closed, and were instructed not to provide feedback to the children during the experiment. Eye tracking was performed at 60 Hz with a Tobii T120 eye tracker (Tobii Technology, Stockholm, Sweden), and left and right eye positions were averaged to reduce noise. The experiment began with a 5-point calibration procedure. Following the eye tracker calibration, toddlers completed one experimental run, which lasted four minutes seven seconds (four TD and three toddlers with ASD completed an additional run, which we included here to increase the power of the analysis. We verified that excluding these additional runs did not alter the outcome of any of the reported analyses).
Data Analysis
The measure of interest was saccadic reaction time (SRT), defined as the time elapsed between the peripheral stimulus onset and the time at which the participant's point of gaze first arrived on the peripheral stimulus. We computed the SRT for each trial, and included in this analysis only trials that met three quality criteria: (i) the child was looking at the central stimulus at the time of the peripheral stimulus onset (anticipatory saccades were removed), (ii) the child made an eye movement to the peripheral stimulus within 2 s after its onset (failures to disengage at all were analyzed separately), and (iii) no more than 50% of gaze measurements were missing (not properly read from the eye tracker) in that trial. The threshold in criterion iii was intentionally liberal: while criteria i-ii ensured that good data was present for the full period of the trial being analyzed, criterion iii ensured that the toddler was looking at the screen during much of the rest of the trial. We occasionally lost the track on a toddler's eyes during a less critical part of a trial (e.g. after the saccade to the peripheral stimulus), and the 50% threshold allowed us to still include such trials. If fewer than six (37.5%) of a participant's trials in either condition met these criteria, the child was excluded from the analysis (seven ASD and seven TD children from a larger original subject pool were excluded on this basis). There was no significant difference in the percentage of trials retained in the analysis from the TD and ASD groups (71.8% vs. 66.5% of trials, respectively; t = 1.70, p = 0.1).
We computed a child's disengagement cost – the extra time it took to disengage attention from the central stimulus when it remained onscreen – as the mean SRT from disengage trials minus the mean SRT from shift trials. We performed significance testing using permutation tests (Pitman, 1937), which characterized the null distribution by randomly assigning the condition or group labels on each of 10,000 iterations. For example, to test for a significant disengagement cost (SRTdisengage – SRTshift), we characterized the null distribution by assuming that the SRTs from shift trials and disengage trials originated from the same distribution, and were thus interchangeable. On each iteration, we randomly assigned a “shift” or “disengage” label to each SRT (maintaining the same overall number of trials assigned each label) and then computed overall disengagement cost. The distribution of disengagement costs computed in this way was the null distribution – the range of disengagement costs that would be expected by chance. We then compared the true disengagement cost from non-shuffled data to the null distribution, computing the proportion of the distribution that was more extreme than the true value. This nonparametric approach avoided making the assumptions entailed in a parametric test, such as normality of the SRT distributions.
We also identified trials in which the child failed to disengage attention from the central stimulus as those “disengage” trials which met three criteria: (i) the child must have looked at the central stimulus at some point before the peripheral stimulus appeared, (ii) after looking at the central stimulus, the child's gaze must have remained on the central stimulus for 90% of the rest of the trial (this allowed for some imprecision in the tracker measurements or briefly leaving the central stimulus but returning quickly), and (iii) the child must not have looked at the peripheral stimulus at any point during the trial. We counted the number of such trials for each participant, and tested for a difference in the frequency of such trials between groups with a permutation test as described above.
Results
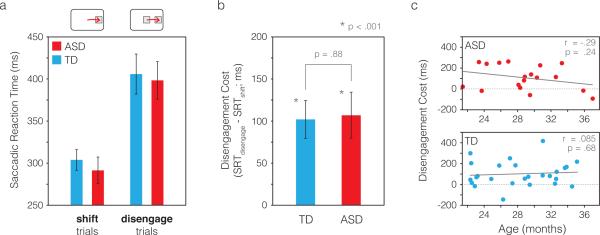
Our central question was whether the attentional disengagement cost (the time it takes to disengage attention from one stimulus in order to move attention to another stimulus) differed between toddlers with and without ASD. Figure 2a shows saccadic reaction times broken down by trial type (shift vs. disengage trials). If toddlers with ASD have impaired attentional disengagement, they should show a larger difference between SRTs in shift vs. disengage trials. That difference, the disengagement cost, is plotted in Figure 2b. While each group individually showed a highly significant disengagement cost (both ps < .001; Cohen's d = 1.26 for ASD and d = 1.04 for TD), there was no difference in disengagement cost between groups (p = .88, d = 0.045).
Figure 2.
Saccadic reaction times and disengagement costs. a) Mean saccadic reaction times are shown for each group, separated by trial type (shift trials vs. disengage trials). If toddlers with ASD have “sticky” attention, they should show a larger difference between SRTs in shift trials and disengage trials than TD toddlers, reflecting more time required to disengage attention. Panel b shows this difference, the disengagement cost, in each group. While each group showed a highly significant disengagement cost individually (p < .001), there was no difference in disengagement costs between groups (p = .88). c) Disengagement cost was not significantly correlated with age in either group. Solid gray lines show least-squares linear fits to the data in each group. Error bars were computed with a bootstrapping procedure (Efron, 1981) in which subjects were subsampled with replacement on each of 10,000 iterations, and the group mean computed on each iteration. The error bars shown are the SD of the resulting bootstrapped distribution, reflecting the standard error of the group mean.
We also tested whether toddlers with ASD were more likely to fail to disengage attention from the central stimulus entirely. Trials where the toddler continued to dwell on the central stimulus for an extended time after the peripheral stimulus appeared were uncommon in both groups (2.3% of disengage trials in the TD group and 3.8% of disengage trials in the ASD group; see Methods), indicating that the onset of the peripheral stimulus was effective in capturing attention and eliciting an eye movement in both groups. There was no significant difference between groups in the frequency of failure to disengage (z = 1.28, p = .20). Similarly, when we included trials with very long SRTs (up to 3 seconds, the full duration of the peripheral stimulus), we still found no difference in disengagement cost between groups (disengagement cost of 121.9±34.3 ms for ASD and 152.4±32.1 ms for TD; p = .52; d = 0.19 for the group difference).
The disengagement cost was not significantly correlated with age in either group (r = −.29, p = .24 for ASD; r = .085, p = .68 for TD; Fig. 2c). Reflecting the gender ratio typically observed in ASD, the ASD group had a smaller proportion of females (11% vs. 58% in the TD group); we examined SRTs when only TD males were included in the analysis, and still found no difference in the disengagement cost between groups (TD: 134.0±37.8 ms, ASD: 125.5±27.5 ms; p = .87, d = .069 for the group difference). We also found no significant correlation between ADOS-2 calibrated severity scores (CSS; Esler et al., 2015; Gotham, Pickles, & Lord, 2009) and disengagement cost within the ASD group (r = .12, p = .64), nor a correlation between ADOS-2 restricted and repetitive behaviors CSSs and disengagement cost (r = .12, p = .63).
Finally, we tested whether the disengagement cost was related to toddlers’ cognitive abilities assessed by the Mullen Scales of Early Learning. We ran a regression of disengagement costs on Mullen Early Learning Composite (ELC) scores across all participants (except for one child with ASD and five TD children, for whom the Mullen test was not administered due to time constraints) and included a Group x ELC interaction term in the model. Mullen ELC scores did not significantly predict disengagement costs (R2 = .003, adjusted R2 = −.056, p = .95), and the Group × ELC interaction was not significant (F1,34 = .014, p = .91). This is perhaps expected given the fast, automatic nature of the exogenous orienting we measured. While top-down attentional abilities are thought to be related to general cognitive ability (Light et al., 2010), the speed of exogenous orienting is independent of top-down attentional abilities (Pinto, van der Leij, Sligte, Lamme, & Scholte, 2013) and likely reflects a lower-level process.
In sum, we find no evidence of a difference between groups in attentional disengagement ability, either in the time it takes to disengage attention, or in the overall rate of successful disengagement.
Discussion
Our results show no evidence for “sticky” attention in toddlers with ASD compared to their typically developing peers. Toddlers with ASD in our study were no more likely to fail to disengage attention from a central image when a peripheral image appeared, and they were just as swift in their disengagement. Given the very similar performance between groups on our disengagement measures, it is important to note that our toddlers with ASD were diagnosed by expert clinicians whose diagnoses were informed by scores above the threshold for ASD on the ADOS-2 (Lord, Rutter, et al., 2012; Lord, Luyster, et al., 2012), the gold standard observational tool for assessing ASD symptoms.
Our findings run counter to some previous reports on attentional disengagement in young children with ASD. For example, Elsabbagh et al. (2009, 2013) reported that infants and toddlers who later received an ASD diagnosis had significantly larger disengagement costs than those who were not ultimately diagnosed with ASD. These findings may in part be due to a motion change at the central stimulus that occurred just as the peripheral stimulus appeared, which likely served as a salient attentional cue. A more general concern is that these studies, along with others frequently cited as evidence for impaired disengagement in children with ASD (R. Landry & Bryson, 2004; Zwaigenbaum et al., 2005) used stimulus sequences with highly predictable image repetitions. In these studies children with ASD may have had more difficulty than their TD peers learning the regularities in the stimulus sequences and forming expectations about what would come next (Pellicano & Burr, 2012; Sinha et al., 2014; Van de Cruys et al., 2014). Because attentional disengagement is slower from novel or unexpected stimuli than anticipated ones (Brockmole & Boot, 2009), if children with ASD experienced repeated stimuli as more novel or surprising than TD children, they would be slower to disengage attention from those stimuli when they remained onscreen. Thus, group differences in prediction ability could give rise to apparent differences in disengagement ability if the stimulus sequence was predictable. This possibility underscores a key strength of our study: each stimulus was presented only once, and thus was novel and unpredictable to children in both groups.
Other accounts have been proposed to explain the conflicting findings on disengagement impairments in ASD, but none has much supporting evidence. For example, Sacrey et al. (2014) suggested that the time elapsed between the onsets of the central and peripheral stimuli in a gap-overlap paradigm (C-P timing) determines whether children with ASD show a disengagement impairment. Studies that found intact disengagement abilities in children with ASD generally reported a C-P timing of one second or more (Fischer et al., 2014; Goldberg et al., 2002; Kikuchi et al., 2011; Mosconi et al., 2009), but most studies reporting impaired disengagement simply failed to report the C-P timing (Elsabbagh et al., 2009, 2013; R. Landry & Bryson, 2004; Zwaigenbaum et al., 2005), leaving little basis for comparing C-P timing among studies. Another variable among gap-overlap experiments is whether they include a blank interval between the central stimulus offset and peripheral stimulus onset (a “gap”), or display the peripheral stimulus at the moment the central stimulus disappears. There is no clear relationship between the inclusion of a stimulus gap and whether a study reported disengagement impairments in ASD; for example, two studies by Elsabbagh et al. used a very similar design with the exception that one employed a stimulus gap (Elsabbagh et al., 2009) while the other did not (Elsabbagh et al., 2013), and the studies yielded consistent results. In the current study we chose not to include a stimulus gap to reduce variability in SRTs that could result from subjects initiating a saccade during the gap period.
We included both social and nonsocial images in our experiment to reflect the diverse stimuli that toddlers encounter. While it was not our goal to compare disengagement from social vs. nonsocial stimuli (there was insufficient power to carry out such an analysis), it is important to note that the inclusion of social stimuli is unlikely to explain why we find no differences in attentional disengagement between groups. Several other gap-overlap studies have required disengagement from social stimuli and reported slower disengagement in children with ASD (Elison et al., 2013; Elsabbagh et al., 2009, 2013; Kawakubo et al., 2007); it is not the case that including social stimuli reduces the ability to detect any disengagement impairment that may be present. That our findings differ from these studies and others that reported impaired disengagement in ASD must be due to other factors besides social content, such as stimulus predictability discussed above.
Our findings agree with the conclusions of a recent meta-analysis by Landry and Parker (2013), who found that young children with ASD showed little or no impairment in non-predictive exogenous orienting tasks like our study. While Landry and Parker emphasize the need for caution in comparing the results of cued orienting tasks (the primary focus of their meta-analysis) with those of uncued tasks like the gap-overlap paradigm, they point out that nearly half of the cued orienting experiments they reviewed had temporally overlapping cues and targets which would be expected to elicit greater impairments in participants with ASD if those participants had slower attentional disengagement. This was not the case, and thus Landry and Parker found no evidence for sticky attention within the studies they reviewed.
Our results join a growing body of recent work showing that many attentional abilities are intact in ASD. Among these abilities are endogenous and exogenous attentional orienting (Grubb, Behrmann, Egan, Minshew, Carrasco, et al., 2013; Grubb, Behrmann, Egan, Minshew, Heeger, et al., 2013), the ability to efficiently direct attention to the learned locations of likely targets (Jiang, Capistrano, Esler, & Swallow, 2013), the ability to attend to global or local stimulus attributes when asked to do so (Koldewyn, Jiang, Weigelt, & Kanwisher, 2013), and visual search, where there is evidence of enhanced abilities in ASD (Kaldy, Kraper, Carter, & Blaser, 2011; O’Riordan, 2004; Plaisted, O’Riordan, & Baron-Cohen, 1998). While there may be some differences in how people with ASD tend to allocate attention (Koldewyn et al., 2013; Robertson, Kravitz, Freyberg, Baron-Cohen, & Baker, 2013), these different allocation strategies do not necessarily reflect impairments per se. However, because attention encompasses a wide variety of functions and only a subset have been carefully characterized in ASD, it will be important to further investigate whether other attentional functions indeed develop on a different trajectory in children with ASD.
Importantly, our results do not imply that attentional disengagement abilities develop normally in all children with ASD; some children with ASD may have impaired disengagement resulting from comorbid conditions or unrelated cognitive difficulties. However, our results, together with previous findings in older children (Fischer et al., 2014), show that slow disengagement is not a pervasive component of ASD. Even as they were beginning to show reliable diagnostic symptoms, toddlers with ASD in our study showed no evidence of sticky attention. It is thus unlikely that impaired disengagement plays a pervasive causal role in the early development of ASD.
Research Highlights.
Impaired attentional disengagement (“sticky” attention) is believed by many to be a root cause of ASD, but evidence for this claim is inconsistent.
We tested toddlers with ASD immediately following diagnosis, tracking their eyes during free viewing of images.
We found no evidence of sticky attention in toddlers with ASD, and strikingly similar performance between the ASD and TD groups.
It is unlikely that general disengagement impairments play a causal role in the early development of ASD.
Acknowledgements
We thank Annalisa Groth for assistance with collecting and organizing the data. This project was supported by a Seed Grant from the Simons Foundation under the auspices of the Simons Center for the Social Brain at MIT (#319294) to Z. Kaldy and N. Kanwisher, Eunice Kennedy Shriver National Institute of Child Health and Human Development Award (#F32-HD075427) to J. Fischer, Autism Speaks Dennis Weatherstone Predoctoral Fellowship Grant (#7415) to F. Martinez Pedraza, and US Department of Health and Human Services, Health Resources and Services Administration (HRSA) (#R40MC26195) to A. S. Carter.
References
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: Comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30(3):237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS. Brief Infant-Toddler Social and Emotional Assessment (BITSEA) manual, version 2.0. Yale University; New Haven, CT: 2002. [Google Scholar]
- Brockmole JR, Boot WR. Should I stay or should I go? Attentional disengagement from visually unique and unexpected items at fixation. Journal of Experimental Psychology: Human Perception and Performance. 2009;35(3):808. doi: 10.1037/a0013707. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40(2):271. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Easterbrook PJ, Gopalan R, Berlin JA, Matthews DR. Publication bias in clinical research. The Lancet. 1991;337(8746):867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- Efron B. Nonparametric estimates of standard error: the jackknife, the bootstrap and other methods. Biometrika. 1981;68(3):589–599. [Google Scholar]
- Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, Piven J. White Matter Microstructure and Atypical Visual Orienting in 7-Month-Olds at Risk for Autism. American Journal of Psychiatry. 2013;170(8):899–908. doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Fernandes J, Webb SJ, Dawson G, Charman T, Johnson MH. Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biological Psychiatry. 2013;74(3):189–194. doi: 10.1016/j.biopsych.2012.11.030. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Holmboe K, Tucker L, Csibra G, Baron-Cohen S, Johnson MH. Visual orienting in the early broader autism phenotype: disengagement and facilitation. Journal of Child Psychology and Psychiatry. 2009;50(5):637–642. doi: 10.1111/j.1469-7610.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler AN, Bal VH, Guthrie W, Wetherby A, Weismer SE, Lord C. The Autism Diagnostic Observation Schedule, Toddler Module: Standardized Severity Scores. Journal of Autism and Developmental Disorders. 2015:1–17. doi: 10.1007/s10803-015-2432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Koldewyn K, Jiang YV, Kanwisher N. Unimpaired Attentional Disengagement and Social Orienting in Children With Autism. Clinical Psychological Science. 2014;2(2):214–223. doi: 10.1177/2167702613496242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MC, Lasker AG, Zee DS, Garth E, Tien A, Landa RJ. Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia. 2002;40(12):2039–2049. doi: 10.1016/s0028-3932(02)00059-3. [DOI] [PubMed] [Google Scholar]
- Goren CC, Sarty M, Wu PY. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56(4):544–549. [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MA, Behrmann M, Egan R, Minshew NJ, Carrasco M, Heeger DJ. Endogenous spatial attention: Evidence for intact functioning in adults with autism. Autism Research. 2013;6(2):108–118. doi: 10.1002/aur.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MA, Behrmann M, Egan R, Minshew NJ, Heeger DJ, Carrasco M. Exogenous spatial attention: Evidence for intact functioning in adults with autism spectrum disorder. Journal of Vision. 2013;13(14):9. doi: 10.1167/13.14.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YV, Capistrano CG, Esler AN, Swallow KM. Directing attention based on incidental learning in children with autism spectrum disorder. Neuropsychology. 2013;27(2):161. doi: 10.1037/a0031648. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40(1):1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Kaldy Z, Kraper C, Carter AS, Blaser E. Toddlers with Autism Spectrum Disorder are more successful at visual search than typically developing toddlers. Developmental Science. 2011;14(5):980–988. doi: 10.1111/j.1467-7687.2011.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakubo Y, Kasai K, Okazaki S, Hosokawa-Kakurai M, Watanabe K, Kuwabara H. Electrophysiological abnormalities of spatial attention in adults with autism during the gap overlap task. Clinical Neurophysiology. 2007;118(7):1464–1471. doi: 10.1016/j.clinph.2007.04.015. others. [DOI] [PubMed] [Google Scholar]
- Keehn B, Müller R-A, Townsend J. Atypical attentional networks and the emergence of autism. Neuroscience & Biobehavioral Reviews. 2013;37(2):164–183. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Senju A, Akechi H, Tojo Y, Osanai H, Hasegawa T. Atypical disengagement from faces and its modulation by the control of eye fixation in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2011;41(5):629–645. doi: 10.1007/s10803-010-1082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Jiang YV, Weigelt S, Kanwisher N. Global/local processing in autism: Not a disability, but a disinclination. Journal of Autism and Developmental Disorders. 2013;43(10):2329–2340. doi: 10.1007/s10803-013-1777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry O, Parker A. A meta-analysis of visual orienting in autism. Frontiers in Human Neuroscience. 2013;7:833. doi: 10.3389/fnhum.2013.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry. 2004;45(6):1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Light KR, Kolata S, Wass C, Denman-Brice A, Zagalsky R, Matzel LD. Working Memory Training Promotes General Cognitive Abilities in Genetically Heterogeneous Mice. Current Biology. 2010;20(8):777–782. doi: 10.1016/j.cub.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Luyster R, Gotham K, Guthrie W. Autism Diagnostic Observation Schedule, 2nd edition (ADOS-2) Manual (Part II): Toddler module. Western Psychological Services; Torrence, CA: 2012. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, 2nd edition (ADOS-2) manual (Part I): Modules 1-4. Western Psychological Services; Torrence, CA: 2012. [Google Scholar]
- Loveland KA, Landry SH. Joint attention and language in autism and developmental language delay. Journal of Autism and Developmental Disorders. 1986;16(3):335–349. doi: 10.1007/BF01531663. [DOI] [PubMed] [Google Scholar]
- Maestro S, Muratori F, Cavallaro MC, Pei F, Stern D, Golse B, Palacio-Espasa F. Attentional skills during the first 6 months of age in autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(10):1239–1245. doi: 10.1097/00004583-200210000-00014. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D'Cruz A-M, Seidenfeld A, Guter S, Stanford LD, Sweeney JA. Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychological Medicine. 2009;39(9):1559–1566. doi: 10.1017/S0033291708004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM. Mullen scales of early learning. AGS; Circle Pines, MN: 1995. pp. 58–64. [Google Scholar]
- O'Riordan MA. Superior Visual Search in Adults with. Autism. 2004;8(3):229–248. doi: 10.1177/1362361304045219. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Burr D. When the world becomes “too real”: a Bayesian explanation of autistic perception. Trends in Cognitive Sciences. 2012;16(10):504–510. doi: 10.1016/j.tics.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Pinto Y, van der Leij AR, Sligte IG, Lamme VAF, Scholte HS. Bottom-up and top-down attention are independent. Journal of Vision. 2013;13(3):16–16. doi: 10.1167/13.3.16. [DOI] [PubMed] [Google Scholar]
- Pitman EJG. Significance Tests Which May be Applied to Samples From any Populations. Supplement to the Journal of the Royal Statistical Society. 1937;4(1):119–130. [Google Scholar]
- Plaisted K, O'Riordan M, Baron-Cohen S. Enhanced Visual Search for a Conjunctive Target in Autism: A Research Note. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39(05):777–783. [PubMed] [Google Scholar]
- Reulen JPH. Latency of visually evoked saccadic eye movements. II. Temporal properties of the facilitation mechanism. Biological Cybernetics. 1984a;50:263–271. doi: 10.1007/BF00337076. [DOI] [PubMed] [Google Scholar]
- Reulen JPH. Latency of visually evoked saccadic eye movements. I. Saccadic latency and the facilitation model. Biological Cybernetics. 1984b;50:251–262. doi: 10.1007/BF00337075. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Hughes HC, Fendrich R. The reduction of saccadic latency by prior offset of the fixation point: an analysis of the gap effect. Perception & Psychophysics. 1991;49(2):167–175. doi: 10.3758/bf03205036. [DOI] [PubMed] [Google Scholar]
- Robertson CE, Kravitz DJ, Freyberg J, Baron-Cohen S, Baker CI. Tunnel vision: sharper gradient of spatial attention in autism. The Journal of Neuroscience. 2013;33(16):6776–6781. doi: 10.1523/JNEUROSCI.5120-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacrey L-AR, Armstrong VL, Bryson SE, Zwaigenbaum L. Impairments to visual disengagement in autism spectrum disorder: A review of experimental studies from infancy to adulthood. Neuroscience & Biobehavioral Reviews. 2014;47:559–577. doi: 10.1016/j.neubiorev.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Salapatek P, Kessen W. Visual scanning of triangles by the human newborn. Journal of Experimental Child Psychology. 1966;3(2):155–167. doi: 10.1016/0022-0965(66)90090-7. [DOI] [PubMed] [Google Scholar]
- Saslow MG. Effects of components of displacement-step stimuli upon latency for saccadic eye movement. JOSA. 1967;57(8):1024–1029. doi: 10.1364/josa.57.001024. [DOI] [PubMed] [Google Scholar]
- Sinha P, Kjelgaard MM, Gandhi TK, Tsourides K, Cardinaux AL, Pantazis D, Held RM. Autism as a disorder of prediction. Proceedings of the National Academy of Sciences. 2014;111(42):15220–15225. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Georgiades S, Bryson S, Zwaigenbaum L, Roberts W, Mahoney W, Tuff L. Investigating the structure of the restricted, repetitive behaviours and interests domain of autism. Journal of Child Psychology and Psychiatry. 2006;47(6):582–590. doi: 10.1111/j.1469-7610.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- Toth K, Munson J, Meltzoff AN, Dawson G. Early predictors of communication development in young children with autism spectrum disorder: Joint attention, imitation, and toy play. Journal of Autism and Developmental Disorders. 2006;36(8):993–1005. doi: 10.1007/s10803-006-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. Annotation: Repetitive behaviour in autism: A review of psychological research. Journal of Child Psychology and Psychiatry. 1999;40(6):839–849. [PubMed] [Google Scholar]
- Van de Cruys S, Evers K, Van der Hallen R, Van Eylen L, Boets B, de-Wit L, Wagemans J. Precise minds in uncertain worlds: Predictive coding in autism. Psychological Review. 2014;121(4):649. doi: 10.1037/a0037665. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]