Abstract
Purpose
Prior ultrasound biomicroscopy (UBM) studies showed that accommodative optical response (AOR) can be predicted from accommodative biometric changes in a young and a pre-presbyopic population from linear relationships between accommodative optical and biometric changes, with a standard deviation of less than 0.55D. Here, paraxial schematic eyes (SE) were constructed from measured accommodative ocular biometry parameters to see if predictions are improved.
Methods
Measured ocular biometry (OCT, A-scan and UBM) parameters from 24 young and 24 pre-presbyopic subjects were used to construct paraxial SEs for each individual subject (individual SEs) for three different lens equivalent refractive index methods. Refraction and AOR calculated from the individual SEs were compared with Grand Seiko (GS) autorefractor measured refraction and AOR. Refraction and AOR were also calculated from individual SEs constructed using the average population accommodative change in UBM measured parameters (average SEs).
Results
Schematic eye calculated and GS measured AOR were linearly related (young subjects: slope = 0.77; r2 = 0.86; pre-presbyopic subjects: slope = 0.64; r2 = 0.55). The mean difference in AOR (GS - individual SEs) for the young subjects was −0.27D and for the pre-presbyopic subjects was 0.33D. For individual SEs, the mean ± SD of the absolute differences in AOR between the GS and SEs was 0.50 ± 0.39D for the young subjects and 0.50 ± 0.37D for the pre-presbyopic subjects. For average SEs, the mean ± SD of the absolute differences in AOR between the GS and the SEs was 0.77 ± 0.88D for the young subjects and 0.51 ± 0.49D for the pre-presbyopic subjects.
Conclusions
Individual paraxial SEs predict AOR, on average, with a standard deviation of 0.50D in young and pre-presbyopic subject populations. Although this prediction is only marginally better than from individual linear regressions, it does consider all the ocular biometric parameters.
Keywords: paraxial schematic eyes, accommodation, refraction, ultrasound biomicroscopy
Objective, clinical measurement of accommodation is becoming increasingly important as clinical studies are undertaken to determine if accommodation restoration concepts are able to restore accommodation to the presbyopic eye. Such accommodation restoration concepts currently under investigation include scleral treatments, pharmacological or laser interventions directed at softening the phakic lens, or so-called accommodative intraocular lenses. Accommodation can be measured clinically either as an optical change in power of the eye (accommodative optical response; AOR) or as the biometric accommodative changes in the ocular anterior segment (such as changes in lens thickness or changes in lens surface curvatures). The optical changes provide a measure of the magnitude of the optical response, but no information on the mechanism of the optical change whereas the biometric changes provide an indication of the mechanism, but no information on the magnitude of the optical response. Measuring both the accommodative optical and biometric changes is important to fully characterize the accommodative response of an eye or of an accommodation restoration concept. However, currently, it is not possible to measure both the accommodative optical and biometric changes with a single clinical instrument and it is challenging and time consuming to measure them concurrently with two different instruments. An ideal scenario would be if the AOR could be accurately predicted or calculated from measured accommodative biometric changes.
Prior studies in young and pre-presbyopic subjects showed that ultrasound biomicroscopy (UBM) measured anterior segment parameters can be used to predict a population AOR based on linear regressions between the individual biometric parameters and the AOR.1, 2 On average, from population data, these predictions can be achieved with a standard deviation of less than 0.55 D in both the young and the pre-presbyopic subject populations. The limitation of using linear regressions from only a single biometric parameter is that this does not utilize all the ocular biometric changes that occur concurrently during accommodation. In phakic eyes, accommodative changes in anterior chamber depth, lens thickness and anterior and posterior lens radii of curvature occur simultaneously and are strongly linearly correlated with each other.2–5 This might suggest that predictions could be strengthened if all the biometric parameters that change with accommodation could be used together. One approach might be to use a multiple regression model. However, because of the strong linear correlations among the individual biometry parameters (multicollinearity), a multiple regression model has large prediction errors and is therefore unsuitable to use.
Another approach to use all the anterior segment biometry parameters together is to construct paraxial schematic eye models. Schematic eyes are constructed using corneal surface radii of curvatures, corneal thickness, anterior chamber depth, lens surface radii of curvatures and thickness, axial length and the refractive indices of the various optical media. Schematic eyes provide information on the optical properties of the eye including refractive state and therefore also on the change in refraction or accommodation. Schematic eye modeling generally includes calculations of surface and equivalent powers of the cornea, lens and the eye and other optical parameters such as cardinal points and entrance and exit pupil positions. Paraxial schematic eyes use simplified Gaussian optics equations, axial biometry parameters and radii of curvatures of the regions of the cornea and lens close to the optical axis; therefore, paraxial schematic eyes are generally useful only for axial optical parameters such as refraction and accommodation, but not for spherical aberration or other aberrations, for example. These simplified paraxial calculations can be performed using four surface (anterior and posterior cornea and anterior and posterior lens) schematic eyes and using a single lens equivalent refractive index value. More broadly, non-paraxial schematic eyes can be useful for understanding optical image quality6, in the design of intraocular implants7 and for customized refractive surgery.8
Prior accommodation dependent schematic eye models have biometric and optical parameters for just a few accommodative states.9, 10 No prior accommodation dependent schematic eye models have been used to calculate refraction and AOR from measured accommodative biometric changes and to compare the calculated values with the measured refractions and AOR from the same subjects. Furthermore, there are no accommodation dependent schematic eye models that have been constructed for older pre-presbyopic eyes with low accommodative amplitudes.
The current study was undertaken to; a) construct schematic eye models for different accommodative states for each individual subject from the prior studies in the young and pre-presbyopic populations, b) use the schematic eyes to calculate the refractive state and the accommodative optical response, c) compare the schematic eye calculated refraction and AOR with Grand Seiko autorefractor measured refraction and AOR from the subjects in these two populations, d) construct individual schematic eyes using average accommodative changes in UBM measured parameters for the young and pre-presbyopic populations to calculate refraction and AOR, and e) compare the prediction errors between schematic eyes and linear regressions from individual biometry parameters in the two subject populations.1, 2 A statistically significant linear relationship between schematic eye refraction and GS measured refraction or AOR with a high r2 value would indicate that the schematic eye might offer accurate predictions of refraction or AOR. Smaller r2 values indicate variability in the data and impact the accuracy of schematic eye prediction. Regression equations with higher r2 values and slopes different from one would still permit the regression equations to be used to predict actual measured refractions and AOR from calculated schematic eyes.
MATERIALS AND METHODS
Biometric Data for Schematic Eye Modeling
This study followed the tenets of the Declaration of Helsinki. Informed consent was obtained from all subjects after explanation of the nature and possible consequences of the study, and the research was performed in accordance with a human subject’s protocol approved by the University of Houston institutional review board (IRB). Ocular biometric data from 24 young and 24 pre-presbyopic subjects from prior studies2, 3, 11 were used to construct accommodative schematic eye models. Briefly, corneal anterior and posterior central radii of curvature and central corneal thickness were measured from Visante anterior segment optical coherence tomography (AS-OCT) images corrected for spatial and optical distortions.11 Since these corneal parameters do not change with accommodation, they were measured only for the unaccommodated state and these constant corneal values were used for all accommodated states in the schematic eye models. Anterior chamber depth (ACD), lens thickness (LT) and anterior and posterior lens radii of curvature (ALRC & PLRC) were measured from distortion corrected UBM images as the eyes accommodated to various stimulus demands.2, 3 Axial length was measured using A-scan ultrasound and axial length values for the unaccommodated state were used for all accommodative states in the schematic eye modeling.2, 3 Standard values for refractive indices for various ocular media were those used in a published schematic eye model (cornea: 1.376; aqueous/vitreous: 1.336 and lens: 1.422).9 All the measured AS-OCT, UBM and A-scan data were stored in Matlab (MathWorks, Natick, MA) structures and saved as Matlab '.mat' files and these structures were used for schematic eye calculations. Static AORs were measured in these subjects using a Grand Seiko (GS) autorefractor as the eyes accommodated to the same stimulus demands as were used for the biometric measures.1, 2 The stimulus demands used were 0 to 6D in 1D steps for the young subjects and from 0 to 2D in 0.25D steps and from 2 to 4D in 0.5D steps and from 4 to 6D in 1D steps for the pre-presbyopic subjects. Subjects with refractive errors were corrected with soft contact lenses to achieve emmetropic refractions for the far target (baseline). Measurements from the GS, UBM and A-scan were recorded separately for the same eye (left eye) for the same accommodative stimulus demands so that comparisons could be made between GS measured and schematic eye calculated refraction and AOR. The design of the metal frame used for performing UBM required that the UBM measurements were constrained to the left eye. For comparisons with the schematic eyes, all the GS refraction measurements were adjusted for the contact lens powers by adding the signed contact lens power to the GS measurements to get the uncorrected, underlying refractive error of the eyes. Henceforth, all GS measured refractions adjusted for contact lens powers will be referred to as adjusted GS refractions.
Individual Paraxial Schematic Eye Models
Individual, four surface paraxial schematic eye models were constructed from the measured ocular biometry parameters for each accommodative stimulus demand for each subject in the young and pre-presbyopic subject populations. A Matlab program was written to read in the ‘.mat’ files containing all the ocular biometry parameters and construct schematic eye models using equations 1 to 22 (listed in the Appendix, available at [LWW insert link]) for each accommodative stimulus demand for each individual in the young and pre-presbyopic subject populations. For each subject, this represents a unique schematic eye calculated for each stimulus demand. Absolute refractions were calculated from the schematic eye models for each accommodative stimulus demand from 0D to 6D and AORs relative to the baseline 0D stimulus demand were calculated for each accommodative stimulus demand from the schematic eye models. Schematic eye calculated refractions and AORs were compared with adjusted GS refractions and AORs. All the calculated schematic eye parameters were stored as Matlab ‘.mat’ files for further analysis.
Lens refractive index is one of the parameters required for the schematic eye calculations and it is potentially variable between subjects but it cannot readily be measured. The natural lens has a gradient refractive index and the lens equivalent refractive index is the single refractive index value that achieves a lens of the same optical power as the lens of the same shape with a gradient refractive index. As mentioned above, the initial lens equivalent refractive index chosen for all the individual schematic eyes was a constant value of 1.422.9 It is unlikely that every eye would have the same lens equivalent refractive index and, furthermore, there is a possibility that the lens equivalent refractive index could change systematically with age and with accommodation.12 For these reasons, in addition to using the constant value of 1.422 described above, individual lens equivalent refractive index values were calculated for each subject, for each accommodative stimulus demand. To do this, the standard lens equivalent refractive index of 1.422 was used as the starting index for the baseline stimulus demand (0D). Schematic eye calculations were performed using the custom developed Matlab program described above. The difference between the schematic eye calculated and adjusted GS refraction for the baseline 0D stimulus was calculated and the starting lens equivalent refractive index was iterated in 0.00001 steps until the schematic eye calculated refraction matched the adjusted GS refraction. The calculated lens refractive index value that achieved the matching schematic eye and adjusted GS refraction at baseline was stored in a Matlab array. This individually calculated lens equivalent refractive index at baseline served as the starting index for the schematic eyes for all the other stimulus demands and the refractive index was iterated to match the adjusted GS refraction as described above for each stimulus demand. The calculated lens equivalent refractive indices for all stimulus demands for each subject were stored in ‘.mat files’ for further analysis.
Average Paraxial Schematic Eye Models
It is also of interest to establish if the average measured accommodative biometry changes from each population as a whole can be used in conjunction with the calculated schematic eyes to try to predict the accommodative response from the younger and pre-presbyopic populations. To do this, the individual schematic eyes were calculated at baseline as described above and to each of them was applied the average accommodative changes in UBM measured biometry parameters for each stimulus demand calculated from the two populations. Schematic eye refractions and AORs were then calculated from these averaged SEs and compared with the adjusted GS refraction and AORs from the two populations. To calculate the average accommodative changes, UBM measured parameters (ACD, LT, ALRC & PLRC) for each accommodative stimulus demand were subtracted from the corresponding baseline (0D) value in each individual subject. The resulting accommodative changes in UBM measured biometry parameters were averaged across all subjects for each stimulus demand in the young (Table 1) and pre-presbyopic populations (Table 2) separately. For the young and the pre-presbyopic populations, individual accommodative schematic eyes for each subject for each stimulus demand were then calculated. This was done using each subject’s individual baseline corneal thickness, corneal surface radii of curvatures and axial length and then the calculated population average accommodative changes in UBM biometry parameters (LT, ACD, ALRC, PLRC) were added to each subjects’ baseline biometry values. The iteratively calculated baseline lens equivalent refractive index for each subject as described above was used for these calculations. Individual schematic eye calculated refractions and AORs were compared with adjusted GS refractions and AORs for both the young and pre-presbyopic populations.
Table 1.
Average accommodative change in UBM measured biometry parameters from 24 young subjects.
| Average Accommodative Change in
UBM Measured Biometry Parameters |
Accommodative Stimulus Demand (D) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| ACD (mm) | 0.00 | −0.04 | −0.11 | −0.18 | −0.23 | −0.26 | −0.26 |
| SD of ACD (mm) | 0.00 | 0.03 | 0.04 | 0.05 | 0.05 | 0.06 | 0.07 |
| LT (mm) | 0.00 | 0.07 | 0.16 | 0.24 | 0.30 | 0.36 | 0.39 |
| SD of LT (mm) | 0.00 | 0.05 | 0.05 | 0.07 | 0.06 | 0.07 | 0.07 |
| ALRC (mm) | 0.00 | −0.59 | −1.82 | −2.68 | −3.42 | −3.98 | −4.26 |
| SD of ALRC (mm) | 0.00 | 0.46 | 0.70 | 0.87 | 1.01 | 1.11 | 1.20 |
| PLRC (mm) | 0.00 | −0.11 | −0.31 | −0.57 | −0.78 | −0.91 | −1.00 |
| SD of PLRC (mm) | 0.00 | 0.14 | 0.20 | 0.25 | 0.26 | 0.36 | 0.38 |
SD: standard deviation; ACD: anterior chamber depth; LT: lens thickness; ALRC: anterior lens radius of curvature; PLRC: posterior lens radius of curvature.
Table 2.
Average accommodative change in UBM measured biometry parameters from 24 pre-presbyopic subjects.
| Average
Accommodative Change in UBM Measured Biometry Parameters |
Accommodative Stimulus Demand (D) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 | 1.50 | 1.75 | 2.0 | 2.5 | 3.0 | 3.5 | 4 | 5 | 6 | |
| ACD (mm) | 0.00 | 0.00 | −0.01 | −0.02 | −0.04 | −0.05 | −0.06 | −0.07 | −0.09 | −0.10 | −0.11 | −0.12 | −0.12 | −0.12 | −0.13 |
| SD of ACD (mm) | 0.00 | 0.02 | 0.03 | 0.03 | 0.04 | 0.04 | 0.05 | 0.05 | 0.06 | 0.06 | 0.06 | 0.07 | 0.07 | 0.09 | 0.09 |
| LT (mm) | 0.00 | 0.02 | 0.03 | 0.04 | 0.06 | 0.07 | 0.09 | 0.10 | 0.11 | 0.13 | 0.15 | 0.16 | 0.17 | 0.19 | 0.21 |
| SD of LT (mm) | 0.00 | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 | 0.04 | 0.03 | 0.04 | 0.06 | 0.06 | 0.07 | 0.07 | 0.08 | 0.09 |
| ALRC (mm) | 0.00 | −0.09 | −0.29 | −0.50 | −0.66 | −0.97 | −1.00 | −1.27 | −1.52 | −1.62 | −1.85 | −2.17 | −2.36 | −2.39 | −2.85 |
| SD of ALRC (mm) | 0.00 | 0.44 | 0.45 | 0.83 | 0.66 | 0.76 | 0.97 | 1.02 | 0.95 | 1.01 | 0.88 | 1.21 | 1.37 | 1.20 | 1.30 |
| PLRC (mm) | 0.00 | 0.08 | 0.08 | 0.11 | 0.04 | 0.05 | −0.03 | −0.05 | −0.08 | −0.15 | −0.10 | −0.13 | −0.12 | −0.08 | −0.18 |
| SD of PLRC (mm) | 0.00 | 0.15 | 0.15 | 0.12 | 0.18 | 0.21 | 0.18 | 0.23 | 0.24 | 0.27 | 0.32 | 0.39 | 0.38 | 0.46 | 0.40 |
The number of subjects whose biometry parameters were averaged for each stimulus demand was: 0 D to 3 D: 24, 3.5 to 4 D: 23, 5 D: 19 and 6 D: 15.
SD: standard deviation; ACD: anterior chamber depth; LT: lens thickness; ALRC: anterior lens radius of curvature; PLRC: posterior lens radius of curvature.
RESULTS
The age ± standard deviation (SD) of the young subjects was mean: 24.15 ± 3.03 years; median: 24 years; range: 21 to 36 years and for the pre-presbyopic subjects was mean: 40.80 ± 3.08; median: 41 years; range: 36 to 46 years.
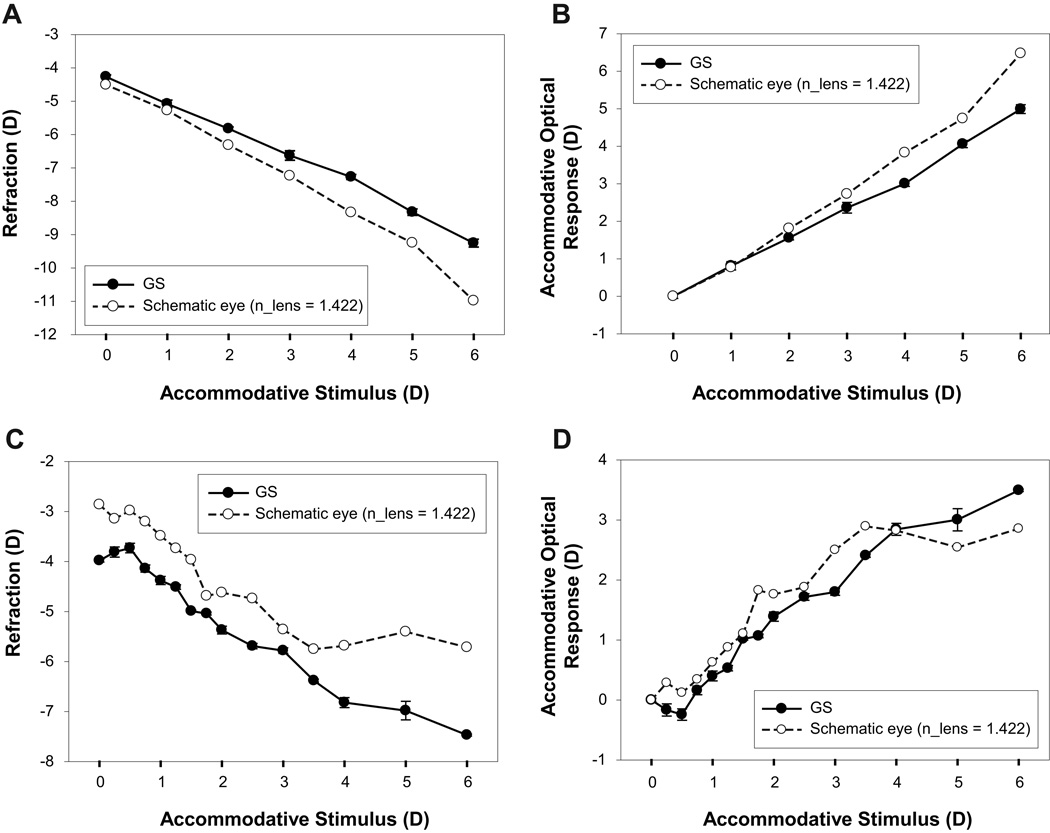
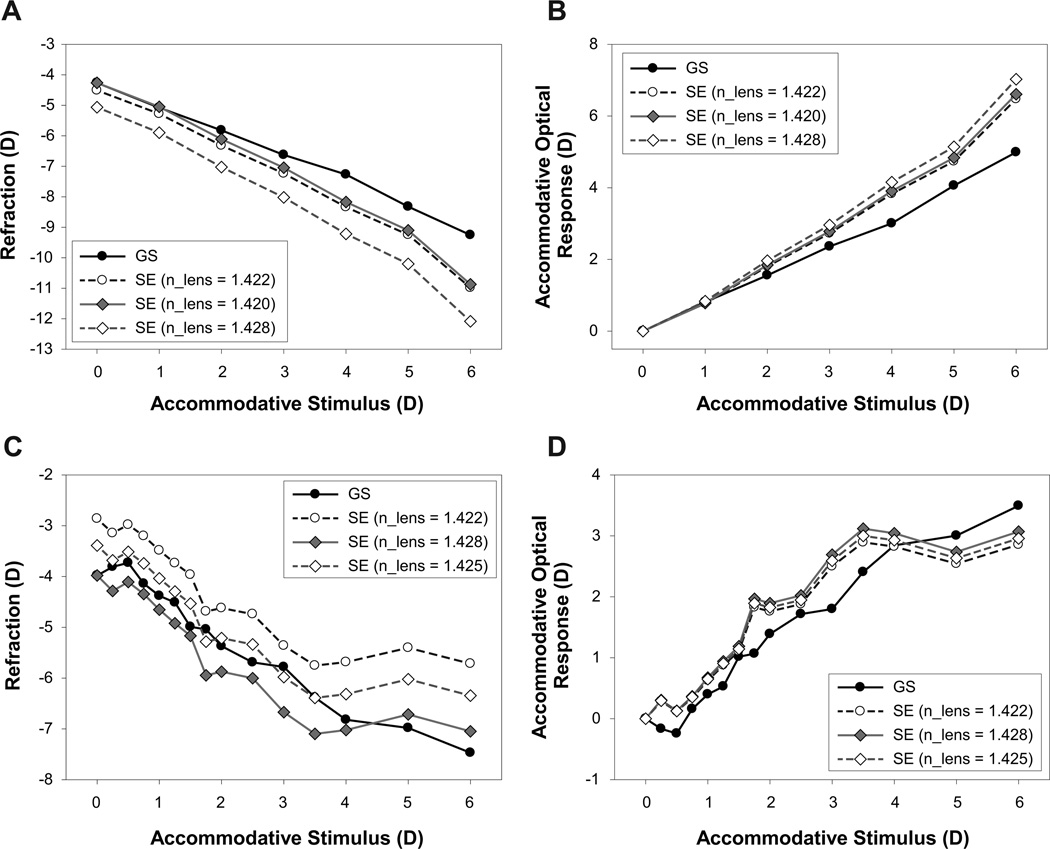
Data from a young and a pre-presbyopic subject showing comparisons between individual schematic eye calculated and adjusted GS measured refraction and AOR is shown in Figure 1. These schematic eyes used the standard lens equivalent refractive index of 1.422. For the young subject, there is a linear change in GS measured refraction with increasing stimulus demand. However, the individual schematic eye calculated refraction is consistently and progressively more myopic than the adjusted GS measurements (Figure 1A). The individual schematic eye AOR increasingly overestimates the GS measured AOR with increasing stimulus demands for this subject (Figure 1B). In this young subject, each GS data point and the measured ocular biometry parameters used for the individual schematic eye are an average of 9 measurements from 3 measurements each in 3 independent trials. For the pre-presbyopic subject, there is a reasonably systematic myopic change in adjusted GS refraction with increasing stimulus demand. The individual schematic eye calculated refraction is less myopic than the adjusted GS measurements and asymptotes at a lower stimulus demands (Figure 1C). The individual schematic eye calculated AOR in the pre-presbyopic subject is initially slightly greater than the GS measured AOR and saturates at a lower stimulus demands (Figure 1D). In this pre-presbyopic subject, each GS data point and the ocular biometry parameters used for the individual schematic eye calculations is an average of 3 measurements from a single trial.
Figure 1.
(A) Comparison of a calculated individual schematic eye and the Grand Seiko (GS) measured refraction from a 23 year old subject. (B) Comparison of the GS measured and the individual schematic eye calculated stimulus-response function. (C) Comparison of the individual schematic eye calculated and the GS measured refractions from a 36 year old pre-presbyopic subject. (D) Comparison of the GS measured and the individual schematic eye calculated stimulus-response function from the same pre-presbyopic subject. The schematic eye data shown here were calculated using the standard equivalent lens refractive index of 1.422. Grand Seiko data error bars represent standard deviations from 9 measurements from 3 different trials.
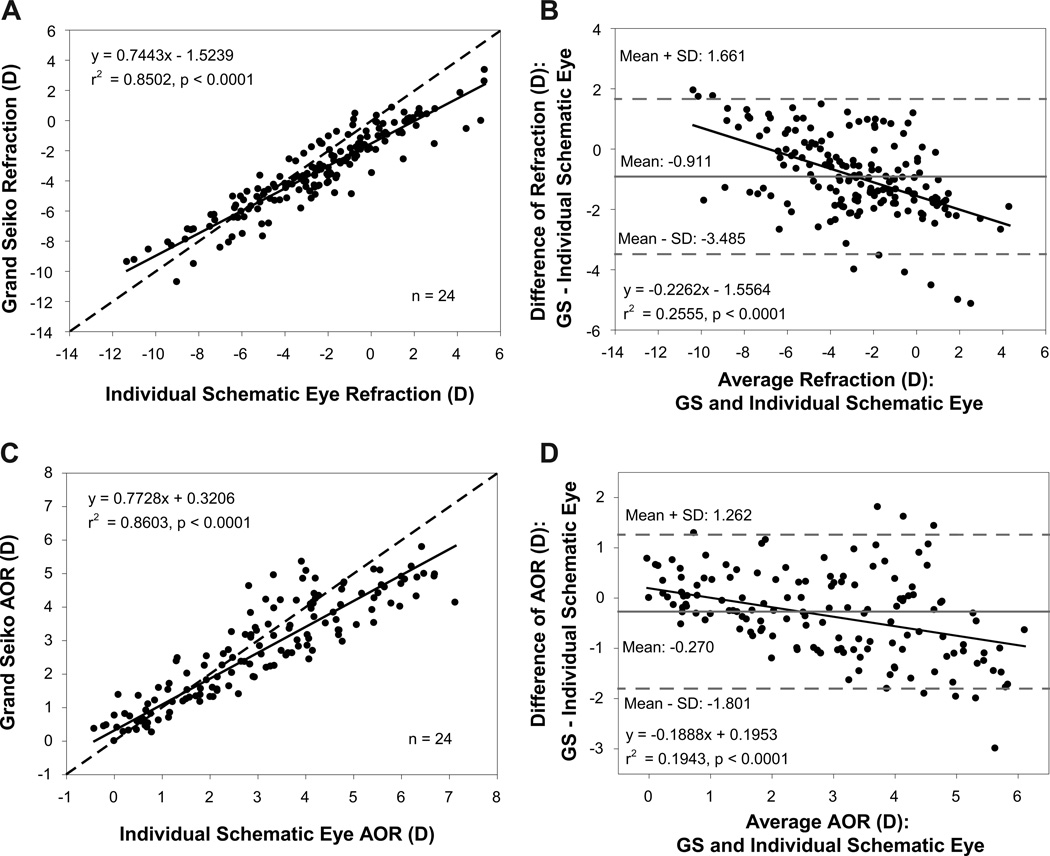
Comparison of individual schematic eye calculated and adjusted GS refractions from all stimulus demands from the 24 young subjects showed a statistically significant linear relationship with an r2 value of 0.85 indicating that the individual schematic eyes, in general, could provide reasonably good predictions of refraction (Figure 2A). Bland-Altman analysis showed that individual schematic eye refractions were on average more hyperopic by 0.91D than the adjusted GS refractions with a statistically significant linear relationship (Figure 2B). The schematic eye calculated accommodative optical response was underestimated at lower accommodation and overestimated at higher accommodation compared to the GS measurements (Figure 2C) and this overestimation showed a linear increase as a function of increasing AOR (Figure 2D).
Figure 2.
(A) Comparison of individual schematic eye calculated and Grand Seiko (GS) measured refractions from 24 young subjects. (B) Bland-Altman comparison of refractions between GS and individual schematic eyes showing a statistically significant linear trend. (C) Comparison of accommodative optical response (AOR) between GS and individual schematic eyes. (D) Bland-Altman comparison of AOR between GS and individual schematic eyes showing a systematic linear overestimation at higher AORs.
In the 24 pre-presbyopic subjects, there was a statistically significant linear relationship between individual schematic eye refractions and GS refractions with an r2 value of 0.83 (Figure 3A). The r2 values for refractions were comparable between the young and pre-presbyopic subjects. Most of the schematic eye measured refractions were more hyperopic than adjusted GS refractions and the Bland-Altman analysis showed a mean difference of −0.77D with a statistically significant linear trend (Figure 3B). The individual schematic eye models underestimated the AOR at lower accommodation and overestimated the AOR at higher accommodation compared to GS measured AOR (Figure 3C). The r2 values for AOR were smaller than for refraction which means that the predictions of AOR are less consistent than for refraction. Bland-Altman analysis showed that the individual schematic eyes underestimated the AOR, on average, by 0.38D or more with a statistically significant linear trend (Figure 3D).
Figure 3.
(A) Comparison of individual schematic eye calculated and Grand Seiko (GS) measured refractions from 24 pre-presbyopic subjects. (B) Bland-Altman comparison of refractions between GS and individual schematic eyes showing a statistically significant linear trend. (C) Comparison of accommodative optical response (AOR) between GS and individual schematic eyes. (D) Bland-Altman comparison of AOR between GS and individual schematic eyes showing a systematic linear overestimation by the individual schematic eyes for higher accommodative responses.
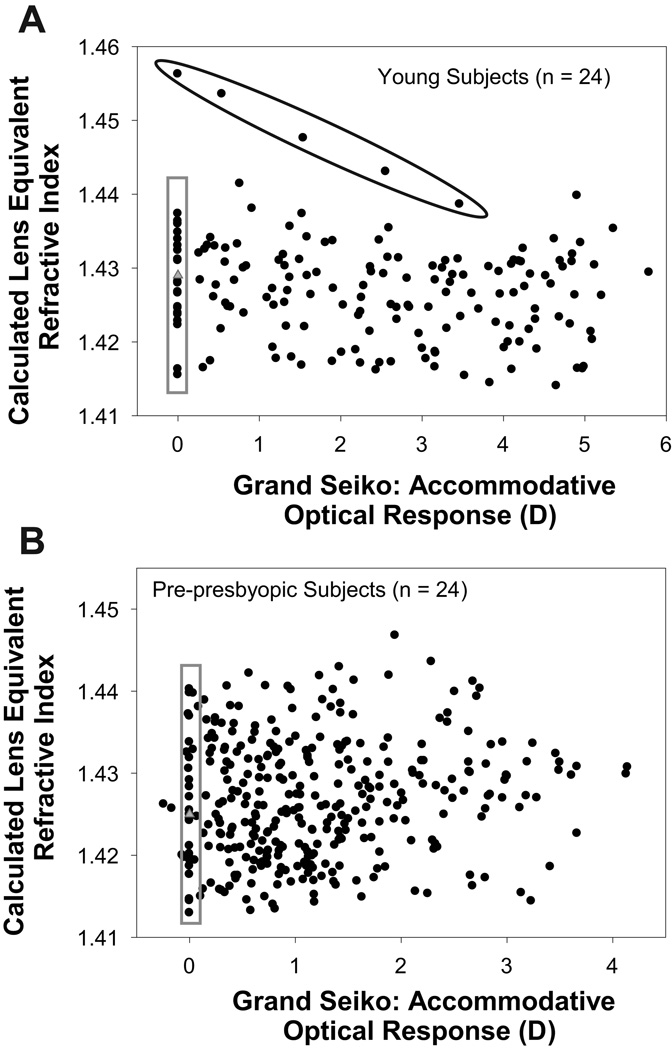
The iteratively calculated lens equivalent refractive index values when plotted as a function of the GS measured AOR showed no trend as a function of the AOR for either the young (Figure 4A) or the pre-presbyopic subjects (Figure 4B). The mean ± SD of the calculated baseline lens refractive index values in the young and pre-presbyopic subject populations was 1.428 ± 0.006 and 1.425 ± 0.008, respectively. The mean ± SD of the calculated lens equivalent refractive index values from all stimulus demands was 1.427 ± 0.007 in both the young and pre-presbyopic subject populations. To determine if altering the lens equivalent refractive index improved the predictive ability of the individual schematic eye models, in addition to using the fixed standard lens equivalent refractive index value of 1.422 as described above, schematic eye calculations were also performed using two additional lens equivalent refractive index methods: i) using the lens equivalent refractive index calculated from each individual subject's baseline refraction for each individual subject and ii) using the average lens equivalent refractive index calculated from all subject's baseline refraction for all subjects (young: 1.428; pre-presbyopic: 1.425).
Figure 4.
Calculated lens equivalent refractive index plotted as a function of GS measured AOR in young (A) and pre-presbyopic subjects (B). Data points circled in black are from the same subject shown in Figure 4 and are not included in the calculation of the average refractive index. Data points within the grey box represent the lens refractive index values for baseline. Grey triangles represent the average calculated refractive index at baseline.
Comparison of adjusted GS refractions and AOR and individual schematic eye calculated refraction and AOR for the different lens equivalent refractive index methods for a young and a pre-presbyopic subject are shown in Figure 5. Data from the same subjects shown in Figure 1 are plotted here for comparison. For the young subject, the refraction for the subject’s calculated baseline lens refractive index of 1.420 is closer to the adjusted GS curves than for other refractive index values (Figure 5A). The AOR curves for the standard lens equivalent refractive index of 1.422 is closer to the adjusted GS curves (Figure 5B). For the mean baseline lens refractive index of 1.428, refraction is more myopic and produces a relatively larger AOR. For the pre-presbyopic subject, the mean baseline lens equivalent refractive index of 1.425, in general, brought the refraction and AOR curves closer to adjusted GS curves than for the other lens refractive index values (Figure 5 C and Figure 5D).
Figure 5.
Comparison of Grand Seiko (GS) measured and individual schematic eye calculated refractions (A) and AORs (B) for three lens refractive index values from a 23 year old subject. Comparison of GS measured and individual schematic eye calculated refractions (C) and AORs (D) from a 36 year old pre-presbyopic subject for three lens refractive index values. Curves with open circles, filled diamonds and open diamonds represent the data from the standard, individual baseline and averaged population baseline lens equivalent refractive index values respectively.
Linear regression parameters and Bland-Altman analyses for comparisons between adjusted GS refractions and AORs and individual schematic eye refractions and AORs calculated using three different lens equivalent index methods for young subjects are shown in Table 3. The r2 values for the linear regressions ranged from 0.85 to 0.89 for refraction. Root mean square (RMS) errors of refraction calculated relative to the 1:1 line were, on average, 1D or more. For AOR, the mean RMS errors calculated relative to the 1:1 line ranged from 0.77 to 1.04D. The RMS errors show that predictions for AOR are, on average, better than for refraction. In pre-presbyopic subjects, the RMS error of refraction calculated relative to the 1:1 line was smaller when the individual subject’s calculated baseline lens equivalent refractive index was used than for other refractive index methods (Table 4). For AOR, all three lens refractive index methods had comparable RMS errors. Overall, in pre-presbyopic subjects, using the individual subject’s calculated baseline lens refractive index offered good predictions of refraction and AOR. The purpose of constructing individual schematic eye models using three different lens equivalent refractive index values was to see if better predictions of refraction and AOR could be achieved. However, no single lens equivalent refractive index method could provide better predictions of both refraction and AOR for young subjects. The three refractive index values considered are similar to each other.
Table 3.
Parameters from linear regressions and Bland-Altman analyses comparing Grand Seiko measured and individual schematic eye calculated refractions and accommodative optical responses (AORs) using the three different lens equivalent refractive index methods for the young subject population.
| Parameters | Grand Seiko vs. Individual Schematic Eye (Young Subjects, n = 24) | |||||
|---|---|---|---|---|---|---|
| Refraction (D) | AOR (D) | |||||
| nlens = 1.422 | nlens =
subject’s baseline index |
nlens = 1.428 | nlens = 1.422 | nlens =
subject’s baseline index |
nlens = 1.428 | |
| Linear Regression | ||||||
| Slope | 0.744 | 0.803 | 0.698 | 0.771 | 0.655 | 0.688 |
| Intercept | −1.524 | −0.212 | −0.653 | 0.326 | 0.446 | 0.367 |
| r2 | 0.858 | 0.886 | 0.847 | 0.859 | 0.810 | 0.843 |
| p value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| Bland - Altman Analysis | ||||||
| Mean Difference (D) | −0.912 | 0.544 | 0.493 | −0.270 | −0.544 | −0.529 |
| Upper limit (D) | 1.662 | 2.674 | 3.403 | 1.262 | 1.587 | 1.356 |
| Lower limit (D) | −3.485 | −1.587 | −2.417 | −1.801 | −2.675 | −2.414 |
| Mean RMS error before correction (D) | 1.42 | 1.04 | 1.28 | 0.77 | 1.04 | 0.98 |
| Mean RMS error after linear regression correction (D) | 0.84 | 0.79 | 0.87 | 0.58 | 0.66 | 0.61 |
RMS: root mean square relative to 1:1 line; nlens = lens equivalent refractive index.
Table 4.
Parameters from linear regressions and Bland-Altman analyses comparing Grand Seiko measured and individual schematic eye calculated refractions and accommodative optical responses (AORs) using the three different lens equivalent refractive index methods for the pre-presbyopic subject population.
| Parameters | Grand Seiko vs. Individual Schematic Eye (Pre-presbyopic Subjects, n = 24) | |||||
|---|---|---|---|---|---|---|
| Refraction (D) | AOR (D) | |||||
| nlens = 1.422 | nlens =
subject’s baseline index |
nlens = 1.425 | nlens = 1.422 | nlens =
subject’s baseline index |
nlens = 1.425 | |
| Linear Regression | ||||||
| Slope | 0.931 | 0.854 | 0.911 | 0.635 | 0.585 | 0.611 |
| Intercept | −0.920 | −0.707 | −0.456 | 0.673 | 0.679 | 0.676 |
| r2 | 0.689 | 0.899 | 0.673 | 0.552 | 0.539 | 0.551 |
| p value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| Bland - Altman Analysis | ||||||
| Mean Difference (D) | −0.770 | −0.326 | −0.215 | 0.383 | 0.328 | 0.353 |
| Upper limit (D) | 1.697 | 1.238 | 2.324 | 1.834 | 1.894 | 1.848 |
| Lower limit (D) | −3.236 | −1.891 | −2.755 | −1.068 | −1.239 | −1.141 |
| Mean RMS error before correction (D) | 1.31 | 0.80 | 1.20 | 0.78 | 0.80 | 0.79 |
| Mean RMS error after linear regression correction (D) | 1.13 | 0.66 | 1.16 | 0.60 | 0.60 | 0.60 |
RMS: root mean square relative to 1:1 line; nlens = lens equivalent refractive index.
A linear regression equation with a high r2 value and a slope different from one can still be used for predictions. A slope different from one simply means a correction factor needs to be applied for the predictions. Individual schematic eye calculated refractions and AORs in individual subjects were corrected using linear regression parameters (slopes and intercepts) for refraction and AOR, respectively, for each of the three lens equivalent refractive index methods in young (Table 3) and pre-presbyopic subjects (Table 4). Root mean square (RMS) errors relative to the 1:1 line after the linear regression correction were calculated for refraction and AOR. For both the young and the pre-presbyopic subjects, the RMS errors after linear regression correction, were in general, smaller than before correction for refraction and AOR using all three lens equivalent refractive index methods.
Mean absolute differences between GS measured and individual schematic eye calculated AORs after linear regression correction for the three different lens equivalent index methods in young subjects are listed in Table 5. Prediction results from individual linear regressions of UBM measured biometry parameters from a prior study are shown for comparison. The mean difference between measured and predicted AOR was smaller from the schematic eyes than from individual biometry parameters, indicating better prediction with schematic eyes in young subjects. For pre-presbyopic eyes, AOR predictions with schematic eyes were comparable to those from individual biometry parameters (Table 6).
Table 5.
Comparison of absolute differences between Grand Seiko (GS) measured accommodative optical response (AOR) and predicted AOR from schematic eyes and using individual linear regressions of UBM biometry parameters in young subjects from a prior study.1
| Prediction Method and Biometry Parameters Used | Absolute Difference between
GS Measured AOR and Predicted AOR (D) in Young Subjects |
|||
|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | |
| Linear Regression | ||||
| ACD | 0.62 | 0.44 | 0.02 | 1.87 |
| LT | 0.56 | 0.46 | 0.00 | 2.57 |
| ALRC | 0.74 | 0.54 | 0.00 | 3.08 |
| PLRC | 0.75 | 0.56 | 0.01 | 3.33 |
| ASL | 0.91 | 0.65 | 0.00 | 3.29 |
| Paraxial Schematic Eyes | ||||
| All
parameters nlens = 1.422 |
0.50 | 0.39 | 0.00 | 2.13 |
| All
parameters nlens = subject’s baseline index |
0.57 | 0.46 | 0.00 | 3.01 |
| All
parameters nlens = 1.428 |
0.52 | 0.41 | 0.00 | 2.15 |
ACD: anterior chamber depth; LT: lens thickness; ALRC: anterior lens radius of curvature; PLRC: posterior lens radius of curvature; ASL: anterior segment length; SD: standard deviation; nlens lens equivalent refractive index.
Table 6.
Comparison of absolute differences between Grand Seiko (GS) measured accommodative optical response (AOR) and predicted AOR from schematic eyes and using individual linear regressions of UBM biometry parameters in pre-presbyopic subjects from a prior study.2
| Prediction Method and
Biometry Parameters Used |
Absolute Difference between GS Measured AOR
and Predicted AOR (D) in Pre-presbyopic Subjects |
|||
|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | |
| Linear Regression | ||||
| ACD | 0.53 | 0.42 | 0.01 | 2.22 |
| LT | 0.41 | 0.33 | 0.00 | 1.70 |
| ALRC | 0.50 | 0.42 | 0.00 | 2.22 |
| PLRC | 0.62 | 0.47 | 0.01 | 2.75 |
| ASL | 0.60 | 0.49 | 0.00 | 2.42 |
| Paraxial Schematic Eyes | ||||
| All
parameters nlens = 1.422 |
0.50 | 0.37 | 0.00 | 2.17 |
| All
parameters nlens = subject’s baseline index |
0.51 | 0.38 | 0.00 | 2.16 |
| All
parameters nlens = 1.425 |
0.50 | 0.37 | 0.00 | 2.17 |
ACD: anterior chamber depth; LT: lens thickness; ALRC: anterior lens radius of curvature; PLRC: posterior lens radius of curvature; ASL: anterior segment length; SD: standard deviation; nlens lens equivalent refractive index.
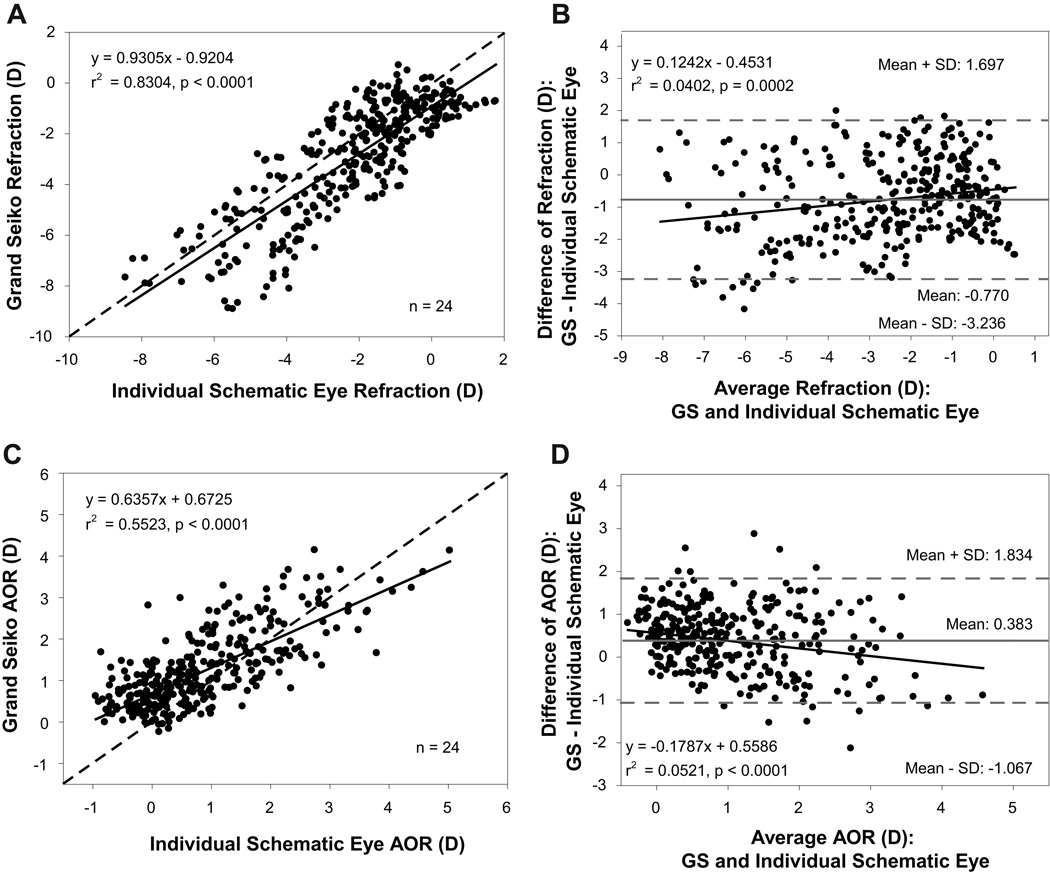
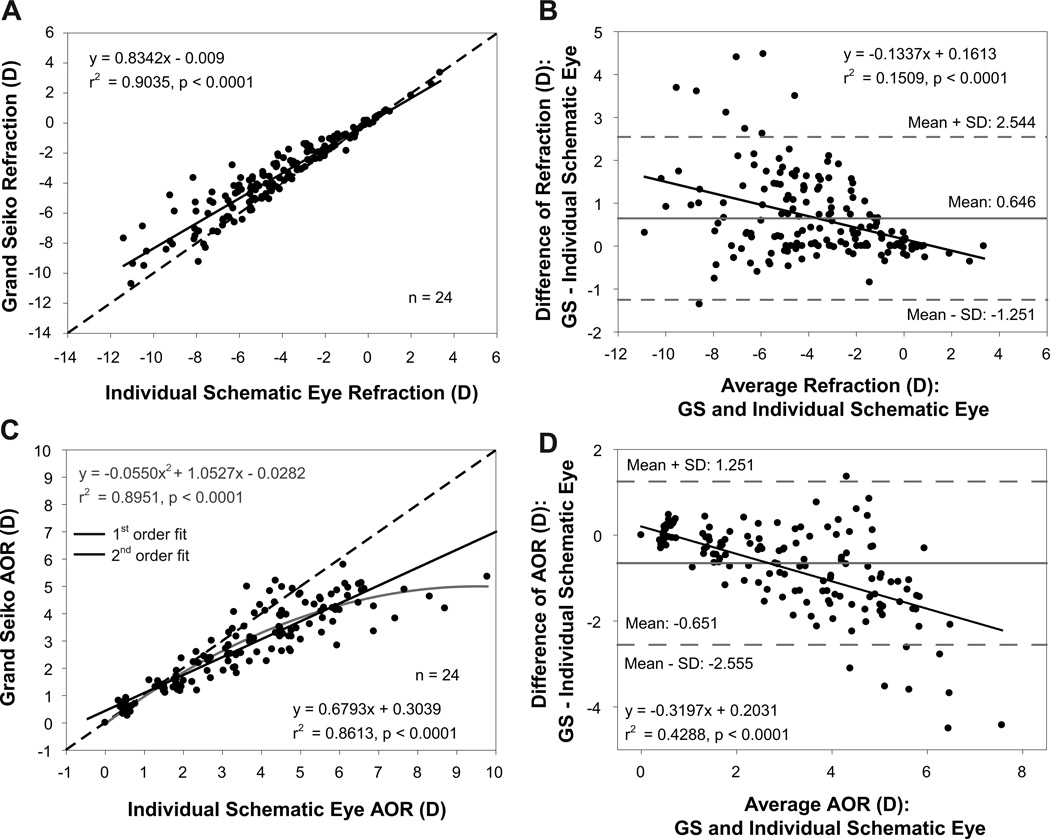
Comparisons of refraction and AOR between individual schematic eyes constructed using the average change in UBM biometry parameters and the GS measurements in young subjects are shown in Figure 6. The individual schematic eye refractions and adjusted GS refractions had a statistically significant linear relationship with an r2 value of 0.90 (Figure 6 A). Bland-Altman analysis showed a mean difference of 0.646D with a statistically significant linear trend (Figure 6 B). The schematic eye calculated AOR was underestimated at lower response levels and overestimated at higher response levels compared to GS measured AOR (Figure 6 C) and this overestimation showed a linear increase as a function of increasing AOR (Figure 6 D). The RMS errors of refraction and AOR relative to the 1:1 line for the young subjects were 0.95D and 0.96D, respectively.
Figure 6.
(A) Comparison of refraction between Grand Seiko (GS) measurements and individual schematic eyes calculated from the average change in UBM biometry measurements from 24 young subjects. (B) Bland-Altman comparison of refraction between GS and individual schematic eyes showing a statistically significant linear trend. (C) Comparison of accommodative optical response (AOR) between GS and individual schematic eyes. (D) Bland-Altman comparison of AOR between GS and individual schematic eyes showing a systematic linear overestimation.
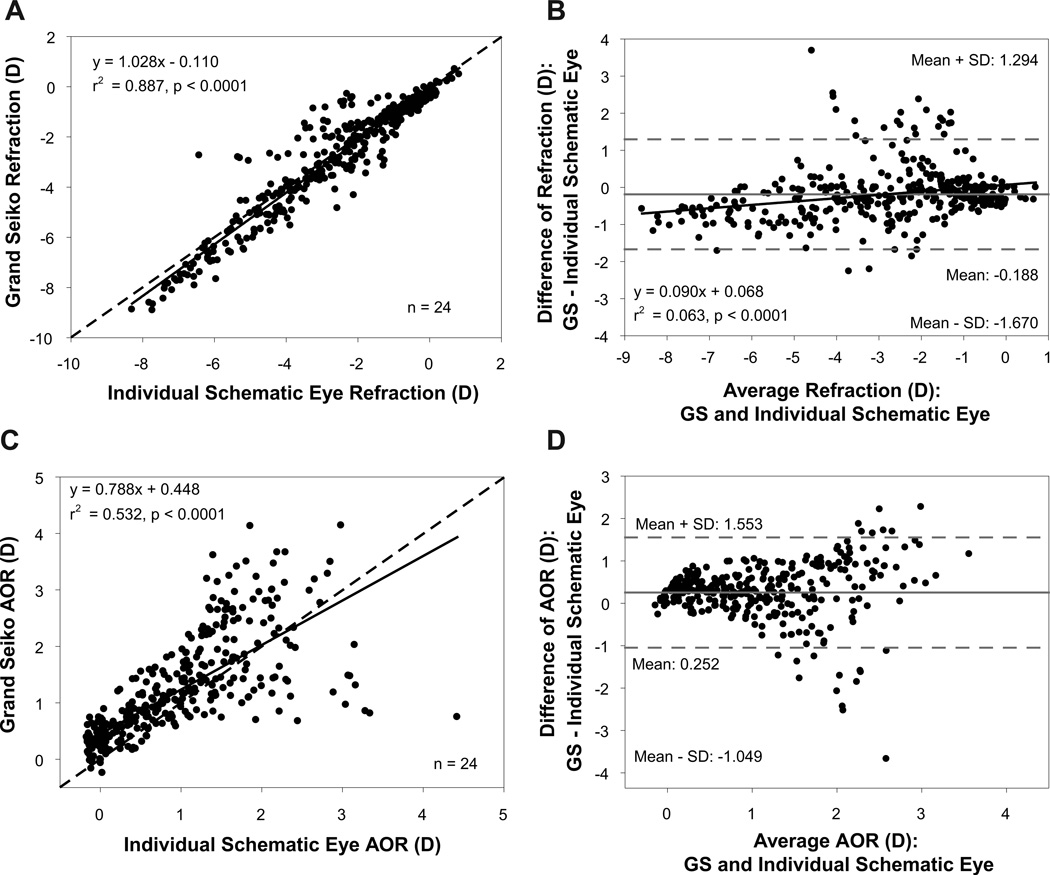
Comparisons of refraction and AOR between individual schematic eyes constructed using the average change in UBM biometry parameters and the GS in pre-presbyopic subjects is shown in Figure 7. The individual schematic eye refractions were linearly correlated with the adjusted GS refractions with a slope 1.02 (Figure 7 A). Bland-Altman analysis showed a mean difference of −0.188D between schematic eye and adjusted GS refractions (Figure 7 B). The schematic eye calculated AOR was overestimated at lower response levels and underestimated at higher response levels compared to GS measured AOR (Figure 7 C). Bland-Altman analysis showed a mean difference of 0.252D between schematic eye and GS AORs (Figure 7 D). The RMS errors of refraction and AOR relative to the 1:1 line for the pre-presbyopic subjects were 0.69D and 0.63D, respectively.
Figure 7.
(A) Comparison of refraction between Grand Seiko (GS) and individual schematic eyes calculated from the average change in UBM biometry measurements from 24 pre-presbyopic subjects. (B) Bland-Altman comparison of refraction between GS and individual schematic eyes showing a statistically significant linear trend. (C) Comparison of accommodative optical response (AOR) between GS and individual schematic eyes. (D) Bland-Altman comparison of AOR between GS and individual schematic eyes.
DISCUSSION
In the current study, the RMS errors relative to the 1:1 line for AOR from individual schematic eyes were in general larger than RMS errors from individual linear regressions of UBM measured biometry parameters for both young and pre-presbyopic subjects from prior studies.1, 2 However, when the individual schematic eye AORs were corrected using linear regression equations (Table 3 and Table 4), the RMS errors after correction were comparable to the RMS errors from individual linear regressions of UBM measured biometry parameters in both young and pre-presbyopic subjects. For refraction, the RMS errors after linear regression corrections were smaller in young subjects than in pre-presbyopes as demonstrated by the higher r2 values. For young subjects, the individual schematic eye offers marginally better AOR predictions than from individual linear regressions of UBM biometry parameters. For pre-presbyopic subjects, both individual schematic eyes and individual linear regressions of UBM biometry parameters offer comparable AOR predictions.1, 2 Overall, linear regression corrections can be applied to the individual schematic eye AORs calculated using either of the three lens equivalent refractive index methods to predict the AOR.
One of the limitations of the current study is that the Grand Seiko, UBM and A-scan measurements were performed sequentially and not simultaneously. Hence, there is a possibility of subjects accommodating to different amplitudes during each procedure. UBM and A-scan are both instruments with relatively low axial resolution and this could have resulted in inaccuracies in obtaining the true measurements of the ocular accommodative biometric changes, thereby contributing to variation in the refraction and AOR predictions. The relatively small number of lens surface pixels from UBM images that were used to calculate lens radius of curvature and indistinct edges of the lens surface in the UBM images3 could have resulted in variable lens curvatures which could have affected the individual schematic eye calculations.
Inaccuracies in the schematic eye calculations could come from inaccuracies in one, several or all of the individual measured parameters used for the schematic eye calculations. In an effort to try to improve the accuracy of individual schematic eyes, individual schematic eye parameters such as ACD, LT, ALRC and PLRC can be iteratively recalculated to achieve a calculated refraction matching the GS measured refraction. An attempt was made to do this by iteratively calculating each of the individual schematic eye biometry parameters independently while using all the other parameters as measured and using a standard lens equivalent refractive index of 1.422. This was done in a similar manner to the iterative calculations described above for the lens equivalent refractive index to obtain schematic eye refractions that matched the adjusted GS refractions for each stimulus demands in all subjects. When the iteratively re-calculated ALRC and PLRC values were plotted as function of GS measured AOR, they tended to show flattening of lens surface curvatures with increasing accommodation. Since this is obviously contrary to what actually happens with accommodation13–16, neither of these parameters alone could account for the inaccuracies in the schematic eyes. Similarly, the iteratively calculated ACD and LT values were impossibly small or showed no change with increasing accommodation. These impossible outcomes for individual parameters demonstrate that the lens surface curvatures, ACD or LT per-se could not be the sole independent cause of the discrepancies between the schematic eye and measure refractions and AOR. Further, a prior UBM study identified a discrepancy between A-scan and UBM measured LT and reported correction factors.3 However, these discrepancies are small and small changes to ACD and LT do not impact the schematic eye calculations markedly. With these corrections applied, although there was the expected systematic shift in calculated refractions, this had no influence on accommodation which is calculated by subtracting out the baseline refraction.
Lens equivalent refractive index is one parameter that provides a significant contribution to the lens power and to the overall power of the eye. It is unlikely that every eye has the same lens equivalent refractive index, therefore this warrants the calculations of individual values. The averaged iterated baseline lens equivalent refractive index values (young: 1.428, pre-presbyopes: 1.425) were close to the standard lens equivalent index of 1.422 used in Bennetts and Rabbetts schematic eye model.9 This suggests that the UBM measured lens biometry parameters are reasonably accurate. However, using the individual baseline lens equivalent refractive index improved the overall accuracy of the refraction and accommodation predictions in pre-presbyopic subjects but not in young subjects. Small changes in lens equivalent refractive index can result in large changes in ocular refraction and although the average lens equivalent refractive index values come out close to the standard value, there is still considerable variation in the individual iteratively calculated values at different stimulus demands. The average calculated lens refractive index for all stimulus demands from both the young and pre-presbyopic subjects was 1.427. This means that a single lens equivalent refractive index value can be justifiably used for baseline and for various accommodative demands to construct accommodative schematic eyes. Calculating this lens equivalent refractive index, requires that the refractive and biometric parameters be measured only for the baseline (0D stimulus) condition. Prior studies on human eyes have reported that the lens equivalent refractive index and the gradient refractive index do not change with accommodation17, 18 and this is in agreement with the findings of the current study. However, trends from individual subjects in the current study showed that the iteratively calculated lens equivalent refractive index decreased with accommodation in 18 out of 24 young subjects and showed no change with accommodation in most presbyopic subjects. Using a lens gradient refractive index for schematic eye calculations would not increase the accuracy of predictions because an appropriate lens equivalent refractive index achieves the same paraxial lens power.
Individual accommodative schematic eyes constructed using the average accommodative change in UBM measured parameters applies the average accommodative changes in UBM parameters from the population to each individual subjects’ measured baseline biometry parameters. If the data from the current study is representative of what would occur in every population, this means that, in the future, the refractions and AOR calculated using this approach do not actually require measuring the accommodative biometric changes in each individual subject to calculate their accommodative responses. This approach would not yield accurate predictions when an individual subjects’ per-diopter accommodative biometric changes are different from the average per-diopter biometric changes in the population. The ideal approach to use for trying to predict the accommodative optical response from an individual subject would be to measure the baseline ocular biometry parameters and the refraction of the eye and to then construct a schematic eye from those measured parameters and to then measure the accommodative changes in the ocular biometry parameters for a variety of increasing stimulus demands and to then apply those measured biometric changes to the schematic eye to then calculate the accommodative optical response for each stimulus demand. Although the study was conducted in a single center in the US, the subjects were from diverse ethnic backgrounds. However, it is important to recognize that the accommodative schematic eye model described in this manuscript may not be representative of specific ethnic populations and may not be representative of more diverse worldwide populations.
CONCLUSIONS
The results from this study demonstrate that individual schematic eyes offer marginally better AOR predictions than with individual linear regressions of UBM measured biometry parameters in young subjects. For pre-presbyopic subjects, AOR predictions from individual linear regression and individual schematic eyes were comparable. Prediction of AOR from schematic eyes considers all of the ocular accommodative biometric changes together as opposed to predictions from individual linear regressions which consider the changes in only one individual biometric parameter.
Acknowledgments
Thanks to Sonomed-Escalon for providing the UBM. This work was funded by Student Vision Research Support Grant (VR), NIH/NEI R01 EY017076 (AG) and NIH/NEI Core Grant P30 EY007551 (UHCO).
APPENDIX
In a four surface paraxial schematic eye, A1, A2, A3 and A4 represent the anterior and posterior surfaces of the cornea and the lens, respectively. All distances are measured from the anterior corneal vertex (A1).
Surface powers and equivalent power of the cornea were calculated using equations 1, 2 and 3.
| (1) |
| (2) |
| (3) |
where FAC and FPC are the surface powers (D) of the anterior and posterior corneal surfaces, respectively, FCornea is the equivalent power of cornea (D), rAC and rPC are the Visante OCT measured radii of curvature (mm) of the anterior and posterior corneal surfaces after distortion correction, respectively, ncornea is the refractive index of cornea (1.376), nair is the refractive index of air (1.0), naqueous is the refractive index of aqueous (1.336) and CT is the Visante OCT measured corneal thickness (mm) after distortion correction.
Distances of the first (A1P1) and second principal points of the cornea from the anterior corneal vertex were calculated using equations 4 and 5.
| (4) |
| (5) |
Surface powers and equivalent powers of the crystalline lens were calculated using equations 6, 7 and 8.
| (6) |
| (7) |
| (8) |
where FAL and FPL are the surface powers (D) of the anterior and posterior lens surfaces, respectively, FLens is the equivalent power of lens (D), rALRC and rPLRC are the UBM measured radii of curvature (mm) of the anterior and posterior lens surfaces, respectively, nlens is the equivalent refractive index of the lens (1.422), nvitreous is the refractive index of vitreous (1.336) and LT is the UBM measured lens thickness (mm).
Distances of the first (A1P2) and second principal points of the lens from the anterior corneal vertex were calculated using equations 9 and 10.
| (9) |
| (10) |
where ACD is the UBM measured anterior chamber depth (mm).
Equivalent power of the eye (FEye) was calculated using equation 11. Distances of the first and second principal points (A1P & A1P′), first and second focal points (A1F & A1F′), first and second nodal points (A1N & A1N′), entrance pupil (A1E) and exit pupil (A1E′) of the eye from the corneal vertex were calculated using equations 12 through 21.
| (11) |
| (12) |
| (13) |
| (14) |
| (15) |
where are the primary and secondary equivalent focal lengths (mm) of the eye.
| (16) |
| (17) |
| (18) |
| (19) |
| (20) |
| (21) |
Refractive state of the eye was calculated using equation 22 for each accommodative demand.
| (22) |
Accommodative optical response was calculated as the difference in refraction between each accommodative state and the baseline.
REFERENCES
- 1.Ramasubramanian V, Glasser A. Can ultrasound biomicroscopy be used to predict accommodation accurately? J Refract Surg. 2015;31:266–273. doi: 10.3928/1081597X-20150319-06. [DOI] [PubMed] [Google Scholar]
- 2.Ramasubramanian V, Glasser A. prediction of accommodative optical response in pre-presbyopes using ultrasound biomicroscopy (UBM) J Cataract Refract Surg. 2015;41:964–980. doi: 10.1016/j.jcrs.2014.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramasubramanian V, Glasser A. Objective measurement of accommodative biometric changes using ultrasound biomicroscopy. J Cataract Refract Surg. 2015;41:511–526. doi: 10.1016/j.jcrs.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolz M, Prinz A, Drexler W, Findl O. Linear relationship of refractive and biometric lenticular changes during accommodation in emmetropic and myopic eyes. Br J Ophthalmol. 2007;91:360–365. doi: 10.1136/bjo.2006.099879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrin L, Kasthurirangan S, Win-Hall D, Glasser A. Simultaneous measurements of refraction and A-scan biometry during accommodation in humans. Optom Vis Sci. 2006;83:657–665. doi: 10.1097/01.opx.0000232810.61191.02. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Zwick H, Stuck B, Lund DJ. On the use of schematic eye models to estimate retinal image quality. J Biomed Opt. 2000;5:307–314. doi: 10.1117/1.430001. [DOI] [PubMed] [Google Scholar]
- 7.Preussner PR, Wahl J, Weitzel D. Topography-based intraocular lens power selection. J Cataract Refract Surg. 2005;31:525–533. doi: 10.1016/j.jcrs.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 8.MacRae S, Schwiegerling J, Snyder RW. Customized and low spherical aberration corneal ablation design. J Refract Surg. 1999;15:S246–S248. doi: 10.3928/1081-597X-19990302-23. [DOI] [PubMed] [Google Scholar]
- 9.Bennett AG, Rabbetts RB. The Schematic Eye. In: Rabbetts RB, editor. Clinical Visual Optics. Philadephia: Elsevier/Butterworth Heinemann; 2007. pp. 221–243. [Google Scholar]
- 10.Navarro R, Santamaria J, Bescos J. Accommodation-dependent model of the human eye with aspherics. J Opt Soc Am (A) 1985;2:1273–1281. doi: 10.1364/josaa.2.001273. [DOI] [PubMed] [Google Scholar]
- 11.Ramasubramanian V, Glasser A. correction of corneal distortions from visante anterior segment optical coherence tomography images. Optom Vis Sci. 2015;92:1170–1181. doi: 10.1097/OPX.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 12.Jones CE, Atchison DA, Pope JM. Changes in lens dimensions and refractive index with age and accommodation. Optom Vis Sci. 2007;84:990–995. doi: 10.1097/OPX.0b013e318157c6b5. [DOI] [PubMed] [Google Scholar]
- 13.Dubbelman M, van der Heijde GL, Weeber HA. Change in shape of the aging human crystalline lens with accommodation. Vision Res. 2005;45:117–132. doi: 10.1016/j.visres.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Rosales P, Dubbelman M, Marcos S, van der Heijde R. Crystalline lens radii of curvature from Purkinje and Scheimpflug imaging. J Vis. 2006;6:1057–1067. doi: 10.1167/6.10.5. [DOI] [PubMed] [Google Scholar]
- 15.Kasthurirangan S, Markwell EL, Atchison DA, Pope JM. MRI study of the changes in crystalline lens shape with accommodation and aging in humans. J Vis. 2011;11:1–16. doi: 10.1167/11.3.19. [DOI] [PubMed] [Google Scholar]
- 16.Sheppard AL, Evans CJ, Singh KD, Wolffsohn JS, Dunne MC, Davies LN. Three-dimensional magnetic resonance imaging of the phakic crystalline lens during accommodation. Invest Ophthalmol Vis Sci. 2011;52:3689–3697. doi: 10.1167/iovs.10-6805. [DOI] [PubMed] [Google Scholar]
- 17.de Castro A, Birkenfeld J, Maceo B, Manns F, Arrieta E, Parel JM, Marcos S. Influence of shape and gradient refractive index in the accommodative changes of spherical aberration in nonhuman primate crystalline lenses. Invest Ophthalmol Vis Sci. 2013;54:6197–6207. doi: 10.1167/iovs.13-11996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasthurirangan S, Markwell EL, Atchison DA, Pope JM. In vivo study of changes in refractive index distribution in the human crystalline lens with age and accommodation. Invest Ophthalmol Vis Sci. 2008;49:2531–2540. doi: 10.1167/iovs.07-1443. [DOI] [PubMed] [Google Scholar]