Abstract
Background
Endometriosis is a common cause of pain including radicular pain. Ectopic endometrial tissue may directly affect peripheral nerves including the sciatic, which has not been modelled in animals.
Methods
We developed a rat model for sciatic endometriosis by grafting a piece of autologous uterine tissue around the sciatic nerve. Control animals underwent a similar surgery but received a graft of pelvic fat tissue.
Results
The uterine grafts survived and developed fluid filled cysts; the adjacent nerve showed signs of swelling and damage. Mechanical and cold hypersensitivity and allodynia of the ipsilateral hindpaw developed gradually over the first two weeks after the surgery, peaked at 2 to 5 weeks, and was almost resolved by 7 weeks. Control animals showed only minor changes in these pain behaviors. Histological signs of inflammation in the uterine graft and in the adjacent nerve were observed at 3 weeks but were resolving by 7 weeks. In vivo fiber recording showed increased spontaneous activity, especially of C fibers, in sciatic nerve proximal to the uterine graft. Several pro-inflammatory cytokines including interluekin-18, VEGF, fractalkine, and MIP-1α, were elevated in the uterine graft plus sciatic nerve samples, compared to samples from normal nerve or nerve plus fat graft. Growth associated protein 43 (GAP43), a marker of regenerating nerve fibers, was observed in the adjacent sciatic nerve as well as in the uterine graft.
Conclusions
This model shared many features with other rat models of endometriosis, but also had some unique features more closely related to neuropathic pain models.
Introduction
In endometriosis, endometrial stromal and glandular cells appear outside the uterus, most often in the pelvic cavity, but also at other sites (Yuen et al., 2001).. It affects 6–10% of women of reproductive age (Burney and Giudice, 2012). Endometriosis is a significant cause of pain. Pain is related to menstrual periods (dysmenorrhea), intercourse (dyspareunia), bowel movements or urination (dyschezia or dysuria), and leg pain, and can be relieved by surgically removing the ectopic lesions (Jones and Sutton, 2003). Endometriosis is an important cause of pelvic pain, including viscera, somatic and referred pain, somatic and visceral hypersensitivity, and generalized hyperalgesia within and remote from the ectopic endometrium (Giamberardino et al., 2014; Howard, 2009).
Preclinical models used to investigate probable mechanisms and treatments of endometriosis involve implanting endometrium in ectopic sites (Alvarez et al.,; Golan et al., 1984; Sharpe et al., 1991; Vernon and Wilson, 1985). Numerous studies have investigated peripheral nerve changes during the establishment and maintenance of endometriosis. Increased nerve fiber density was confirmed in endometriosis lesions in humans (Tokushige et al., 2006a; Tokushige et al., 2006b). Inflammation also plays a role in clinical and experimental endometriosis. Increases in macrophages and pro-inflammatory cytokines were found in peritoneal fluid of endometriosis patients (Harada et al., 2001; Khorram et al., 1993; McLaren et al., 1996; Montagna et al., 2008). Local inflammation and abnormal nerve fiber growth also play important roles in preclinical models of endometriosis (see Discussion). Pain behaviors are observed in preclinical models of endometriosis, including vaginal hypersensitivity (Berkley et al., 2001), enhanced visceral and muscle pain in response to ureter stones remote from the endometrium explant sites (Giamberardino et al., 2002; Lopopolo et al., 2014) and mechanical hypersensitivity of endometrium implants in extrapelvic muscle sites (Alvarez et al., 2012).
Leg pain including sciatica is significantly more common in endometriosis patients, with an incidence of 50% (Missmer and Bove, 2011; Walch et al., 2014). Conversely, in women referred for sacral radiculopathy of nonspinal origin, endometriosis was the most common cause (Possover et al., 2011). Clinical case reports show that sciatica can be caused by endometriosis of the sciatic nerve, often fluctuating with the menstrual cycle (Dhote et al., 1996; Head et al., 1962; Papapietro et al., 2002; Torkelson et al., 1988; Vaisberg, 1964). In patients with sciatic nerve endometriosis the endometriosis lesions were found near the sciatic notch, around the sciatic nerve (Cottier et al., 1995; Floyd et al., 2011; Pham et al., 2010; Vercellini et al., 2003) or even under the nerve sheath (Baker et al., 1966). In this study we examined possible mechanisms for pain induced by a rat model of sciatic endometriosis. More generally, this new model may be useful for understanding conditions in which nerves are directly affected by ectopic endometrium. Endometriotic implants are proposed to cause pain via 3 mechanisms: production of pro-analgesic cytokines and growth factors; direct irritation or invasion of pelvic nerves; and effects of active bleeding (Howard, 2009; Medicine, 2014; Morotti et al., 2014). The model described here examines the first two of these mechanisms.
Methods
Animals
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Cincinnati. Experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. Female Sprague Dawley rats (Harlan, Indianapolis, USA) weighing 180–220g at the start of the experiment were used for all experiments.
Surgical induction of sciatic nerve endometriosis
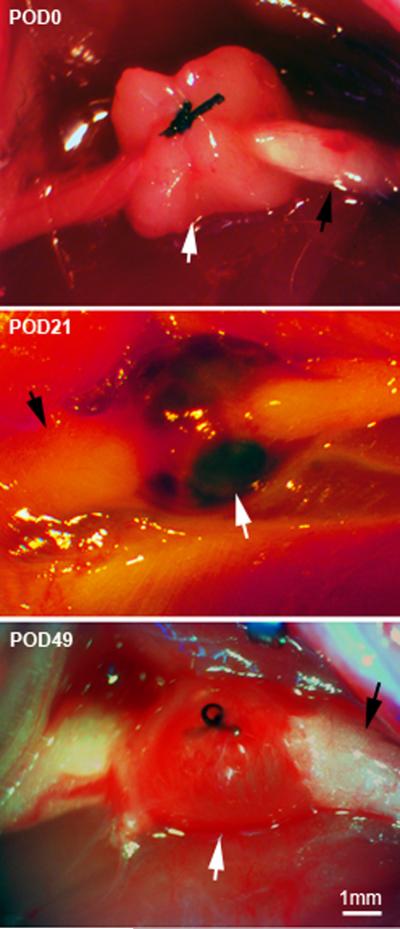
Female rats in the experimental group all received autologous uterine tissue implantation to the sciatic nerve, regardless of which phase of the estrous cycle rats were in at the time of surgery (unless indicated). For the control group, rats underwent a similar transplantation procedure using fat tissue from around the uterus. Under isoflurane anesthesia, a media ñ1 cm incision of the lower abdomen exposed the double-horned uterus. A piece of uterus about 5 mm long was cut and removed, and then put into 50 ml phosphate-buffered saline (PBS) containing penicillin plus streptomycin (100 I.U./ml and 100 μg/ml, respectively). Rats were then positioned on the left side and the right sciatic nerve exposed with a 0.5cm-long incision along the thigh bone. The sciatic nerve was carefully freed from adjacent connective tissues, and the piece of uterus tissue was wrapped around the nerve with the endometrium adjacent to the nerve and loosely sutured in place using 6–0 silk, without compressing the sciatic nerve (Fig. 1, top).
Figure 1.
Dissecting microscope images of sciatic endometriosis model. Top: view of the grafting method, taken during the implementation of the model on postoperative day (POD) 0. The sciatic nerve was exposed (black arrow), and a piece of uterine tissue (white arrow) wrapped around the nerve and fastened with a single suture. Middle: re-exposing the surgery site 21 days later (POD21), when mechanical pain is at a peak, shows that the graft has developed fluid filled cysts (white arrow). Black arrow, sciatic nerve. Bottom: Re-exposing the surgery site on POD49, when mechanical pain is beginning to resolve, shows that the graft is still intact but the fluid is no longer dark red.
In some experiments, on postoperative day (POD) 21, a second surgery to remove the uterine or fat graft was performed. Under isoflurane anesthesia, the sciatic surgery site was re-exposed. The uterine cysts and connective tissues that had formed were carefully dissected free from adjacent tissues, and then the implant was removed taking care not to damage the sciatic nerve. A similar process was performed to remove the fat tissue from sciatic nerve.
Behavioral testing
Mechanical sensitivity was tested by applying a series of von Frey filaments to the heel region of the paws, using the up-and-down method (Chaplan et al., 1994) using a maximum cutoff value of 15 g. A wisp of cotton was stroked mediolaterally across the plantar surface of the hindpaw to score the presence or absence of a brisk withdrawal response to a normally innocuous mechanical stimulus (light touch-evoked tactile allodynia). Cold sensitivity was scored as withdrawal responses to a drop of acetone applied to the ventral surface of the hind paw.
Microscopy
H&E staining
Standard hematoxylin and eosin (H&E) staining was used to verify histological features of lesions and to test for inflammation of the sciatic surgery site. Grafts, proximal and distal sciatic nerve, adjacent muscle and the portions of the sciatic nerve which were wrapped by grafts were fixed in 4% paraformaldehyde (in 0.1 M phosphate buffer, pH 7.4) for 2 h prior to incubation in 30% sucrose PBS at 4°C. These fixed specimens were used to make cross sections of graft plus nerve. For viewing longitudinal sections along the nerve axis, the grafts were dissected away from the nerve after fixation. Sections (4–5 μm, paraffin embedded) were cut and processed for H&E staining by the Pathology Research Core at the Cincinnati Children's Hospital Medical Center, Cincinnati, OH, USA).
Immunohistochemistry
Uterine or fat grafts with the portion of sciatic nerve wrapped by graft were fixed in 4% paraformaldehyde (in 0.1 M phosphate buffer, pH 7.4) for 2 h prior to sectioning and incubation in primary and secondary antibodies. Frozen sections were cut at 30–40 μm, either cross sections (nerve plus graft) or longitudinal sections (nerve only). Tissue sections were first blocked with 10% normal goat serum in PBS for 60 min. To verify that implanted tissues were from endometrium, sections were incubated in antibody to cytokeratin 18 (1:1000, catalog number ab668, Abcam, Cambridge, MA, USA), a marker of endometrial epithelial cells. To detect regenerating nerve fibers, sections were incubated in antibody to GAP43 (1:500, catalog number ab16053, Abcam). Sections were incubated in primary antibodies for 24 h at 4°C, then were incubated either in goat anti-mouse or goat anti-rabbit secondary antibodies for 60 min at room temperature. Triton-X (0.3%) was used in all reaction solutions to enhance antibody penetration.
Multiplex Cytokine Measurement
Cytokine expression profiles were evaluated using a multiplex antibody-based method. A total of 21 rat cytokines was measured simultaneously from a single well according to the manufacturer's protocols and as in our previous study (Xie et al., 2006). Full names, systemic names, and alternative names of the cytokines examined are given in Table 1. Additional details are given in Supplemental Methods.
Table 1.
Complete names and alternative names of cytokines
| Name in text | Full Name | Alternative and Systemic Names |
|---|---|---|
| EGF | Epidermal Growth Factor | |
| Eotaxin | Eotaxin | Ccl11 |
| Fractalkine | Fractalkine | Cx3cl1 |
| GRO/KC | Growth related oncogene/keratine-derived chemokine | Cxcl1; CINC-1; plays a role similar to human IL-8 |
| IFNγ | Interferon γ | |
| IL-10 | lnterleukin-10 | Cytokine synthesis inhibitory factor |
| IL-12p70 | lnterleukin-12 | p70 refers to the active heterodimer |
| IL-18 | Interleukin 18 | interferon-gamma inducing factor |
| IL-1α | Interleukin 1α | |
| IL-1β | Interleukin 1β | |
| IL-2 | lnterleukin-2 | |
| IL-4 | lnterleukin-4 | |
| IL-6 | lnterleukin-6 | |
| IP-10 | Interferon γ induced Protein 10 | Cxcl10 |
| Leptin | Leptin | Ob |
| MCP-1 | Monocyte chemotactic protein 1 | Ccl2 |
| MIP-1α | Macrophage Inflammatory Protein 1α | Ccl3 |
| MIP-2 | macrophage inflammatory protein 2 | Cxcl2 |
| RANTES | regulated on activation, normal T cell expressed and secreted | CCL5 |
| TNFα | Tumor necrosis factor α | |
| VEGF | Vascular endothelial growth factor |
Data analysis
Behavioral time course data from experimental (uterine graft) and control (fat graft) groups were compared using 2-way repeated measures ANOVA, with Bonferroni post-test to determine on which day the groups were significantly different. Within each individual group, 1-way ANOVA with Dunnett's post-test was used to determine on which day behavior was significantly different from baseline. For cytokine data, concentrations of cytokine normalized by protein content were compared using a ratio t-test (i.e., a t-test on the log-transformed values). For the log transform, concentrations detected by the software as being out of range (low) were set equal to the lowest detectable concentration as specified by the detection kit manufacturer instead of being set to zero.
Significance was ascribed for P<0.05. The number of animals or samples per experimental group is given in the figure legends. Data are presented as mean ± SEM.
Results
Uterine grafts survived and formed cysts around the sciatic nerve but did not invade it
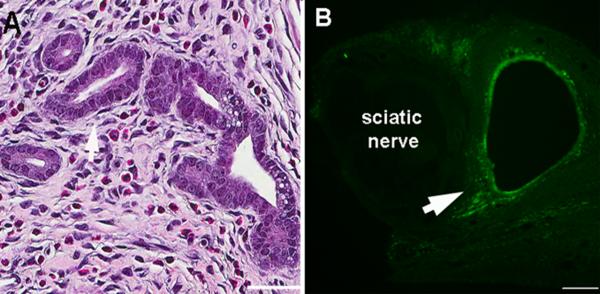
All rats survived the surgery with no complications. A lesion with a single fluid-filled cyst ~ 4– 5 mm in diameter could be observed upon surgically re-exposing the implant site on postoperative day (POD) 21, in most rats examined in the uterine graft group (Fig. 1), while fat grafts appeared unchanged from the initial day of surgery. Newly established blood vessels were evident at the uterine graft sites. Uterine grafts and cysts could still be observed on POD 49 although the fluid was no longer dark (Fig. 1). H&E staining of cross sections of the sciatic nerve and adjacent uterine graft on POD 21 showed that the graft contained characteristic gland formations with columnar cells, such as are seen in normal uterus (Fig. 2A). Staining for cytokeratin-18, a marker for glandular epithelial cells, showed that this marker was present in the graft but that the epithelial cells did not enter the sciatic nerve (Fig. 2B). Low power examination of complete graft cross sections processed in the same way as that shown in Fig. 2B obtained on POD 21 to 28 showed that 18 out of 18 rats had only a single cyst (approximately 30 sections processed per rat), though occasionally multiple cysts were observed during gross dissection.
Figure 2.
Grafts had histological features of uterine tissue. Examples of cross sections of the sciatic nerve and adjacent uterine graft on POD21. A: H&E staining showed characteristic gland formations with columnar cells such as are seen in normal uterus. Scale bar = 50μm. B: Staining for cytokeratin-18, a marker for glandular epithelial cells. Note that the marker is not seen inside the sciatic nerve. Arrows indicate labeled tissue. Scale bar = 100μm.
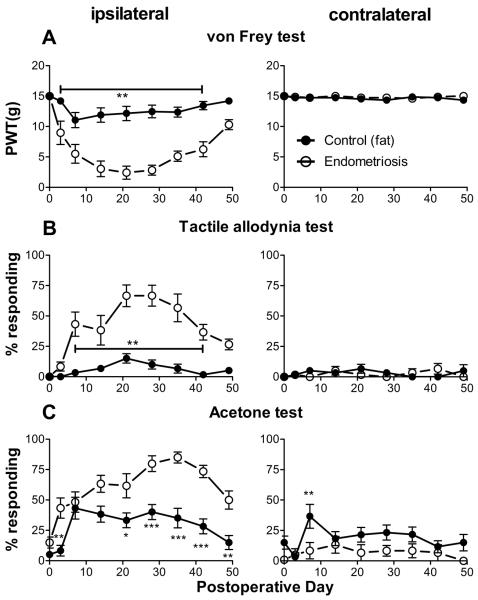
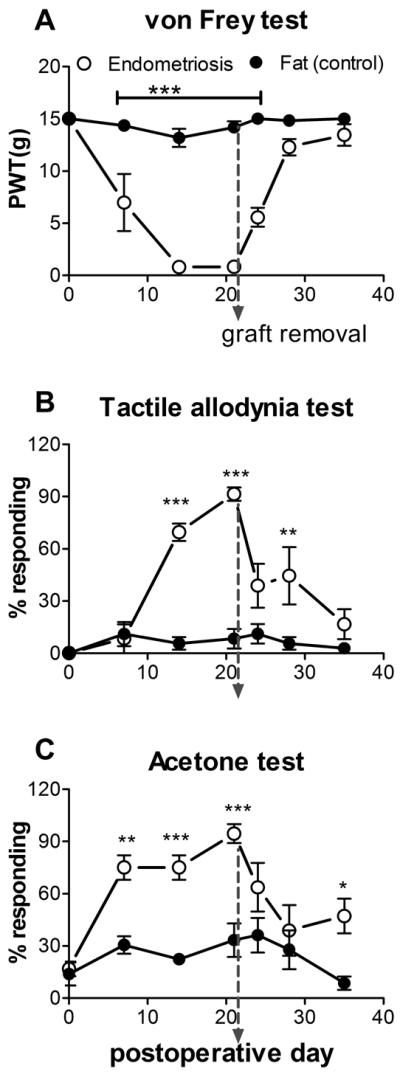
Sciatic nerve endometriosis induced mechanical and cold hypersensitivity of the ipsilateral hindpaw
No obvious motor deficits were observed in either group. Rats from the uterine graft group developed significant ipsilateral mechanical hyperalgesia, and light touch and cold allodynia compared to the fat graft group (Fig. 3). The mechanical hypersensitivity and allodynia developed over the first week and were maintained over several weeks, then began to resolve. On POD 49 the differences between the 2 groups were no longer significant. In the uterine graft group, ipsilateral mechanical sensitivity was significantly different from baseline on all post-surgical days measured, and mechanical allodynia was significantly different from baseline on all post-surgical days measured except the last (POD 49; one way ANOVAs with Dunnett's posttest). The fat graft group showed a much smaller ipsilateral response in both the mechanical pain tests shown in Fig. 3, and in fact these values were not significantly different from baseline on any postoperative day except for POD7 (von Frey test) and POD 21 (mechanical allodynia test). Contralateral von Frey and mechanical allodynia responses never differed significantly from baseline or between the two groups.
Figure 3.
Behavioral effects of sciatic endometriosis model. Presurgical baseline values (average of two trials) are plotted on postoperative day 0. Data from the ipsilateral hindpaw are on the left, contralateral on the right. A: Mechanical sensitivity as determined by the von Frey method. PWT, paw withdrawal threshold B. Tactile allodynia, quantified as the percent of withdrawal responses (out of 6 trials) to a normally innocuous stroking of the paw with a fine cotton wisp. C. Cold allodynia, measured as percent of withdrawal responses (out of 6 trials) to a drop of acetone placed on the paw. *, p<0.05; **, p<0.01; ***, p<0.001; significant difference between the two groups at the indicated time points (2-way repeated measures ANOVA with Bonferroni post-test). N = 10 animals per group. Animals received uterine tissue graft (“Endometriosis”) or fat tissue from around the uterus (“Control (fat)”).
Both fat graft and uterine graft groups developed cold allodynia which was significantly different from baseline on most days tested. The ipsilateral cold allodynia response was higher in the uterine graft group, with differences between the groups being significant on POD3 and POD21 through 49. Contralateral acetone responding did not differ significantly from baseline in either group on any day (1 way ANOVA with Dunnett's post-test). The fat graft group had significantly higher contralateral acetone responses than the uterine graft group on POD7 only; however, this result was not replicated in other experiments (e.g. Figs. 4 and 5, below). This test has a higher variability and rate of baseline responding than the mechanical tests used.
Figure 4.
Behavioral effects of graft removal in the sciatic endometriosis model. Presurgical baseline values (average of two tests) are plotted on postoperative day 0. Fat or uterine grafts were established on postoperative day 0 and removed on postoperative day 21 after behavior testing was completed for that day (dotted vertical line). Data are from the ipsilateral hindpaw. A: Mechanical sensitivity as determined by the von Frey method. PWT, paw withdrawal threshold. B. Tactile allodynia. C. Cold allodynia. *, p<0.05; **, p<0.01; ***, p<0.001; significant difference between the two groups at the indicated time points (2-way repeated measures ANOVA, with Bonferroni post-test). N = 6 animals per group. Experimenter was blinded to the fat/endometrium graft status on POD 0.
Figure 5.
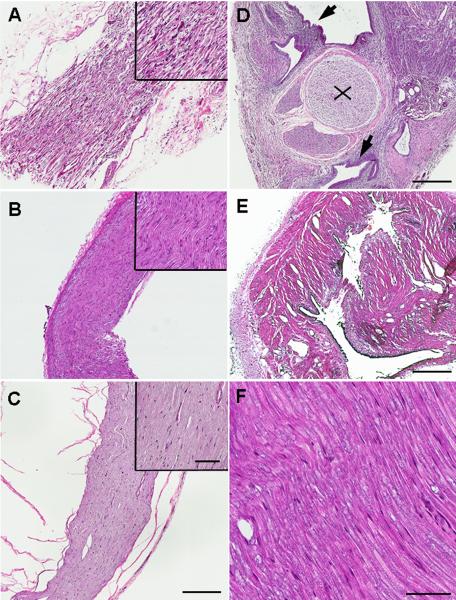
H&E staining of nerve and uterine graft showed signs of inflammation. A. Longitudinal section of nerve adjacent to uterine implant on POD21. Note inflammatory cell infiltration, swelling leading to spaces between nerve fibers, and destruction of nerve fibers. B. Longitudinal section of nerve adjacent to uterine graft on POD49. Fewer inflammatory cells and amelioration of swelling between nerve fibers were observed. C. Inflammatory signs disappeared when the implant was removed on POD21 (image taken 14 days after removal). Longitudinal section of nerve adjacent to uterine implant. D. Cross-section of sciatic nerve adjacent to uterine graft showing sciatic nerve (x) and endometrium (arrows) around the sciatic nerve on POD21. Note extensive inflammatory cell infiltration of the endometrium. E. Cross section of uterine implant tissue on POD49. Fewer inflammatory cells were observed. F. Longitudinal section of nerve adjacent to fat tissue implant on POD21 does not show inflammation or nerve damage. Figure A–E under 4× objective, upper right areas and figure F under 20× objective. Scale bar A–E = 400μm, upper right areas and F = 70μm.
In a separate experiment, in order to determine whether pain responses varied with the rat estrus cycle, pain behaviors and the phase of the estrus cycle were tested daily for two weeks postoperatively. As shown in Fig. S1, there were no overt cycle-related fluctuations in the measured pain behaviors.
In order to mimic the clinical situation, in which symptoms of endometriosis are sometimes relieved by surgical removal of ectopic endometrium, a separate group of animals underwent a second surgery 21 days after the initial graft, to remove the fat or uterine graft. Continuing behavior testing showed that mechanical hyperalgesia, tactile and cold allodynia reversed more quickly after uterine graft removal surgery (Fig. 4). For the von Frey test, the two groups no longer differed significantly after POD24 (compared POD 42 in Fig. 3 without removal), and the uterine graft group did not differ from baseline after POD 24 (compared to Fig. 3, where values were different from baseline through POD49). In the fat graft group, von Frey responses differed from baseline only on POD14. In the mechanical and cold allodynia tests, sensitivity of the uterine graft group declined sharply within 3 days of graft removal though it remained significantly above baseline until POD 25 (cold) or POD28 (mechanical). In the fat graft group the cold and mechanical allodynia responses were never significantly different from baseline.
Uterine grafts showed signs of inflammation and nerve damage
H&E staining on POD 21 showed signs of inflammation both in uterine grafts and the adjacent sciatic nerve. Many inflammatory cells was observed in longitudinal sections of sciatic nerve adjacent to uterine grafts, and swelling and nerve damage were observed as indicated by abnormal gaps between the fibers(Fig. 5-A). These changes were not observed in nerve adjacent to fat grafts (Fig. 5-F). Cross sections on POD21 also showed a large number of inflammatory cells in the uterine graft as well as in the adjacent nerve (Fig. 5-D). Correlating with the behavioral experiments, reduced signs of inflammation were observed on POD49 (Fig. 5-B) or after removal of the uterine graft (Fig. 5-E).
Longitudinal sections of nerve adjacent to the uterine or fat grafts were also examined for GAP43, a marker of regenerating nerve. Consistent with the nerve damage observed in the H&E stained sections, GAP43 positive fibers were observed in the nerve from uterine graft samples on POD21, but little staining was seen adjacent to fat grafts at the same time point (Fig. S2) or in normal nerve (data not shown).
In other rat endometriosis models, GAP43 positive nerve fibers have been observed to invade the ectopic uterine tissue. Such fibers were also observed in the uterine grafts in this model on POD21, as shown in Fig. S3.
Cytokine profiles showed a pro-inflammatory pattern after uterine grafts
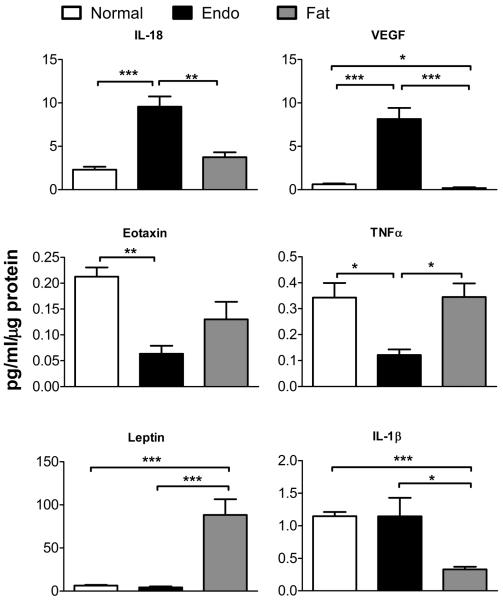
In order to provide a broad picture of the cytokine changes induced by the model, cytokines present in protein samples from normal sciatic nerve, sciatic nerve with uterine graft, and sciatic nerve with fat graft were examined with a multiplex antibody-based method. Samples were obtained on POD 30, when pain behaviors were well established. Twenty one cytokines were measured (Table 1) and data is presented from 14; others were present only in low concentrations or omitted for other reasons (see Supplemental Methods).
The 14 cytokines showed expression patterns that broadly speaking fell into 3 categories of response (although in some cases the differences did not reach significance). Some examples are shown in Fig. 6 and the entire set of data is presented in Table 2. The first group consisted of 8 cytokines that were upregulated in the uterine graft samples compared to normal nerve samples and fat graft samples. This group (IL-18, VEGF, fractalkine, MIP-1α, IP-10, RANTES, IL-1α, and MCP-1) consisted primarily of pro-inflammatory cytokines, including several chemotaxic proteins that attract monocytes and neutrophils; some pro-inflammatory cytokines that can be derived from macrophages; and some pleotropic cytokines that are considered master regulators of inflammation. The second group contained eotaxin and TNFα, which were down-regulated in the endometriosis group. The third group (IL-1β, Leptin, IL-6, GRO/KC) were similarly expressed in endometriosis samples and normal samples. Of these, IL-1β in the fat graft sample was significantly lower than in normal nerve and leptin was significantly higher.
Figure 6.
Examples of cytokine profiles measured in samples from normal sciatic nerve (“normal”), sciatic nerve plus uterine tissue graft (“endo”) and sciatic nerve plus fat graft (“fat”). Concentrations measured via multiplex method in pg/mL were divided by the μg of protein present in each 25 μL sample. Top: Examples of cytokines in group 1, that were elevated in endometriosis samples but partially or completely normalized in fat samples. IL, interleukin; VEGF, Vascular endothelial growth factor. Middle: The two cytokines from group 2, that were lowest in endometriosis samples. TNF, tumor necrosis factor. Bottom: Examples of cytokines in group 3, that were similar in normal and endometriosis samples with varying results in the fat samples. *, p<0.05; **, p<0.01; ***, p<0.001, significant difference between the indicated groups (ratio t-test). See also Table 2.
Table 2.
Cytokine measurements
| Cytokine | Normal nerve concentration a | Endometriosis fold change b | Fat fold change b | |
|---|---|---|---|---|
| Group 1 | IL-18 | 2.30 ± 0.34 | 4.16 ± 0.51***## | 1.41 ± 0.29 |
| VEGF | 0.63 ± 0.09 | 12.97 ± 2.01***### | 0.65 ± 0.36* | |
| Fractalkine | 0.12 ± 0.02 | 7.14 ± 1.80***## | 1.34 ± 0.16 | |
| MIP-1α | 0.07 ± 0.01 | 6.94 ± 1.65**# | 1.91 ± 0.52 | |
| IP-10 | 0.44 ± 0.06 | 2.45 ± 0.50*# | 0.88 ± 0.24 | |
| RANTES | 0.04 ± 0.00 | 6.60 ± 2.32** | 3.64 ± 1.02* | |
| IL-1α | 0.58 ± 0.14 | 3.00 ± 1.55 | 0.57 ± 0.27 | |
| MCP-1 | 1.3 ± 0.28 | 2.5 ± 1.12 | 0.9 ± 0.24 | |
| Group 2 | Eotaxin | 0.21 ± 0.02 | 0.29 ± 0.07** | 0.50 ± 0.16 |
| TNFα | 0.3 ± 0.06 | 0.4 ± 0.06*# | 0.8 ± 0.22 | |
| Group 3 | IL-1β | 1.1 ± 0.06 | 1.0 ± 0.25 # | 0.3 ± 0.03*** |
| Leptin | 6.4 ± 0.79 | 0.7 ± 0.17 ### | 11.1 ± 3.53*** | |
| IL-6 | 1.0 ± 0.53 | 0.5 ± 0.18 | 0.4 ± 0.15 | |
| GRO/KC | 0.36 ± 0.08 | 0.98 ± 0.58 | 1.80 ± 0.63 |
Average of concentrations in the sample (pg/mL) divided by μg protein
fold change was determined by normalizing values to the averge value in normal nerve.
p<0.05,
p<0.01,
p<0.001 significantly different from normal (log t test),
p<0.05,
p<0.01,
p<0.001 significantly different from fat (log t test)
N = 4 samples for normal nerve group, 6 samples for nerve + uterine graft group, and 4 samples for nerve plus fat graft group.
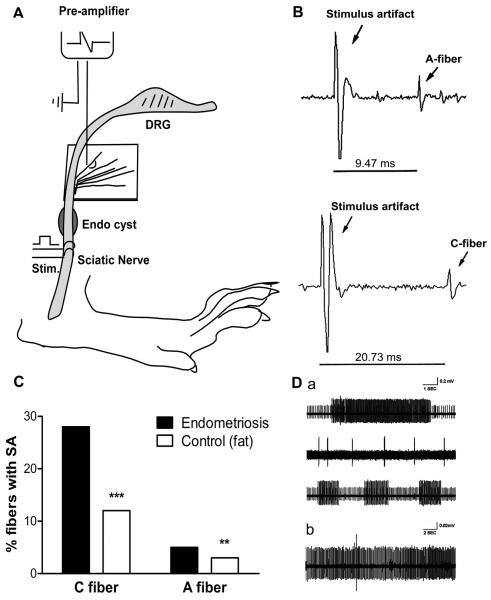
In vivo fiber recording shows spontaneous activity in sciatic nerve with uterine grafts
In vivo fiber recording examined abnormal spontaneous activity in sciatic nerve proximal to the graft site on POD 21 – 28. Significantly higher levels of spontaneous activity were observed in A- and especially C-fibers in rats with uterine grafts compared to those with fat grafts (Fig. 7). A-fiber spontaneous activity increased from 3 to 5%, and C-fiber from 12 to 28%. The percentages of fibers recorded that were C fibers did not differ significantly between the 2 groups (26% from uterine graft group, 25% from fat graft group; p = 0.6, Chi-Square test). The incidence of C-fiber spontaneous activity even in the fat graft group was higher than we have previously observed in normal rats (Xie et al., 2006); this may be due to the specific properties of this “control” tissue which also elevated local leptin and induced some pain behaviors, albeit much less so than uterine grafts (see Discussion).
Figure 7.
In vivo spontaneous activity increased in sciatic nerve with uterine grafts. A. Schematic of the recording setup: sciatic nerve fiber bundles were teased out from the sciatic nerve proximal to the graft site and placed on a stimulating electrode to record spontaneous activity; then the nerve was stimulated distal to the graft site to record conduction velocity and total number of fibers. B. Examples of conduction velocity from an A fiber and a C fiber (note different time bases). C. Summary data showing spontaneous activity was significantly higher in both A fiber and C fiber subsets on postoperative day 30. Significant difference between fat and uterine graft groups, **, p<0.01, Chi square test; ***, p<0.001, Fisher's exact test. D. Examples of spontaneous activity patterns observed in A fibers (a) and C fibers (b). Data obtained from recordings in 6 uterine graft animals (469 C fibers and 1351 A fibers total) and 4 fat graft animals (438 C fibers and 1342 A fibers total).
Discussion
We found that uterine tissue grafts around the sciatic nerve survived, developed fluid-filled cysts, and induced mechanical and cold pain behaviors in the ipsilateral hindpaw lasting up to 6 weeks. Similarities to other rat endometriosis models include: inflammation, GAP43 positive nerve fibers, and increased pro-inflammatory cytokines in the graft (Berkley et al., 2005; Grummer, 2006; McKinnon et al., 2015; Story and Kennedy, 2004; Umezawa et al., 2008).
Many endometriosis pain symptoms involve mechanical hypersensitivity, which is also observed in a model in which uterine tissue is implanted over the gastrocnemius muscle, allowing direct stimulation of the implant (Alvarez et al.), and in models with pelvic cavity implants, which cause secondary hyperalgesia to vaginal distension (Berkley et al., 2001; Grummer, 2006). We observed some compression, damage, and inflammation in the sciatic nerve adjacent to the uterine graft. The compression was likely due to growth of the graft, since none was initially applied. The time course of mechanical hypersensitivity was most closely related to the time course of nerve damage, inflammation and regeneration: all had abated after 6 weeks, even though the uterine graft was still intact. Indeed, cysts survived for over a year in a sciatic endometriosis model using a similar approach(Bove and Weissner, 2005). This may be a primary difference between our model of sciatic endometriosis and other rat endometriosis models: the primary cause of pain as measured by hindpaw testing may be essentially neuropathic. This could account for the faster resolution of the pain compared to the gastrocnemius muscle model, in which mechanical pain is undiminished at 16 weeks (Alvarez et al., 2015). In that model, the implant itself is mechanically stimulated whereas in our study the heel region innervated by the affected nerve was tested. Using heel stimulation, we would not have observed pain induced by stimulation of newly grown sensory fibers in the implant.
We also observed cold allodynia in our model, consistent with the idea that the model shares features with neuropathic pain models such as chronic sciatic nerve constriction. Other rodent endometriosis models are not suitable for measuring cold allodynia.
Pain behaviors developed gradually over a 10 day period, somewhat slower than we observe with sciatic nerve constriction (Xie et al., 2005). This may reflect the time required for the graft to vascularize and grow before affecting the adjacent nerve. However, pain develops even more slowly in models of pelvic endometriosis (Cason et al., 2003; McAllister et al., 2012); this may reflect time to develop secondary hyperalgesia or central sensitization (Alvarez et al.).
In endometriosis patients, surgical removal of ectopic foci often relieves pain symptoms. We observed that removing the uterine graft during the peak of the pain behavior response escalated recovery of pain behaviors. Normalized pain behaviors were also observed following implant removal in the gastrocnemius muscle model, although only a transient effect was observed when removal was performed at earlier time points (at 2 or 8 weeks in this much longer lasting model). One suggested explanation for the transience was the difficulty in removing all of the endometrial and stromal cells, which invade the muscle in this model (Alvarez et al., 2015). In contrast, in our model there was no infiltration of the adjacent nerve by endometrial cells, perhaps facilitating a more complete removal.
We did not observe marked effects of estrus cycle phase on observed pain behaviors. In human patients pain often fluctuates with the menstrual cycle. Lack of cycle effects in our model could reflect differences between the human menstrual cycle and the shorter, rat estrus cycle; or perhaps our model is anatomically less constricted, reducing effects of cyclical graft size changes. Hence our model may not be useful for understanding effects of menstrual cycle on pain. Cycle effects are also absent in the gastrocnemius muscle model; perhaps because high local estrogen at the test site masks systemic cyclical changes (Alvarez et al.). In contrast, in a pelvic model of endometriosis, vaginal hypersensitivity was absent during estrus and most marked during proestrus (McAllister et al., 2009). In this model the test site (vagina) is remote from the implant sites (mesenteric arteries); cycle effects may be due to estrogen effects on sensitized sensory or CNS neurons.
Increased levels of pro-inflammatory cytokines have been observed in endometriosis patients, rat models, and cultured cells from ectopic human endometrium. We observed elevated levels of several pro-inflammatory cytokines from the endometriosis model nerve + graft samples compared to normal nerve or fat graft samples. MCP-1 and RANTES, which were elevated in our study, have been observed in human endometriosis cells and peritoneal fluid (McKinnon et al., 2015), and were elevated in a rat peritoneal endometriosis model (Umezawa et al., 2008). These two papers also reported increased eotaxin, while we observed a decrease, possibly because we studied a later time point than Umezawa et al. Similarly, we found that the pleotropic pro-inflammatory cytokines IL-6 and IL-1β were not significantly altered and TNF-α decreased. These are upregulated in some studies and can induce many of the cytokines that we did observe to be upregulated; possibly they were elevated at earlier time points than the one we studied.
The original rat pelvic endometriosis model and many subsequent models used fat tissue as a sham control for the uterine transplant procedure (Alvarez et al.,; Berkley et al., 2001; Vernon and Wilson, 1985). However, recent studies show adipose tissue is not an inert control; it may produce inflammatory mediators. Leptin was originally identified as a signal of adiposity from fat tissue to brain, but has since been found to be pro-inflammatory (La Cava et al., 2004). It has been implicated in endometriosis in both human and animal studies (Alvarez et al., 2014; Oh et al., 2013). In our study, we observed upregulation of leptin in nerves with fat grafts but not uterine grafts, presumably consistent with survival of the fat tissue. These results appear to be at odds with the finding that leptin was upregulated in the gastrocnemius muscle endometriosis model (Alvarez et al., 2014). However, in that study, the comparison was to normal uterus, not to fat implants on the muscle. In the same study, injection of leptin into the muscle also caused mechanical pain. However, in the same model as originally described, placing a sham fat tissue implant onto the muscle did not cause pain (Alvarez et al.), similar to our behavioral results. Possibly these differences reflect availability of leptin to nearby nerves; if the fat cells did not release much of their leptin content this might account for the lack of pain behaviors induced by the “control” fat grafts in our study.
In summary, in the model we have described, uterine tissues placed near the sciatic nerve induce pain behaviors in the rat without infiltration of the nerve, consistent with clinical reports of sciatic endometriosis which only rarely involve growth of tissue within the nerve sheath. The model had many similarities to other models of endometriosis, such as histological and biochemical signs of inflammation and of newly generated nerve fibers. However, the uterine graft also compressed and damaged the adjacent nerve, so that the model has some unique features that may be more closely related to neuropathic pain models invoked by peripheral nerve damage. The model may shed light on the relatively rare condition of isolated sciatic endometriosis which has been described primarily in case reports(Baker et al., 1966; Cottier et al., 1995; Floyd et al., 2011; Papapietro et al., 2002; Pham et al., 2010; Vercellini et al., 2003). In addition, the model may be relevant for more common cases of endometriosis in the abdomino-pelvic cavity directly affecting nerves (including the sciatic, pudendal, obturator and femoral) or their spinal roots, often in conjunction with other sites (Ceccaroni et al., 2010a; Ceccaroni et al., 2011; Ceccaroni et al., 2010b; Lemos et al., 2012; Possover, 2009; Possover et al., 2007; Possover and Chiantera, 2007; Possover et al., 2011; Waer et al., 2012; Zager et al., 1998). In particular, deep infiltrating endometriosis, observed in 1/3 of laparoscopies performed to investigate severe pelvic pain, has been shown to be associated with intraneurial and perineurial invasion and nerve encapsulation; greater nerve involvement correlated with higher pain levels (Anaf et al., 2000; Ceccaroni et al., 2012; Fraser, 2010; Morotti et al., 2014; Wang et al., 2009). In patients with deep infiltrating endometriosis of the sigmoid and rectum, over 50% of lesions were in direct contact with nerves; it was proposed that ectopic endometrium infiltrated the bowel wall preferentially along nerves (Anaf et al., 2004). In women referred for sacral radiculopathy of unknown origin, endometriosis affecting pelvic nerves was found to be the cause in 82%(Possover et al., 2011). Thus understanding the interactions between ectopic endometrium and adjacent nerves may shed light on some of the most painful and intractable manifestations of endometriosis. These conditions involving nerve-associated endometriosis often involve mechanically evoked pain or pain that, while initially catamenial, often becomes noncyclical and constant as the disease progresses; our model may be more useful in understanding these conditions.
Supplementary Material
Bulleted statements.
What's already known about this subject
Some especially painful forms of endometriosis are essentially neuropathic, because peripheral nerves are directly affected by nearby ectopic endometrial tissue.
What does this study add?
We modelled endometriosis by implanting autologous uterine tissue around rat sciatic nerve.
We observed mechanical and cold pain behaviors along with signs of inflammation and nerve damage and increased pro-inflammatory cytokines at the implant site. Pain behaviors correlated with signs of nerve inflammation and damage rather than with cyst survival.
Acknowledgments
Funding sources: Funding was provided by the Department of Anesthesiology, University of Cincinnati, College of Medicine and by NIH grant NS55860 to J.-M.Z. This research was also partly funded by the National Natural Science Foundation of China (81372808 to J.J.) and 81173614 (to Qing Tao Lv).
Footnotes
None of the authors has any conflicts of interest to report.
Author contributions:
S. Chen: developed project idea, experimental design, conducted experiments, analyzed data. W. Xie, conducted experiments, analyzed data. J. A. Strong, experimental design, analyzed data, drafted the manuscript. J. Jiang, developed project idea, experimental design. J.-M. Zhang, developed project idea, experimental design. All authors discussed the results and commented on and edited the manuscript.
References
- Alvarez P, Bogen O, Chen X, Giudice LC, Levine JD. Ectopic endometrium-derived leptin produces estrogen-dependent chronic pain in a rat model of endometriosis. Neuroscience. 2014;258:111–120. doi: 10.1016/j.neuroscience.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Chen X, Hendrich J, Irwin JC, Green PG, Giudice LC, Levine JD. Ectopic uterine tissue as a chronic pain generator. Neuroscience. 2012;225:269–282. doi: 10.1016/j.neuroscience.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Giudice LC, Levine JD. Impact of surgical excision of lesions on pain in a rat model of endometriosis. Eur J Pain. 2015;19:103–110. doi: 10.1002/ejp.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaf V, El Nakadi I, Simon P, Van de Stadt J, Fayt I, Simonart T, Noel JC. Preferential infiltration of large bowel endometriosis along the nerves of the colon. Hum Reprod. 2004;19:996–1002. doi: 10.1093/humrep/deh150. [DOI] [PubMed] [Google Scholar]
- Anaf V, Simon P, El Nakadi I, Fayt I, Buxant F, Simonart T, Peny MO, Noel JC. Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum Reprod. 2000;15:1744–1750. doi: 10.1093/humrep/15.8.1744. [DOI] [PubMed] [Google Scholar]
- Baker GS, Parsons WR, Welch JS. Endometriosis within the sheath of the sciatic nerve. Report of two patients with progressive paralysis. J Neurosurg. 1966;25:652–655. doi: 10.3171/jns.1966.25.6.0652. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Cason A, Jacobs H, Bradshaw H, Wood E. Vaginal hyperalgesia in a rat model of endometriosis. Neurosci Lett. 2001;306:185–188. doi: 10.1016/s0304-3940(01)01906-1. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308:1587–1589. doi: 10.1126/science.1111445. [DOI] [PubMed] [Google Scholar]
- Bove GM, Weissner W. A rat model of neuritis associated with endometriosis. Program No 16918 Society for Neuroscience Abstracts: Neuroscience Meeting Planner; 2005. online. [Google Scholar]
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Samuelsen CL, Berkley KJ. Estrous changes in vaginal nociception in a rat model of endometriosis. Horm Behav. 2003;44:123–131. doi: 10.1016/s0018-506x(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Ceccaroni M, Clarizia R, Alboni C, Ruffo G, Bruni F, Roviglione G, Scioscia M, Peters I, De Placido G, Minelli L. Laparoscopic nerve-sparing transperitoneal approach for endometriosis infiltrating the pelvic wall and somatic nerves: anatomical considerations and surgical technique. Surg Radiol Anat. 2010a;32:601–604. doi: 10.1007/s00276-010-0624-6. [DOI] [PubMed] [Google Scholar]
- Ceccaroni M, Clarizia R, Bruni F, D'Urso E, Gagliardi ML, Roviglione G, Minelli L, Ruffo G. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc. 2012;26:2029–2045. doi: 10.1007/s00464-012-2153-3. [DOI] [PubMed] [Google Scholar]
- Ceccaroni M, Clarizia R, Cosma S, Pesci A, Pontrelli G, Minelli L. Cyclic sciatica in a patient with deep monolateral endometriosis infiltrating the right sciatic nerve. J Spinal Disord Tech. 2011;24:474–478. doi: 10.1097/BSD.0b013e31820fc53b. [DOI] [PubMed] [Google Scholar]
- Ceccaroni M, Clarizia R, Roviglione G, Bruni F, Ruffo G, Peters I, De Placido G, Minelli L. Deep rectal and parametrial infiltrating endometriosis with monolateral pudendal nerve involvement: case report and laparoscopic nerve-sparing approach. Eur J Obstet Gynecol Reprod Biol. 2010b;153:227–229. doi: 10.1016/j.ejogrb.2010.07.032. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cottier JP, Descamps P, Sonier CB, Rosset P. Sciatic endometriosis: MR evaluation. AJNR Am J Neuroradiol. 1995;16:1399–1401. [PMC free article] [PubMed] [Google Scholar]
- Dhote R, Tudoret L, Bachmeyer C, Legmann P, Christoforov B. Cyclic sciatica. A manifestation of compression of the sciatic nerve by endometriosis. A case report. Spine (Phila Pa 1976) 1996;21:2277–2279. doi: 10.1097/00007632-199610010-00021. [DOI] [PubMed] [Google Scholar]
- Floyd JR, 2nd, Keeler ER, Euscher ED, McCutcheon IE. Cyclic sciatica from extrapelvic endometriosis affecting the sciatic nerve. J Neurosurg Spine. 2011;14:281–289. doi: 10.3171/2010.10.SPINE09162. [DOI] [PubMed] [Google Scholar]
- Fraser IS. Mysteries of endometriosis pain: Chien-Tien Hsu Memorial Lecture 2009. J Obstet Gynaecol Res. 2010;36:1–10. doi: 10.1111/j.1447-0756.2010.01181.x. [DOI] [PubMed] [Google Scholar]
- Giamberardino MA, Berkley KJ, Affaitati G, Lerza R, Centurione L, Lapenna D, Vecchiet L. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95:247–257. doi: 10.1016/S0304-3959(01)00405-5. [DOI] [PubMed] [Google Scholar]
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26:253–259. doi: 10.1097/GCO.0000000000000083. [DOI] [PubMed] [Google Scholar]
- Golan A, Winston RM, Dargenio R. Experimental endometriosis: a microsurgical animal model in rats. Isr J Med Sci. 1984;20:1094–1096. [PubMed] [Google Scholar]
- Grummer R. Animal models in endometriosis research. Hum Reprod Update. 2006;12:641–649. doi: 10.1093/humupd/dml026. [DOI] [PubMed] [Google Scholar]
- Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril. 2001;76:1–10. doi: 10.1016/s0015-0282(01)01816-7. [DOI] [PubMed] [Google Scholar]
- Head HB, Welch JS, Mussey E, Espinosa RE. Cyclic sciatica. Report of case with introduction of a new surgical sign. Jama. 1962;180:521–524. doi: 10.1001/jama.1962.03050200005002. [DOI] [PubMed] [Google Scholar]
- Howard FM. Endometriosis and mechanisms of pelvic pain. J Minim Invasive Gynecol. 2009;16:540–550. doi: 10.1016/j.jmig.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Jones KD, Sutton C. Patient satisfaction and changes in pain scores after ablative laparoscopic surgery for stage III–IV endometriosis and endometriotic cysts. Fertil Steril. 2003;79:1086–1090. doi: 10.1016/s0015-0282(02)04957-9. [DOI] [PubMed] [Google Scholar]
- Khorram O, Taylor RN, Ryan IP, Schall TJ, Landers DV. Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am J Obstet Gynecol. 1993;169:1545–1549. doi: 10.1016/0002-9378(93)90433-j. [DOI] [PubMed] [Google Scholar]
- La Cava A, Alviggi C, Matarese G. Unraveling the multiple roles of leptin in inflammation and autoimmunity. J Mol Med (Berl) 2004;82:4–11. doi: 10.1007/s00109-003-0492-1. [DOI] [PubMed] [Google Scholar]
- Lemos N, Kamergorodsky G, Ploger C, Castro R, Schor E, Girao M. Sacral nerve infiltrative endometriosis presenting as perimenstrual right-sided sciatica and bladder atonia: case report and description of surgical technique. J Minim Invasive Gynecol. 2012;19:396–400. doi: 10.1016/j.jmig.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Lopopolo M, Affaitati G, Fabrizio A, Massimini F, Lapenna D, Giamberardino MA, Costantini R. Effects of tramadol on viscero-visceral hyperalgesia in a rat model of endometriosis plus ureteral calculosis. Fundam Clin Pharmacol. 2014;28:331–341. doi: 10.1111/fcp.12038. [DOI] [PubMed] [Google Scholar]
- McAllister SL, Dmitrieva N, Berkley KJ. Sprouted innervation into uterine transplants contributes to the development of hyperalgesia in a rat model of endometriosis. PLoS One. 2012;7:e31758. doi: 10.1371/journal.pone.0031758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147:255–264. doi: 10.1016/j.pain.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon BD, Bertschi D, Bersinger NA, Mueller MD. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol Metab. 2015;26:1–10. doi: 10.1016/j.tem.2014.10.003. [DOI] [PubMed] [Google Scholar]
- McLaren J, Prentice A, Charnock-Jones DS, Smith SK. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod. 1996;11:220–223. doi: 10.1093/oxfordjournals.humrep.a019023. [DOI] [PubMed] [Google Scholar]
- Medicine, P. C. o. t. A. S. f. R. Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101:927–935. doi: 10.1016/j.fertnstert.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Bove GM. A pilot study of the prevalence of leg pain among women with endometriosis. J Bodyw Mov Ther. 2011;15:304–308. doi: 10.1016/j.jbmt.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna P, Capellino S, Villaggio B, Remorgida V, Ragni N, Cutolo M, Ferrero S. Peritoneal fluid macrophages in endometriosis: correlation between the expression of estrogen receptors and inflammation. Fertil Steril. 2008;90:156–164. doi: 10.1016/j.fertnstert.2006.11.200. [DOI] [PubMed] [Google Scholar]
- Morotti M, Vincent K, Brawn J, Zondervan KT, Becker CM. Peripheral changes in endometriosis-associated pain. Hum Reprod Update. 2014;20:717–736. doi: 10.1093/humupd/dmu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh HK, Choi YS, Yang YI, Kim JH, Leung PC, Choi JH. Leptin receptor is induced in endometriosis and leptin stimulates the growth of endometriotic epithelial cells through the JAK2/STAT3 and ERK pathways. Mol Hum Reprod. 2013;19:160–168. doi: 10.1093/molehr/gas055. [DOI] [PubMed] [Google Scholar]
- Papapietro N, Gulino G, Zobel BB, Di Martino A, Denaro V. Cyclic sciatica related to an extrapelvic endometriosis of the sciatic nerve: new concepts in surgical therapy. J Spinal Disord Tech. 2002;15:436–439. doi: 10.1097/00024720-200210000-00016. [DOI] [PubMed] [Google Scholar]
- Pham M, Sommer C, Wessig C, Monoranu CM, Perez J, Stoll G, Bendszus M. Magnetic resonance neurography for the diagnosis of extrapelvic sciatic endometriosis. Fertil Steril. 2010;94:351, e311–354. doi: 10.1016/j.fertnstert.2009.12.046. [DOI] [PubMed] [Google Scholar]
- Possover M. Laparoscopic management of endopelvic etiologies of pudendal pain in 134 consecutive patients. J Urol. 2009;181:1732–1736. doi: 10.1016/j.juro.2008.11.096. [DOI] [PubMed] [Google Scholar]
- Possover M, Baekelandt J, Flaskamp C, Li D, Chiantera V. Laparoscopic neurolysis of the sacral plexus and the sciatic nerve for extensive endometriosis of the pelvic wall. Minim Invasive Neurosurg. 2007;50:33–36. doi: 10.1055/s-2007-970075. [DOI] [PubMed] [Google Scholar]
- Possover M, Chiantera V. Isolated infiltrative endometriosis of the sciatic nerve: a report of three patients. Fertil Steril. 2007;87:417, e417–419. doi: 10.1016/j.fertnstert.2006.05.084. [DOI] [PubMed] [Google Scholar]
- Possover M, Schneider T, Henle KP. Laparoscopic therapy for endometriosis and vascular entrapment of sacral plexus. Fertil Steril. 2011;95:756–758. doi: 10.1016/j.fertnstert.2010.08.048. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Bertero MC, Muse KN, Vernon MW. Spontaneous and steroid-induced recurrence of endometriosis after suppression by a gonadotropin-releasing hormone antagonist in the rat. Am J Obstet Gynecol. 1991;164:187–194. doi: 10.1016/0002-9378(91)90652-8. [DOI] [PubMed] [Google Scholar]
- Story L, Kennedy S. Animal studies in endometriosis: a review. Ilar J. 2004;45:132–138. doi: 10.1093/ilar.45.2.132. [DOI] [PubMed] [Google Scholar]
- Tokushige N, Markham R, Russell P, Fraser IS. High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Hum Reprod. 2006a;21:782–787. doi: 10.1093/humrep/dei368. [DOI] [PubMed] [Google Scholar]
- Tokushige N, Markham R, Russell P, Fraser IS. Nerve fibres in peritoneal endometriosis. Hum Reprod. 2006b;21:3001–3007. doi: 10.1093/humrep/del260. [DOI] [PubMed] [Google Scholar]
- Torkelson SJ, Lee RA, Hildahl DB. Endometriosis of the sciatic nerve: a report of two cases and a review of the literature. Obstet Gynecol. 1988;71:473–477. [PubMed] [Google Scholar]
- Umezawa M, Sakata C, Tanaka N, Kudo S, Tabata M, Takeda K, Ihara T, Sugamata M. Cytokine and chemokine expression in a rat endometriosis is similar to that in human endometriosis. Cytokine. 2008;43:105–109. doi: 10.1016/j.cyto.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Vaisberg M. Cyclic Sciatica Due to Endometriosis. N Y State J Med. 1964;64:1983–1987. [PubMed] [Google Scholar]
- Vercellini P, Chapron C, Fedele L, Frontino G, Zaina B, Crosignani PG. Evidence for asymmetric distribution of sciatic nerve endometriosis. Obstet Gynecol. 2003;102:383–387. doi: 10.1016/s0029-7844(03)00532-5. [DOI] [PubMed] [Google Scholar]
- Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44:684–694. [PubMed] [Google Scholar]
- Waer P, Samson I, Sinnaeve F, Sciot R, Pans S. Perineural spread of endometriosis along the obturator nerve into the adductor thigh compartment. Jpn J Radiol. 2012;30:446–449. doi: 10.1007/s11604-012-0060-0. [DOI] [PubMed] [Google Scholar]
- Walch K, Kernstock T, Poschalko-Hammerle G, Gleiss A, Staudigl C, Wenzl R. Prevalence and severity of cyclic leg pain in women with endometriosis and in controls - effect of laparoscopic surgery. Eur J Obstet Gynecol Reprod Biol. 2014;179:51–57. doi: 10.1016/j.ejogrb.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Wang G, Tokushige N, Markham R, Fraser IS. Rich innervation of deep infiltrating endometriosis. Hum Reprod. 2009;24:827–834. doi: 10.1093/humrep/den464. [DOI] [PubMed] [Google Scholar]
- Xie W, Strong JA, Meij JT, Zhang J-M, Yu L. Neuropathic pain: Early spontaneous afferent activity is the trigger. Pain. 2005;116:243–256. doi: 10.1016/j.pain.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang J-M. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142:809–822. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen JS, Chow PK, Koong HN, Ho JM, Girija R. Unusual sites (thorax and umbilical hernial sac) of endometriosis. J R Coll Surg Edinb. 2001;46:313–315. [PubMed] [Google Scholar]
- Zager EL, Pfeifer SM, Brown MJ, Torosian MH, Hackney DB. Catamenial mononeuropathy and radiculopathy: a treatable neuropathic disorder. J Neurosurg. 1998;88:827–830. doi: 10.3171/jns.1998.88.5.0827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.