Abstract
Background
Despite significant developments in transurethral surgery for benign prostatic hyperplasia, simple prostatectomy remains an excellent option for patients with severely enlarged glands. The objective is to describe our results of robot-assisted simple prostatectomy (RASP) with a modified urethrovesical anastomosis (UVA).
Methods
From May 2011 to February 2014, RASP with UVA was performed in 34 patients by a single surgeon (O.C.) using the da Vinci S-HD surgical system. The UVA was performed between the bladder neck and urethral margin using the Van Velthoven technique. Demographic, perioperative, and outcome data were recorded. Complications were recorded with the Clavien–Dindo system.
Results
The mean (standard deviation) age was 68 years (62–74 years). The median preoperative prostate volume (interquartile range) was 117 cc (99–146 cc). Operative time was 96 minutes (78–126 minutes), estimate blood loss was 200 mL (100–300 mL), and two (5.8%) patients required a blood transfusion. No conversion to open surgery was needed. The median specimen weight on pathological examination was 76 g (58–100 g). The average hospital stay was 2.2 days (1–4 days) and average Foley catheter time was 4.6 days (4–6 days). No intraoperative complications were recorded. There were seven (20.5%) postoperative complications, most of them Clavien less than or equal to Grade II.
Conclusion
The results of our study show that RASP with UVA is a feasible, secure, and reproducible procedure with low morbidity. Additional series with larger patient cohorts are needed to validate this approach.
Keywords: Benign prostatic hyperplasia, Enlarged prostate, Robotic simple prostatectomy
1. Introduction
With the development of new surgical techniques and energy sources, options for the endoscopic management of men with moderately enlarged prostates have widened over the past years. However, despite these advances, open simple prostatectomy (OSP) remains particularly well suited for patients with large glands (> 100 cc).1 Newer options for minimally-invasive treatment of large glands include laparoscopic simple prostatectomy and holmium laser enucleation. While both of them showed comparable outcomes to the open approach,2, 3, 4 they require a steep learning curve thus preventing wider acceptance among urologists. The robotic platform is an attractive alternative as it potentially overcomes these constraints by providing stereoscopic three-dimensional vision and exceptional dexterity to facilitate the more technically demanding steps of the simple prostatectomy procedure.5 Robot-assisted simple prostatectomy (RASP) is a novel procedure not yet widely performed, even in high volume robotic centers with no more than a couple of hundred cases reported worldwide. With this paucity of information, the experience and results of high volume centers is still valuable.
The objective of this report is to describe our results with RASP in a contemporary cohort of men with lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH).
2. Materials and methods
Between May 2011 and February 2014, 34 patients with BPH-related LUTS underwent RASP by a single surgeon (O.C.). Peri- and intraoperative data were prospectively collected and retrospectively analyzed. Indications for surgery included LUTS refractory to medical treatment, urinary retention, and BPH-related consequences to the upper tract. Besides regular preoperative testing, all patients were specifically evaluated with digital rectal examination, prostate-specific antigen, renal and pelvic ultrasound, International Prostate Symptom Score (IPSS), and maximum urine flow (Qmax). Prostate cancer was ruled out with transrectal ultrasound-guided biopsies in patients with elevated prostate-specific antigen and/or abnormal digital rectal examination. Complications were classified with the Dindo–Clavien classification.6
2.1. Surgical technique
All of the procedures were performed by a single surgeon (O.C.) using the Da Vinci S-HD Surgical System (Intuitive Surgical, Sunnyvale, CA, USA).
2.1.1. Patient position and port placement
After induction, the patient was placed in a lithotomy position and a steep Trendelenburg position, over an antisliding foam with padding for all pressure points. Pneumatic compressors were used on the lower extremities to prevent postoperative deep venous thrombosis. We placed four trocars as follows: 12-mm camera port supraumbilically, two 8-mm robotic ports bilaterally on a line between the camera port and the iliac crest at least 9 cm from the camera port, and a lateral 12-mm port cranial to the right iliac crest for the assistant. Three robotic instruments were used: hot shears monopolar curved scissors, fenestrated bipolar forceps, and a large needle driver. A 0-degree camera was used throughout the whole procedure.
2.1.2. Bladder dissection and opening
Firstly, the median and medial umbilical ligaments were taken down giving full access to the preperitoneal space and prostate. The periprostatic fat was then completely removed to gain full access to the prostatic capsule and vesicoprostatic junction. An anterior opening was made in the bladder before the junction and continued distally along the prostatic capsule. Both edges of the bladder were then sutured to the Cooper's ligament at each side to achieve optimal visualization of the adenoma (Fig. 1).
Fig. 1.
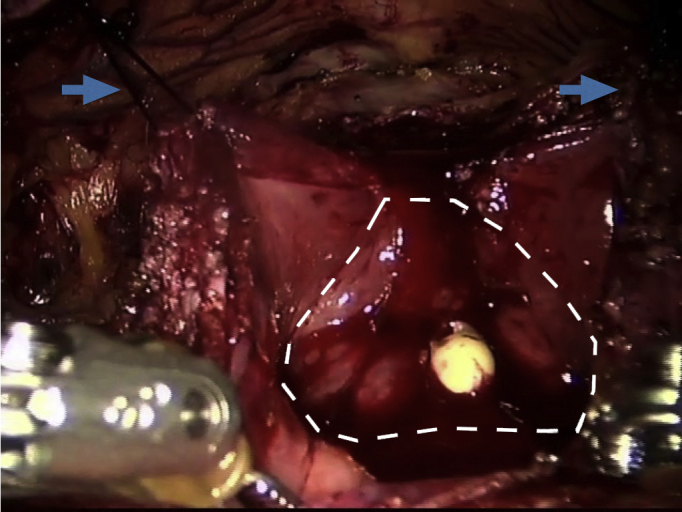
A longitudinal incision was made in the bladder anteriorly and through the vesicoprostatic junction. The contour of the adenoma was then visualized (broken line). Stay sutures were placed on the lateral edges of the bladder (arrows) and then sutured to the Cooper's ligament at each side to achieve optimal visualization of the adenoma.
2.1.3. Dissection of the adenoma
The ureteral orifices were identified first. The correct plane of dissection between the prostatic capsule and the adenoma was first identified circumferentially on both sides of the prostate. Dissection starts at the lower half of the contour with counter traction given by the assistant with the suction cannula (Fig. 2). Dissection then continued towards the anterior half. Finally, the catheter was identified at the apex and the urethra was sectioned under direct vision with care not to risk the sphincteric complex. The adenoma was collected in an endoscopic bag.
Fig. 2.
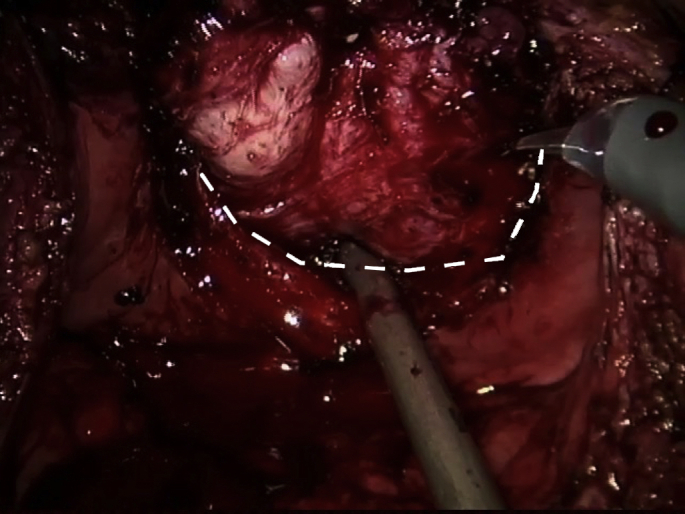
Excision of the adenoma started at the lower half of the contour by identifying the plane between the adenoma and prostatic capsule (broken line), while the assistant gave counter traction with the suction cannula. Dissection was then continued anteriorly towards the apex. Finally, the urethra was incised under direct vision.
2.1.4. Modified urethrovesical anastomosis
After careful revision of hemostasia, a double-needle barbed suture was used to create a posterior urethorvesical anastomosis using the Van Velthoven technique. Being careful not to include the ureteral orifices, the posterior bladder neck and urethra were sown between Hour 3 and Hour 9 to create a halfway urethrovesical anastomosis (Fig. 3).
Fig. 3.
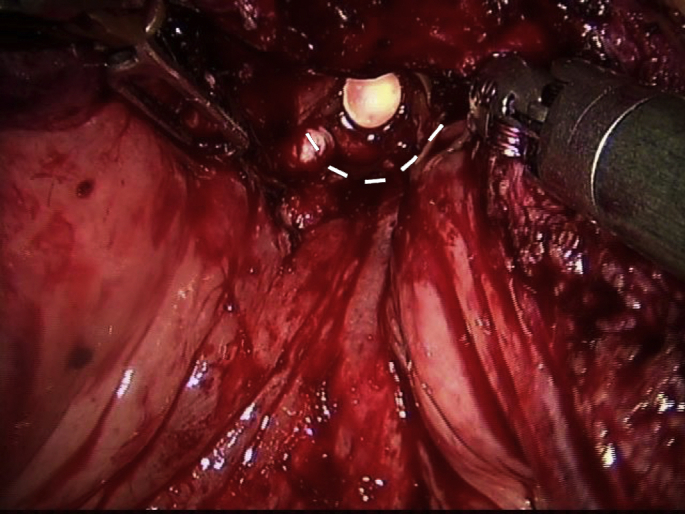
A double-needle barbed suture was used to create a posterior urethrovesical anastomosis using the Van Velthoven technique. Being careful not to include the ureteral orifices, the posterior bladder neck and urethra were sown between Hour 3 and Hour 9 to create a halfway urethrovesical anastomosis (broken line).
2.1.5. Bladder closure and postoperative care
A 22-Fr three-way Foley catheter was placed and the prostatic capsule and anterior bladder were sutured in a running fashion using a vicryl 2-0 suture. The bladder was then filled with 200 cc of saline to verify watertight closure. A percutaneous drain was left and bladder irrigation was started and left for 24 hours. Specimen was sent for pathological analysis.
2.2. Statistical analysis
Normally distributed quantitative data were summarized as means, and measures of variability were reported as standard deviations, whereas non-normally distributed data were summarized as median and variability reported as interquartile range (IQR). Qualitative data were reported as percentages. A Kaplan–Meier curve was designed to present changes in IPSS and Qmax after surgery.
3. Results
Patient characteristics are presented in Table 1. All patients failed previous medical therapy with α-blockers and/or 5-α-reductase inhibitors. Median (IQR) preoperative prostate volume by transrectal ultrasound was 117 (99–146) cc, while 12 patients (35%) had an indwelling urethral catheter. Table 2 summarizes the intraoperative results. Notably, all procedures were successfully completed robotically. Median (IQR) operative time was 96 minutes (78–126 minutes), estimated blood loss 200 cc (100–300 cc), and two patients (5.8%) had a blood transfusion. Table 3 describes postoperative complications: seven complications (20.5%) were reported, most of them (6/7) being low grade (Clavien I or II). One patient had a bladder neck contracture and was treated with endoscopic incision (Clavien IIIa). Median (IQR) specimen weight was 76 g (58–100 g). A small focus of prostate cancer Gleason 3+3 was identified in one case and the patient is currently under active surveillance with no evidence of disease progression.
Table 1.
Patient characteristics.
| Patients | 34 |
| Average age (y) | 68 (8.5) |
| Mean BMI (IQR) | 27.5 (23–30) |
| Abdominal surgery | 14 (41.2) |
| Bladder stones | 2 (4.88) |
| Indwelling urethral catheter | 12 (35.3) |
| Median prostate volume (IQR), cc | 117 (99–146) |
| Median IPSS (IQR) | 23.5 (22–27) |
| Mean PSA | 7.3 (9.5) |
| Median SHIM score (IQR) | 18 (12–23) |
Data are presented as n (%) or mean ± SD.
BMI, body mass index; IQR, interquartile range; SHIM, Sexual Health Inventory for Men.
Table 2.
Operative results.
| Median operative time (IQR) | 96 min (78–126) |
| Median EBL (IQR) | 200 min (100–300) |
| Blood transfusion | 2 (5.8) |
| Conversion | 0 |
| Length of hospital stay (IQR) | 2 d (1–4) |
| Median time-to-catheter removal (IQR) | 5 d (4–6) |
| Mean time-to-drain removal | 1.42 (0.47) |
Data are presented as n (%) or mean ± SD.
EBL, estimated blood loss; IQR, interquartile range.
Table 3.
Postoperative complications.
| n (%) | 7 (20.5) | Clavien | Management |
|---|---|---|---|
| Urinary infection (%) | 3 (8.8) | I | Antibiotics |
| Meatal stenosis | 1 (2.9) | I | Bedside dilation |
| Urinary retention | 2 (5.9) | II | Urinary catheter |
| Bladder neck contracture | 1 (2.9) | IIIa | Endoscopic incision |
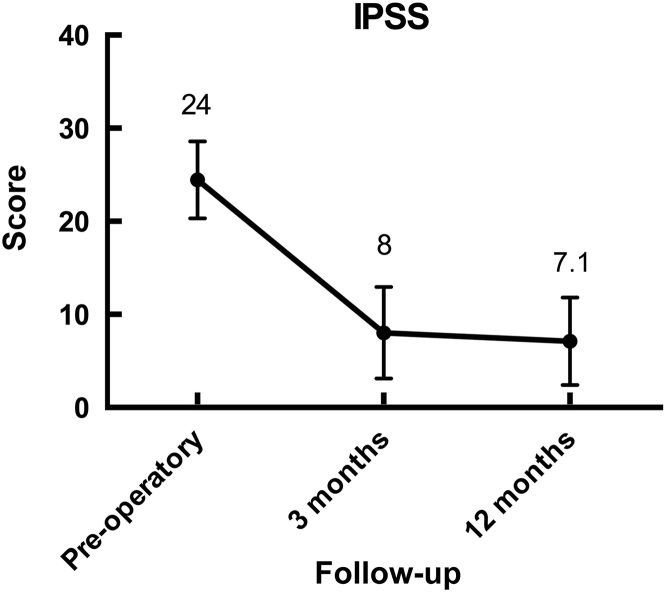
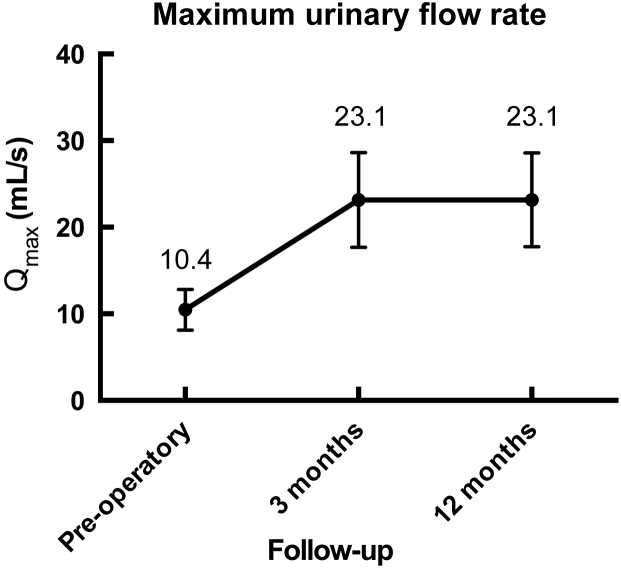
Urinary symptoms and Qmax improved significantly at 3 months, with the effectiveness was maintained during follow-up at 12 months (Fig. 4, Fig. 5). None of the patients had de-novo urinary incontinence or erectile dysfunction resulting from the procedure.
Fig. 4.
Significant decrease in urinary symptoms was seen after surgery. IPSS, International Prostate Symptom Score.
Fig. 5.
Significant increase in flow strength is seen after surgery.
4. Discussion
Open simple prostatectomy is still the standard for patients with LUTS caused by large prostatic adenomas.7 Nevertheless, this procedure is also associated with significant perioperative morbidity and a long convalescence. Laparoscopic simple prostatectomy has emerged as an alternative for OSP, offering lower blood loss, less pain, shorter postoperative catheterization period, and shorter hospital stay.8 However, it is technically a highly demanding procedure requiring a steep learning curve and advanced laparoscopic skills, thus it remained limited to a selected population of highly expert laparoscopic urologists. The robotic platform provides increased magnification, better visualization, and wristed instrumentation, and has been shown to alleviate the stiff learning curve associated with complex minimally invasive reconstructive procedures.9
The largest multi-institutional analysis of minimally invasive simple prostatectomy was recently published.10 Overall, 1,330 consecutive cases were analyzed, including 487 RASPs (36.6%) and 843 laparoscopic simple prostatectomies (63.4%). The median overall prostate volume was 100 mL (range, 89–128 mL) and estimated blood loss was 200 mL (range, 150–300 mL). Intraoperative transfusion was required in 3.5% of cases, intraoperative complications were reported in 2.2% of cases, and the conversion rate to open surgery was 3%. The median length of stay was 4 days (range, 3–5 days) and the overall postoperative complication rate was 10.6%, mostly of low grade (i.e., Clavien I or II). At a median follow-up of 12 months, significant improvement was observed for Qmax and IPSS (P < 0.001). Interestingly, a time trend comparing laparoscopic and robotic simple prostatectomy showed that while in 2006–2008 only 11% of the cases were done robotically—this changed to 74% during 2012–2014.
Several technical modifications to the standard open prostatectomy techniques have been described for RSP, probably reflecting a novel technique that is still under development. Sotelo et al's11 original report on RSP consisted in a horizontal cystotomy proximal to the vesicoprostatic junction. Coelho et al12 reported a technique in which a continuous vesicourethral anastomosis was performed as during a radical prostatectomy with optimal intraoperative and postoperative outcomes, but with the drawback that this results in complete exclusion of the prostatic bed from further transurethral access.
However, others perform the operation through a capsular incision mimicking the classic technique reported by Sutherland et al.13
Our technique is a combination of the above. We perform a longitudinal capsular and vesical incision as it gives complete access to the adenoma and facilitates enucleation. After excision of the adenoma and hemostasis, we performed plication of the posterior capsule as described by Coelho et al12 followed by a modification of their original vesicourethral anastomosis technique, which we perform only at the posterior leap. The advantage of our technique is that it provides hemostasis to the prostatic bed while allowing endoscopic access to the prostatic lodge if needed.
Our cohort represents one of the largest single center experiences in RASP reported to date. There was a significant improvement in the baseline IPSS and maximum urinary flow (Fig. 4, Fig. 5).
Two patients (5.9%) required a blood transfusion and the overall complication rate was 20.5% with only one patient requiring a secondary procedure. Median length of stay was 2 days (1–4 days). These results are comparable to two recent reports on RASP from centers of excellence from Europe and the United States reporting overall complications of 30% and 20%, and transfusion rates of 1.5% and 4%, respectively.14, 15 Conversely, a recent analysis of the United States Nationwide Inpatient Sample demonstrated a clinically significant transfusion rate of 21% among 34,418 OSPs performed between 1998 and 2010.16
In an attempt to amplify the benefits seen with minimally invasive surgery, Fareed et al17 reported the first series of single-port RSP in nine patients. Despite improvements in postoperative urine flow, perioperative complications were significant with two patients requiring blood transfusions and two others developing significant hematuria requiring endoscopic evacuation and coagulation. Although single-port RSP is feasible, the high complication rates indicate that further refinements are necessary before it can be more widely endorsed.
The main limitations of this study are its retrospective nature and lack of a control group. However, we wanted to show our experience with a procedure that is still under development and not widely performed even among robotic urologists. Single center reports are still necessary to increase knowledge about the feasibility and technical aspects of this novel technique. Only randomized trials comparing different treatments for large prostates will tell us where RSP really stands.
In conclusion, RASP with UVA is safe and feasible with low morbidity and excellent short-term functional results.
Conflicts of interest
All authors have no conflict of interest to declare.
References
- 1.Suer E., Gokce I., Yaman O., Anafarta K., Gogus O. Open prostatectomy is still a valid option for large prostates: a high-volume, single-center experience. Urology. 2008;72:90–94. doi: 10.1016/j.urology.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 2.McCullough T.C., Heldwein F.L., Soon S.J., Galiano M., Barret E., Cathelineau X. Laparoscopic versus open simple prostatectomy: an evaluation of morbidity. J Endourol. 2009;23:129–133. doi: 10.1089/end.2008.0401. [DOI] [PubMed] [Google Scholar]
- 3.Salonia A., Suardi N., Naspro R., Mazzoccoli B., Zanni G., Gallina A. Holmium laser enucleation versus open prostatectomy for benign prostatic hyperplasia: an inpatient cost analysis. Urology. 2006;68:302–306. doi: 10.1016/j.urology.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Elzayat E.A., Elhilali M.M. Holmium laser enucleation of the prostate (HoLEP): long-term results, reoperation rate, and possible impact of the learning curve. Eur Urol. 2007;52:1465–1471. doi: 10.1016/j.eururo.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 5.Vora A., Mittal S., Hwang J., Bandi G. Robot-assisted simple prostatectomy: multi-institutional outcomes for glands larger than 100 grams. J Endourol. 2012;26:499–502. doi: 10.1089/end.2011.0562. [DOI] [PubMed] [Google Scholar]
- 6.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McVary K.T., Roehrborn C.G., Avins A.L., Barry M.J., Bruskewitz R.C., Donnell R.F. Update on AUA guideline on the management of benign prostatic hiperplasia. J Urol. 2011;185:1793–1803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 8.Baumert H., Ballaro A., Dugardin F., Kaisary A.V. Laparoscopic versus open simple prostatectomy: a comparative study. J Urol. 2006;175:1691–1694. doi: 10.1016/S0022-5347(05)00986-9. [DOI] [PubMed] [Google Scholar]
- 9.Mottrie A., De Naeyer G., Schatteman P., Carpentier P., Sangalli M., Ficarra V. Impact of the learning curve on perioperative outcomes in patients who underwent robotic partial nephrectomy for parenchymal renal tumors. Eur Urol. 2010;58:127–132. doi: 10.1016/j.eururo.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 10.Autorino R., Zargar H., Mariano M.B., Sanchez-Salas R., Sotelo R.J., Chlosta P.L. Perioperative outcomes of robotic and laparoscopic simple prostatectomy: A European-American multi-institutional analysis. Eur Urol. 2015;68:86–94. doi: 10.1016/j.eururo.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 11.Sotelo R., Clavijo R., Carmona O., Garcia A., Banda E., Miranda M. Robotic simple prostatectomy. J Urol. 2008;179:513–515. doi: 10.1016/j.juro.2007.09.065. [DOI] [PubMed] [Google Scholar]
- 12.Coelho R.F., Chauhan S., Sivaraman A., Palmer K.J., Orvieto M.A., Rocco B. Modified technique of robotic-assisted simple prostatectomy: advantages of a vesico-urethral anastomosis. BJU Int. 2012;109:426–433. doi: 10.1111/j.1464-410X.2011.010401.x. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland D.E., Perez D., Weeks D. Robot-assisted simple prostatectomy for severe benign prostatic hyperplasia. J Endourol. 2011;25:641–644. doi: 10.1089/end.2010.0528. [DOI] [PubMed] [Google Scholar]
- 14.Pokorny M., Novara G., Geurts N., Dovey Z., De Groote R., Ploumidis A. Robot-assisted simple prostatectomy for treatment of lower urinary tract symptoms secondary to benign prostatic enlargement: surgical technique and outcomes in a high-volume robotic center. Eur Urol. 2015;68:451–457. doi: 10.1016/j.eururo.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Leslie S., Abreu A.L., Chopra S., Ramos P., Park D., Berger A.K. Transvesical robotic simple prostatectomy: initial clinical experience. Eur Urol. 2014;66:321–329. doi: 10.1016/j.eururo.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Parsons J.K., Rangarajan S.S., Palazzi K., Chang D. A national, comparative analysis of perioperative outcomes of open and minimally invasive simple prostatectomy. J Endourol. 2015;29:919–924. doi: 10.1089/end.2014.0879. [DOI] [PubMed] [Google Scholar]
- 17.Fareed K., Zaytoun O.M., Autorino R., White W.M., Crouzet S., Yakoubi R. Robotic single port suprapubictransvesicalenucleation of the prostate: initial experience. BJU Int. 2012;110:732–737. doi: 10.1111/j.1464-410X.2012.10954.x. [DOI] [PubMed] [Google Scholar]