Abstract
Background
To investigate the relationship between age, prostate specific antigen (PSA), and prostate volume (PV) in Indonesian men with histologically proven benign prostatic hyperplasia.
Methods
Data were generated from our BPH database from June 1994 until December 2013. Subjects were men with a minimum age of 40 years with chief complaint of LUTS or urinary retention, diagnosed with BPH. All patients underwent TRUS-guided prostate biopsy. Patients with PSA level >10 ng/mL were excluded from the study to exclude the possibility of occult prostate cancer. PV was measured with TRUS. Appropriate statistical tests were employed for data analysis.
Results
In all, 1638 patients were enrolled in our study. There was a statistically significant difference in PSA (P = 0.03) and PV (P < 0.0001) between age groups. Overall correlation between age, PSA, and PV were: i). Age and PV (r = 0.12, P < 0.0001); ii). Age and PSA (r = 0.07, P = 0.008); iii). PSA and PV (r = 0.26, P < 0.0001). Subgroup analysis in terms of indwelling catheter use versus without: i). Age 66.09 ± 8 years versus 65.38 ± 7.66 years (P = 0.158); ii). PSA 4.93 ± 2.62 ng/mL versus 4.68 ± 2.82 ng/mL (P = 0.038); iii). PV 47.58 ± 21.33 mL versus 41.43 ± 20.55 mL (P < 0.0001). Correlation between age, PSA, and PV in patients were similar in patients with and without indwelling catheter.
Conclusion
In Indonesian men with biopsy-proven BPH, both PV and PSA increased with ageing. Prostate volume was significantly correlated with PSA. Even though the results were weaker, these results are consistent with results in other sets of population. The results vary between different countries and thus, ethnicities. Indonesia is a populous a sociocultural and ethnically diverse country. Therefore, aside from PSA, age, and PV, when investigating men with BPH, ethnicity may also need to be taken into account.
Keywords: Age, Benign prostatic hyperplasia, Indonesian men, Prostate specific antigen, Prostate volume
1. Introduction
Benign prostatic hyperplasia (BPH) is a common progressive disease in the male aging population.1 Although aging and androgens are established risk factors, the cause of BPH remains uncertain.2, 3 Several mechanisms were hypothesized to be involved in the progression of BPH including hormonal or vascular alterations, inflammation, epithelial/stromal interactions, and luminal/epithelial cell interactions.2, 3
In the aging male, there is significant tissue remodeling taking place within the prostate. It was postulated that prostate growth is the result of a disturbed balance between apoptotic and proliferative activities with a net reduction in apoptotic activity. Histologic analysis showed a decreased apoptotic activity in glandular and basal epithelial cells of the prostate.2, 3, 4 Thus, with increasing age there is a tendency of increasing prostate volume (PV).
Prostate-specific antigen (PSA) is a widely used tumor marker for prostate cancer.5, 6 Although it is well known that PSA is prostate specific, it is not a disease-specific biomarker. Several studies have examined the relationship between PSA and PV.5, 7, 8, 9 These studies consistently showed a positive correlation between PSA level and PV. However, these results were derived from Western and East Asian populations, and thus may not accurately reflect the conditions in an Indonesian population. Differences in ethnicity and geographical factors may exert differences in BPH characteristics in men.10, 11 The exact relationship between age, serum PSA, and PV in Indonesian men with histologically proven BPH has yet to be established. Thus, the aim of this study was to investigate the relationship between age, PSA level, and PV in Indonesian men with histologically proven BPH.
2. Materials and methods
Data were generated from our BPH database from June 1994 until December 2013. These involved patients whose chief complaint was lower urinary tract symptoms or urinary retention who visited the Department of Urology of the “CiptoMangunkusumo” Hospital. The inclusion criteria were a minimum age of 40 years and a diagnosis of BPH (histopathologically proven). Patients with indwelling catheter were those with a history of urinary retention who failed trial without catheter with α blocker. All patients underwent standard clinical evaluation and PSA testing. Indication for prostate biopsy in our department was a PSA value of greater than or equal to 4 ng/mL or abnormal findings in digital rectal examination. Core biopsy was done with an 18-gauge needle, TRUS guided, using a spring-loaded biopsy gun (Bard Magnum). Our patients underwent a 6- to 12-core biopsy. Those patients with biopsy results of prostate cancer, atypical acinus, atypical small acinar proliferation, atypical adenomatous hyperplasia, and prostatic intraepithelial neoplasia were excluded from the study. Those who consumed 5α-reductase inhibitors and those with a PSA level of greater than 10 ng/mL (in order to avoid the possibility of occult prostate cancer) were also excluded from the study.
Patients were divided into three age groups: 60 years of age or younger, between 61 and 69 years of age, and 70 years of age and older. Based on the measurements obtained using TRUS, PV was calculated using the following formula: PV = height × width × length × 0.52. PV was categorized into Less than 30 mL, 31–40 mL, 41–50 mL, 51–100 mL, and greater than 100 mL.12
Descriptive statistics were used to characterize all variables. Prior to statistical analysis, numerical data were log-transformed for normalization. One-way analysis of variance (ANOVA) test and independent t test were used to analyze the differences in numerical data (age, PV, and PSA) among the different age groups and catheter use groups. Pearson's test for correlation was used to analyze the linear correlation between age, PSA, and PV. A P value of less than 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics (IBM Corp., New York, United States; www.ibm.com/SPSS_Statistics) version 20.
3. Results
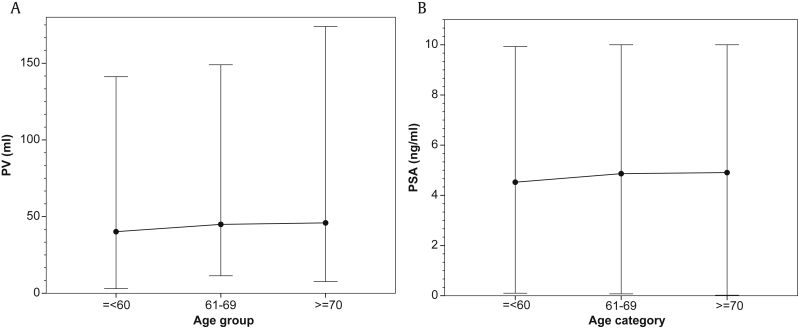
A total of 1,638 patients were included in our study. The characteristics of these patients are presented in Table 1. The median (range) PSA and PV in age groups ≤ 60 years, 61–69 years, and ≥ 70 years were 4.29 (0.1–9.93) ng/mL and 30.68 (3–141.29) mL, 4.61 (0.07–10) ng/mL and 38.92 (11.4–149) mL, and 4.8 (0.02–10) ng/mL and 40.48 (3–174) mL, respectively. There was a statistically significant difference in PSA (P = 0.03, one-way ANOVA test) and PV (P < 0.001, one-way ANOVA test) between age groups. These results are illustrated in Figs. 1A and 1B.
Table 1.
Characteristics of 1638 patients.
| Age (y) | 65.67 ± 7.81 |
| Age group (y) | |
| ≤ 60 | 448 (27.4) |
| 61–69 | 664 (40.5) |
| ≥ 70 | 526 (32.1) |
| PSA (ng/mL) | 4.78 ± 2.74 |
| 0.02–10 | |
| PV (mL) | 43.93 ± 21.08 |
| 3–174 | |
| Indwelling catheter | |
| Yes | 666 (40.7) |
| No | 972 (59.3) |
Data are presented as n (%) or mean ± SD.
PSA, prostate-specific antigen; PV, prostate volume.
Fig. 1.
Median and range values, by age group. (A) Prostate volume (PV). (B) Prostate-specific antigen (PSA).
PSA was < 4 ng/mL in 715 (43.65%) patients. PV was ≤ 30 mL in 436 (26.6%) patients, 31–40 mL in 442 (27%), 41–50 mL in 296 (18.1%), 51–100 mL in 430 (26.3%), and > 100 mL in 34 (2.1%).
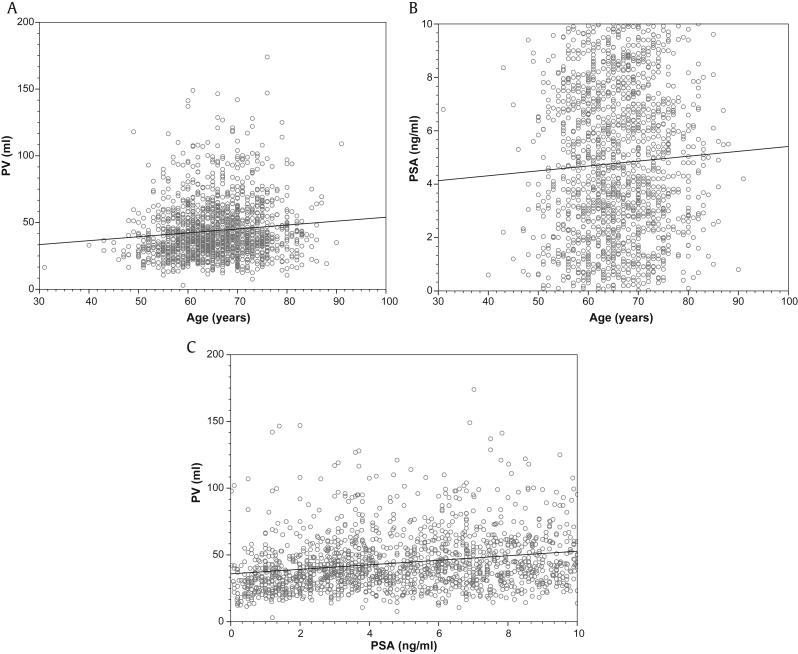
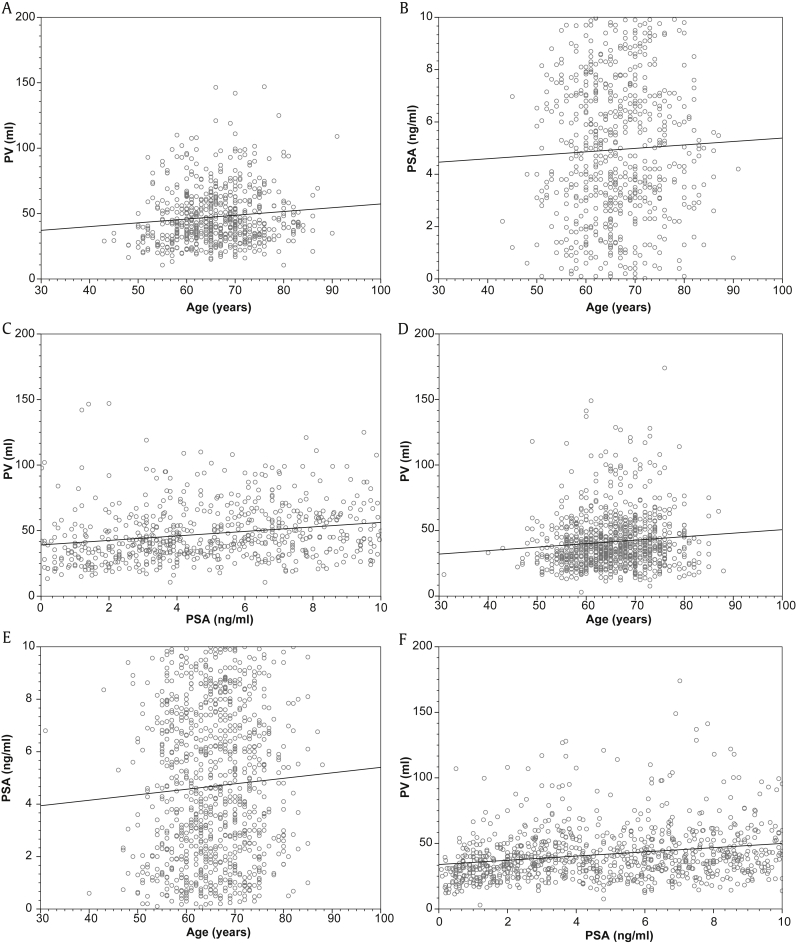
The correlation between age, PSA, and PV are illustrated in Fig. 2. The results of the subgroup analysis based on indwelling catheter use are presented in Table 2. The correlation between age, PSA, and PV in patients with and without indwelling catheter is illustrated in Fig. 3.
Fig. 2.
Pearson's correlation coefficient. (A) Between age and PV (r = 0.12, P < 0.001). (B) Between age and PSA (r = 0.07, P = 0.008). (C) Between PSA and PV (r = 0.26, P < 0.001). PSA, prostate-specific antigen; PV, prostate volume.
Table 2.
Subgroup analysis based on catheter usage.
| Variable | Indwelling catheter |
P | |
|---|---|---|---|
| Yes | No | ||
| Overall (y) | 666 (40.7) | 972 (59.3) | – |
| ≤ 60 | 171 (10.4) | 277 (16.9) | – |
| 61–69 | 267 (16.3) | 397 (24.2) | – |
| ≥ 70 | 228 (13.9) | 298 (18.2) | – |
| Age (y) | 66.09 ± 8 | 65.38 ± 7.66 | 0.158 a) |
| PSA (ng/mL) | 4.93 ± 2.62 | 4.68 ± 2.82 | 0.038 a) |
| PV (mL) | 47.58 ± 21.33 | 41.43 ± 20.55 | < 0.001 a) |
Data are presented as n (%) or mean ± SD.
PSA, prostate-specific antigen; PV, prostate volume.
Independent t test.
Fig. 3.
Pearson's correlation coefficient in patients with (A–C) and without (D–F) indwelling catheter. (A) Between age and PV (r = 0.13, P = 0.001). (B) Between age and PSA (r = 0.04, P = 0.267). (C) Between PSA and PV (r = 0.23, P < 0.001). (D) Between age and PV (r = 0.11, P = 0.001). (E) Between age and PSA (r = 0.08, P = 0.016). (F) Between PSA and PV (r = 0.28, P < 0.001). PSA, prostate-specific antigen; PV, prostate volume.
4. Discussion
BPH is age-related, and the prevalence increases with increasing age.13, 14 Among many factors that contribute to prostate enlargement in BPH, the two most well-known etiologic factors were aging and androgen.3 Consistent with the theory that aging is an etiologic factor of BPH, our results showed a trend of increasing median PV with advancing age, with the highest PV recorded in the ≥ 70 years group and the lowest PV in the ≤ 60 years group. This increasing PV with aging is accompanied with an increasing trend of PSA with age. This result is consistent with studies in Indian, South Korean, Taiwanese, and Swedish populations (Table 3).15, 16, 17, 18, 19 The correlation between age and PV in our study is the weakest (r = 0.12, P < 0.001). Consistent with results from other studies, PSA was positively correlated with age in our study. However, the correlation is the weakest (r = 0.07, P < 0.008) (Table 4) compared with results from other studies.15, 16, 17 PSA has been suggested as an estimator for PV.7, 20, 21 This is supported by the fact that prostate epithelial cells are responsible for circulating PSA, and several studies had documented a positive correlation between PSA and PV.8, 17, 22, 23 Hochberg et al8 reported a correlation coefficient of 0.33–0.41 in a series of white patients. Studies in Japanese, South Korean, and Indian patients showed a positive significant correlation coefficient between PSA and PV of 0.65, 0.41, and 0.78, respectively.15, 17, 23 Our study showed a similar but weaker correlation with a correlation coefficient of 0.26 (P < 0.001). The corresponding studies had a similar study design. The difference between the degree of correlation of age with PV and age with PSA is probably attributable to the ethnic or geographical factors that may influence prostatic growth.10, 11
Table 3.
Correlation of age and prostate volume in different countries from various studies.
Table 4.
Correlation of age and prostate-specific antigen in different countries from various studies.
We have performed a subanalysis in patients with and without indwelling catheter, comparing age, serum PSA, and PV. Patients with indwelling catheter tend to have a higher serum PSA (P = 0.038) and PV (P < 0.001). PSA is an organ-specific biomarker of the prostate. Disruption of the normal anatomic prostatic tissue results in an increase in serum PSA. This increase may result from malignant or benign prostatic diseases or prostatic manipulation including catheterization.24 It is also known that patients with higher PSA and PV tend have a greater risk of urinary retention and thus, catheterization.25, 26 Although aging is related to a higher risk for urinary retention,27 results from our study showed that there was no difference in age in terms of indwelling catheter use. In both groups, PV was significantly correlated with age and PSA. Both groups showed similar coefficient correlation values.
Elevated serum PSA is observed in men with BPH, prostatitis, or prostate cancer. In another study conducted earlier in our center, from January 1995 to December 2014, the overall prostate cancer detection rate was 28.7%. With specific levels of PSA 4.0–9.9 ng/mL and 10.0–19.9 ng/mL, the prostate cancer detection rates were 9.3% and 13.1%, respectively. Meanwhile, the overall detection rate for PSA level 4.0–20 ng/mL was 11.3%, which was similar to that reported in a study by Shahab et al.28, 29 The indications for prostate biopsy in our center were PSA > 4.0 ng/mL or abnormal digital rectal examination (DRE) findings. We believed that the possibility of unintentionally including patients with prostate cancer had been minimized as much as possible as we enrolled only individuals with a PSA of ≤ 10.0 ng/mL and histopathologically proven BPH from either a transrectal prostate biopsy or a transurethral resection of the prostate (TURP) specimen.
The limitation of our study was that the correlations between PSA, age, and PV found in this study were weaker than those found in similar studies from other centers. This difference could be attributable to methodological differences between this and other studies; or there was a fundamental difference in the biology of prostate (including PV and PSA) in various ethnicities.10, 30, 31 Indonesia, the most populous country in Southeast Asia, is a socioculturally and ethnically diverse country. The heterogeneity of our study population may account for the weak correlation coefficient between the investigated variables. Future studies may be necessary to explore the correlations in specific ethnicities. A prospective, multicenter, long-term longitudinal study is warranted to address this issue.
In Indonesian men with biopsy-proven BPH, both PV and PSA increased with aging. PV was significantly correlated with PSA. Even though the results were weaker, these results are consistent with the results in other population groups. Thus, aside from PSA, age, and PV, when investigating men with BPH, ethnicity may also need to be taken into account.
Conflicts of interest
None declared.
References
- 1.Fitzpatrick J.M. The natural history of benign prostatic hyperplasia. BJU Int. 2006;97:3–6. doi: 10.1111/j.1464-410X.2006.06097.x. [DOI] [PubMed] [Google Scholar]
- 2.Briganti A., Capitanio U., Suardi N., Gallina A., Salonia A., Bianchi M. Benign prostatic hyperplasia and its aetiologies. Eur Urol Suppl. 2009;8:865–871. [Google Scholar]
- 3.Untergasser G., Madersbacher S., Berger P. Benign prostatic hyperplasia: age-related tissue-remodeling. Exp Gerontol. 2005;40:121–128. doi: 10.1016/j.exger.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Konwar R., Chattopadhyay N., Bid H.K. Genetic polymorphism and pathogenesis of benign prostatic hyperplasia. BJU Int. 2008;102:536–544. doi: 10.1111/j.1464-410X.2008.07667.x. [DOI] [PubMed] [Google Scholar]
- 5.Pinsky P.F., Kramer B.S., Crawford E.D., Grubb R.L., Urban D.A., Andriole G.L. Prostate volume and prostate-specific antigen levels in men enrolled in a large screening trial. Urology. 2006;68:352–356. doi: 10.1016/j.urology.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick J.M. PSA measurement in the treatment of BPH. BJU Int. 2004;93:2–4. doi: 10.1111/j.1464-410x.2003.04632.x. [DOI] [PubMed] [Google Scholar]
- 7.Mochtar C.A., Kiemeney L.A., van Riemsdijk M.M., Barnett G.S., Laguna M.P., Debruyne F.M. Prostate-specific antigen as an estimator of prostate volume in the management of patients with symptomatic benign prostatic hyperplasia. Eur Urol. 2003;44:695–700. doi: 10.1016/s0302-2838(03)00384-1. [DOI] [PubMed] [Google Scholar]
- 8.Hochberg D.A., Armenakas N.A., Fracchia J.A. Relationship of prostate-specific antigen and prostate volume in patients with biopsy proven benign prostatic hyperplasia. Prostate. 2000;45:315–319. doi: 10.1002/1097-0045(20001201)45:4<315::aid-pros5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Park D.S., Hong J.Y., Hong Y.K., Lee S.R., Hwang J.H., Kang M.H. Correlation between serum prostate specific antigen level and prostate volume in a community-based cohort: large-scale screening of 35,223 Korean men. Urology. 2013;82:1394–1399. doi: 10.1016/j.urology.2013.07.071. [DOI] [PubMed] [Google Scholar]
- 10.Jin B., Turner L., Zhou Z., Zhou E.L., Handelsman D.J. Ethnicity and migration as determinants of human prostate size. J Clin Endocrinol Metab. 1999;84:3613–3619. doi: 10.1210/jcem.84.10.6041. [DOI] [PubMed] [Google Scholar]
- 11.Platz E.A., Kawachi I., Rimm E.B., Willett W.C., Giovannucci E. Race, ethnicity and benign prostatic hyperplasia in the health professionals follow-up study. J Urol. 2000;163:490–495. [PubMed] [Google Scholar]
- 12.Hedelin H., Johansson N., Stroberg P. Relationship between benign prostatic hyperplasia and lower urinary tract symptoms and correlation between prostate volume and serum prostate-specific antigen in clinical routine. Scand J Urol Nephrol. 2005;39:154–159. doi: 10.1080/00365590510007685. [DOI] [PubMed] [Google Scholar]
- 13.Bushman W. Etiology, epidemiology, and natural history of benign prostatic hyperplasia. Urol Clin North Am. 2009;36:403–415. doi: 10.1016/j.ucl.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Lee S.H., Lee J.Y. Current role of treatment in men with lower urinary tract symptoms combined with overactive bladder. Prostate Int. 2014;2:43–49. doi: 10.12954/PI.14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baruah S.K., Nath S.J., Puthenveetil R.T., Baruah S.J., Deka P.M., Bawri B. Correlation of age, prostate volume, serum prostate-specific antigen, and serum testosterone in Indian, benign prostatic hyperplasia patients. UroToday Int J. 2012;5:1–6. [Google Scholar]
- 16.Vesely S., Knutson T., Damber J.E., Dicuio M., Dahlstrand C. Relationship between age, prostate volume, prostate-specific antigen, symptom score and uroflowmetry in men with lower urinary tract symptoms. Scand J Urol Nephrol. 2003;37:322–328. doi: 10.1080/00365590310014760. [DOI] [PubMed] [Google Scholar]
- 17.Lee S.E., Chung J.S., Han B.K., Moon K.H., Hwang S.I., Lee H.J. Relationship of prostate-specific antigen and prostate volume in Korean men with biopsy-proven benign prostatic hyperplasia. Urology. 2008;71:395–398. doi: 10.1016/j.urology.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Chung B.H., Hong S.J., Cho J.S., Seong D.H. Relationship between serum prostate-specific antigen and prostate volume in Korean men with benign prostatic hyperplasia: a multicentre study. BJU Int. 2006;97:742–746. doi: 10.1111/j.1464-410X.2006.06016.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu C.C., Huang S.P., Li W.M., Wang C.J., Chou Y.H., Li C.C. Relationship between serum testosterone and measures of benign prostatic hyperplasia in aging men. Urology. 2007;70:677–680. doi: 10.1016/j.urology.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Roehrborn C.G., Boyle P., Gould A.L., Waldstreicher J. Serum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasia. Urology. 1999;53:581–589. doi: 10.1016/s0090-4295(98)00655-4. [DOI] [PubMed] [Google Scholar]
- 21.Bohnen A.M., Groeneveld F.P., Bosch J.L. Serum prostate-specific antigen as a predictor of prostate volume in the community: the Krimpen study. Eur Urol. 2007;51:1645–1652. doi: 10.1016/j.eururo.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 22.Morote J., Encabo G., Lopez M., de Torres I.M. Prediction of prostate volume based on total and free serum prostate-specific antigen: is it reliable? Eur Urol. 2000;38:91–95. doi: 10.1159/000020258. [DOI] [PubMed] [Google Scholar]
- 23.Tsukamoto T., Masumori N., Rahman M., Crane M.M. Change in International Prostate Symptom Score, prostrate-specific antigen and prostate volume in patients with benign prostatic hyperplasia followed longitudinally. Int J Urol. 2007;14:321–324. doi: 10.1111/j.1442-2042.2007.01596.x. [DOI] [PubMed] [Google Scholar]
- 24.Payne H., Cornford P. Prostate-specific antigen: an evolving role in diagnosis, monitoring, and treatment evaluation in prostate cancer. Urol Oncol. 2011;29:593–601. doi: 10.1016/j.urolonc.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Marberger M.J., Andersen J.T., Nickel J.C., Malice M.P., Gabriel M., Pappas F. Prostate volume and serum prostate-specific antigen as predictors of acute urinary retention. Combined experience from three large multinational placebo-controlled trials. Eur Urol. 2000;38:563–568. doi: 10.1159/000020356. [DOI] [PubMed] [Google Scholar]
- 26.Lieber M.M., Jacobsen S.J., Roberts R.O., Rhodes T., Girman C.J. Prostate volume and prostate-specific antigen in the absence of prostate cancer: a review of the relationship and prediction of long-term outcomes. Prostate. 2001;49:208–212. doi: 10.1002/pros.1136. [DOI] [PubMed] [Google Scholar]
- 27.Roehrborn C.G. Acute urinary retention: risks and management. Rev Urol. 2005;7(Suppl 4):S31–S41. [PMC free article] [PubMed] [Google Scholar]
- 28.Atmoko W., Hamid A.R.A.H., Mochtar C.A., Umbas R. Prostate cancer detection rate in Indonesian men. BJU Int. 2015;116:2–29. doi: 10.1016/j.asjsur.2017.01.001. Abstract #177. [DOI] [PubMed] [Google Scholar]
- 29.Shahab A.A., Soebadi D.M., Djatisoesanto W., Hardjowijoto S., Soetojo S., Hakim L. Prostate-specific antigen and prostate-specific antigen density cutoff points among Indonesian population suspected for prostate cancer. Prostate Int. 2013;1:23–30. doi: 10.12954/PI.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He D., Wang M., Chen X., Gao Z., He H., Zhau H.E. Ethnic differences in distribution of serum prostate-specific antigen: a study in a healthy Chinese male population. Urology. 2004;63:722–726. doi: 10.1016/j.urology.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 31.Lee S.E., Kwak C., Park M.S., Lee C.H., Kang W., Oh S.J. Ethnic differences in the age-related distribution of serum prostate-specific antigen values: a study in a healthy Korean male population. Urology. 2000;56:1007–1010. doi: 10.1016/s0090-4295(00)00837-2. [DOI] [PubMed] [Google Scholar]