Abstract
Background
While it is assumed that neuraxial analgesia and pain management may beneficially influence perioperative hemodynamics, few studies provided data quantifying such effects and none have assessed the potential contribution of the addition of a nerve block.
Questions/Purposes
This clinical trial compared the visual analog scale (VAS) scores and measurement of arterial tone using augmentation index of patients who received combined spinal–epidural (CSE) only to patients who received both CSE and lumbar plexus block.
Methods
After obtaining written consent, 92 patients undergoing total hip arthroplasty were randomized to receive either CSE or CSE with lumbar plexus block (LPB). Perioperative pain and arterial tone were measured using VAS scores and augmentation index (AI) respectively, at baseline and at various times postoperatively.
Results
After the exclusion of 2 patients, 44 patients received CSE alone and 46 patients received CSE and LPB. Patient demographics and perioperative characteristics were similar in both groups. AI continuously decreased after placement of a CSE with or without LBP, beyond full resolution of neuraxial and peripheral blockade. Although the LPB group demonstrated a statistically significant reduction of VAS pain scores in the postanesthesia care unit (PACU; P < 0.05), overall, the addition of a LPB did not significantly reduce the AI when compared to the control group.
Conclusion
The addition of a LPB provided better pain control in the PACU but did not reduce the AI, compared to the control group. We conclude that the addition of a LPB may have limited ability to affect arterial tone in the presence of a continuous infusion of epidural analgesics. In summary, the addition of a LPB in patients undergoing total hip arthroplasty is clinically effective and provided better pain control, especially in the immediate postoperative period. The continuous decrease on the AI in both groups beyond the full resolution of the neuroaxial and LPB will require further studies.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-015-9477-1) contains supplementary material, which is available to authorized users.
Keywords: hip arthroplasty, arterial stiffness, vascular resistance, lumbar plexus block
Introduction
Pain causes activation of the autonomic nervous system, thus increasing vascular tone, which may potentially contribute to the risk of adverse perioperative cardiovascular events [12, 13]. It has been well established that regional anesthetic techniques are effective in reducing postoperative pain. As such, epidural coupled with or without peripheral nerve blocks has been widely utilized to provide optimal analgesia to patients [2, 20].
Although some authors suggest that this improved control of pain relates to superior cardiovascular outcomes [4, 11], few studies have actually provided data linking pain control to an improved hemodynamic profile and none have measured the effects on large vessel arterial tone, a major determinant of cardiac afterload [25]. Optimizing perioperative hemodynamics is of utmost importance in patients with limited cardiac reserve. In this context, patients undergoing total hip arthroplasty represent an especially important target for intervention, as they frequently present with significant cardiovascular compromise [16].
In an attempt to maximize pain control, it has become common practice at our institution to combine patient-controlled epidural analgesia with a single-injection lumbar plexus block (LPB) for patients undergoing total hip arthroplasty.
However, to date, it has been difficult and impractical to measure the effect of regional analgesia on arterial vascular tone. With the advent of new, non-invasive technology, we were able to design a study to address this question. Arterial tone or vascular stiffness can be measured with the Pulsecor® monitor using a simple blood pressure cuff which can measure changes in arterial pulse wave reflections. This allows calculation of the augmentation index (AI) as a measure of arterial tone [15, 17].
The goal of this study was to measure the effect of combined spinal–epidural (CSE) anesthesia with or without the addition of a LPB on arterial tone and pain. We hypothesized that (1) the use of regional anesthesia would decrease the arterial tone compared to baseline, (2) the addition of a LPB to CSE anesthesia alone would result in a further decrease in vascular tone, and (3) the addition of LBP would lead to superior pain control compared to CSE alone.
Patients and Methods
This randomized controlled clinical trial received approval from the Institutional Review Board of Hospital for Special Surgery (IRB #28098) [6]. Informed consent was obtained from all individual participants included in the study. Using Excel-based randomizing software, all treatment assignments were prepared prior to study commencement by unaffiliated research personnel. Randomized treatments were placed in sealed opaque envelopes and were opened only after the treating anesthesiologist obtained written informed consent from the patient. After verifying the randomized assignment, the treating anesthesiologist proceeded with administration of the control (CSE alone) or treatment (CSE with the addition of a LPB) anesthetic protocol. Study patients and data assessors remained blinded throughout the patients’ participation. Enrollment for primary outcome was completed on January 14, 2011.
Patients scheduled for primary total hip arthroplasty (THA) were eligible for participation if they were between the ages of 60 and 100 and could safely undergo neuraxial anesthesia and whose health status was rated as 1–3, as per the American Society of Anesthesiologists (ASA) Physical Classification System [1]. Exclusion criteria were patient refusal, patients not within IRB-approved age range, surgery other than primary THR, chronic opioid use (defined as daily use of opioids for more than 3 months), allergy to study medications, and contraindication to CSE anesthesia or LPB (history of lumbar spinal fusion, bleeding disorder, use of clinically relevant anticoagulant or antiplatelet medications, anatomic abnormalities, infection at a potential injection site).
Intraoperatively, all patients received standard monitoring, a radial arterial line, oxygen via nasal cannula, and sedation with 5 mg of intravenous midazolam. For CSE, all patients received an intrathecal injection consisting of 60 mg of 1.5% mepivacaine [24]. A 20-gauge epidural catheter was passed into the epidural space. Intraoperative sedation was provided with intravenous propofol (2–4 mg per kilogram body weight per hour), titrated to achieve sedation while maintaining adequate ventilation. As needed, the epidural was dosed with 2% lidocaine in 3-ml aliquots in order to achieve hypotensive anesthesia at a target mean arterial blood pressure of 55 mmHg [21]. During surgical closure, 4 mg of intravenous ondansetron was administered.
For blinding purposes, control patients were prepared as if they were receiving LPB, and a bandage was placed on the patients’ backs in the appropriate site for LPB.
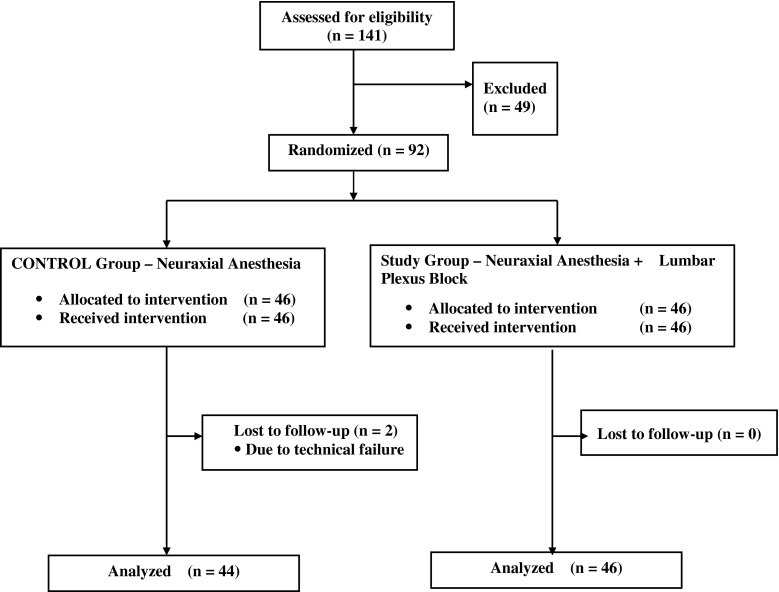
Ninety-two patients were enrolled after obtaining informed consent. Of those, 90 were included in the final data analysis. Forty-six patients received a CSE (control), and 46 patients received both CSE and LPB (treatment). Two patients from the control group had to be excluded due to equipment failure (see Fig. 1 for the CONSORT flow diagram). No adverse events attributable to either technique occurred. There was no significant difference in patient demographics, ASA status, preoperative use of antiinflammatory medication, length of surgery, blood loss, incidence of transfusion, or wound drainage (Table 1).
Fig. 1.
The CONSORT flow diagram is presented.
Table 1.
Perioperative characteristics
| Control (CSE only) | Treatment (CSE with LPB) | P value | |
|---|---|---|---|
| N | 44 | 46 | N/A |
| Sex (female, male) | 23 (52%), 21(48%) | 23 (50%), 23 (50%) | 0.829 |
| Age (years) | 70 (8.00) | 70 ± 11 | 0.933 |
| Height (cm) | 168 (18) | 166 ± 13 | 0.650 |
| Weight (kg) | 77 (22) | 78 (24) | 0.971 |
| BMI (kg/m2) | 26 (5) | 28 (5) | 0.580 |
| ASA (n ASA 1 (%), n ASA 2 (%), n ASA 3 (%)) | 1 (2%), 34 (77%), 9 (20%) | 1 (2%), 40 (87%), 5 (11%) | 0.707 |
| Use of preoperative anti-inflammatory drugs (meloxicam and/or dexamethasone) | 20 (45%) | 21 (46%) | 0.985 |
| Length of surgery (min) | 28 (19) | 31 (15) | 0.132 |
| Estimated blood loss (ml) | 150 (50) | 150 (50) | 0.498 |
| Patients with 1 or more postoperative blood transfusions | 11 (25%) | 14 (30%) | 0.565 |
| Postoperative drainage (ml) | 135 (300) | 180 (272) | 0.597 |
Perioperative characteristics of patients undergoing total hip arthroplasty by groups. Data presented as median (interquartile range) for continuous variables, and n (percent of group total) for categorical variables. POD-1/POD-2 = postoperative day 1/2. P values calculated using Mann–Whitney U test (continuous variables) and chi-square test (categorical variables)
CSE combined spinal–epidural anesthesia, LPB lumbar plexus block
The management of patients in the treatment group differed only in the addition of a LPB performed prior to the neuraxial anesthetic, but after sedation as previously described [3]. After sterile preparation and local anesthesia to the skin, a 21-gauge Stimuplex needle (B. Braun, Bethlehem, PA) was inserted with initial nerve stimulator settings of 2 mA and 2 Hz. After obtaining quadriceps stimulation at less than 1.0 mA, 30 ml of 0.5% bupivacaine was given in incremental doses of 5 ml.
Other than the study intervention (LPB), the postoperative analgesia regimen was identical for both groups. All patients received epidural analgesia consisting of a combination of 0.06% bupivacaine and 10 μg/ml hydromorphone. The patient-controlled epidural analgesia (PCEA) was started immediately upon arrival in the recovery room. Patients received a continuous infusion of 2 ml/h, with an additional bolus of 4 ml on demand and a lockout of 10 min with a 20 ml/h maximum. The epidural continuous infusion was switched to demand dose (basal rate 0 ml/h) by noon of postoperative day 1, the epidural catheter was removed at noon the same day.
Arterial tone was measured with the Pulsecor® monitor, using a simple blood pressure cuff by assessing changes in arterial pulse wave reflections as published previously [15, 17, 23]. The augmentation index (AI) was used as the primary measure of arterial tone. A blinded research assistant recorded three measurements at each time point of interest, which were the following:
In the holding area before transfer to the operating room (baseline)
Upon arrival in the operating room, but before sedation was administered (OR)
In the postanesthesia care unit (PACU) after resolution of the neuraxial motor blockade as assessed by foot flexion/extension (PACU-1)
Two hours after point 3 (PACU-2)
In the morning of postoperative day 1 (POD-1)
In the morning of postoperative day 2 (POD-2)
The final AI for each time point was the average of the three AI measurements. Pain scores at rest were assessed at all postoperative visits using a visual analog scale (VAS). The final time point was used to determine whether any measurement effects prevailed beyond the expected duration of the lumbar plexus block. To allow adjustment for confounding factors with potential influence on cardiovascular function and accuracy of the AI measurement, additional systemic parameters were obtained at every time point: Body temperature was measured using a Genius™ 3000A tympanic thermometer (Covidien, Mansfield, MA); a complete blood count was performed to determine potential influencing variables (white blood cell count, hemoglobin level, and hematocrit).
The primary outcome was the AI at PACU-1 in patients who received lumbar plexus block with CSE, compared to patients who received CSE alone. Secondary outcomes included changes of AI over time, as well as intergroup differences in pain scores, cumulative epidural medication, opioid consumption, incidence of postoperative itching, nausea, antiemetic consumption, body temperature, white blood cell count, hemoglobin, and hematocrit.
The sample size was computed to detect an effect size (a difference in mean AI between the two groups) at PACU-1 of 30% with an estimated variability of 50. Using an alpha level of 0.1, we calculated that a sample size of 45 patients per group will yield to 80% power.
Patient demographics and clinical outcomes are summarized with descriptive statistics. Results are expressed as the median (interquartile range), mean ± standard deviation (SD), or number (%) as appropriate and compared using chi-square test for categorical and Mann–Whitney U test for continuous variables. Independent two-group Mann–Whitney U test was also performed to compare the mean AI index for CSE and CSE+LPB groups at time point PACU-1.
Secondary analyses included a longitudinal assessment of VAS score differences over time and an analysis of trend of AI over time. Normality of the data was assessed using descriptive tools such as normality qq plots. Longitudinal analysis of VAS score and AI changes over time was performed using linear mixed-effects regression modeling. In each model, VAS or AI was the response variable with time as the repeated effect and patient as a random effect. Missing data was handled using multiple imputation. All P values were two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses were performed in R: A Language and Environment for Statistical Computing, R Development Core Team, Vienna, Austria, 2011.
Results
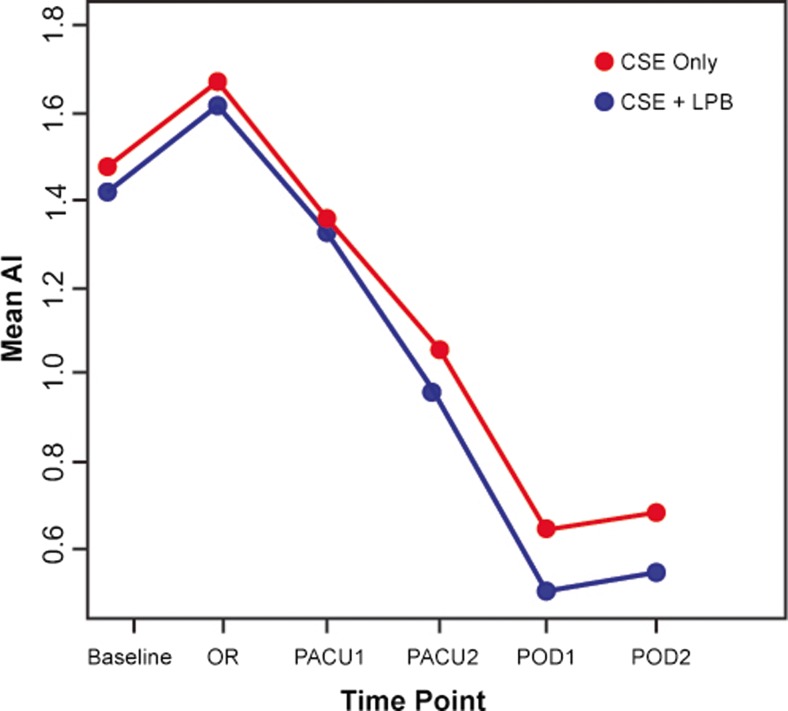
No significant difference in AI as measured by the Pulsecor® device became apparent between groups at time point PACU-1 (CSE only, 1.13 (interquartile range 0.94); CSE+LPB, 1.14 (interquartile range 0.93); P = 0.778). In the first linear mixed-effects regression model (longitudinal analysis of AI), time was associated with a highly significant impact as a repeated effect (P < 0.0001), while the influence of the group effect was not statistically significant (P = 0.542). Please refer to Fig. 2 for a presentation of temporal development of mean AI.
Fig. 2.
The temporal development of augmentation index (AI) across different time points. Baseline = holding area before OR; OR = in the OR, before block placement; PACU-1 = in the PACU, after resolution of residual motor blockade; PACU-2 = 2 h after PACU-1; POD 1 = morning of postoperative day 1; POD 2 = morning of postoperative day 2. CSE = control (combined spinal–epidural anesthesia only), CSE+LPB = treatment (combined spinal–epidural anesthesia and lumbar plexus block).
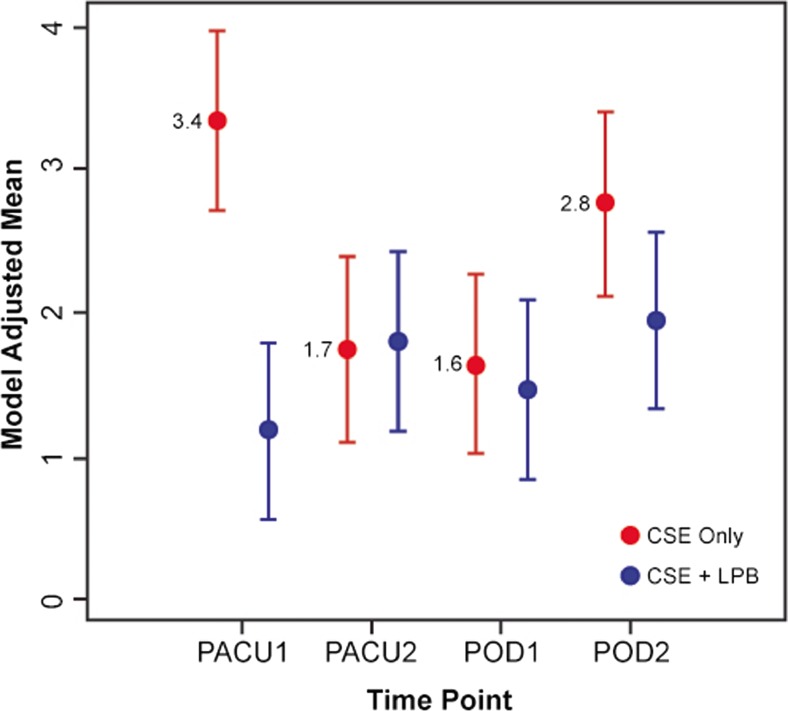
A longitudinal analysis of VAS indicates that the treatment group exhibited significantly lower pain scores compared to the control group, particularly at time point PACU-1 (group effect, P = 0.0077). Please refer to Table 2 for VAS scores. Figure 3 displays model-adjusted mean VAS scores for each of the two groups. There were no differences in total opioid consumption (both oral and via PCA) calculated as morphine equivalents, incidence of postoperative itching, nausea, or requirement of antiemetic medication across groups (Table 3). Moreover, the potential confounders (temperature, white blood cell count, hemoglobin, and hematocrit) are not statistically different.
Table 2.
Total opioid consumption
| Control (CSE only) | Treatment (CSE with LPB) | P value | |
|---|---|---|---|
| Total morphine equivalent—oral opioid use only (mg) | 47 ± 30 | 41 ± 25 | 0.251 |
| Total morphine equivalent—PCA use only (mg) | 23 ± 14 | 19 ± 10 | 0.113 |
| Total morphine equivalent—PCA and oral opioid use (mg) | 71 ± 37 | 60 ± 33 | 0.150 |
| Number of patients using antiemetics over 48 h following surgery | 19 (43%) | 20 (43%) | 0.977 |
| Number of patients using antipruitics over 48 h following surgery | 10 (23%) | 15 (33%) | 0.295 |
| Number of patients reporting nausea (POD-1) | 11 (25%) | 14 (30%) | 0.565 |
| Number of patients reporting nausea (POD-2) | 7 (16%) | 7 (15%) | 0.928 |
| Number of patients reporting itching (POD-1) | 14 (32%) | 14 (30%) | 0.887 |
| Number of patients reporting itching (POD-2) | 5 (11%) | 5 (11%) | 0.941 |
Consumption of opioids, antiemetics, antipruritics, and incidence of nausea and itching among patients undergoing total hip arthroplasty by groups. Data presented as mean ± standard deviation. P values calculated using Mann–Whitney U test (continuous variables) and chi-square test (categorical variables)
CE combined spinal–epidural anesthesia, LPB lumbar plexus block
Fig. 3.
Model-adjusted visual analog scale (VAS) pain score means for the two groups. CSE = combined spinal–epidural anesthesia, LPB = lumbar plexus block. Time points: PACU-1 = in the PACU, after resolution of residual motor blockade; PACU-2 = 2 h after PACU-1; POD 1 = morning of postoperative day 1; POD 2 = morning of postoperative day 2.
Table 3.
Postoperative pain scores
| Control (CSE only, N = 44) | Treatment (CSE with LPB, N = 46) | P value | |
|---|---|---|---|
| PACU-1 | 2.75 (7.00) | 0.00 (2.50) | 0.0077 |
| PACU-2 | 2.00 (2.25) | 1.25 (3.00) | |
| Postoperative day 1 | 1.00 (2.50) | 1.00 (2.0) | |
| Postoperative day 2 | 2.50 (3.50) | 1.50 (2.0) |
This table details postoperative visual analog scale (VAS) pain scores at different time points. PACU-1 = PACU, after resolution of residual motor blockade; PACU-2 = 2 h after PACU-1; POD-1 = morning of postoperative day 1; POD-2 = morning of postoperative day 2. VAS scores are presented as median (interquartile range). P value calculated using linear mixed effects regression modeling
Discussion
In this study, we compared combined spinal–epidural (CSE) alone to CSE in conjunction with lumbar plexus block (LPB) in patients undergoing total hip arthroplasty. The specific aims were to assess whether (1) the use of regional anesthesia would decrease the arterial tone compared to baseline, (2) the addition of a LPB to neuraxial anesthesia alone would result in a further decrease in vascular tone, and (3) the addition of LBP would lead to superior pain control compared to LPB alone.
Our study is limited by a number of factors. First, we applied continuous epidural infusion in both groups, which represents standard of care at the study institution. Yet this may be the reason for reduced differences in AI found between groups by overriding the effects of the LPB on arterial tone. Presumably, a more pronounced difference would become apparent when comparing patients with neuraxial anesthesia to those receiving general anesthesia, respectively. Second, as pain scores were not our primary outcome, VAS sampling was not carried out at time points prior to surgery. Therefore, no assertions about comparative preoperative and postoperative pain scores can be made. However, as participants were randomly assigned to either treatment or control group, and as no differences in their demographics or surgical parameters become apparent, existence of a significant sampling bias is unlikely.
We found a significant postoperative drop in the vascular tone (AI) compared to the preoperative baseline reading. The decreasing trend lasted well beyond resolution of neuraxial and peripheral nerve blockade. Looking at the temporal development of AI in our sample, after an initial slight increase, a continuous decline can be observed starting at the first postoperative measurement, when the motor blockade had already worn off. This development may in part be attributable to a decrease in vascular tone triggered by neuraxial and/or regional anesthesia. Decrease in sympathetic outflow (through concomitant blockade of ganglia and nerves of the autonomic nervous system) is a well-studied effect of regional anesthetic techniques. This partial sympathectomy and subsequent vasodilatation was observed in spinal and epidural analgesia as well as after performance of peripheral nerve blocks; yet, whether there is an influence of different local anesthetic agents and concentrations is not fully understood [5, 8, 22].
Interestingly, AI reached its peak in the operating room, shortly before the procedure started, and it kept decreasing steadily until the last day of sampling on postoperative day two (POD-2), even after the neuraxial analgesia and the peripheral nerve block (in the intervention group) had worn off. This finding indicates postoperative changes in vascular tone outlasting the direct effects of sympathetic blockade and possibly prevailing for a much longer time than previously anticipated. Jans et al. recently found a high incidence of symptomatic postoperative orthostatic intolerance after fast-track total hip arthroplasty under spinal anesthesia, at 6 and 24 h postoperatively [10]. The authors controlled for a number of factors, possibly causing the observed poor cardiovascular response to orthostatic challenge during mobilization, among them hypovolemia, anemia, opioid administration, postoperative pain, and residual spinal blockade. However, none of these findings seems to sufficiently explain the frequent occurrence of orthostatic intolerance. Consequently, other factors might exist that exert significant influence on the reactivity of the vascular system, and subsequently on AI and orthostatic intolerance. On the one hand, surgery-related metabolic injury and increased postoperative discharge of pro-inflammatory cytokines are known to cause short-term reductions in arterial tone through immune-system-related mechanisms [18, 19]. On the other hand, psychological stress and anxiety preceding surgery follows a similarly decreasing pattern in most patients. Indeed, a study by Logan et al. linked increased psychological stress to increased arterial stiffness, measured using carotid–femoral pulse wave velocity sampling [14]. Although this study was not carried out in surgical patients, similar occurrences in a perioperative setting seem intuitive.
However, no further reduction of AI became apparent in those patients receiving a lumbar plexus block in addition to the neuraxial anesthesia. The resulting effect of the lumbar plexus block had a non-significant impact on the AI. This non-significant comparative intergroup difference in AI we observed in our sample can likely be attributed to the pronounced effect of neuraxial analgesia alone on arterial tone, overriding the impact of the LPB to a certain degree.
Furthermore, LPB did significantly reduce the pain scores in the recovery room compared to controls. Patients receiving a LPB in addition to CSE had lower pain levels when compared to controls, particularly in the early postoperative phase, when there is no other form af analgesia but the LPB. In the late postoperative phase, other forms of analgesia are administered. These results are consistent with numerous recent publications demonstrating the significance of peripheral regional anesthesia in total hip arthroplasty [7, 9]. Moreover, LPB can be a valuable additional approach to analgesia in patients where neuraxial anesthesia is contraindicated.
There is a theoretical increase on the fall rate on the patients with LP; this can be avoided with a careful assessment of the strength by the physical therapist before each session. In fact, we did not have any falls during the study.
In summary, the addition of a LPB in patients undergoing total hip arthroplasty provided better pain control, especially in the immediate postoperative period. However, the highly significant decline of AI affected both groups equally. Given the known sympatholytic effects of regional anesthesia, further research could focus on the comparative impact of general anesthesia or intravenous analgesia versus regional anesthesia on AI to identify potential benefits of the latter. Furthermore, as AI continued to decrease after the sympathetic blockade had resolved and probably even further beyond our last measurement, research into other factors potentially influencing vascular reactivity could serve to clarify mechanisms involved in the development of postoperative dizziness and other symptoms of orthostatic intolerance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
Acknowledgments
We thank Ms. Tara Thompson, RN, BN, for her help with late-night follow-up examinations.
Compliance with Ethical Standards
ᅟ
Conflict of Interest
Enrique A. Goytizolo, MD, Ottokar Stundner, MD, Sandra Hurtado Rúa, PhD, Dorothy Marcello, BA, Valeria Buschiazzo, Ansara M Vaz, MD and Stavros G Memtsoudis, MD, PhD have declared that they have no conflict of interest.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent
Informed consent was obtained from all patients for being included in the study.
Funding
This study was supported with funds from the Research and Education Fund of the Department of Anesthesiology at the Hospital for Special Surgery. Dr. Sandra Hurtado Rúa was partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1- RR024996).
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Level of Evidence: Therapeutic study, level II.
Work performed at Hospital for Special Surgery.
References
- 1.American Society of Anesthesiologists. ASA physical status classification system. http://www.asahq.org/Home/For-Members/Clinical-Information/ASA-Physical-Status-Classification-System.
- 2.Block BM, Liu SS, Rowlingson AJ, et al. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA. 2003;18:2455–2463. doi: 10.1001/jama.290.18.2455. [DOI] [PubMed] [Google Scholar]
- 3.Capdevila X, Coimbra C, Choquet O. Approaches to the lumbar plexus: success, risks, and outcome. Reg Anesth Pain Med. 2005;2:150–6213. doi: 10.1016/j.rapm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 4.de Oliveira RM, Tenorio SB, Tanaka PP, et al. Control of pain trough epidura328 l block and incidence of cardiac dysrhythmias in postoperative period of thoracic and major abdominal surgical procedures: a comparative study. Rev Bras Anestesiol. 2012;1:10–18. doi: 10.1016/S0034-7094(12)70098-3. [DOI] [PubMed] [Google Scholar]
- 5.Ginosar Y, Weiniger CF, Kurz V, et al. Sympathectomy-mediated vasodilatation: a randomized concentration ranging study of epidural bupivacaine. Can J Anaesth. 2009;3:213–221. doi: 10.1007/s12630-008-9036-z. [DOI] [PubMed] [Google Scholar]
- 6.Goytizolo E, Marcello D. Hospital for special surgery clinical trial registry: effect of optimal regional analgesia on arterial tone after total hip arthroplasty. http://www.hss.edu/clinical-trials_arthroplasty-hip-arterial-tone-optimal-regional-analgesia.asp. Accessed July 2012.
- 7.Horlocker TT. Pain management in total joint arthroplasty: a historical review. Orthopedics. 2010;9(Suppl):14–19. doi: 10.3928/01477447-20100722-65. [DOI] [PubMed] [Google Scholar]
- 8.Ilfeld BM. Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg. 2011;4:904–925. doi: 10.1213/ANE.0b013e3182285e01. [DOI] [PubMed] [Google Scholar]
- 9.Ilfeld BM, Mariano ER, Madison SJ, et al. Continuous femoral versus posterior lumbar plexus nerve blocks for analgesia after hip arthroplasty: a randomized, controlled study. Anesth Analg. 2011;4:897–903. doi: 10.1213/ANE.0b013e318212495b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jans O, Bundgaard-Nielsen M, Solgaard S, et al. Orthostatic intolerance during early mobilization after fast-track hip arthroplasty. Br J Anaesth. 2012;3:436–443. doi: 10.1093/bja/aer403. [DOI] [PubMed] [Google Scholar]
- 11.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;6:630–641. doi: 10.1016/S0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 12.Ledowski T, Reimer M, Chavez V, et al. Effects of acute postoperative pain on catecholamine plasma levels, hemodynamic parameters, and cardiac autonomic control. Pain. 2012;4:759–764. doi: 10.1016/j.pain.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Ledowski T, Stein J, Albus S, et al. The influence of age and sex on the relationship between heart rate variability, haemodynamic variables and subjective measures of acute post-operative pain. Eur J Anaesthesiol. 2011;6:433–437. doi: 10.1097/EJA.0b013e328343d524. [DOI] [PubMed] [Google Scholar]
- 14.Logan JG, Barksdale DJ, Carlson J, et al. Psychological stress and arterial stiffness in Korean Americans. J Psychosom Res. 2012;1:53–58. doi: 10.1016/j.jpsychores.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowe A, Harrison W, El-Aklouk E, et al. Non-invasive model342 based estimation of aortic pulse pressure using suprasystolic brachial pressure waveforms. J Biomech. 2009;13:2111–2115. doi: 10.1016/j.jbiomech.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Memtsoudis SG, Ma Y, Gonzalez Della Valle A, et al. Demographics, outcomes, and risk factors for adverse events associated with primary and revision total hip arthroplasties in the United States. Am J Orthop. 2010;8:E72–E77. [PubMed] [Google Scholar]
- 17.Munir S, Guilcher A, Kamalesh T, et al. Peripheral augmentation index defines the relationship between central and peripheral pulse pressure. Hypertension. 2008;1:112–118. doi: 10.1161/HYPERTENSIONAHA.107.096016. [DOI] [PubMed] [Google Scholar]
- 18.Ni Choileain N, Redmond HP. Cell response to surgery. Arch Surg. 2006;11:1132–1140. doi: 10.1001/archsurg.141.11.1132. [DOI] [PubMed] [Google Scholar]
- 19.Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;2:258–261. doi: 10.3349/ymj.2012.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;1:248–257. doi: 10.1213/01.ANE.0000181289.09675.7D. [DOI] [PubMed] [Google Scholar]
- 21.Sharrock NE, Salvati EA. Hypotensive epidural anesthesia for total hip arthroplasty: a review. Acta Orthop Scand. 1996;1:91–107. doi: 10.3109/17453679608995620. [DOI] [PubMed] [Google Scholar]
- 22.Stevens RA, Frey K, Liu SS, et al. Sympathetic block during spinal anesthesia in volunteers using lidocaine, tetracaine, and bupivacaine. Reg Anesth. 1997;4:325–331. doi: 10.1016/S1098-7339(97)80006-5. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson IB, MacCallum H, Flint L, et al. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000; 263–70. [DOI] [PMC free article] [PubMed]
- 24.YaDeau JT, Liguori GA, Zayas VM. The incidence of transient neurologic symptoms after spinal anesthesia with mepivacaine. Anesth Analg. 2005;3:661–665. doi: 10.1213/01.ane.0000167636.94707.d3. [DOI] [PubMed] [Google Scholar]
- 25.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;5:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)